Effect of Water Jet Cavitation Peening on Short-Period Oxidation Behavior of Alloy 600 in PWR Primary Water
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of 600 MA after the WJCP Treatment
3.1.1. Residual Stress, Hardness, and Surface Roughness
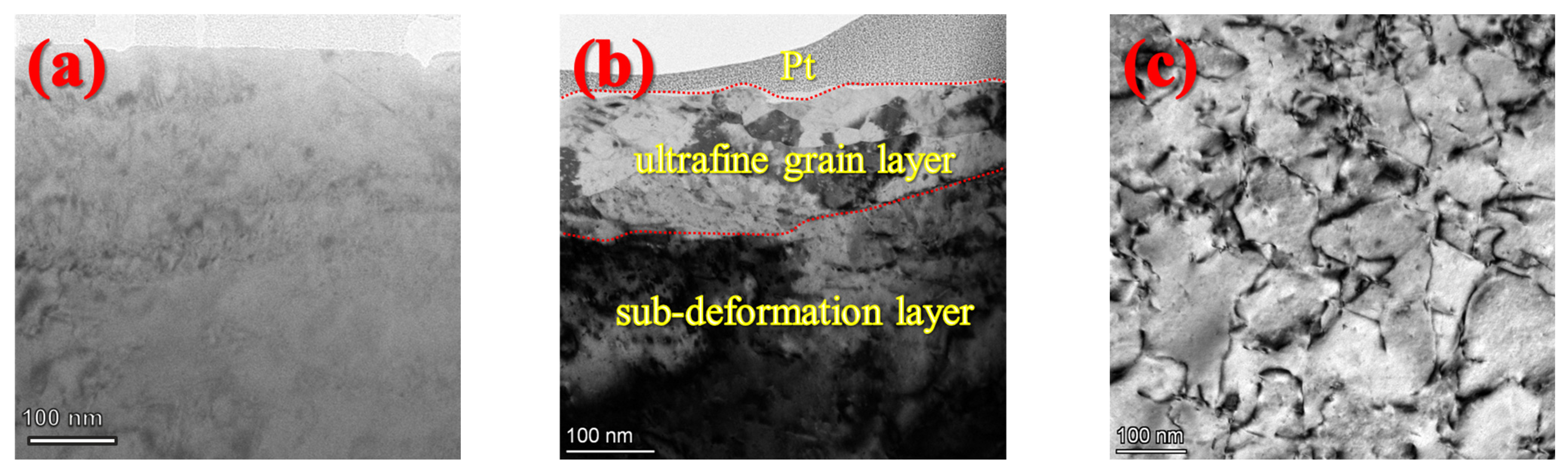
3.1.2. Microstructure
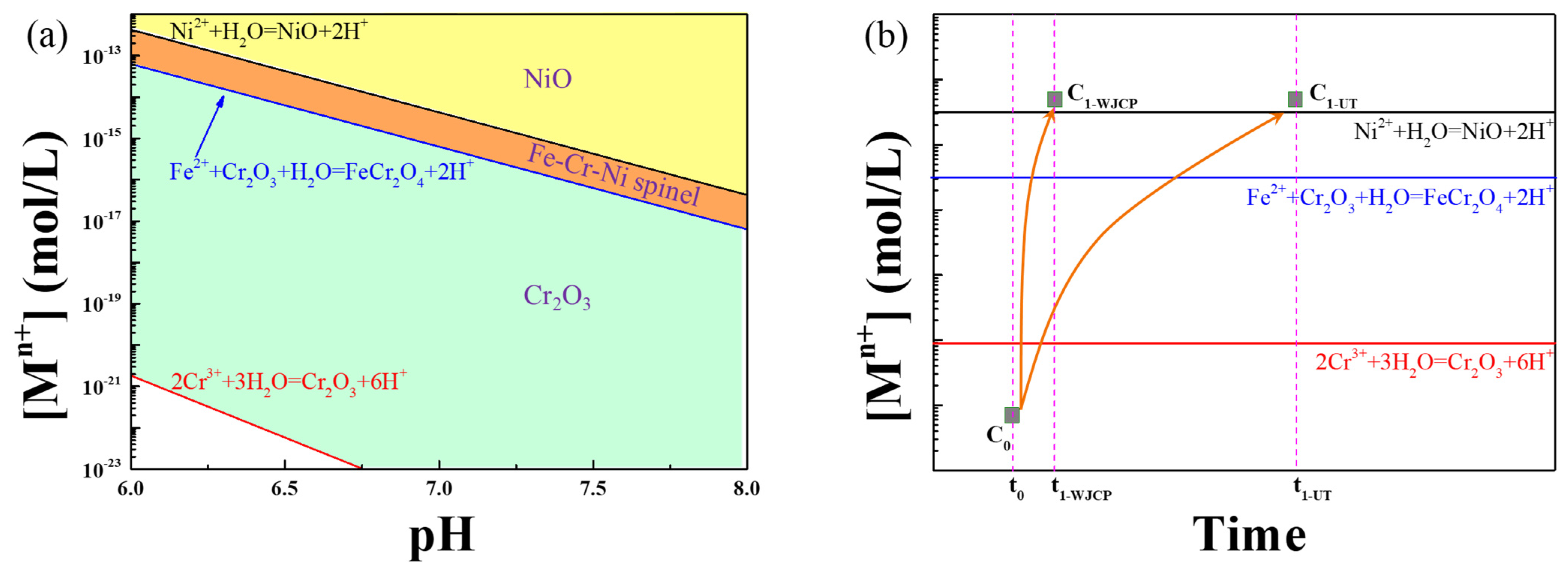
3.2. Characterization of Oxide Scales after the Exposure Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, Y.S.; Hwang, S.S.; Kim, S.W.; Kim, H.P. Primary water stress corrosion cracking behavior of an alloy 600/182 weld. Corros. Sci. 2015, 100, 12–22. [Google Scholar] [CrossRef]
- Min, S.; Liu, H.; Wang, J.; Hou, J. Early oxidation behavior of Ni-based Alloy 690 in simulated pressurized water reactor environment with varying surface conditions. Corros. Commun. 2021, 4, 68–81. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhang, Z.; Tan, J.; Han, E.; Ke, W. Some fundamental understandings of Zn-injection water chemistry on material corrosion in pressurized water reactor primary circuit. Corros. Commun. 2022, 6, 52–61. [Google Scholar] [CrossRef]
- Persaud, S.Y.; Langelier, B.; Korinek, A.; Ramamurthy, S.; Botton, G.A.; Newman, R.C. Characterization of initial intergranular oxidation processes in alloy 600 at a sub-nanometer scale. Corros. Sci. 2018, 133, 36–47. [Google Scholar] [CrossRef]
- Wang, Z.H.; Takeda, Y. Mechanistic understanding of the roles of hydrogen in modification of oxide film of alloy 600 in high temperature high pressure water environment. Corros. Sci. 2020, 170, 108656. [Google Scholar] [CrossRef]
- Wang, Z.H.; Takeda, Y. Amorphization and structural modification of the oxide film of Ni-based alloy by in-situ H charging in high temperature high pressure water environment. Corros. Sci. 2020, 166, 108474. [Google Scholar] [CrossRef]
- Telang, A.; Gill, A.S.; Teysseyre, S.; Mannava, S.R.; Qian, D.; Vasudevan, V.K. Effects of laser shock peening on SCC behavior of alloy 600 in tetrathionate solution. Corros. Sci. 2015, 90, 434–444. [Google Scholar] [CrossRef]
- Nishikawa, S.; Ooi, K.; Takahashi, M.; Furukawa, T. Influence of shot peening and thermal ageing treatment on resistance to intergranular corrosion in shielded metal arc weld metal for type 600 nickel base alloy. Weld. Int. 2017, 31, 837–845. [Google Scholar] [CrossRef]
- Lu, J.Z.; Luo, K.Y.; Yang, D.K.; Cheng, X.N.; Hu, J.L.; Dai, F.Z.; Qi, H.; Zhang, L.; Zhong, J.S.; Wang, Q.W.; et al. Effects of laser peening on stress corrosion cracking (SCC) of AISI 304 austenitic stainless steel. Corros. Sci. 2012, 60, 145–152. [Google Scholar] [CrossRef]
- Lu, Z.M.; Xu, F.; Tang, C.; Cui, Y.; Xu, H.; Mao, J.F. Stress corrosion cracking susceptibility of 304 stainless steel subjected to laser shock peening without coating. J. Mater. Eng. Perform. 2021, 30, 7163–7170. [Google Scholar] [CrossRef]
- Zhou, J.; Retraint, D.; Sun, Z.; Kanouté, P. Comparative study of the effects of surface mechanical attrition treatment and conventional shot peening on low cycle fatigue of a 316L stainless steel. Surf. Coat. Technol. 2022, 349, 556–566. [Google Scholar] [CrossRef]
- Lopez-Ruiz, P.; Garcia-Blanco, M.B.; Vara, G.; Fernández-Pariente, I.; Guagliano, M.; Bagherifard, S. Obtaining tailored surface characteristics by combining shot peening and electropolishing on 316L stainless steel. Appl. Surf. Sci. 2019, 492, 1–7. [Google Scholar] [CrossRef]
- Karthik, D.; Swaroop, S. Laser shock peening enhanced corrosion properties in a nickel based Inconel 600 superalloy. J. Alloy Compd. 2017, 694, 1309–1319. [Google Scholar] [CrossRef]
- Ming, T.Y.; Xue, H.; Zhang, T.; Han, Y.L.; Peng, Q.J. Improving the corrosion and stress corrosion cracking resistance of 316L stainless steel in high temperature water by water jet cavitation peening. Surf. Coat. Tech. 2022, 438, 128420. [Google Scholar] [CrossRef]
- Ming, T.Y.; Peng, Q.J.; Han, Y.L.; Zhang, T. Effect of water jet cavitation peening on electrochemical corrosion behavior of nickel-based alloy 600 in NaCl solution. Mater. Chem. Phys. 2023, 295, 127122. [Google Scholar] [CrossRef]
- Kuang, W.J.; Wu, X.Q.; Han, E.H.; Rao, J.C. The mechanism of oxide film formation on alloy 690 in oxygenated high temperature water. Corros. Sci. 2011, 53, 3853–3860. [Google Scholar] [CrossRef]
- Feng, H.; Dai, J.; Li, H.B.; Jiang, Z.H.; Qu, J.D.; Zhao, Y.; Zhang, S.C.; Zhang, T. Sn microalloying enhances corrosion resistance of stainless steel by accelerating heterogeneous nucleation of passive film. Corros. Sci. 2022, 201, 110279. [Google Scholar] [CrossRef]
- Huang, R.; Han, Y. The effect of SMAT-induced grain refinement and dislocation on the corrosion behavior of Ti-25Nb-3Mo-3Zr-2Sn alloy. Mater. Sci. Eng. C 2013, 33, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Li, Y.S.; Zhu, Y.T.; Bai, Y.K.; Yang, B. Improved corrosion resistance of 316LN stainless steel performed by rotationally accelerated shot peening. Appl. Surf. Sci. 2019, 481, 1305–1312. [Google Scholar] [CrossRef]


| Element | Cr | Fe | C | S | Mn | Si | Cu | Ni |
|---|---|---|---|---|---|---|---|---|
| Composition | 16.79 | 8.29 | 0.033 | <0.010 | 0.46 | 0.11 | <0.010 | Bal. |
| Specimen | Residual Stress (MPa) | Hardness (HV) | Surface Roughness (μm) |
|---|---|---|---|
| UT | 55 ± 18 | 162.8 ± 3.5 | 1.3 ± 0.2 |
| WJCP | −535 ± 25 | 201.2 ± 5.8 | 2.1 ± 0.3 |
| Position | Fe | Cr | Ni | O | Structure |
|---|---|---|---|---|---|
| A | 4.13 | 0.26 | 47.05 | 48.56 | NiO |
| B | 5.69 | 16.54 | 18.94 | 58.83 | Fe-Cr-Ni spinel + Cr2O3 |
| C | 3.55 | 0.32 | 47.31 | 48.81 | NiO |
| D | 4.20 | 16.24 | 19.83 | 59.74 | Fe-Cr-Ni spinel + Cr2O3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, T.; Peng, Q.; Han, Y.; Zhang, T. Effect of Water Jet Cavitation Peening on Short-Period Oxidation Behavior of Alloy 600 in PWR Primary Water. Metals 2023, 13, 336. https://doi.org/10.3390/met13020336
Ming T, Peng Q, Han Y, Zhang T. Effect of Water Jet Cavitation Peening on Short-Period Oxidation Behavior of Alloy 600 in PWR Primary Water. Metals. 2023; 13(2):336. https://doi.org/10.3390/met13020336
Chicago/Turabian StyleMing, Tingyun, Qunjia Peng, Yaolei Han, and Tao Zhang. 2023. "Effect of Water Jet Cavitation Peening on Short-Period Oxidation Behavior of Alloy 600 in PWR Primary Water" Metals 13, no. 2: 336. https://doi.org/10.3390/met13020336
APA StyleMing, T., Peng, Q., Han, Y., & Zhang, T. (2023). Effect of Water Jet Cavitation Peening on Short-Period Oxidation Behavior of Alloy 600 in PWR Primary Water. Metals, 13(2), 336. https://doi.org/10.3390/met13020336






