Recovery of Valuable Metals by Roasting of Jarosite in Cement Kiln
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussion
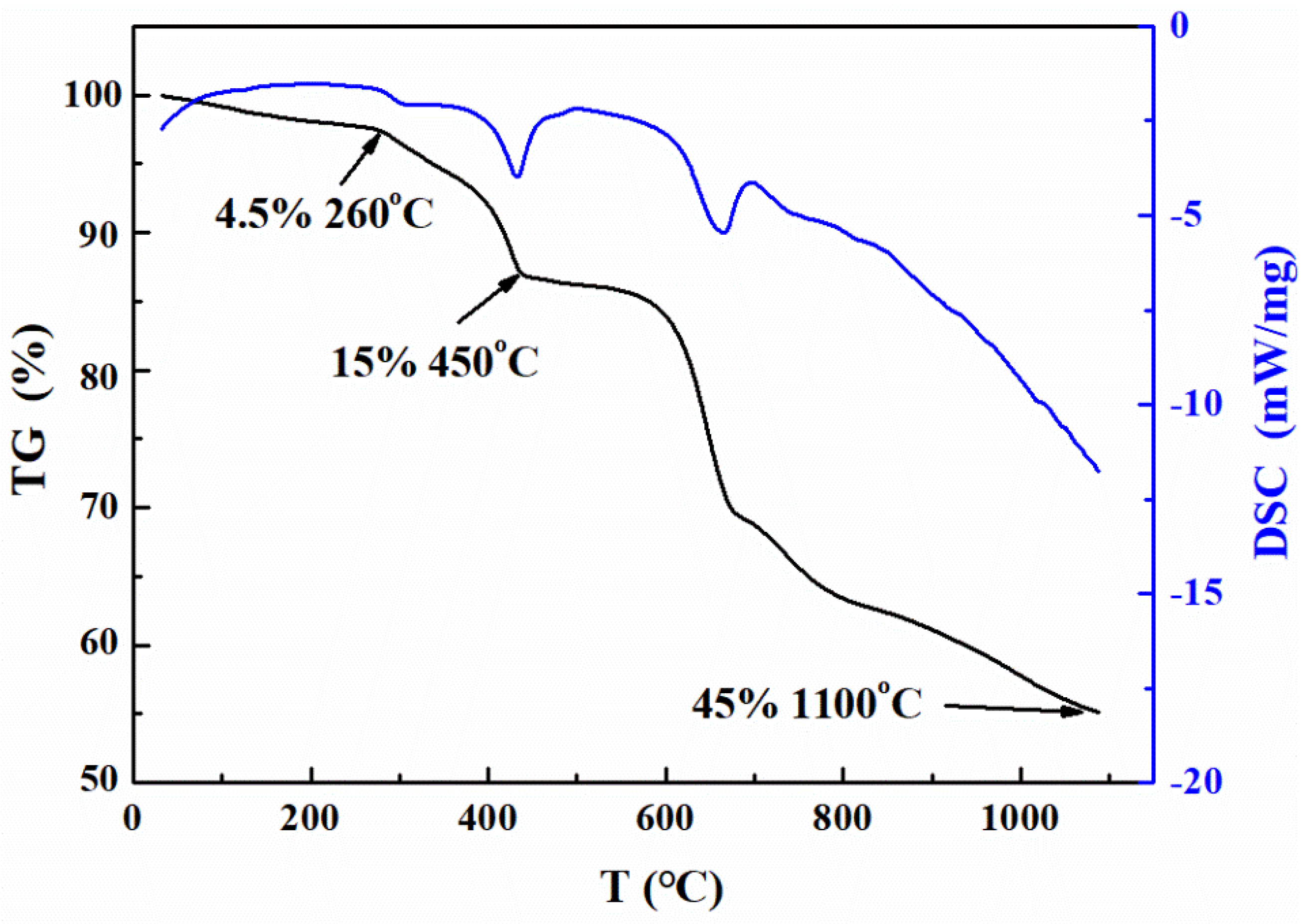
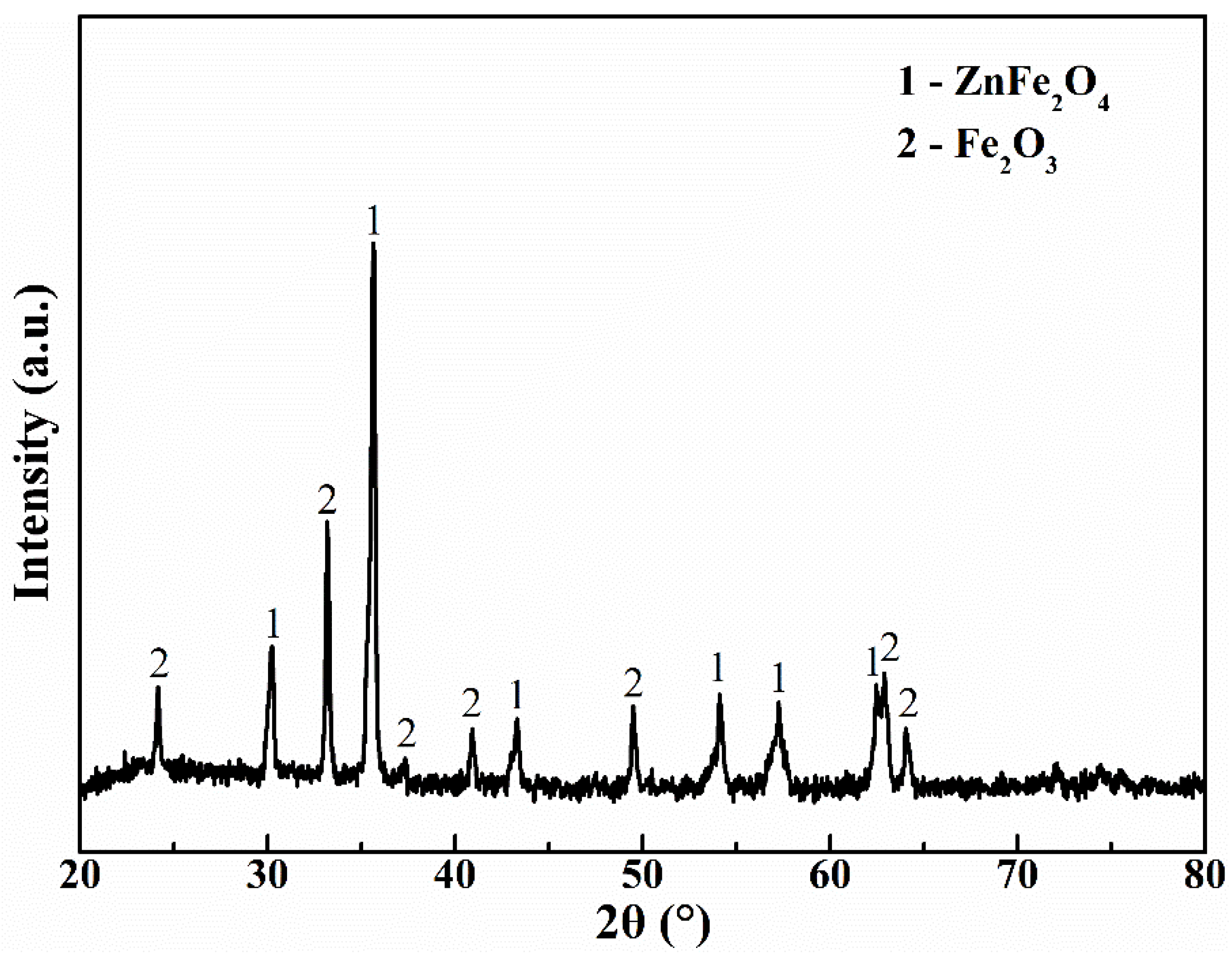
3.1. Properties of the Jarosite Residue
3.2. Thermodynamic Analysis
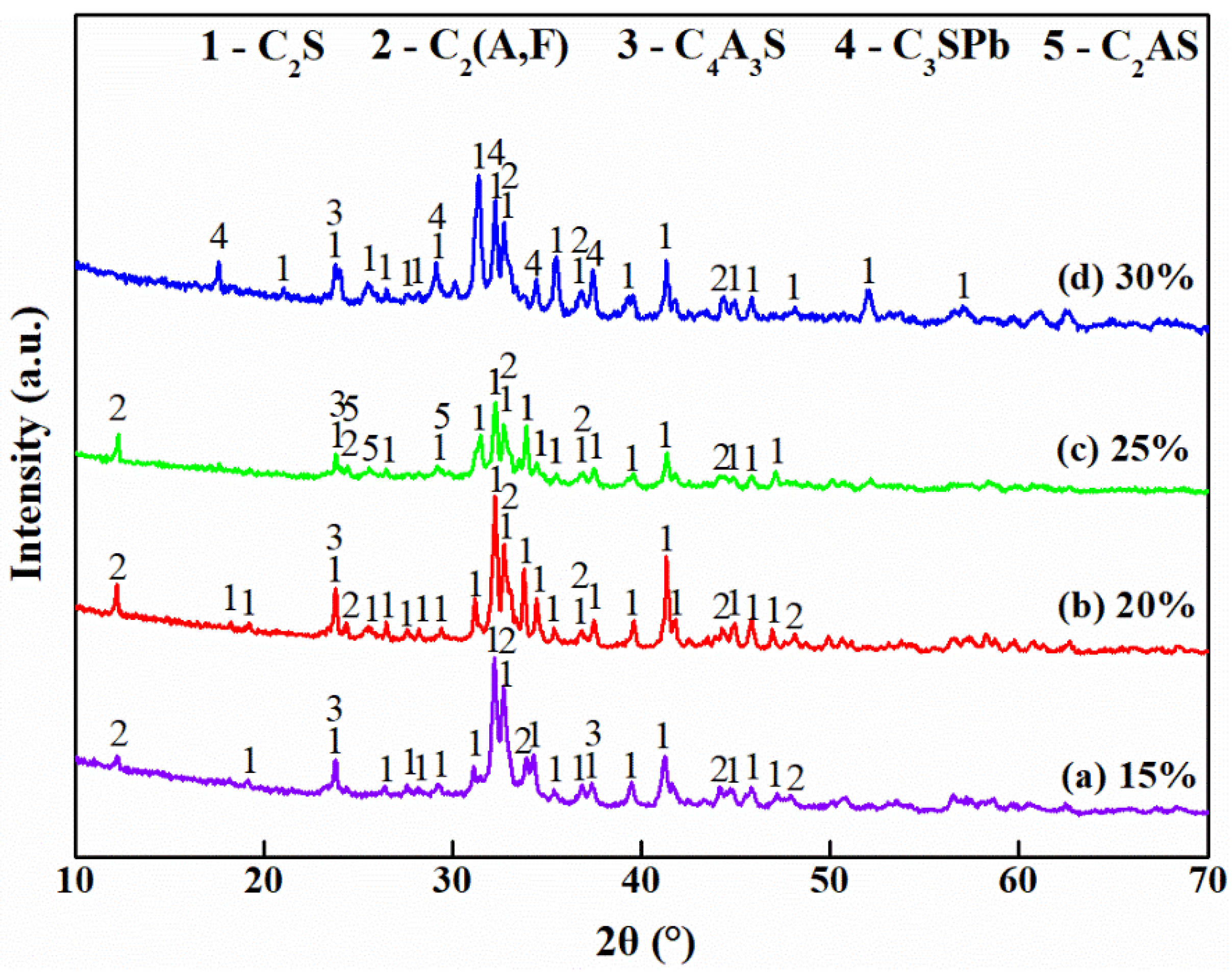
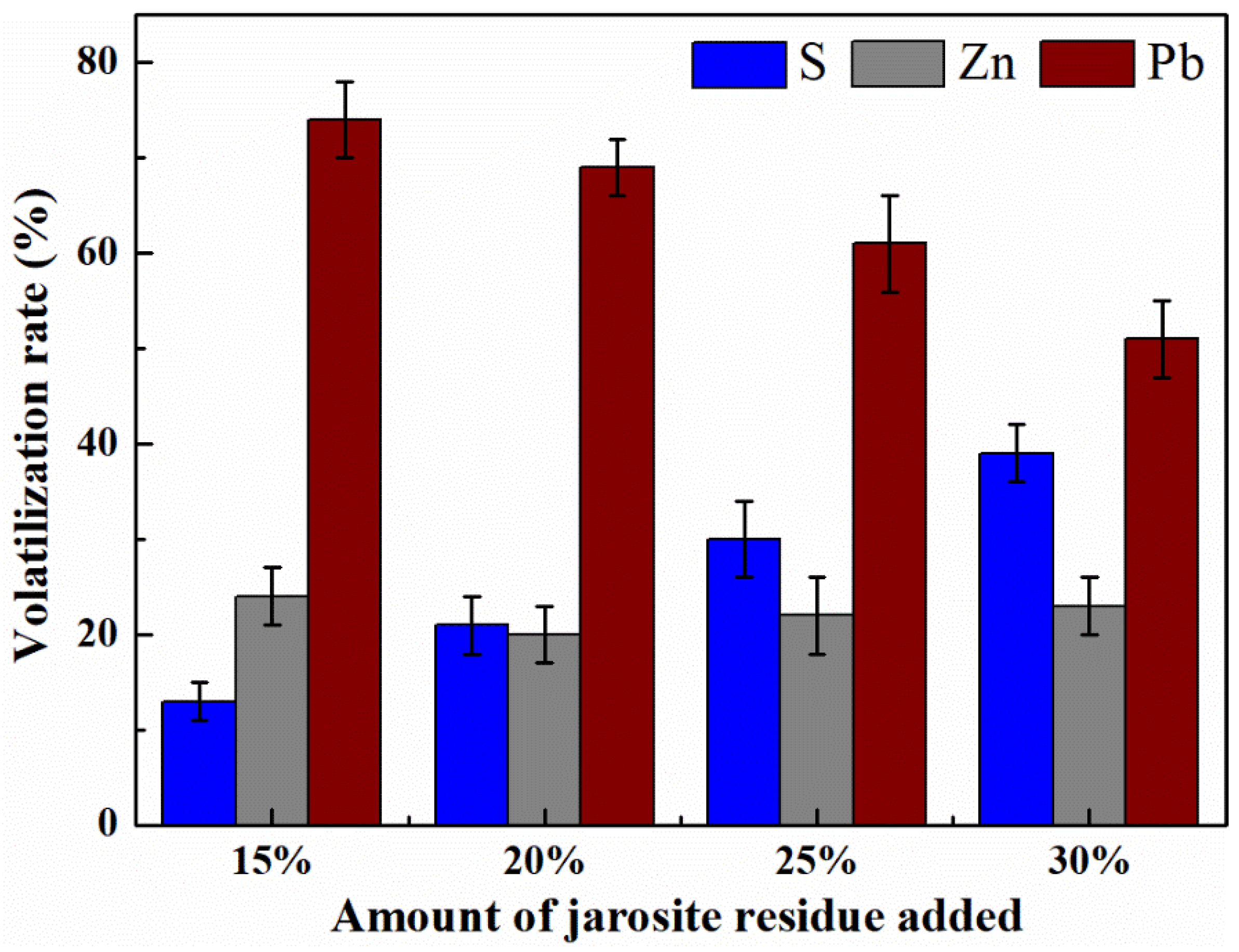
3.3. Effect of Jarosite Addition
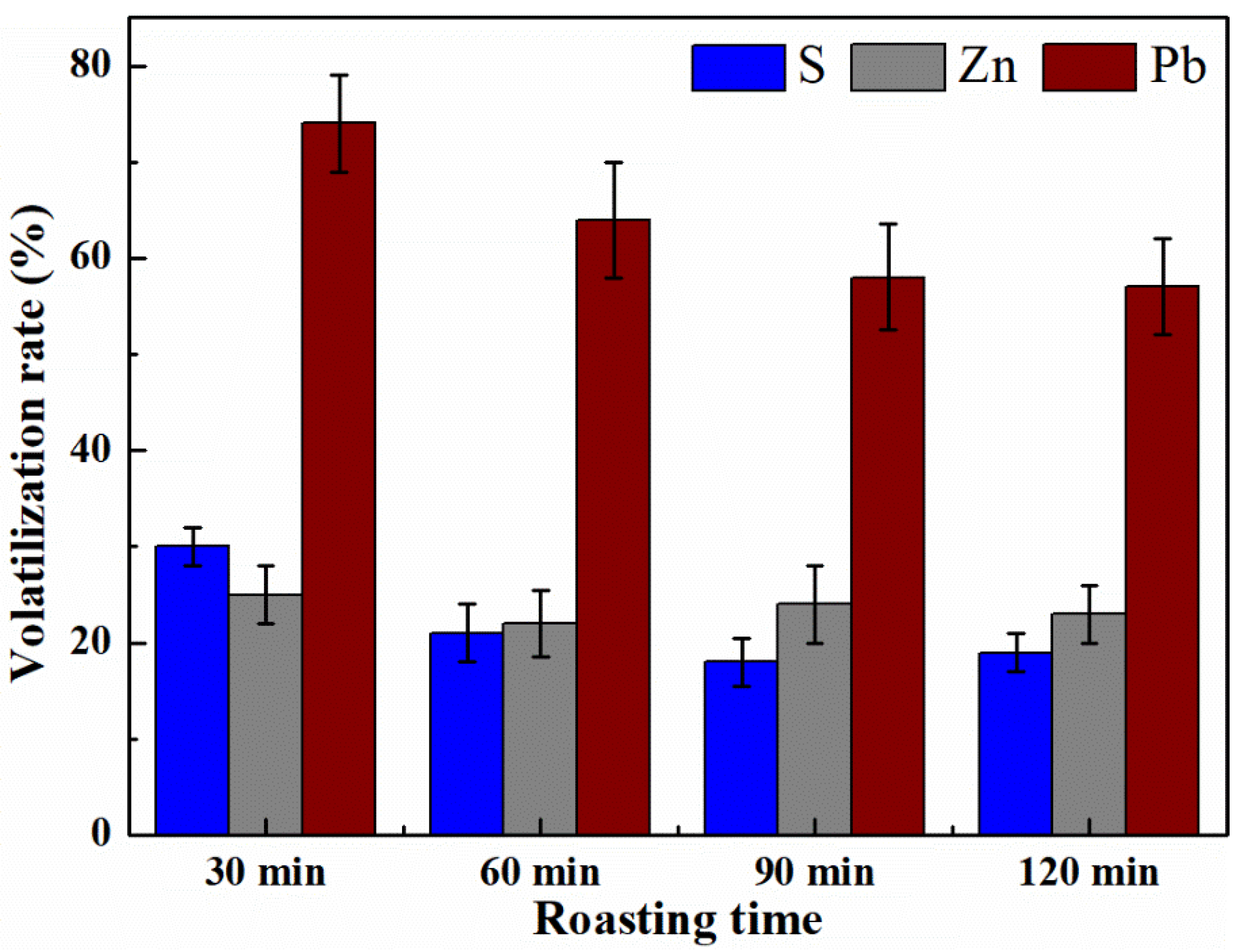
3.4. Effect of Roasting Time
3.5. Effect of Mineralizer CaCl2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappu, M.A.; Saxena, S.R. Asolekar, Jarosite characteristics and its utilisation potentials. Total Environ. 2006, 359, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.T.; Scott, T.R. Removal of iron from leach liquors by the “Goethite” process. Hydrometallurgy 1976, 2, 25–33. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The effectiveness of jarosite species for precipitating sodium jarosite. J. Oper. Manag. 1999, 51, 30–32. [Google Scholar] [CrossRef]
- Coetzee, R.; Dorfling, C.; Bradshaw, S.M. Characterization of precipitate formed during the removal of iron and precious metals from sulphate leach solutions. S. Afr. Inst. Min. Metall. 2017, 117, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, A.M.; Pons, M.N.; Ricoux, Q.; Issa, S.; Goettmann, F.; Lapicque, F. New pathway for utilization of jarosite, an industrial waste of zinc hydrometallurgy. Miner. Eng. 2021, 170, 107030. [Google Scholar] [CrossRef]
- Strbac, N.; Mihajlovi´c, I.; Andri´c, V.; Živkovi´c, Ž.; Rosi´c, A. Kinetic investigations of two processes for zinc recovery from zinc plant residue. Can. Metall. Q. 2011, 50, 28–36. [Google Scholar] [CrossRef]
- Hoeber, L.; Steinlechner, S. A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy. Clean. Eng. Technol. 2021, 4, 100214. [Google Scholar] [CrossRef]
- Calla-Choque, D.; Nava-Alonso, F.; Fuentes-Aceituno, J.C. Acid decomposition and thiourea leaching of silver from hazardous jarosite residues: Effect of some cations on the stability of the thiourea system. J. Hazard. Mater. 2016, 317, 440–448. [Google Scholar] [CrossRef]
- Malenga, E.N.; Mulaba-Bafubiandi, A.; Nheta, W. Alkaline leaching of nickel bearing ammonium jarosite precipitate using KOH, NaOH and NH4OH in the presence of EDTA and Na2S. Hydrometallurgy 2015, 155, 69–78. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tang, M.T.; Yang, S.H.; He, J.; Tang, C.; Yang, J.; Lu, J. Novel technique of decomposition of ammonium jarosite bearing indium in NaOH medium. Chin. J. Nonferrous Met. 2009, 7, 28. [Google Scholar]
- De-La-Cruz-Moreno, J.E.; Ceniceros-Gómez, A.E.; Morton-Bermea, O.; Hernández-Álvarez, E. Recovery of indium from jarosite residues of zinc refinery by a hydrometallurgical process. Hydrometallurgy 2021, 203, 105697. [Google Scholar] [CrossRef]
- Hu, H.; Deng, Q.; Li, C.; Xie, Y.; Dong, Z.; Zhang, W. The recovery of Zn and Pb and the manufacture of lightweight bricks from zinc smelting slag and clay. Hazard. Mater. 2014, 271, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, D.; Mapelli, C.; Di Cecca, C.; Barella, S.; Gruttadauria, A.; Ragona, M.; Pisu, M.; Viola, A. Characterization of cast iron and slag produced by jarosite sludges reduction via Arc Transferred Plasma (ATP) reactor. J. Environ. Chem. Eng. 2018, 6, 773–783. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, C.; Pan, J.; Guo, Z.; Li, S. New pyrometallurgical route for separation and recovery of Fe, Zn, In, Ga and S from jarosite residues. J. Clean. Prod. 2018, 205, 781–788. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, Y.; Zhang, Y.; Xue, P.; Wang, Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J. Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef]
- Mehra, P.; Gupta, R.C.; Thomas, B.S. Properties of concrete containing jarosite as a partial substitute for fine aggregate. Clean. Prod. 2016, 120, 241–248. [Google Scholar] [CrossRef]
- Ray, S.; Daudi, L.; Yadav, H.; Ransinchung, G. Utilization of Jarosite waste for the development of sustainable concrete by reducing the cement content. J. Clean. Prod. 2020, 272, 122546. [Google Scholar] [CrossRef]
- Mehra, P.; Gupta, R.C.; Thomas, B.S. Assessment of durability characteristics of cement concrete containing jarosite. J. Clean. Prod. 2016, 119, 59–65. [Google Scholar] [CrossRef]
- Gared, O.; Gaur, A. Feasibility study of Jarosite as cement replacement in rigid pavement. Mater. Today Proc. 2020, 10, 554. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Sorrentino, F.; Damidot, D. Effect of the clinker composition on the threshold limits for Cu, Sn or Zn. Cement Concrete Res. 2012, 42, 1088–1093. [Google Scholar] [CrossRef]
- Andrade, F.R.D.; Maringolo, V.; Kihara, Y. Incorporation of V, Zn and Pb into the crystalline phases of Portland clinker. Cem. Concr. Res. 2003, 33, 63–71. [Google Scholar] [CrossRef]
- Stephan, D.; Maleki, H.; Knöfel, D.; Eber, B.; Härdtl, R. Influence of Cr, Ni, and Zn on the properties of pure clinker phases Part, I. C3S. Cem. Concr. Res. 1999, 29, 545–552. [Google Scholar] [CrossRef]
- Frost, R.L.; Weier, M.L.; Martens, W. Thermal decomposition of jarosites of potassium, sodium and lead. Therm. Anal. Calorim. 2005, 82, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Steinlechner, S.; Antrekowitsch, J. Thermodynamic considerations for a pyrometallurgical extraction of indium and silver from a jarosite residue. Metals 2018, 8, 335. [Google Scholar] [CrossRef]






| Element | Fe (%) | S (%) | Zn (%) | Pb (%) | Ag (g/t) |
|---|---|---|---|---|---|
| Content | 22.42 | 12.54 | 5.35 | 3.17 | 238 |
| Reaction | Equations |
|---|---|
| Fe2O3 + 2CaO = 2CaO·Fe2O3 | (11) |
| Fe2O3 + CaO = CaO·Fe2O3 | (12) |
| 2CaO + 2SO2 + O2 = 2CaSO4 | (13) |
| CaO + SO3 = CaSO4 | (14) |
| PbO + CaCl2 + SO2(g) + 0.5O2(g) = PbCl2(g) + CaSO4 | (15) |
| PbO + CaCl2 + SO3(g) = PbCl2(g) + CaSO4 | (16) |
| PbS + CaCl2 + 2O2(g) = PbCl2(g) + CaSO4 | (17) |
| PbSO4 + CaCl2 = PbCl2(g) + CaSO4 | (18) |
| PbSO4 = PbO(g) + SO3 | (19) |
| ZnSO4 + CaCl2 = ZnCl2(g) + CaSO4 | (20) |
| ZnS + CaCl2 + 2O2 = ZnCl2(g) + CaSO4 | (21) |
| ZnFe2O4 = ZnO + Fe2O3 | (22) |
| ZnFe2O4 + CaCl2 + 2CaO = ZnCl2(g) + 2CaO·Fe2O3 | (23) |
| Addition Amount | Silicate (%) | Aluminate and Impurity (%) | Ferroaluminate and Aluminosulfate (%) | Free-CaO (%) |
|---|---|---|---|---|
| 15% | 65.50 | 27.83 | 6.67 | 0.63 |
| 20% | 57.58 | 34.25 | 8.17 | 0.55 |
| 25% | 54.43 | 35.16 | 10.41 | 0.88 |
| 30% | 41.23 | 46.42 | 12.35 | 0.98 |
| Roasting Time | Silicate (%) | Aluminate and Impurity (%) | Ferroaluminate and Aluminosulfate (%) | Free-CaO (%) |
|---|---|---|---|---|
| 30 min | 54.43 | 35.16 | 10.41 | 0.88 |
| 60 min | 49.82 | 36.95 | 13.23 | 0.65 |
| 90 min | 50.6 | 35.95 | 13.45 | 0.84 |
| 120 min | 47.28 | 41.50 | 11.22 | 0.74 |
| Amount of CaCl2 Added | Silicate (%) | Aluminate and Impurity (%) | Ferroaluminate and Aluminosulfate (%) | Free-CaO (%) |
|---|---|---|---|---|
| 50% | 47.65 | 33.83 | 18.52 | 0.54 |
| 75% | 45.73 | 38.22 | 16.05 | 0.72 |
| 100% | 40.81 | 40.95 | 18.24 | 0.69 |
| 125% | 30.51 | 46.85 | 22.64 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, H.; Pan, Z.; Xie, F.; Lu, D.; Wang, W.; Wu, S. Recovery of Valuable Metals by Roasting of Jarosite in Cement Kiln. Metals 2023, 13, 250. https://doi.org/10.3390/met13020250
Ge H, Pan Z, Xie F, Lu D, Wang W, Wu S. Recovery of Valuable Metals by Roasting of Jarosite in Cement Kiln. Metals. 2023; 13(2):250. https://doi.org/10.3390/met13020250
Chicago/Turabian StyleGe, Hui, Zhigang Pan, Feng Xie, Diankun Lu, Wei Wang, and Shulin Wu. 2023. "Recovery of Valuable Metals by Roasting of Jarosite in Cement Kiln" Metals 13, no. 2: 250. https://doi.org/10.3390/met13020250
APA StyleGe, H., Pan, Z., Xie, F., Lu, D., Wang, W., & Wu, S. (2023). Recovery of Valuable Metals by Roasting of Jarosite in Cement Kiln. Metals, 13(2), 250. https://doi.org/10.3390/met13020250





