High Gradient Magnetic Separation of Pure Gd2O3 Particles from Pure La2O3 Particles
Abstract
1. Introduction
2. Experiment
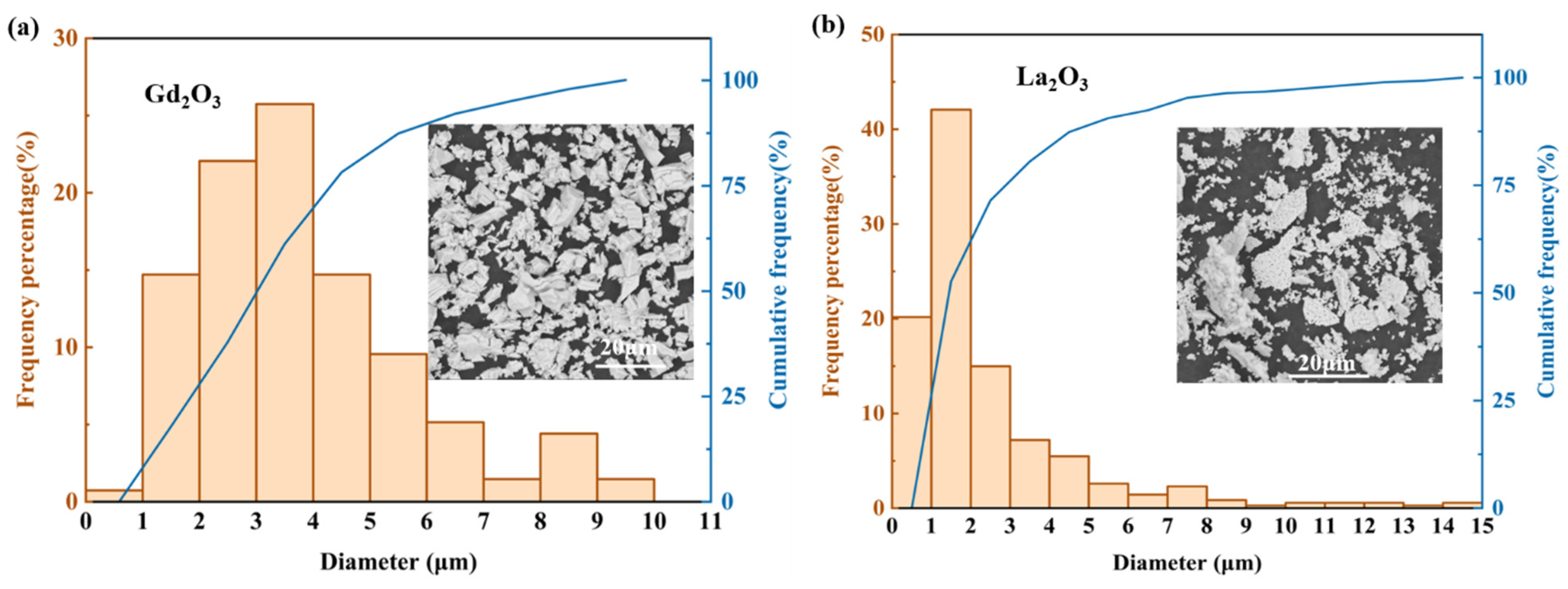
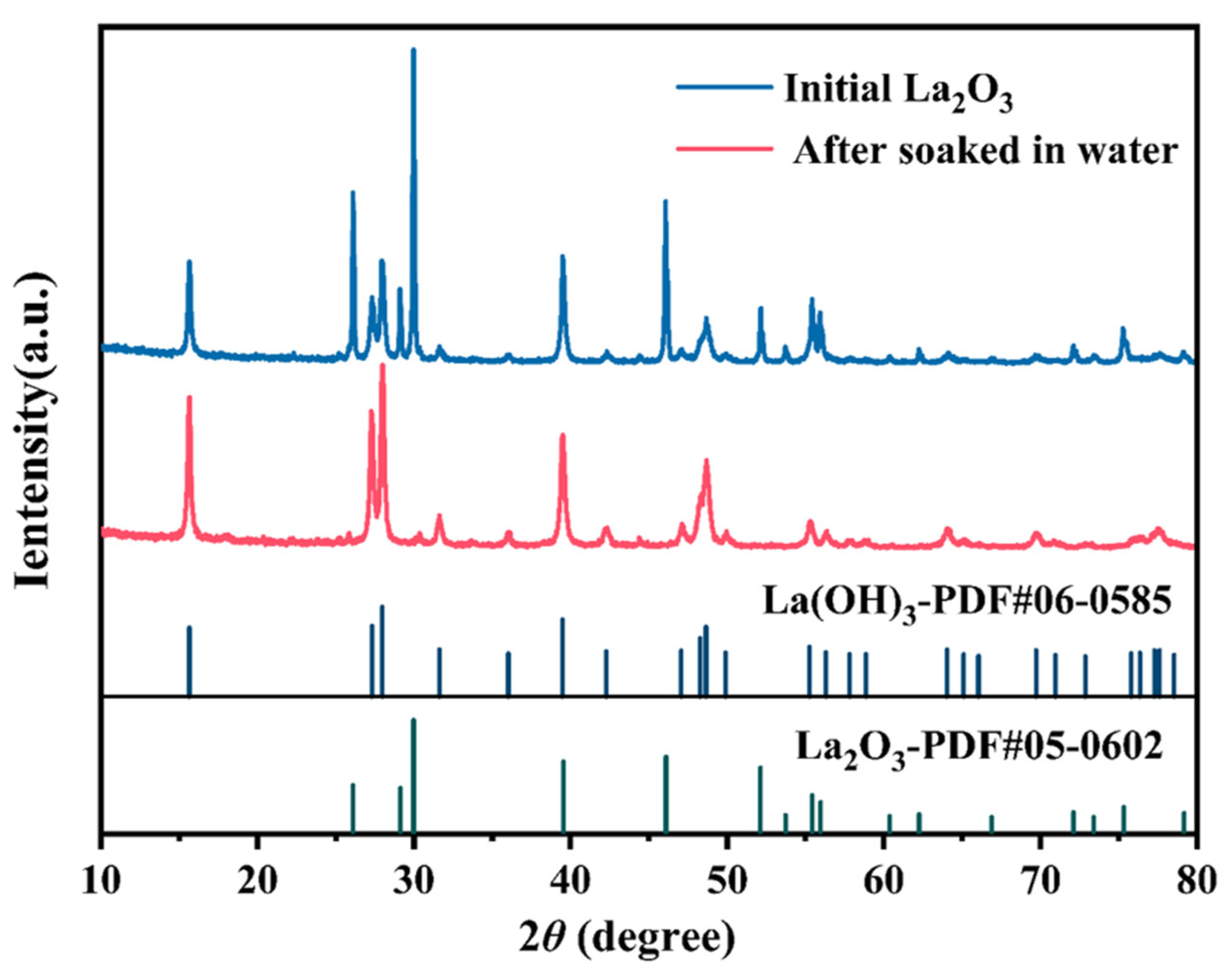
2.1. Materials and Their Characteristics
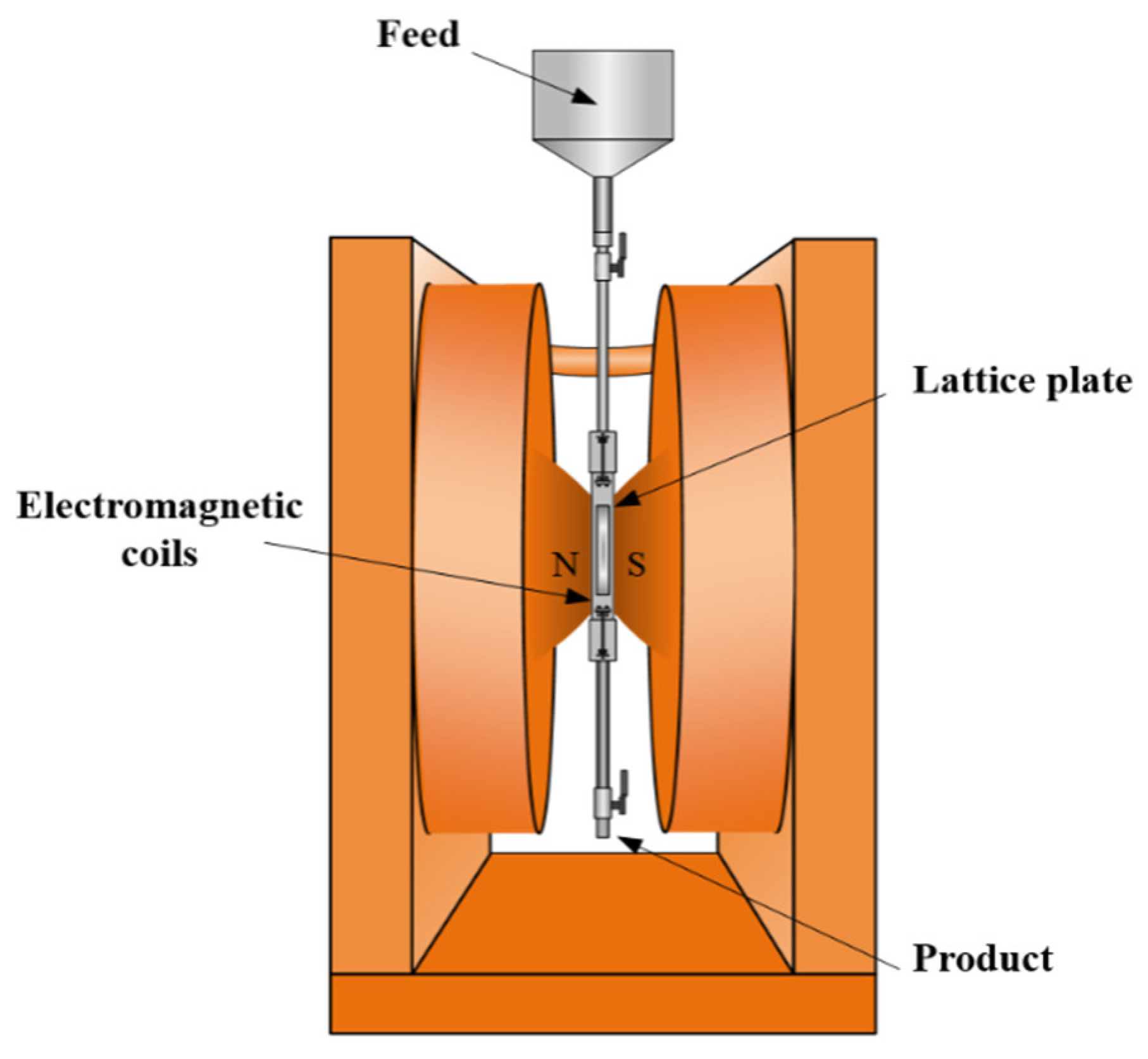
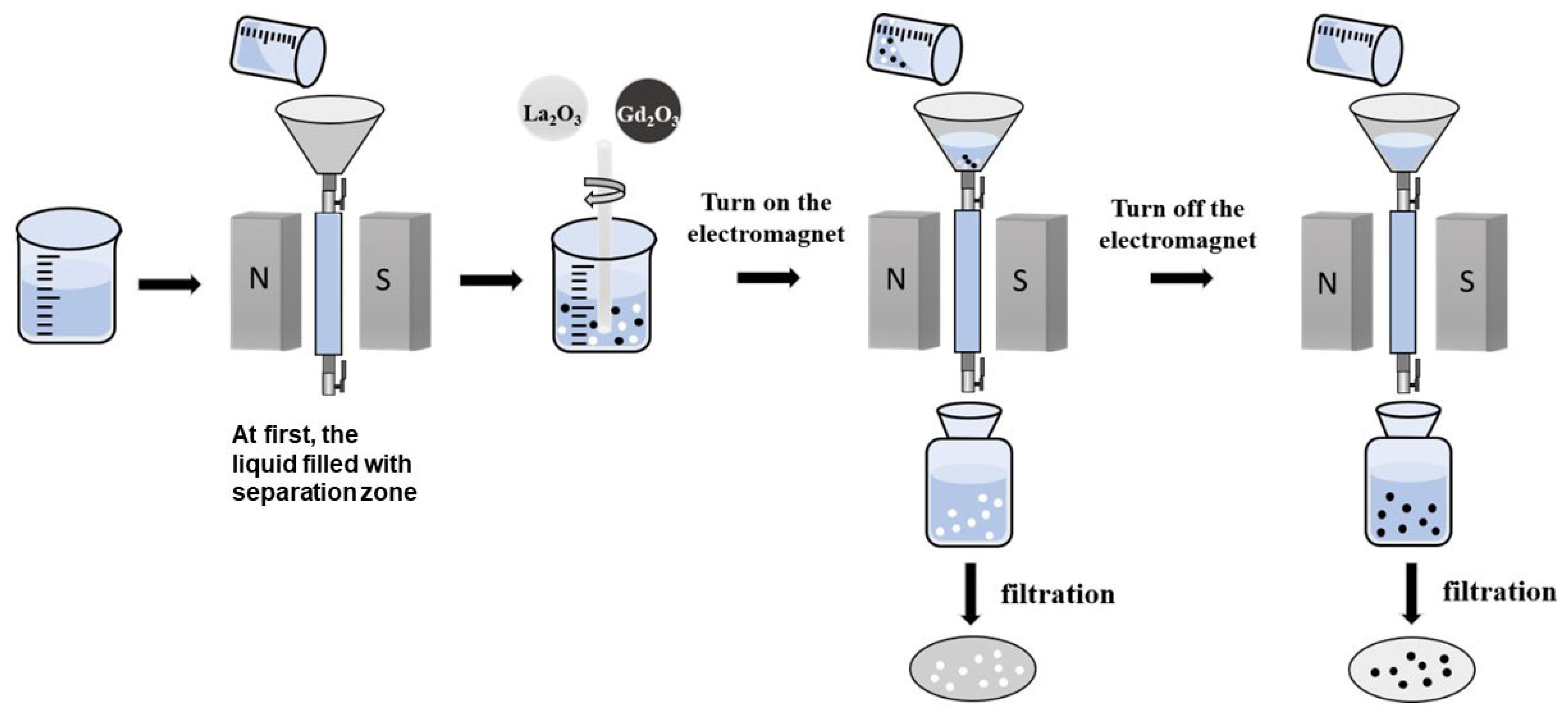
2.2. Methods
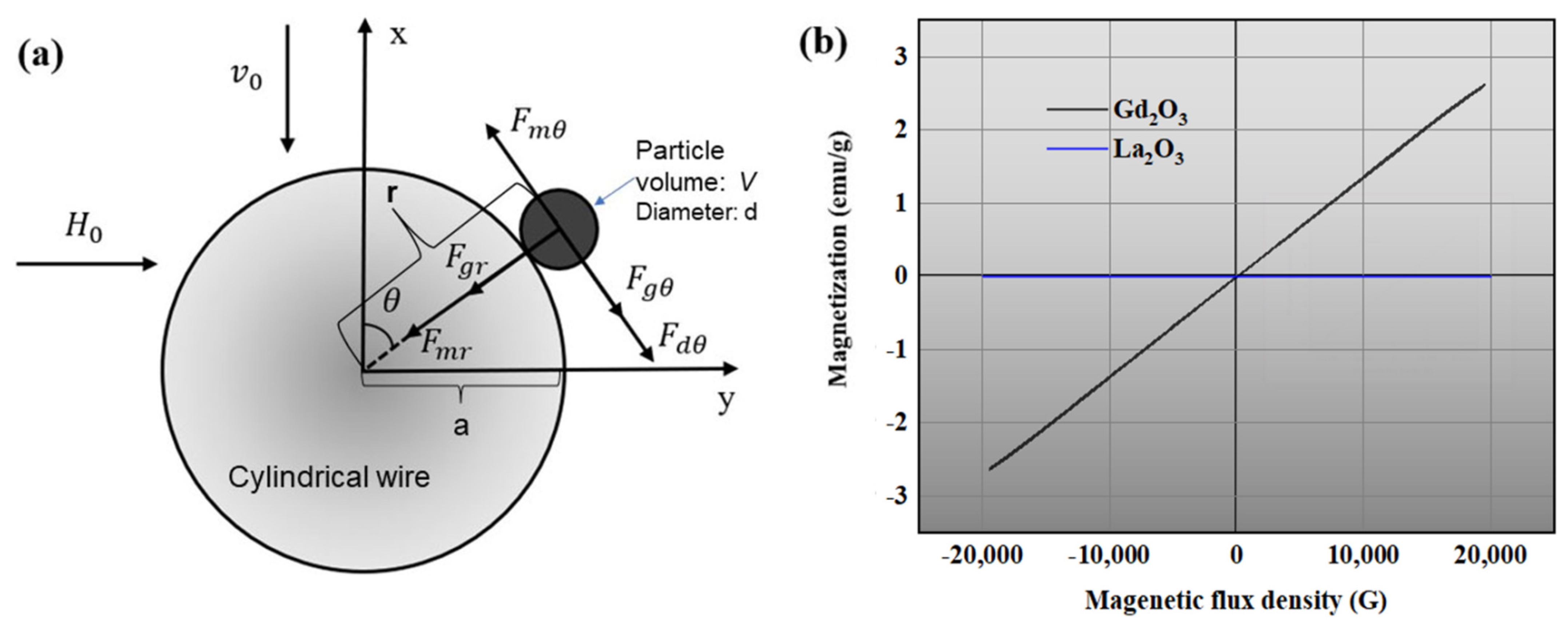
2.3. Theoretical Basis
2.3.1. Magnetic Force
2.3.2. Fluid Drag Force
2.3.3. Gravitational Force
2.3.4. Surface Force
3. Results and Discussion
3.1. Ethanol as a Fluid Medium
3.2. Different Dispersants
3.2.1. Magnetic Separation Results, Zeta Potential, and Infrared Analysis
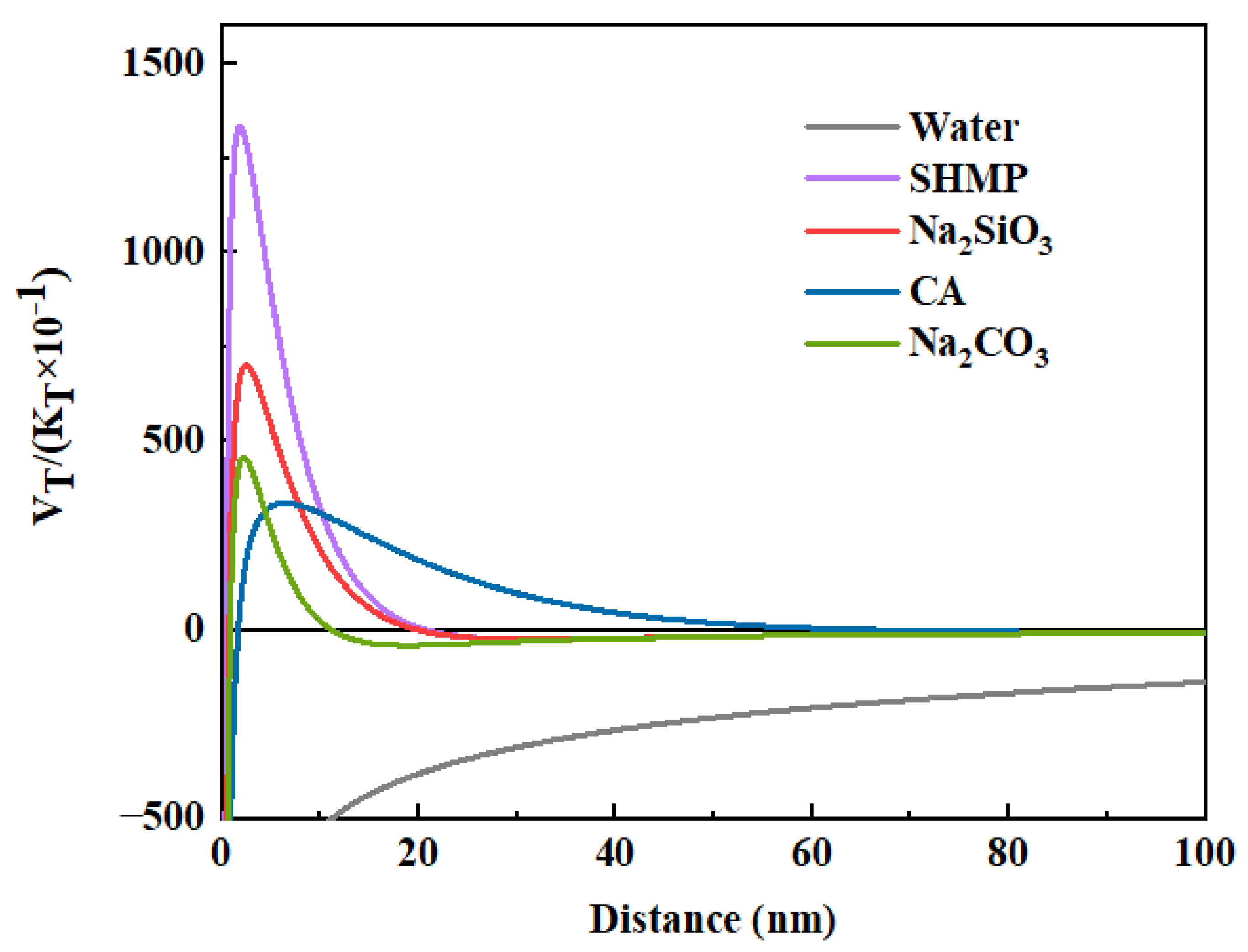
3.2.2. DLVO Theory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheisson, T.B.; Eric, J. Rare earth elements: Mendeleev’s bane, modern marvels. Science 2019, 363, 489–493. [Google Scholar] [CrossRef]
- Archambo, M.S.; Kawatra, S.K. Extraction of Rare Earths from Red Mud Iron Nugget Slags with Oxalic Acid Precipitation. Miner. Process. Extr. Metall. Rev. 2022, 43, 656–663. [Google Scholar] [CrossRef]
- Xi, L.; Gao, J.T.; Du, Y.; Guo, Z.C. A novel method of selectively enriching and separating rare earth elements from rare-earth concentrate under super gravity. Miner. Eng. 2019, 133, 27–34. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhang, L.Q.; Lei, P.X.; Chi, M.Y. Rare earth extraction and separation from mixed bastnaesite-monazite concentrate by stepwise carbochlorination-chemical vapor transport. Metall. Mater. Trans. B 2002, 33, 661–668. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, K.M.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Gunn, A.G. (Ed.) Critical Metals Handbook; John Wiley & Sons: London, UK, 2014; pp. 312–339. ISBN 978-0-470-67171-9. [Google Scholar]
- Jiang, Y.; Shibayama, A.; Liu, K.; Fujita, T. A hydrometallurgical process for extraction of lanthanum, yttrium and gadolinium from spent optical glass. Hydrometallurgy 2005, 76, 1–9. [Google Scholar] [CrossRef]
- Ashour, R.M.; El-Sayed, R.; Abdel-Magied, A.F.; Abdel-Khalek, A.A.; Ali, M.; Forsberg, K.; Uheida, A.; Muhammed, M.; Dutta, J. Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: Kinetic and thermodynamic studies. Chem. Eng. J. 2017, 327, 286–296. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wu, J.M.; Liu, X.Y.; Wang, W.; Li, Z.W.; Xu, R.J.; Ding, Y.; Wang, J.L. Recovery of neodymium, dysprosium, and cobalt from NdFeB magnet leachate using an unsymmetrical dialkylphosphinic acid extractant, INET-3. J. Rare Earths 2020, 38, 1114–1118. [Google Scholar] [CrossRef]
- Kuang, S.T.; Zhang, Z.F.; Li, Y.L.; Wei, H.Q.; Liao, W.P. Synergistic extraction and separation of rare earths from chloride medium by the mixture of HEHAPP and D2EHPA. Hydrometallurgy 2017, 174, 78–83. [Google Scholar] [CrossRef]
- Batchu, N.K.; Vander, H.T.; Banerjee, D.; Koen, B. Separation of rare-earth ions from ethylene glycol (+LiCl) solutions by non-aqueous solvent extraction with Cyanex 923. RSC Adv. 2017, 7, 45351–45362. [Google Scholar] [CrossRef]
- Na, S.; Huang, K. Separation of rare earths using solvent extraction consisting of three phases. Hydrometallurgy 2019, 188, 112–122. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths 2022, in press. [Google Scholar] [CrossRef]
- Cotruvo, J.A.; Featherston, E.R.; Mattocks, J.A.; Ho, J.V.; Laremore, T.N. Lanmodulin: A Highly Selective Lanthanide-Binding Protein from a Lanthanide-Utilizing Bacterium. J. Am. Chem. Soc. 2018, 140, 15056–15061. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cole, B.E.; Qiao, Y.; Bogart, J.A.; Cheisson, T.; Manor, B.C.; Carroll, P.J.; Schelter, E.J. Electro-kinetic Separation of Rare Earth Elements Using a Redox Active Ligand. Angew. Chem. 2017, 129, 13635–13639. [Google Scholar] [CrossRef]
- Balassone, G.; Manfredi, C.; Vasca, E.; Bianco, M.; Boni, M.; Di Nunzio, A.; Lombardo, F.; Mozzillo, R.; Marino, A.; Mormone, A.; et al. Recycling REEs from the Waste Products of Silius Mine (SE Sardinia, Italy): A Preliminary Study. Sustainability 2021, 13, 14000. [Google Scholar] [CrossRef]
- Ashour, R.M.; Samouhos, M.; Legaria, E.P.; Svärd, M.; Högblom, J.; Forsberg, K.; Palmlöf, M.; Kessler, V.G.; Seisenbaeva, G.A.; Rasmuson, C. DTPA-Functionalized Silica Nano- and Microparticles for Adsorption and Chromatographic Separation of Rare Earth Elements. ACS Sustain. Chem. Eng. 2018, 6, 6889–6900. [Google Scholar] [CrossRef]
- Hu, A.H.; Guo, J.J.; Pan, H.; Zuo, Z.W. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Am. Assoc. Adv. Sci. 2018, 361, 668–672. [Google Scholar] [CrossRef]
- Joris, B.; Binnemans, K. Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef]
- Svoboda, J. Magnetic Techniques for the Treatment of Materials; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Wills, B.A. Wills’ Mineral Processing Technology, 7th ed.; Napier-Munn, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 364–365. [Google Scholar]
- Zhang, X.; Tan, X.; Yi, Y.; Liu, W.; Li, C. Recovery of Manganese Ore Tailings by High-Gradient Magnetic Separation and Hydrometallurgical Method. JOM 2017, 69, 2352–2357. [Google Scholar] [CrossRef]
- Xu, B.; Wen, S.M.; Feng, Q.C.; Liu, J.; Lin, Y.L. Utilization of high-gradient magnetic separation–secondary grinding–leaching to improve the copper recovery from refractory copper oxide ores. Miner. Eng. 2019, 136, 77–80. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Wang, Y.H.; Lu, D.F. Effect of Matrix Shape on the Capture of Fine Weakly Magnetic Minerals in High-Gradient Magnetic Separation. IEEE Trans. Magn. 2016, 52, 7005111. [Google Scholar] [CrossRef]
- Gao, L.K.; Chen, Y. A study on the rare earth ore containing scandium by high gradient magnetic separation. J. Rare Earths 2010, 28, 622–626. [Google Scholar] [CrossRef]
- Yang, C.Q.; Li, S.Q.; Zhang, C.Q.; Bai, J.X.; Guo, Z.J. Application of Superconducting High Gradient Magnetic Separation Technology on Silica Extraction from Iron Ore Beneficiation Tailings. Miner. Process. Extr. Metall. Rev. 2018, 39, 1272–1276. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, G.Q.; Sun, Z.S.; Yan, G.H.; Hao, Y. Fine coal desulfurization and modeling based on high-gradient magnetic separation by microwave energy. Fuel 2018, 217, 434–443. [Google Scholar] [CrossRef]
- Li, S.Q.; Wang, M.F.; Zhu, Z.A.; Wang, Q.; Zhang, X.; Song, H.Q.; Cang, D.Q. Application of superconducting HGMS technology on turbid wastewater treatment from converter. Sep. Purif. Technol. 2012, 84, 56–62. [Google Scholar] [CrossRef]
- George, F.; Pankhurst, Q.A. Biomedical applications of high gradient magnetic separation: Progress towards therapeutic haeomofiltration. Biomed. Tech. 2015, 60, 393–404. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, J.; Lan, X.; Zhao, X.; Mou, H.; Mu, T. A strategy for the dissolution and separation of rare earth oxides by novel Brønsted acidic deep eutectic solvents. Green Chem. 2019, 21, 4748–4756. [Google Scholar] [CrossRef]
- Su, T.; Chen, T.; Zhang, Y.; Hu, P. Selective Flocculation Enhanced Magnetic Separation of Ultrafine Disseminated Magnetite Ores. Minerals 2016, 6, 86. [Google Scholar] [CrossRef]
- Hao, H.Q.; Li, L.X.; Yuan, Z.T.; Liu, J.T. Comparative effects of sodium silicate and citric acid on the dispersion and flotation of carbonate-bearing iron ore. J. Mol. Liq. 2018, 271, 16–23. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Role of sodium carbonate in scheelite flotation—A multi-faceted reagent. Miner. Eng. 2018, 129, 120–128. [Google Scholar] [CrossRef]
- Rumble, J.R. (Ed.) CRC Handbook of Chemistry and Physics, 101st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Zheng, X.Y.; Wang, Y.H.; Lu, D.F. A realistic description of influence of the magnetic field strength on high gradient magnetic separation. Miner. Eng. 2015, 79, 94–101. [Google Scholar] [CrossRef]
- Tsoutsou, D.; Scarel, G.; Debernardi, A.; Capelli, S.; Volkos, S.; Lamagna, L.; Schamm, S.; Coulon, P.; Fanciulli, M. Infrared spectroscopy and X-ray diffraction studies on the crystallographic evolution of La2O3 films upon annealing. Microelectron. Eng. 2008, 85, 2411–2413. [Google Scholar] [CrossRef]
- Jin, J.X.; Gao, H.M.; Chen, X.M.; Peng, Y.J. The separation of kyanite from quartz by flotation at acidic pH. Miner. Eng. 2016, 92, 221–228. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.C. Theory of the Stability of Lyophobic Colloids; Elsevier: New York, NY, USA, 1948. [Google Scholar]
- Derjaguin, B.V.; Landau, L. Theory of the Stability of Strongly Charged Lyophobic Sols and of the Adhesion of Strongly Charged Particles in Solutions of Electrolytes. Acta Physicochim. 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.H.; Hu, X.D.; Zhang, W.; Zeng, R.; Liu, P.D.; Zhang, H.Q. Synthesis and characteristics of nanorods of gadolinium hydroxide and gadolinium oxide. J. Alloys Compd. 2016, 664, 311–316. [Google Scholar] [CrossRef]



| Dispersant | Product * | Weight (g) | Grade (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| La2O3 | Gd2O3 | La2O3 | Gd2O3 | |||
| SHMP | M | 0.437 | 4.53 | 95.47 | 3.78 | 91.31 |
| NM | 0.615 | 92.71 | 7.29 | 96.22 | 8.69 | |
| Na2SiO3 | M | 0.8245 | 3.65 | 96.35 | 4.18 | 99.08 |
| NM | 0.8247 | 98.94 | 1.06 | 95.82 | 0.92 | |
| CA | M | 1.1 | 19.83 | 80.17 | 27.96 | 97.48 |
| NM | 0.603 | 96.11 | 3.89 | 72.04 | 2.52 | |
| Na2CO3 | M | 0.8345 | 5.43 | 94.57 | 5.63 | 94.39 |
| NM | 1.01 | 94.19 | 5.81 | 94.37 | 5.61 | |
| pH and Particle Size | SHMP | Na2SiO3 | CA | Na2CO3 | Water |
|---|---|---|---|---|---|
| Initial pH | 6.62 | 11.25 | 3.4 | 10.68 | 6.7 |
| pH after adding the oxide particle mixture | 10.94 | 11.25 | 6.75 | 10.59 | 7.11 |
| Size of particle mixture at pH = 11 (μm) | 1.21 | 2.51 | 1.91 | 2.37 | 3.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wu, Y.; Nhung, N.T.H.; He, C.; Chen, H.; Dodbiba, G.; Otsuki, A.; Fujita, T. High Gradient Magnetic Separation of Pure Gd2O3 Particles from Pure La2O3 Particles. Metals 2023, 13, 241. https://doi.org/10.3390/met13020241
Chen L, Wu Y, Nhung NTH, He C, Chen H, Dodbiba G, Otsuki A, Fujita T. High Gradient Magnetic Separation of Pure Gd2O3 Particles from Pure La2O3 Particles. Metals. 2023; 13(2):241. https://doi.org/10.3390/met13020241
Chicago/Turabian StyleChen, Liu, Yongxiang Wu, Nguyen Thi Hong Nhung, Chunlin He, Hao Chen, Gjergj Dodbiba, Akira Otsuki, and Toyohisa Fujita. 2023. "High Gradient Magnetic Separation of Pure Gd2O3 Particles from Pure La2O3 Particles" Metals 13, no. 2: 241. https://doi.org/10.3390/met13020241
APA StyleChen, L., Wu, Y., Nhung, N. T. H., He, C., Chen, H., Dodbiba, G., Otsuki, A., & Fujita, T. (2023). High Gradient Magnetic Separation of Pure Gd2O3 Particles from Pure La2O3 Particles. Metals, 13(2), 241. https://doi.org/10.3390/met13020241








