First-Principles Study on the Effect of H, C, and N at the Interface on Austenite/Ferrite Homojunction
Abstract
:1. Introduction
2. Calculation Details
3. Results and Discussion
3.1. One Atom at the Interface
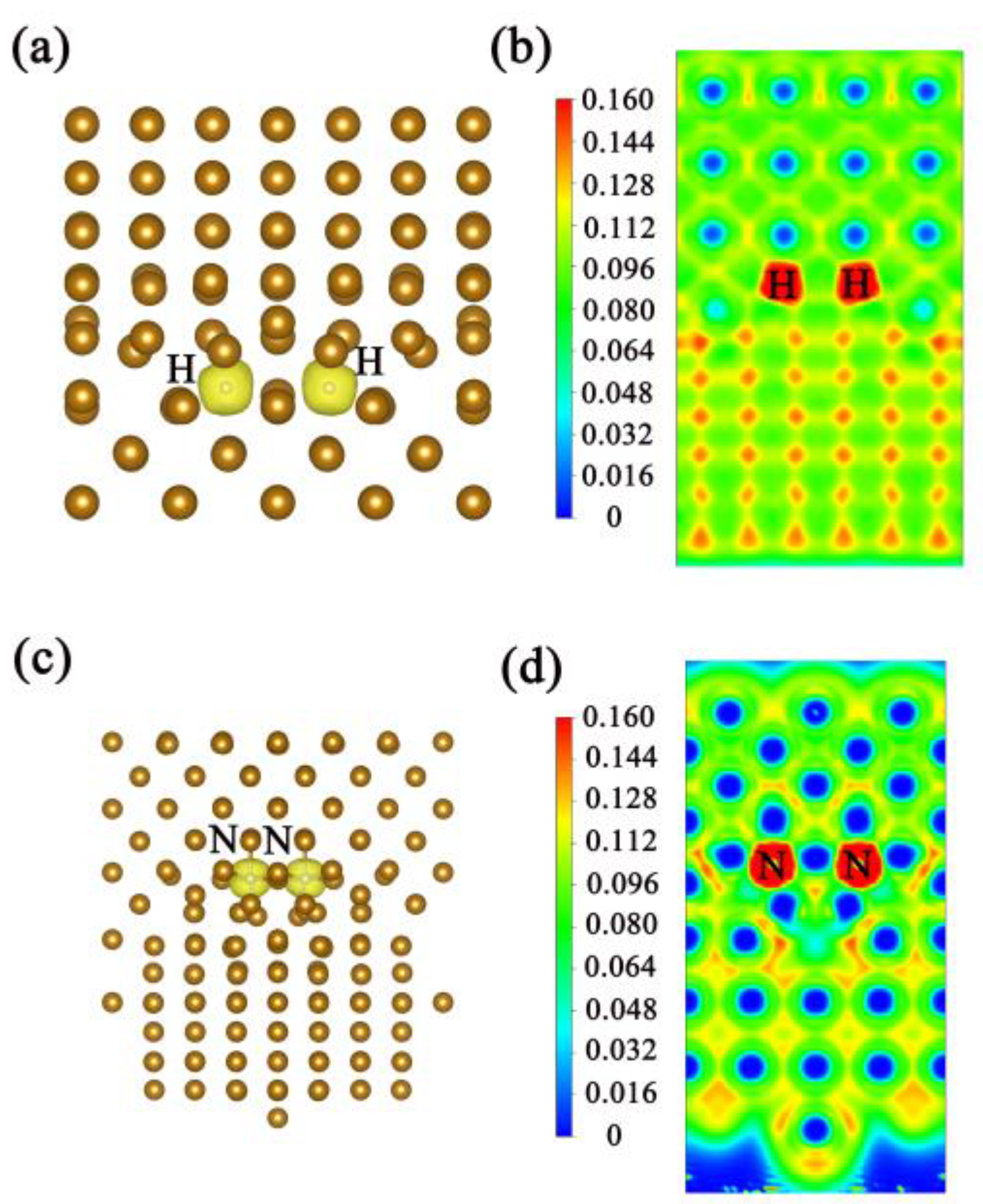
3.2. Two Atoms at the Interface
4. Conclusions
- (1)
- Carbon and nitrogen can effectively strengthen austenite at low concentration, and the local distortion produced in the process is conducive to the combination and stable existence of the interface.
- (2)
- At low concentration, the bonding energy and stability of the C/N-containing system are better than those of the hydrogen-containing system.
- (3)
- A high concentration of hydrogen and nitrogen will reduce the binding energy of the interface. However, the hydrogen can partially act as the interfacial binding medium, which makes the binding energy decrease more slowly.
- (4)
- The high concentration of nitrogen causes the austenite to over-strengthen, which leads to a sharp decline in interface matching. Therefore, the binding energy of the interface and the stability of the whole system are reduced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Was, G.S.; Ukai, S. Chapter 8-Austenitic Stainless Steels. In Structural Alloys for Nuclear Energy Applications; Odette, G.R., Zinkle, S.J., Eds.; Elsevier: Boston, MA, USA, 2019; pp. 293–347. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Lula, R.A. Stainless Steel; American Society for Metals: Metals Park, OH, USA, 1985. [Google Scholar]
- Beddoes, J.; Parr, J. Introduction to Stainless Steels, 3rd ed.; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Şahin, S.; Übeyli, M. A Review on the Potential Use of Austenitic Stainless Steels in Nuclear Fusion Reactors. J. Fusion Energy 2008, 27, 271–277. [Google Scholar] [CrossRef]
- Avner, S.H. Introduction to Physical Metallurgy; McGraw-Hill, Inc: 1325 Avenue of the Americas, New York, NY, USA, 1964. [Google Scholar]
- Handbook of Stainless Steels. Br. Corros. J. 1978, 13, 56. [CrossRef]
- Ooi, S.W.; Hill, P.; Rawson, M.; Bhadeshia, H.K.D.H. Effect of retained austenite and high temperature Laves phase on the work hardening of an experimental maraging steel. Mater. Sci. Eng. A 2013, 564, 485–492. [Google Scholar] [CrossRef]
- Viswanathan, U.K.; Dey, G.K.; Asundi, M.K. Precipitation Hardening in 350 Grade Maraging Steel. MMTA 1993, 24, 2429–2442. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Li, W.; Fu, H.; Liu, S.; Wang, Y.; Li, J. Effect of hydrogen trapping on hydrogen permeation in a 2205 duplex stainless steel: Role of austenite–ferrite interface. Corros. Sci. 2022, 202, 110332. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R. Modelling of hydrogen diffusion and hydrogen induced cracking in supermartensitic and duplex stainless steels. Mater. Des. 2008, 29, 1934–1948. [Google Scholar] [CrossRef]
- Jackson, H.F.; Nibur, K.A.; San Marchi, C.; Puskar, J.D.; Somerday, B.P. Hydrogen-assisted crack propagation in 304L/308L and 21Cr–6Ni–9Mn/308L austenitic stainless steel fusion welds. Corros. Sci. 2012, 60, 136–144. [Google Scholar] [CrossRef]
- Owczarek, E.; Zakroczymski, T. Hydrogen transport in a duplex stainless steel. Acta Mater. 2000, 48, 3059–3070. [Google Scholar] [CrossRef]
- Zakroczymski, T.; Owczarek, E. Electrochemical investigation of hydrogen absorption in a duplex stainless steel. Acta Mater. 2002, 50, 2701–2713. [Google Scholar] [CrossRef]
- Turk, A.; Pu, S.D.; Bombač, D.; Rivera-Díaz-del-Castillo, P.E.J.; Galindo-Nava, E.I. Quantification of hydrogen trapping in multiphase steels: Part II – Effect of austenite morphology. Acta Mater. 2020, 197, 253–268. [Google Scholar] [CrossRef]
- Borchers, C.; Michler, T.; Pundt, A. Effect of Hydrogen on the Mechanical Properties of Stainless Steels. Adv. Eng. Mater. 2008, 10, 11–23. [Google Scholar] [CrossRef]
- Whiteman, M.B.; Troiano, A.R. Hydrogen Embrittlement Of Austenitic Stainless Steel. Corrosion 2013, 21, 53–56. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Matsuoka, S. Hydrogen Embrittlement Mechanism in Fatigue of Austenitic Stainless Steels. MMTA 2008, 39, 1327–1339. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Shivanyuk, V.N.; Foct, J. Diagnostic experimental results on the hydrogen embrittlement of austenitic steels. Acta Mater. 2003, 51, 1293–1305. [Google Scholar] [CrossRef]
- Ferreira, P.J.; Robertson, I.M.; Birnbaum, H.K. Hydrogen effects on the interaction between dislocations. Acta Mater. 1998, 46, 1749–1757. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Electrochemical nanoindentation: A new approach to probe hydrogen/deformation interaction. Scr. Mater. 2006, 55, 195–198. [Google Scholar] [CrossRef]
- Bugaev, V.N.; Gavriljuk, V.G.; Petrov, Y.N.; Tarasenko, A.V. Mechanism of hydrogen-induced phase transformations in metals and alloys. Int. J. Hydrogen Energy 1997, 22, 213–218. [Google Scholar] [CrossRef]
- Vakhney, A.G.; Yaresko, A.N.; Antonov, V.N.; Nemoshkalenko, V.V. The effect of hydrogen on the electronic structure and cohesive properties of iron-based alloys doped by chromium and nickel. Int. J. Hydrogen Energy 2001, 26, 453–456. [Google Scholar] [CrossRef]
- Tsay, L.W.; Yu, S.C.; Huang, R.T. Effect of austenite instability on the hydrogen-enhanced crack growth of austenitic stainless steels. Corros. Sci. 2007, 49, 2973–2984. [Google Scholar] [CrossRef]
- Turnbull, A.; Hutchings, R.B. Analysis of hydrogen atom transport in a two-phase alloy. Mater. Sci. Eng. A 1994, 177, 161–171. [Google Scholar] [CrossRef]
- Haynes, W. CRC Handbook of Chemistry and Physics; CRC: Boca Raton, FL, USA, 2012; Volume 94. [Google Scholar]
- Byrnes, M.L.G.; Grujicic, M.; Owen, W.S. Nitrogen strengthening of a stable austenitic stainless steel. Acta Metall. 1987, 35, 1853–1862. [Google Scholar] [CrossRef]
- Reed, R.P. Nitrogen in austenitic stainless steels. JOM 1989, 41, 16–21. [Google Scholar] [CrossRef]
- Mingolo, N.; Tschiptschin, A.P.; Pinedo, C.E. On the formation of expanded austenite during plasma nitriding of an AISI 316L austenitic stainless steel. Surf. Coat. Technol. 2006, 201, 4215–4218. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Yu, Z.; Qiang, J.; Hei, Z. Study of microstructure of low-temperature plasma-nitrided AISI 304 stainless steel. MMTA 2000, 31, 1193–1199. [Google Scholar] [CrossRef]
- Martinavičius, A.; Abrasonis, G.; Scheinost, A.C.; Danoix, R.; Danoix, F.; Stinville, J.C.; Talut, G.; Templier, C.; Liedke, O.; Gemming, S.; et al. Nitrogen interstitial diffusion induced decomposition in AISI 304L austenitic stainless steel. Acta Mater. 2012, 60, 4065–4076. [Google Scholar] [CrossRef]
- Michal, G.M.; Ernst, F.; Heuer, A.H. Carbon paraequilibrium in austenitic stainless steel. MMTA 2006, 37, 1819–1824. [Google Scholar] [CrossRef]
- Cao, Y.; Ernst, F.; Michal, G.M. Colossal carbon supersaturation in austenitic stainless steels carburized at low temperature. Acta Mater. 2003, 51, 4171–4181. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Bell, T. Low temperature plasma carburising of austenitic stainless steels for improved wear and corrosion resistance. Surf. Eng. 1999, 15, 49–54. [Google Scholar] [CrossRef]
- Vitos, L.; Korzhavyi, P.A.; Johansson, B. Elastic Property Maps of Austenitic Stainless Steels. Phys. Rev. Lett. 2002, 88, 155501. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Oh, C.-S.; Kim, S.-J. Effects of Carbon on the Corrosion Behaviour in Fe-18Cr-10Mn-N-C Stainless Steels. Steel Res. Int. 2009, 80, 488–492. [Google Scholar]
- Sun, Y. Depth-profiling electrochemical measurements of low temperature plasma carburised 316L stainless steel in 1M H2SO4 solution. Surf. Coat. Technol. 2010, 204, 2789–2796. [Google Scholar] [CrossRef]
- Niu, W.; Lillard, R.S.; Li, Z.; Ernst, F. Properties of the Passive Film Formed on Interstitially Hardened AISI 316L Stainless Steel. Electrochim. Acta 2015, 176, 410–419. [Google Scholar] [CrossRef]
- Niu, W.; Li, Z.; Ernst, F.; Lillard, R.S. The passivity of low-temperature carburized austenitic stainless steel AISI-316L in a simulated boiling-water-reactor environment. J. Nucl. Mater. 2020, 537, 152197. [Google Scholar] [CrossRef]
- Tedmon, C.S.; Vermilyea, D.A.; Rosolowski, J.H. Intergranular Corrosion of Austenitic Stainless Steel. J. Electrochem. Soc. 1971, 118, 192. [Google Scholar] [CrossRef]
- Mengzhe, H.; Weigang, C.; Ying, X.; Fucheng, Z.; Bo, L. First-principles study of the effect of N on the ∑5 (210) [001] grain boundary of γ-Fe. Mater. Today Commun. 2022, 33, 104496. [Google Scholar] [CrossRef]
- Liu, T.; Hui, J.; Zhang, B.; He, X.; Liu, M.; Qiu, J.; Liu, W. Corrosion mechanism of lead-bismuth eutectic at grain boundary in ferritic steels and the effect of alloying elements: A first-principles study. J. Nucl. Mater. 2022, 569, 153915. [Google Scholar] [CrossRef]
- Wang, J.; Enomoto, M.; Shang, C. First-principles study on the P-induced embrittlement and de-embrittling effect of B and C in ferritic steels. Acta Mater. 2021, 219, 117260. [Google Scholar] [CrossRef]
- Suutala, N.; Takalo, T.; Moisio, T. Ferritic-austenitic solidification mode in austenitic stainless steel welds. Metall. Trans. A 1980, 11, 717–725. [Google Scholar] [CrossRef]
- Agarwala, R.P.; Naik, M.C.; Anand, M.S.; Paul, A.R. Diffusion of carbon in stainless steels. J. Nucl. Mater. 1970, 36, 41–47. [Google Scholar] [CrossRef]
- Graf, B.; Gumenyuk, A.; Rethmeier, M. Laser Metal Deposition as Repair Technology for Stainless Steel and Titanium Alloys. Phys. Procedia 2012, 39, 376–381. [Google Scholar] [CrossRef]
- AghaAli, I.; Farzam, M.; Golozar, M.A.; Danaee, I. The effect of repeated repair welding on mechanical and corrosion properties of stainless steel 316L. Mater. Des. (1980-2015) 2014, 54, 331–341. [Google Scholar] [CrossRef]
- Mo, D.-f.; Song, T.-f.; Fang, Y.-j.; Jiang, X.-s.; Luo, C.Q.; Simpson, M.D.; Luo, Z.-p. A Review on Diffusion Bonding between Titanium Alloys and Stainless Steels. Adv. Mater. Sci. Eng. 2018, 2018, 8701890. [Google Scholar] [CrossRef]
- Orhan, N.; Khan, T.I.; Eroğlu, M. Diffusion bonding of a microduplex stainless steel to Ti–6Al–4V. Scr. Mater. 2001, 45, 441–446. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y. Unusual structural and electronic properties of porous silicene and germanene: Insights from first-principles calculations. Nanoscale Res. Lett. 2015, 10, 13. [Google Scholar] [CrossRef]
- Bai, H.; Zhu, Y.; Qiao, W.; Huang, Y. Structures, stabilities and electronic properties of graphdiyne nanoribbons. RSC Adv. 2011, 1, 768–775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. PhRvB 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. PhRvB 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Young, M.C.; Chan, S.L.I.; Tsay, L.W.; Shin, C.S. Hydrogen-enhanced cracking of 2205 duplex stainless steel welds. Mater. Chem. Phys. 2005, 91, 21–27. [Google Scholar] [CrossRef]
- da Silva, B.R.S.; Salvio, F.; Santos, D.S.d. Hydrogen induced stress cracking in UNS S32750 super duplex stainless steel tube weld joint. Int. J. Hydrogen Energy 2015, 40, 17091–17101. [Google Scholar] [CrossRef]
- Chan, S.L.I.; Lee, H.L.; Yang, J.R. Effect of retained austenite on the hydrogen content and effective diffusivity of martensitic structure. Metall. Trans. A 1991, 22, 2579–2586. [Google Scholar] [CrossRef]
- Oda, K.; Kondo, N.; Shibata, K. X-ray Absorption Fine Structure Analysis of Interstitial (C, N)-Substitutional (Cr) Complexes in Austenitic Stainless Steels. ISIJ Int. 1990, 30, 625–631. [Google Scholar] [CrossRef]
- Mente, T.; Bollinghaus, T. Modeling Of Hydrogen Distributionin A Duplex Stainless Steel. Weld. World 2012, 56, 66–78. [Google Scholar] [CrossRef]
- Silverstein, R.; Sobol, O.; Boellinghaus, T.; Unger, W.; Eliezer, D. Hydrogen behavior in SAF 2205 duplex stainless steel. J. Alloys Compd. 2017, 695, 2689–2695. [Google Scholar] [CrossRef]
- Dabah, E.; Lisitsyn, V.; Eliezer, D. Performance of hydrogen trapping and phase transformation in hydrogenated duplex stainless steels. Mater. Sci. Eng. A 2010, 527, 4851–4857. [Google Scholar] [CrossRef]
- Li, T.; Chien, S.-C.; Ren, Z.; Windl, W.; Ernst, F.; Frankel, G.S. Understanding the efficacy of concentrated interstitial carbon in enhancing the pitting corrosion resistance of stainless steel. Acta Mater. 2021, 221, 117433. [Google Scholar] [CrossRef]
- Berns, H.; Gavriljuk, V.; Shanina, B. Intensive Interstitial Strengthening of Stainless Steels. Adv. Eng. Mater. 2008, 10, 1083–1093. [Google Scholar] [CrossRef]
- Shankar, P.; Sundararaman, D.; Ranganathan, S. Clustering and ordering of nitrogen in nuclear grade 316LN austenitic stainless steel. J. Nucl. Mater. 1998, 254, 1–8. [Google Scholar] [CrossRef]
- Ledbetter, H.M.; Austin, M.W. Effects of carbon and nitrogen on the elastic constants of AISI type 304 stainless steel. MSEng 1985, 70, 143–149. [Google Scholar] [CrossRef]
- Westin, E.M. Microstructure and Properties of Welds in the Lean Duplex Stainless Steel LDX 2101. Ph.D. Thesis, Comprehensive Summary. KTH, Stockholm, Sweden, 2010. [Google Scholar]
- Bobadilla, M.; Tschiptschin, A. On the Nitrogen Diffusion in a Duplex Stainless Steel. Mater. Res. 2015, 18, 390–394. [Google Scholar] [CrossRef] [Green Version]
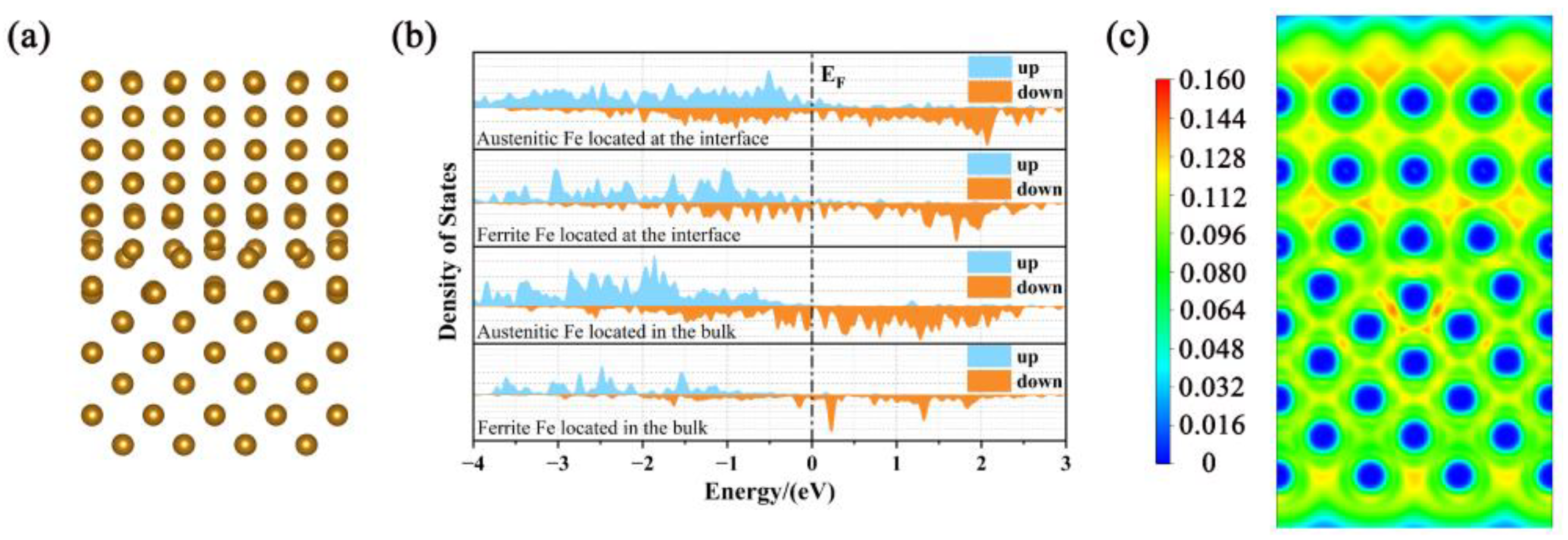


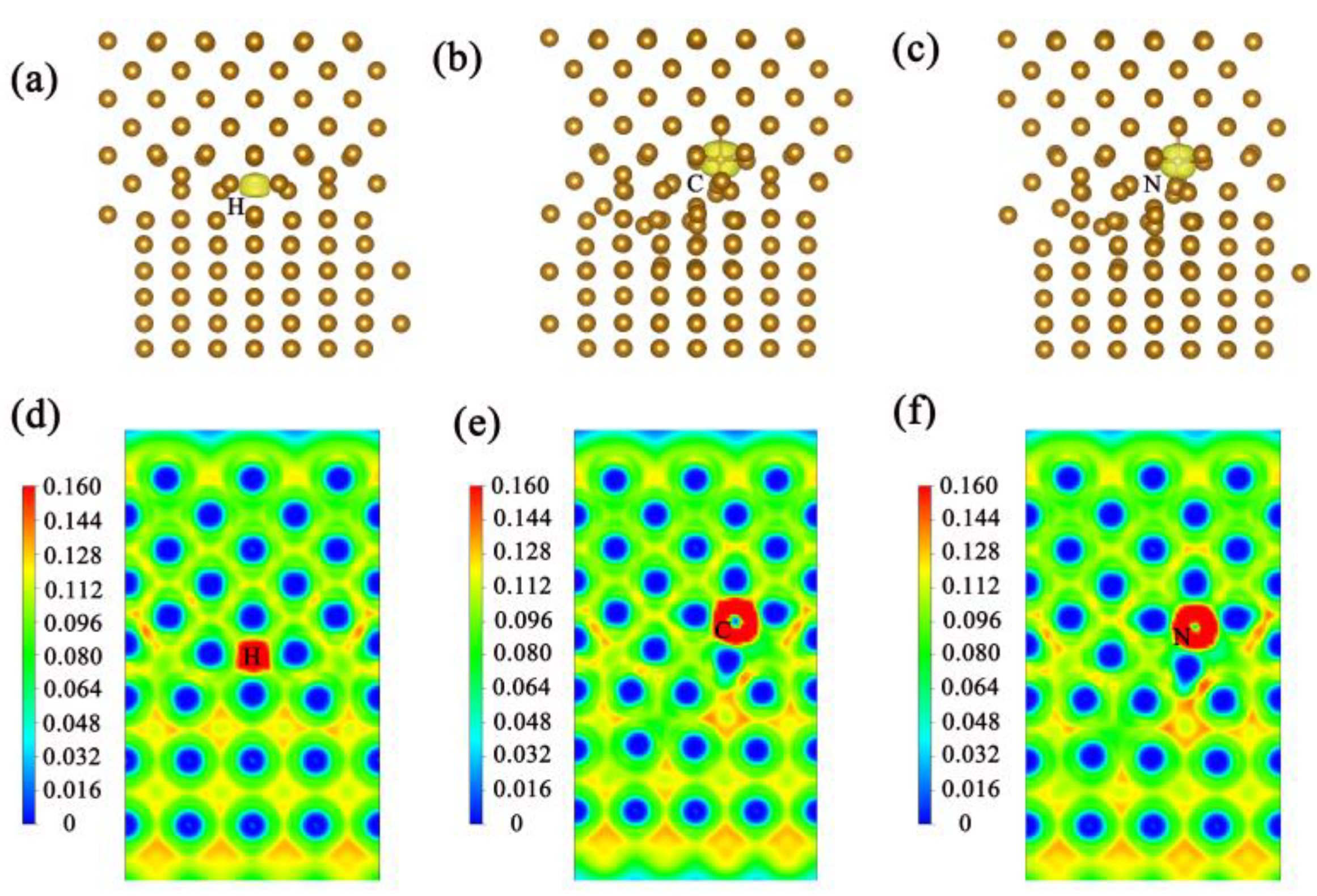
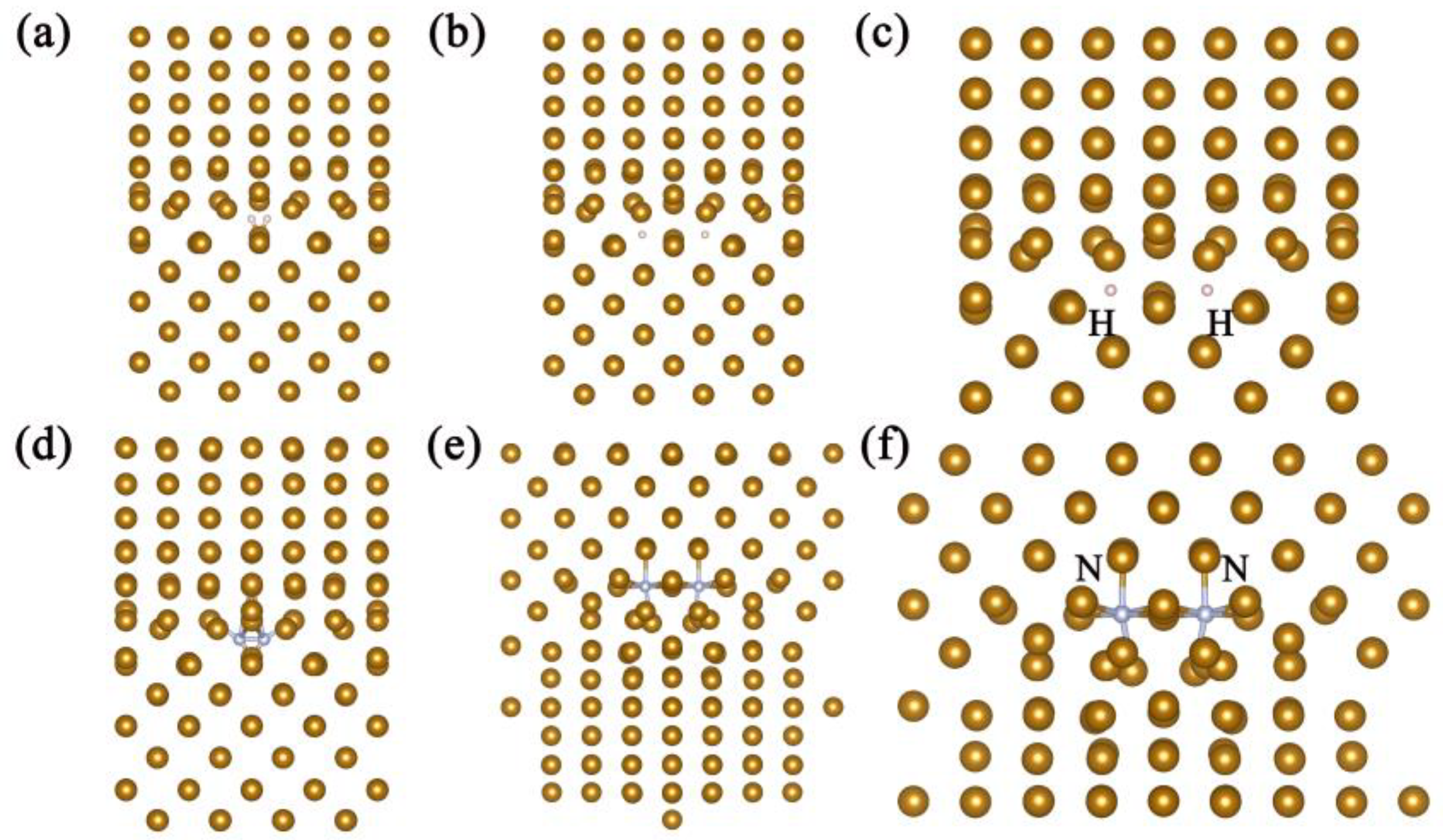
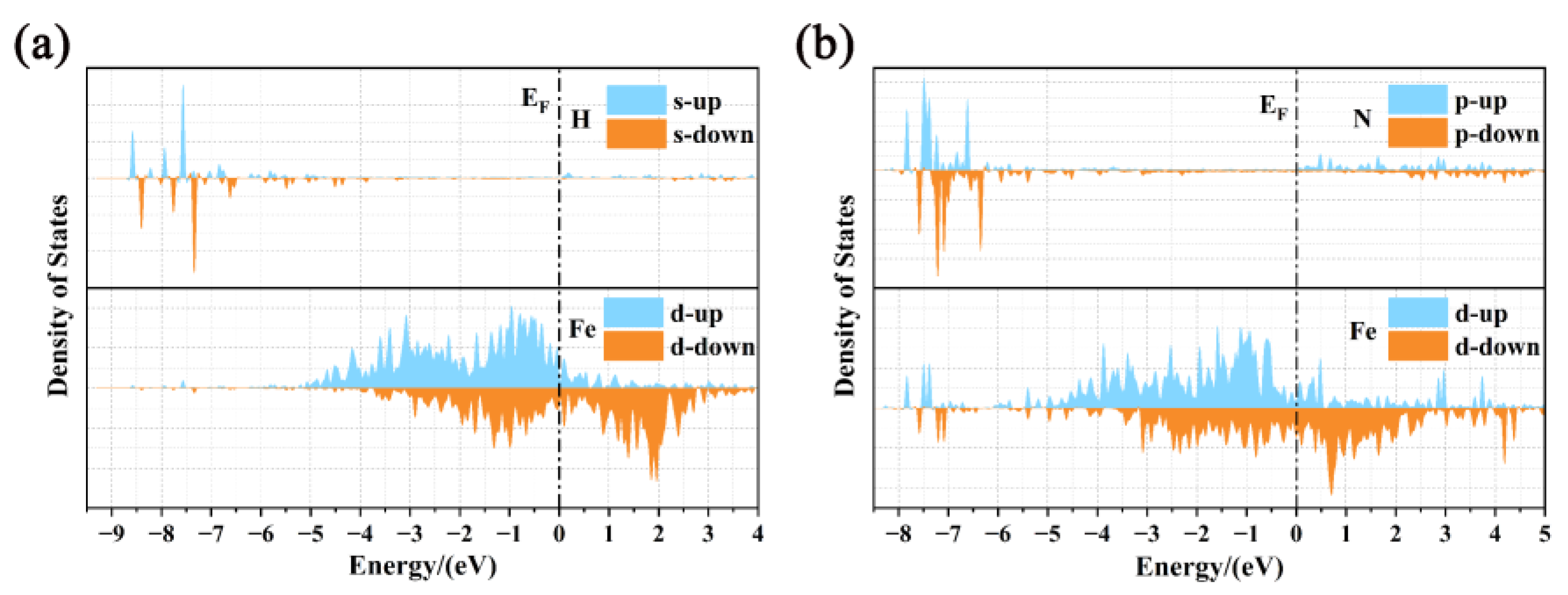
| Pure homojunction | −29.4801 | −4.5601 | 0.3003 |
| Homojunction with H | −29.2527 | −4.5486 | 0.2991 |
| Homojunction with C | −32.3280 | −4.5686 | 0.3057 |
| Homojunction with N | −32.4214 | −4.5585 | 0.2782 |
| Homojunction with H | −29.1385 | −4.5396 | 0.2957 |
| Homojunction with N | −29.0240 | −4.5912 | 0.2220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Chen, B.; Feng, Q.; Xiao, L.; Zhu, X.; Huang, Z.; He, J.; Xu, Y. First-Principles Study on the Effect of H, C, and N at the Interface on Austenite/Ferrite Homojunction. Metals 2023, 13, 317. https://doi.org/10.3390/met13020317
Zhu X, Chen B, Feng Q, Xiao L, Zhu X, Huang Z, He J, Xu Y. First-Principles Study on the Effect of H, C, and N at the Interface on Austenite/Ferrite Homojunction. Metals. 2023; 13(2):317. https://doi.org/10.3390/met13020317
Chicago/Turabian StyleZhu, Xinghua, Bowen Chen, Qingguo Feng, Lei Xiao, Xiaoyang Zhu, Zhiyong Huang, Jianguo He, and Yi Xu. 2023. "First-Principles Study on the Effect of H, C, and N at the Interface on Austenite/Ferrite Homojunction" Metals 13, no. 2: 317. https://doi.org/10.3390/met13020317
APA StyleZhu, X., Chen, B., Feng, Q., Xiao, L., Zhu, X., Huang, Z., He, J., & Xu, Y. (2023). First-Principles Study on the Effect of H, C, and N at the Interface on Austenite/Ferrite Homojunction. Metals, 13(2), 317. https://doi.org/10.3390/met13020317






