Effect of Cr on the Microstructure and Mechanical Properties of the Al-Cu-Y-Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloys Preparation
2.2. Microstructure and Phase Composition Analyses
2.3. Sample Preparation for Microstructure Investigation
2.4. Heat Treatment Processing
2.5. Thermodynamic Calculations
2.6. Mechanical Properties Measurements and Calculations
3. Results and Discussion
3.1. As-Cast Microstructure and Phase Composition
3.2. Evaluation of the Microstructure under Solution Treatment
3.3. Phase Transformation under Solution and Aging Treatment
3.4. Yield Strength and Hardness Calculations
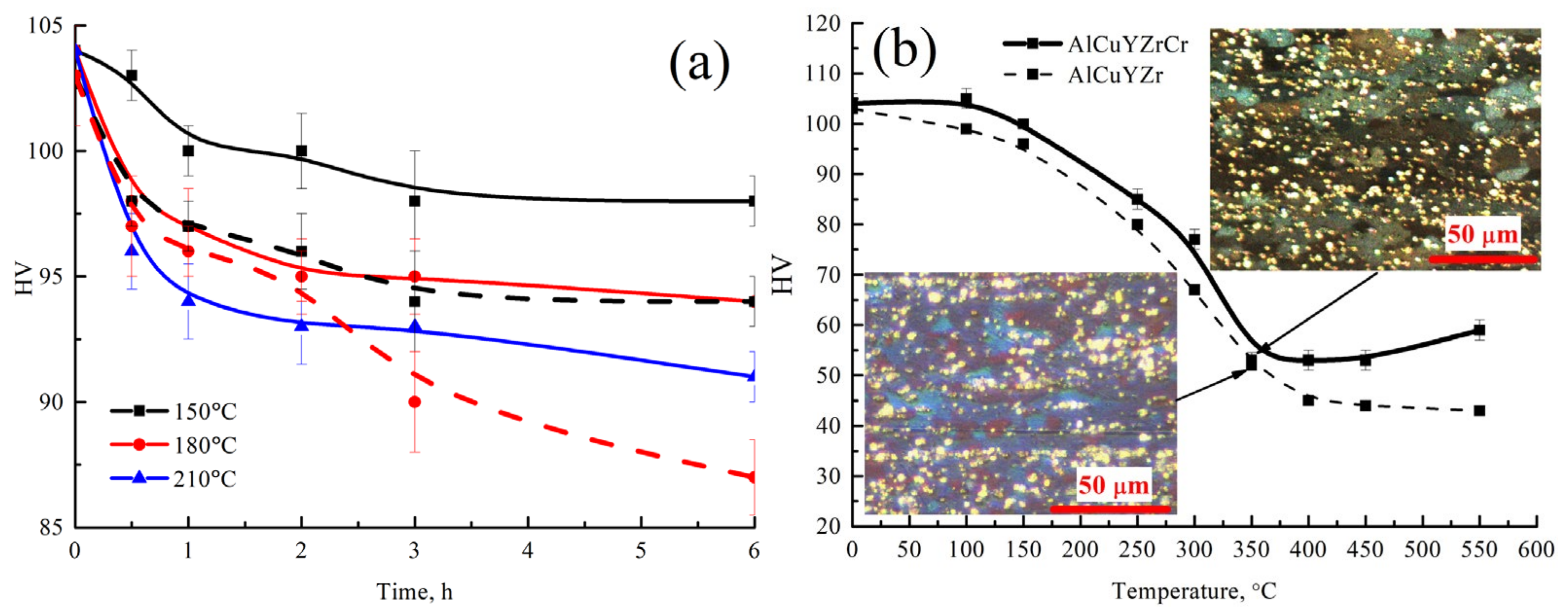
3.5. Microstructure and Hardness Evaluation after Rolling and Annealing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ASM International Handbook Committee. ASM Handbook Volume 2: Properties and Selection—Nonferrous Alloys and Special-Purpose Materials; Materials Park: Novelty, OH, USA, 2001; ISBN 0871700077. [Google Scholar]
- Zolotorevsky, V.S.; Belov, N.A.; Glazoff, M.V. Casting Aluminum Alloys; Alcoa Technical Center: New Kensington, PA, USA, 2007; ISBN 9780080453705. [Google Scholar]
- Eskin, D.G.; Suyitno; Katgerman, L. Mechanical properties in the semi-solid state and hot tearing of aluminium alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Zolotorevskii, V.S.; Pozdnyakov, A.V.; Churyumov, A.Y. Search for promising compositions for developing new multiphase casting alloys based on Al-Cu-Mg matrix using thermodynamic calculations and mathematic simulation. Phys. Met. Metallogr. 2012, 113, 1052–1060. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Zolotorevskiy, V.S. Determining hot cracking index of Al–Si–Cu–Mg casting alloys calculated using effective solidification range. Int. J. Cast Met. Res. 2014, 27, 193–198. [Google Scholar] [CrossRef]
- Krachan, T.; Stel’makhovych, B.; Kuz’ma, Y. The Y-Cu-Al system. J. Alloys Compd. 2003, 349, 134–139. [Google Scholar] [CrossRef]
- Zhang, L.; Masset, P.J.; Tao, X.; Huang, G.; Luo, H.; Liu, L.; Jin, Z. Thermodynamic description of the Al-Cu-Y ternary system. Calphad Comput. Coupling Phase Diagrams Thermochem. 2011, 35, 574–579. [Google Scholar] [CrossRef]
- Zhang, L.G.; Liu, L.B.; Huang, G.X.; Qi, H.Y.; Jia, B.R.; Jin, Z.P. Thermodynamic assessment of the Al-Cu-Er system. Calphad Comput. Coupling Phase Diagrams Thermochem. 2008, 32, 527–534. [Google Scholar] [CrossRef]
- Zhang, L.; Masset, P.J.; Cao, F.; Meng, F.; Liu, L.; Jin, Z. Phase relationships in the Al-rich region of the Al-Cu-Er system. J. Alloys Compd. 2011, 509, 3822–3831. [Google Scholar] [CrossRef]
- Huang, G.; Liu, L.; Zhang, L.; Jin, Z. Thermodynamic description of the Al-Cu-Yb ternary system supported by first-principles calculations. J. Min. Metall. Sect. B Metall. 2016, 52, 177–183. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V.; Alabin, A.N. Microstructure and phase composition of Al-Ce-Cu alloys in the Al-rich corner. Mater. Sci. Forum 2006, 519, 395–400. [Google Scholar] [CrossRef]
- Belov, N.A.; Khvan, A.V. The ternary Al-Ce-Cu phase diagram in the aluminum-rich corner. Acta Mater. 2007, 55, 5473–5482. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y. Microstructure and materials characterisation of the novel Al–Cu–Y alloy. Mater. Sci. Technol. 2018, 34, 1489–1496. [Google Scholar] [CrossRef]
- Pozdnyakov, A.V.; Barkov, R.Y.; Sarsenbaev, Z.; Amer, S.M.; Prosviryakov, A.S. Evolution of Microstructure and Mechanical Properties of a New Al–Cu–Er Wrought Alloy. Phys. Met. Metallogr. 2019, 120, 614–619. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Yakovtseva, O.A.; Pozdniakov, A.V. Comparative Analysis of Structure and Properties of Quasibinary Al–6.5Cu–2.3Y and Al–6Cu–4.05Er Alloys. Phys. Met. Metallogr. 2020, 121, 476–482. [Google Scholar] [CrossRef]
- Amer, S.; Barkov, R.; Pozdniakov, A. Microstructure and mechanical properties of novel quasibinary al-cu-yb and al-cu-gd alloys. Metals 2021, 11, 476. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Yakovtseva, O.A.; Loginova, I.S.; Pozdniakov, A.V. Effect of Zr on microstructure and mechanical properties of the Al–Cu–Er alloy. Mater. Sci. Technol. 2020, 36, 453–459. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y.; Amer, S.M.; Levchenko, V.S.; Kotov, A.D.; Mikhaylovskaya, A.V. Microstructure, mechanical properties and superplasticity of the Al–Cu–Y–Zr alloy. Mater. Sci. Eng. A 2019, 758, 28–35. [Google Scholar] [CrossRef]
- Amer, S.M.; Mikhaylovskaya, A.V.; Barkov, R.Y.; Kotov, A.D.; Mochugovskiy, A.G.; Yakovtseva, O.A.; Glavatskikh, M.V.; Loginova, I.S.; Medvedeva, S.V.; Pozdniakov, A.V. Effect of Homogenization Treatment Regime on Microstructure, Recrystallization Behavior, Mechanical Properties, and Superplasticity of Al-Cu-Er-Zr Alloy. JOM 2021, 73, 3092–3101. [Google Scholar] [CrossRef]
- Mamzurina, O.I.; Amer, S.M.; Loginova, I.S.; Glavatskikh, M.V.; Mochugovskiy, A.G.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Zr on Microstructure and Mechanical Properties of the Al–Cu–Yb and Al–Cu–Gd Alloys. Metals 2022, 12, 479. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Prosviryakov, A.S.; Pozdniakov, A.V. Structure and Properties of New Heat-Resistant Cast Alloys Based on the Al–Cu–Y and Al–Cu–Er Systems. Phys. Met. Metallogr. 2021, 122, 908–914. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Prosviryakov, A.S.; Pozdniakov, A. V Structure and Properties of New Wrought Al–Cu–Y- and Al–Cu–Er-Based Alloys. Phys. Met. Metallogr. 2021, 122, 915–922. [Google Scholar] [CrossRef]
- Mamzurina, O.I.; Amer, S.M.; Glavatskikh, M.V.; Barkov, R.Y.; Loginova, I.S.; Pozdniakov, A.V. Microstructure and Mechanical Properties of Novel Heat Resistant Cast Al-Cu-Yb(Gd)-Mg-Mn-Zr Alloys. Metals 2022, 12, 2079. [Google Scholar] [CrossRef]
- Karnesky, R.A.; van Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Effects of substituting rare-earth elements for scandium in a precipitation-strengthened Al-0.08 at.%Sc alloy. Scr. Mater. 2006, 55, 437–440. [Google Scholar] [CrossRef]
- Harada, Y.; Dunand, D. Microstructure of Al3Sc with ternary transition-metal additions. Mater. Sci. Eng. A 2002, 329–331, 686–695. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y. Microstructure and mechanical properties of novel Al-Y-Sc alloys with high thermal stability and electrical conductivity. J. Mater. Sci. Technol. 2020, 36, 1–6. [Google Scholar] [CrossRef]
- Gao, H.; Feng, W.; Wang, Y.; Gu, J.; Zhang, Y.; Wang, J.; Sun, B. Structural and compositional evolution of Al3(Zr,Y) precipitates in Al-Zr-Y alloy. Mater. Charact. 2016, 121, 195–198. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y.; Prosviryakov, A.S.; Churyumov, A.Y.; Golovin, I.S.; Zolotorevskiy, V.S. Effect of Zr on the microstructure, recrystallization behavior, mechanical properties and electrical conductivity of the novel Al-Er-Y alloy. J. Alloys Compd. 2018, 765, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Tian, Y.; Gao, H.; Wang, J.; Sun, B. Microstructural evolution and mechanical property of Al–Zr and Al–Zr–Y alloys. Mater. Sci. Eng. A 2014, 616, 132–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, H.; Kuai, Y.; Han, Y.; Wang, J.; Sun, B.; Gu, S.; You, W. Effects of Y additions on the precipitation and recrystallization of Al–Zr alloys. Mater. Charact. 2013, 86, 1–8. [Google Scholar] [CrossRef]
- Cao, F.; Zhu, X.; Wang, S.; Shi, L.; Xu, G.; Wen, J. Quasi-superplasticity of a banded-grained Al-Mg-Y alloy processed by continuous casting-extrusion. Mater. Sci. Eng. A 2017, 690, 433–445. [Google Scholar] [CrossRef]
- Barkov, R.Y.; Pozdniakov, A.V.; Tkachuk, E.; Zolotorevskiy, V.S. Effect of Y on microstructure and mechanical properties of Al-Mg-Mn-Zr-Sc alloy with low Sc content. Mater. Lett. 2018, 217, 135–138. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Wei, Z.; Zhu, Z. The effect of Y on the hot-tearing resistance of Al–5wt.% Cu based alloy. Mater. Des. 2010, 31, 2483–2487. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, F.; Zhang, H.; Wang, S.; Fang, C.; Hao, H. Effects of Gd and Y additions on microstructure and properties of Al–Zn–Mg–Cu–Zr alloys. Mater. Sci. Eng. A 2012, 552, 230–235. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Li, M.; Hu, Y.; Zeng, Q.; Zhang, P. Effect of combined addition of Zr, Ti and Y on microstructure and tensile properties of an Al-Zn-Mg-Cu alloy. Mater. Des. 2022, 223, 111129. [Google Scholar] [CrossRef]
- Chen, K.H.; Fang, H.C.; Zhang, Z.; Chen, X.; Liu, G. Effect of of Yb, Cr and Zr additions on recrystallization and corrosion resistance of Al-Zn-Mg-Cu alloys. Mater. Sci. Eng. A 2008, 497, 426–431. [Google Scholar] [CrossRef]
- Glavatskikh, M.V.; Barkov, R.Y.; Khomutov, M.G.; Pozdniakov, A.V. The Effects of Yttrium and Erbium on the Phase Composition and Aging of the Al–Zn–Mg–Cu–Zr Alloy with a High Copper Content. Phys. Met. Metallogr. 2022, 123, 617–623. [Google Scholar] [CrossRef]
- Mao, G.; Liu, S.; Wu, Z.; Zhu, C.; Gao, W. The effects of Y on primary α-Al and precipitation of hypoeutectic Al-Si alloy. Mater. Lett. 2020, 271, 127795. [Google Scholar] [CrossRef]
- Mao, G.; Yan, H.; Zhu, C.; Wu, Z.; Gao, W. The varied mechanisms of yttrium (Y) modifying a hypoeutectic Al–Si alloy under conditions of different cooling rates. J. Alloys Compd. 2019, 806, 909–916. [Google Scholar] [CrossRef]
- Barkov, R.Y.; Khomutov, M.G.; Glavatskikh, M.V.; Pozdniakov, A.V. The Effect of Yittrium and Zirconium on the Structure and Properties of the Al–5Si–1.3Cu–0.5Mg Alloy. Phys. Met. Metallogr. 2022, 123, 598–603. [Google Scholar] [CrossRef]
- Fang, H.C.; Luo, F.H.; Chen, K.H. Effect of intermetallic phases and recrystallization on the corrosion and fracture behavior of an Al-Zn-Mg-Cu-Zr-Yb-Cr alloy. Mater. Sci. Eng. A 2017, 684, 480–490. [Google Scholar] [CrossRef]
- Fang, H.C.; Chen, K.H.; Chen, X.; Chao, H.; Peng, G.S. Effect of Cr, Yb and Zr additions on localized corrosion of Al–Zn–Mg–Cu alloy. Corros. Sci. 2009, 51, 2872–2877. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Fang, H.; Chen, S. Effect of Cr and Yb additions on microstructure and properties of low copper Al–Zn–Mg–Cu–Zr alloy. Mater. Des. 2012, 36, 279–283. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of Zr, Er and Cr additions on microstructures and properties of Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2014, 610, 10–16. [Google Scholar] [CrossRef]
- Fang, H.C.; Chen, K.H.; Chen, X.; Huang, L.P.; Peng, G.S.; Huang, B.Y. Effect of Zr, Cr and Pr additions on microstructures and properties of ultra-high strength Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2011, 528, 7606–7615. [Google Scholar] [CrossRef]
- MENG, Y.; ZHAO, Z.; CUI, J. Effect of minor Zr and Sc on microstructures and mechanical properties of Al–Mg–Si–Cu–Cr–V alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 1882–1889. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al–Zn–Mg–Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef]
- Deschamps, A.; Brechet, Y. Influence of predeformation and ageing of an Al–Zn–Mg alloy—II. Modeling of precipitation kinetics and yield stress. Acta Mater. 1998, 47, 293–305. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Dong, J.; Gao, N.; Chen, Y.; Cao, L.; Song, H.; Fröck, H.; Milkereit, B.; Starink, M.J. Achieving ultra-high strength of Al-Cu-Li alloys by the combination of high pressure torsion and age-hardening. Mater. Sci. Eng. A 2022, 832, 142504. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, N.; Sha, G.; Ringer, S.P.; Starink, M.J. Microstructural evolution, strengthening and thermal stability of an ultrafine-grained Al–Cu–Mg alloy. Acta Mater. 2016, 109, 202–212. [Google Scholar] [CrossRef]
- Weakley-Bollin, S.C.; Donlon, W.; Donlon, W.; Wolverton, C.; Allison, J.E.; Jones, J.W. Modeling the age-hardening behavior of Al-Si-Cu alloys. Metall. Mater. Trans. A 2004, 35, 2407–2418. [Google Scholar] [CrossRef]
- Desch, P.B.; Schwarz, R.B.; Nash, P. Formation of metastable L12 phases in Al3Zr and Al-12.5%X-25%Zr (X ≡ Li, Cr, Fe, Ni, Cu). J. Less Com. Met. 1991, 69, 168. [Google Scholar] [CrossRef]
- Chen, H.L.; Lin, L.; Mao, P.L.; Liu, Z. Phase stability, electronic, elastic and thermodynamic properties of Al-RE intermetallics in Mg-Al-RE alloy: A first principles study. J. Magnes. Alloys 2015, 3, 197–202. [Google Scholar] [CrossRef]
- Amer, S.M.; Barkov, R.Y.; Pozdniakov, A.V. Effect of Mn on the Phase Composition and Properties of Al–Cu–Y–Zr Alloy. Phys. Met. Metallogr. 2020, 121, 1227–1232. [Google Scholar] [CrossRef]
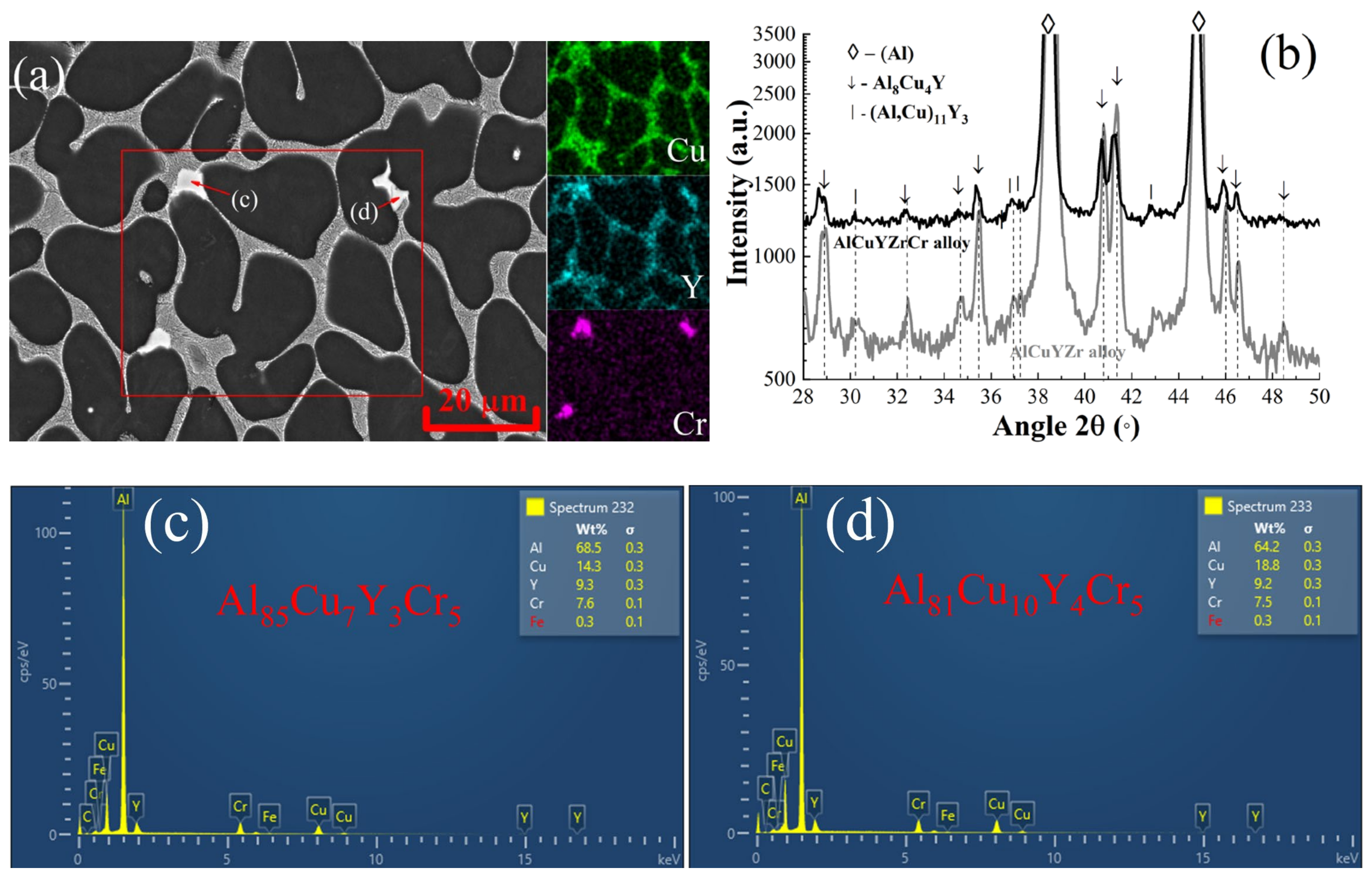
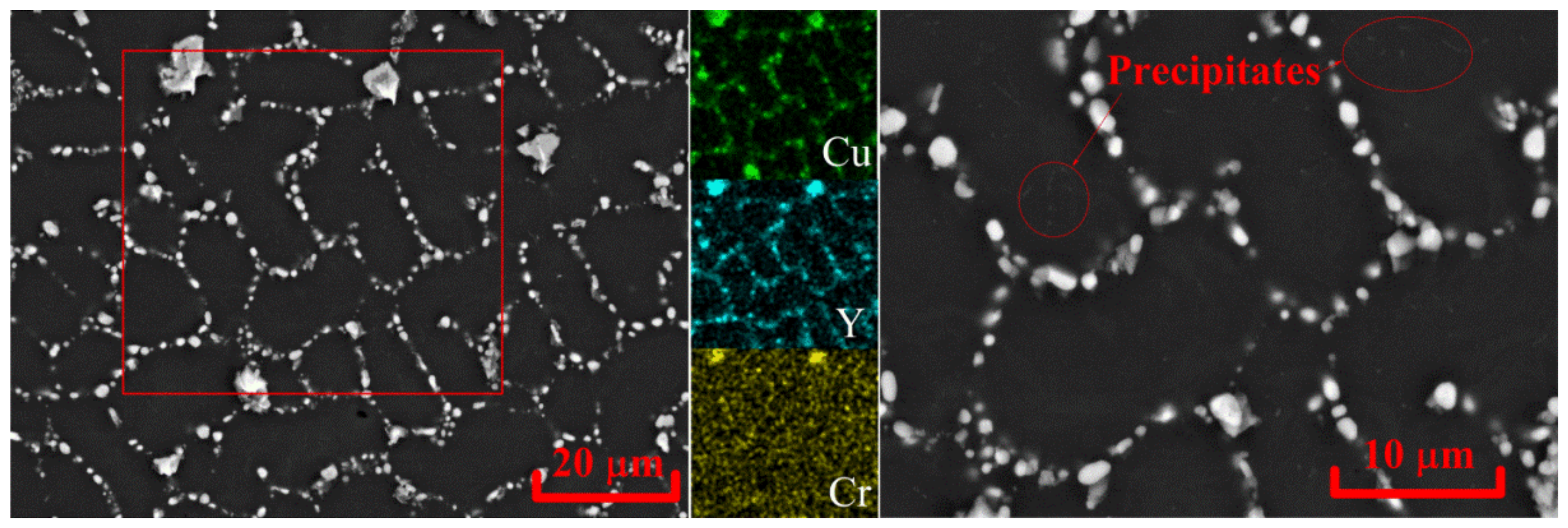
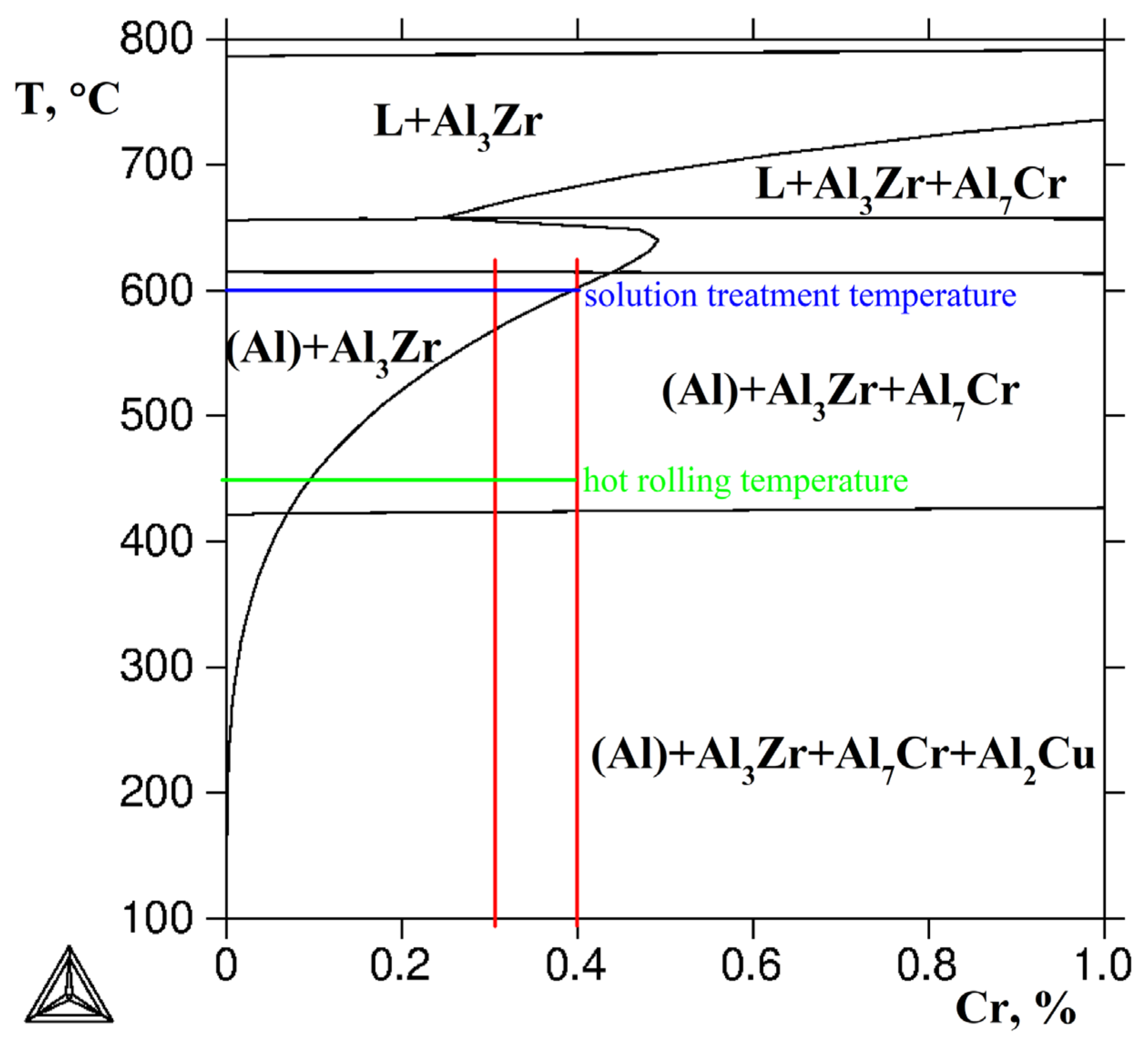

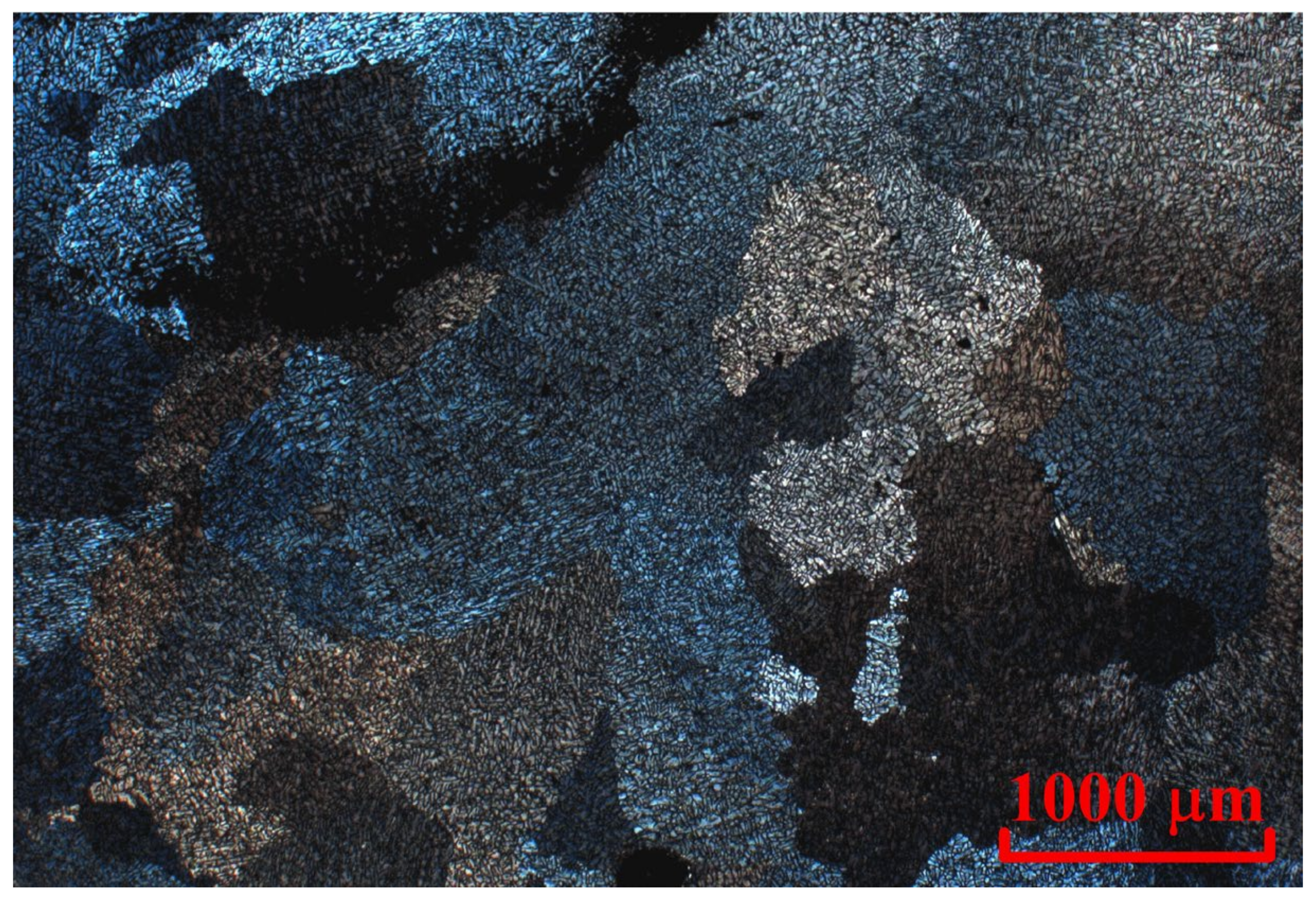

| Alloy | Al | Cu | Y | Zr | Cr | Cu/Y | |
|---|---|---|---|---|---|---|---|
| AlCuYZrCr | wt.% | bal. | 5.1 | 1.7 | 0.3 | 0.3 | - |
| at.% | bal. | 2.27 | 0.54 | 0.09 | 0.16 | 4.2/1 | |
| AlCuYZr [18] | wt.% | bal. | 4.7 | 1.6 | 0.3 | - | - |
| at.% | bal. | 2.08 | 0.51 | 0.09 | - | 4.1/1 | |
| State | Al | Cu | Y | Zr | Cr |
|---|---|---|---|---|---|
| As-cast | bal. | 1.6 | 0.2 | 0.3–0.4 | 0.3–0.4 |
| 600 °C for 1 h | bal. | 1.6 | 0.2 | 0.4 | 0.4 |
| 600 °C for 3 h | bal. | 1.8 | 0.2 | 0.4 | 0.4 |
| 600 °C for 6 h | bal. | 1.8 | 0.2 | 0.3 | 0.4 |
| Equation | Structure Parameters | Contribution, MPa |
|---|---|---|
| = [47,48,49,50] | , k = 0.065 MPa/m−2, d = 500 ± 80 µm | 12.9 |
| [47] | = 109 sm−2 [4] | 21 |
| = 13.8CCu [49] | CCu = 0.1% | 2.5 |
| (Orovan equation [49]) | Eutectic particles (r = 500 nm, f = 0.08) | 12.3 |
| (Orovan equations for disc-shaped particles [52]) | L12 (r = 25 nm, f = 0.005) | 32.4 |
| θ’(Al2Cu) (d = 120 nm, h = 6 nm, f = 0.02 | 29.1 | |
| 110 | ||
| HVcalc = 0.18·+ 41.7 [4] | 61.5 | |
| HVexp | 56 | |
| State | YS, МPа | UTS, МPа | El., % |
|---|---|---|---|
| AlCuYZrCr | |||
| As rolled | 315 ± 5 | 335 ± 9 | 2 ± 1 |
| Annealed at 150 °C for 3 h | 308 ± 2 | 323 ± 3 | 3.3 ± 0.6 |
| Annealed at 180 °C for 3 h | 302 ± 1 | 312 ± 6 | 0.9 ± 0.3 |
| Annealed at 210 °C for 2 h | 265 ± 7 | 292 ± 5 | 1.2 ± 0.2 |
| AlCuYZr [18] | |||
| As rolled | 303 ± 2 | 328 ± 3 | 5.0 ± 0.3 |
| Annealed at 100 °C for 1 h | 292 ± 3 | 320 ± 5 | 5.3 ± 0.2 |
| Annealed at 100 °C for 3 h | 287 ± 1 | 317 ± 3 | 4.8 ± 0.6 |
| Annealed at 150 °C for 1 h | 270 ± 4 | 302 ± 1 | 4.8 ± 0.7 |
| Annealed at 150 °C for 3 h | 270 ± 3 | 299 ± 3 | 6.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, S.M.; Glavatskikh, M.V.; Barkov, R.Y.; Loginova, I.S.; Pozdniakov, A.V. Effect of Cr on the Microstructure and Mechanical Properties of the Al-Cu-Y-Zr Alloy. Metals 2023, 13, 349. https://doi.org/10.3390/met13020349
Amer SM, Glavatskikh MV, Barkov RY, Loginova IS, Pozdniakov AV. Effect of Cr on the Microstructure and Mechanical Properties of the Al-Cu-Y-Zr Alloy. Metals. 2023; 13(2):349. https://doi.org/10.3390/met13020349
Chicago/Turabian StyleAmer, Sayed M., Maria V. Glavatskikh, Ruslan Yu. Barkov, Irina S. Loginova, and Andrey V. Pozdniakov. 2023. "Effect of Cr on the Microstructure and Mechanical Properties of the Al-Cu-Y-Zr Alloy" Metals 13, no. 2: 349. https://doi.org/10.3390/met13020349








