Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract
Abstract
1. Introduction
2. Experimental
2.1. Preparation of 304 Stainless Steel Samples
2.2. Preparation of Terminalia bellirica Fruit Extract
2.3. Corrosion Testing
2.3.1. Potentiodynamic Polarization
2.3.2. Scanning Electron Microscopy
2.4. Fourier-Transform Infrared Spectrophotometric Measurements
3. Results and Discussion
3.1. Potentiodynamic Polarization
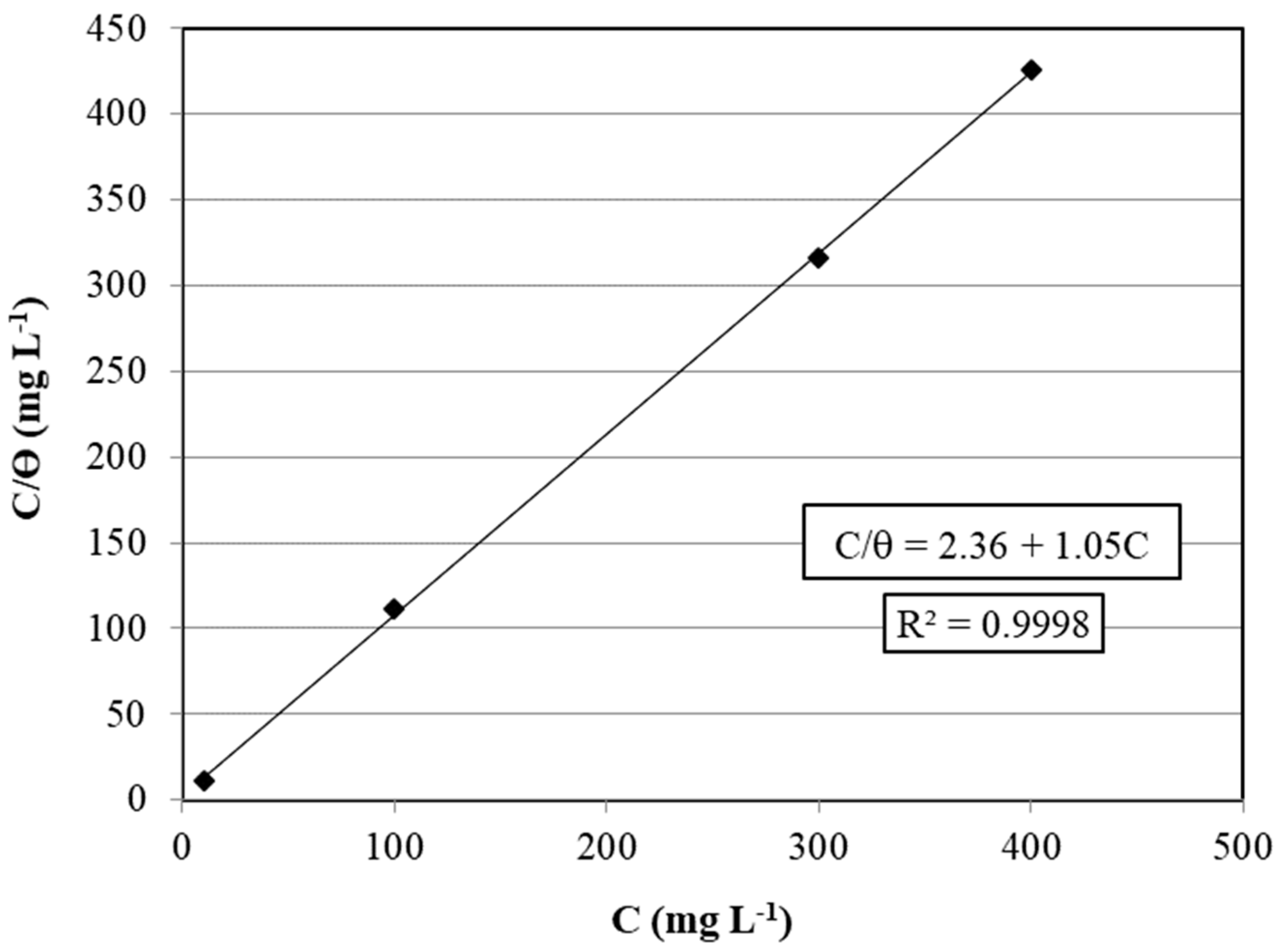
3.2. Terminalia bellirica Fruit Extract Adsorption
3.3. Pitting Potential Improvement
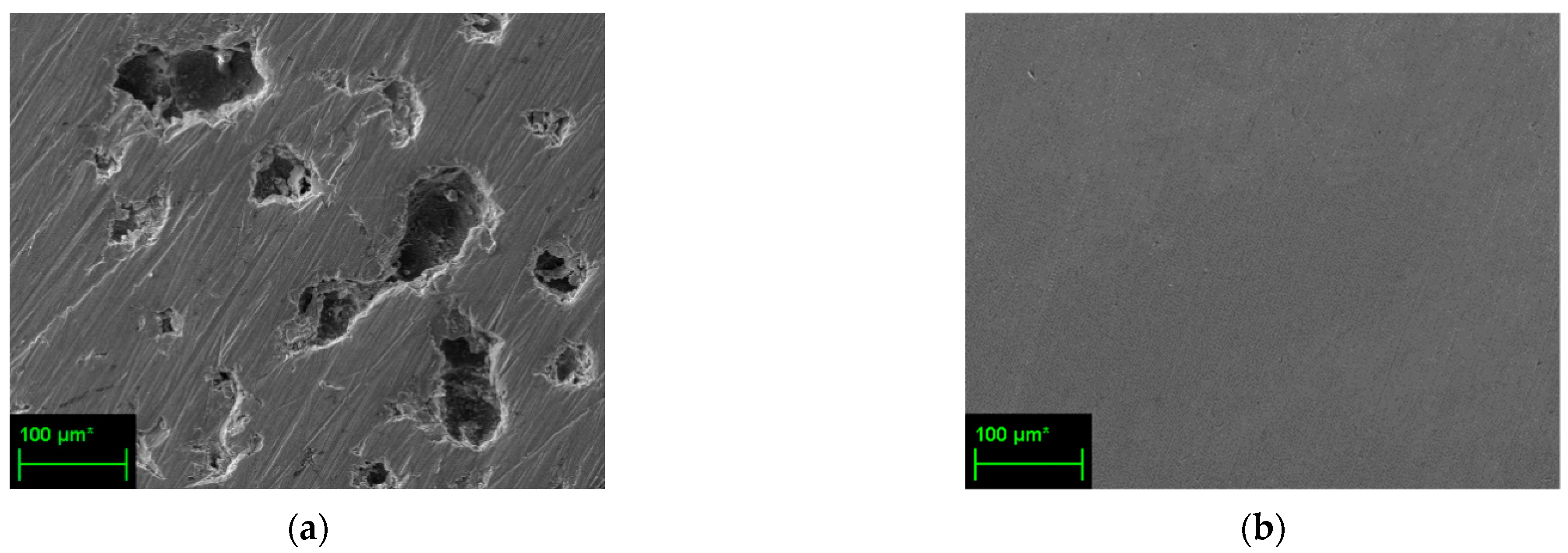
3.4. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, G.; Gupta, S.S.; Bhatia, A.; Sidhu, O.P.; Rawat, A.K.S.; Rao, C.V. Grilling enhances antidiarrheal activity of Terminalia bellerica Roxb. fruits. J. Ethnopharm. 2017, 202, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, M.P.J.; Manoraj, A.; Thevanesam, V.; Ekanayake, A.; Kumar, N.S.; Liyanapathirana, V.; Abeyratne, E.; Bandara, B.M.R. Terminalia bellirica fruit extracts: In-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells. BMC Complement. Altern. Med. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Tong, J.; Ge, L.L.; Ma, B.X.; He, J.S.; Wang, Y.W. Ethyl acetate fraction of Terminalia bellirica fruit inhibits rat hepatic stellate cell proliferation and induces apoptosis. Ind. Crop. Prod. 2015, 76, 364–373. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Comm. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- Wang, Y.; Qiang, Y.; Zhi, H.; Ran, B.; Zhang, D. Evaluating the synergistic effect of maple leaves extract and iodide ions on corrosion inhibition of Q235 steel in H2SO4 solution. J. Ind. Eng. Chem. 2023, 117, 422–433. [Google Scholar] [CrossRef]
- Shang, Z.; Zhu, J. Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol. 2021, 15, 5078–5094. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, I.; Byeon, J.-W. Corrosion inhibition of mild steel and 304 stainless steel in 1 M hydrochloric acid solution by tea tree extract and its main constituents. Materials 2021, 14, 5016. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, W.M.I.W.M.; Fekeri, M.F.M.; Nasir, N.A.M.; Hamidi, N.A.S.M.; Baharom, M.Z.; Adnan, A.; Shaifudin, M.S.; Abdullah, W.R.W.; Wan Nik, W.M.N.; Suhailin, F.H.; et al. Anticorrosive and microbial inhibition performance of a coating loaded with Andrographis paniculata on stainless steel in seawater. Molecules 2021, 26, 3379. [Google Scholar] [CrossRef] [PubMed]
- Asfia, M.P.; Rezaei, M. A study on localized corrosion behavior of 304 stainless steel in the presence of Allium Sativum extract inhibitor using electrochemical noise analysis. Mater. Chem. Phys. 2021, 274, 125158. [Google Scholar] [CrossRef]
- Asfia, M.P.; Rezaei, M.; Bahlakeh, G. Corrosion prevention of AISI 304 stainless steel in hydrochloric acid medium using garlic extract as a green corrosion inhibitor: Electrochemical and theoretical studies. J. Mol. Liq. 2020, 315, 113679. [Google Scholar] [CrossRef]
- Schmuki, P. From Bacon to barriers: A review on the passivity of metals and alloys. J. Solid State Electrochem. 2002, 6, 145–164. [Google Scholar] [CrossRef]
- Pedeferri, P. Pitting Corrosion. In Corrosion Science and Engineering; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Messinese, E.; Casanova, L.; Paterlini, L.; Capelli, F.; Bolzoni, F.; Ormellese, M.; Brenna, A. A Comprehensive investigation on the effects of surface finishing on the resistance of stainless steel to localized corrosion. Metals 2022, 12, 1751. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Bhuyan, P.; Mandal, S. Influence of the individual microstructural features on pitting corrosion in type 304 austenitic stainless steel. Corros. Sci. 2019, 158, 108091. [Google Scholar] [CrossRef]
- Krell, P.D.; Li, S.; Cong, H. Synergistic effect of temperature and HCl concentration on the degradation of AISI 410 stainless steel. Corros. Sci. 2017, 122, 41–52. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Sun, X.; Qiang, Y.; Hou, B.; Zhu, H.; Tian, H. Cabbage extract as an eco-friendly corrosion inhibitor for X70 steel in hydrochloric acid medium. J. Mol. Liq. 2022, 362, 119733. [Google Scholar] [CrossRef]
- Chaubey, N.; Singh, V.K.; Quraishi, M.A. Electrochemical approach of Kalmegh leaf extract on the corrosion behavior of aluminium alloy in alkaline solution. Int. J. Ind. Chem. 2017, 8, 75–82. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Chaouiki, A.; Alahmari, J.M.; Al-hammadi, W.A.; Noor, E.A.; Al-Ghamdi, A.A.; Ko, Y.G. Development of natural plant extracts as sustainable inhibitors for efficient protection of mild steel: Experimental and first-principles multi-level computational methods. Materials 2022, 15, 8688. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Hao, L.; Du, H.; Pan, R.; Huang, G.; Liu, H. Cucumber (Cucumis sativus L.) leaf extract as a green corrosion inhibitor for carbon steel in acidic solution: Electrochemical, functional and molecular analysis. Molecules 2022, 27, 3826. [Google Scholar] [CrossRef]
- Nan, H.Y.; Zhu, L.Q.; Liu, H.C.; Li, W.P. Phytate as efficient and green corrosion inhibitor for NdFeB magnets in aqueous salt solution. Corros. Eng. Sci. Technol. 2015, 50, 589–594. [Google Scholar] [CrossRef]
- Oli, H.B.; Thapa Magar, J.; Khadka, N.; Subedee, A.; Bhattarai, D.P.; Pant, B. Coriaria nepalensis stem alkaloid as a green inhibitor for mild steel corrosion in 1 M H2SO4 solution. Electrochem 2022, 3, 713–727. [Google Scholar] [CrossRef]
- Baskar, P.; Rathinapriya, P.; Prabakaran, M. Use of Trochodendron Aralioides extract as green corrosion inhibitor for mild steel in 1M HCl solutions. Processes 2022, 10, 1480. [Google Scholar] [CrossRef]
- An, L.; Cao, J.; Wu, L.; Mao, H.; Yang, Y. Effects of Mo and Mn on pitting behavior of duplex stainless steel. J. Iron. Steel. Res. Int. 2016, 23, 1333–1341. [Google Scholar] [CrossRef]
- Tang, Y.; Zuo, Y.; Wang, J.; Zhao, X.; Niu, B.; Lin, B. The metastable pitting potential and its relation to the pitting potential for four materials in chloride solutions. Corros. Sci. 2014, 80, 111–119. [Google Scholar] [CrossRef]
- Patra, S.; Panda, P.K.; Naik, P.P.; Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.K.; Mahapatra, K.K.; Padhi, P.; Jena, M.; Patil, S.; et al. Terminalia bellirica extract induces anticancer activity through modulation of apoptosis and autophagy in oral squamous cell carcinoma. Food Chem. Toxicol. 2020, 136, 111073. [Google Scholar] [CrossRef]
- Sales, M.S.; Roy, A.; Antony, L.; Banu, S.K.; Jeyaraman, S.; Manikkam, R. Octyl gallate and gallic acid isolated from Terminalia bellarica regulates normal cell cycle in human breast cancer cell lines. Biomed. Pharmacol. 2018, 103, 1577–1584. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, Y.; Saita, E.; Suzuki-Sugihara, N.; Kamiya, T.; Taguchi, C.; Iida, K.; Kondo, K. Terminalia bellirica extract inhibits low-density lipoprotein oxidation and macrophage inflammatory response in vitro. Antioxidants 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Keny, S.J.; Kumbhar, A.G.; Thinaharan, C.; Venkateswaran, G. Gallic acid as a corrosion inhibitor of carbon steel in chemical decontamination formulation. Corros. Sci. 2008, 50, 411–419. [Google Scholar] [CrossRef]
- Shibata, Y.; Shiromoto, T.; Yuasa, M.; Sekine, I.; Imahama, T.; Wake, T. Corrosion inhibition of mild steel by gallic acid in neutral solution. J. Surf. Finish. Soc. Jpn. 1993, 44, 347–352. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, α-d-glucose and tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Yashonath, S.; Basu, P.K.; Srinivasan, A.; Hedge, M.S.; Rao, C.N.R. Photoelectron spectroscopic studies of the adsorption of organic molecules with lone pair orbitals on transition metal surfaces. J. Chem. Sci. 1982, 91, 101–128. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures—A technical review. J. Loss Prev. Process Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; Wiley: West Sussex, UK, 2004. [Google Scholar]
- Singh, A.; Dayu, X.; Ituen, E.; Ansari, K.; Quraishi, M.A.; Kaya, S.; Lin, Y. Tobacco extracted from the discarded cigarettes as an inhibitor of copper and zinc corrosion in an ASTM standard D1141–98(2013) artificial seawater solution. Mater. Res. Technol. 2020, 9, 5161–5173. [Google Scholar] [CrossRef]
- Loto, R.T. Evaluation of the corrosion inhibition effect of the combined admixture of rosemary and cinnamon cassia oil on mild steel in weak acid electrolyte. Sustain. Chem. Pharm. 2020, 17, 100298. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández–Domene, R.M.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; García–Antón, J. Green approach to corrosion inhibition of stainless steel in phosphoric acid of Artemesia herba albamedium using plant extract. J. Mater. Res. Technol. 2019, 8, 5763–5773. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Res. Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Pojtanabuntoeng, T.; Saiwan, C.; Sutthiruangwong, S.; Gallup, D.L. Effect of mercury on corrosion in production wells in Gulf of Thailand. Corros. Eng. Sci. Technol. 2011, 46, 547–553. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Mori, G.; Kösters, R. Passivity and pseudopassivity of cemented carbides. Int. J. Refrac. Met. Hard Mater. 2005, 23, 129–136. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; NACE: Houston, TX, USA, 1974. [Google Scholar]





| C | Si | Mn | P | S | N | Cr | Ni |
|---|---|---|---|---|---|---|---|
| ≤0.07 | ≤0.75 | ≤2.00 | ≤0.045 | ≤0.03 | ≤0.10 | ≤17.5–19.5 | ≤8.0–10.5 |
| Extract Concentration (mg L−1) | pH of the Solution | Ecorr (VSCE) | icorr (A cm−2) | Epit (VSCE) | %IE | %PPI |
|---|---|---|---|---|---|---|
| 0 | 2.30 | −0.387 | 7.90 × 10−6 | 0.027 | – | – |
| 10 | 2.30 | −0.331 | 7.51 × 10−7 | 0.126 | 90 | 12 |
| 100 | 2.29 | −0.334 | 7.76 × 10−7 | 0.156 | 90 | 16 |
| 300 | 2.28 | −0.372 | 4.17 × 10−7 | 0.263 | 95 | 29 |
| 400 | 2.28 | −0.401 | 4.91 × 10−7 | 0.251 | 94 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutthiruangwong, S.; Wongpaiboon, C.; Sritha, N.; Anukulkich, N. Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract. Metals 2023, 13, 262. https://doi.org/10.3390/met13020262
Sutthiruangwong S, Wongpaiboon C, Sritha N, Anukulkich N. Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract. Metals. 2023; 13(2):262. https://doi.org/10.3390/met13020262
Chicago/Turabian StyleSutthiruangwong, Sutha, Chutikan Wongpaiboon, Nathatida Sritha, and Nattha Anukulkich. 2023. "Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract" Metals 13, no. 2: 262. https://doi.org/10.3390/met13020262
APA StyleSutthiruangwong, S., Wongpaiboon, C., Sritha, N., & Anukulkich, N. (2023). Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract. Metals, 13(2), 262. https://doi.org/10.3390/met13020262






