Role of Grain Size and Recrystallization Texture in the Corrosion Behavior of Pure Iron in Acidic Medium
Abstract
:1. Introduction
2. Experimental Details
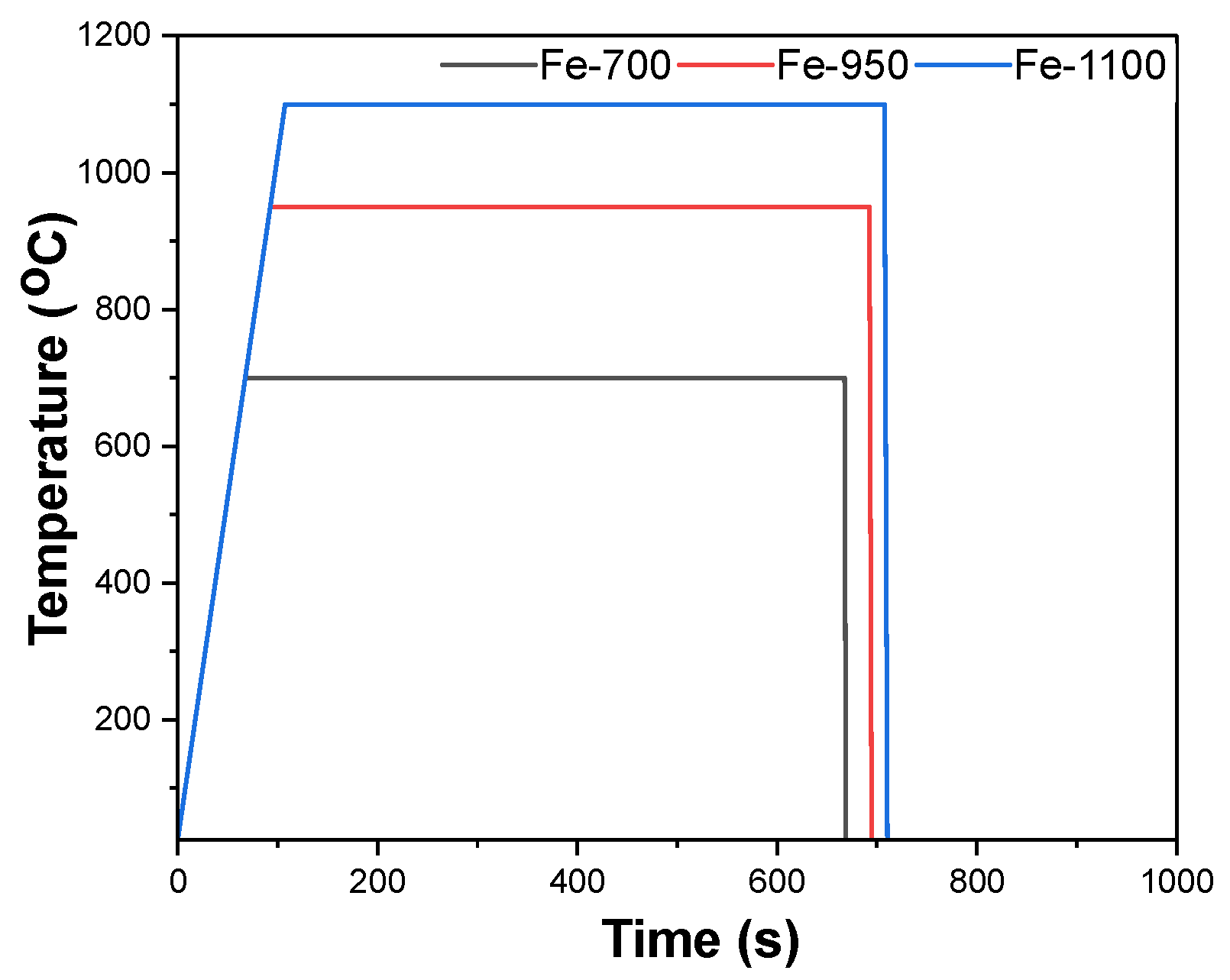
2.1. Material Processing
2.2. Microstructure Characterization
2.3. Electrochemical Characterization
3. Results
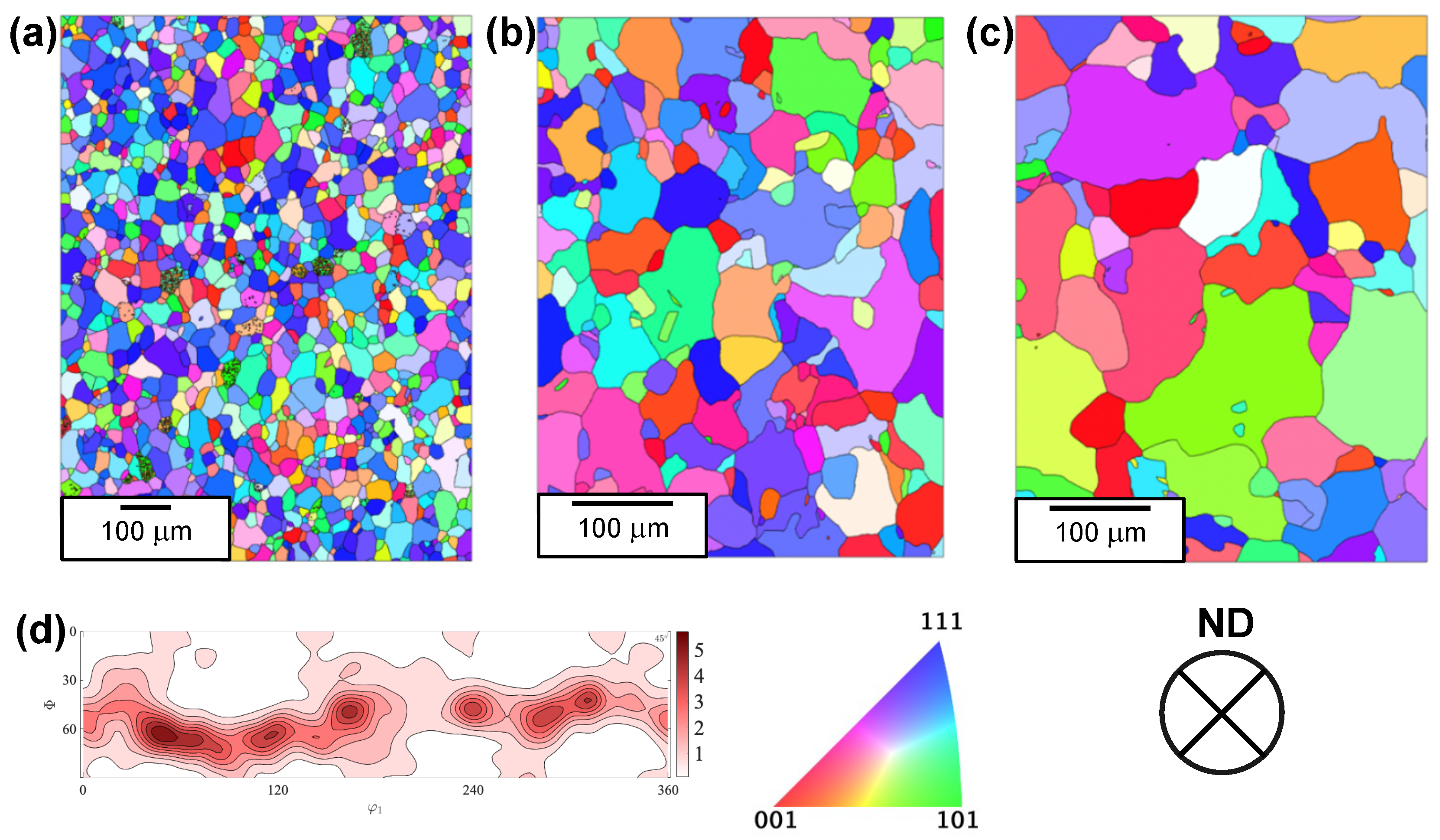
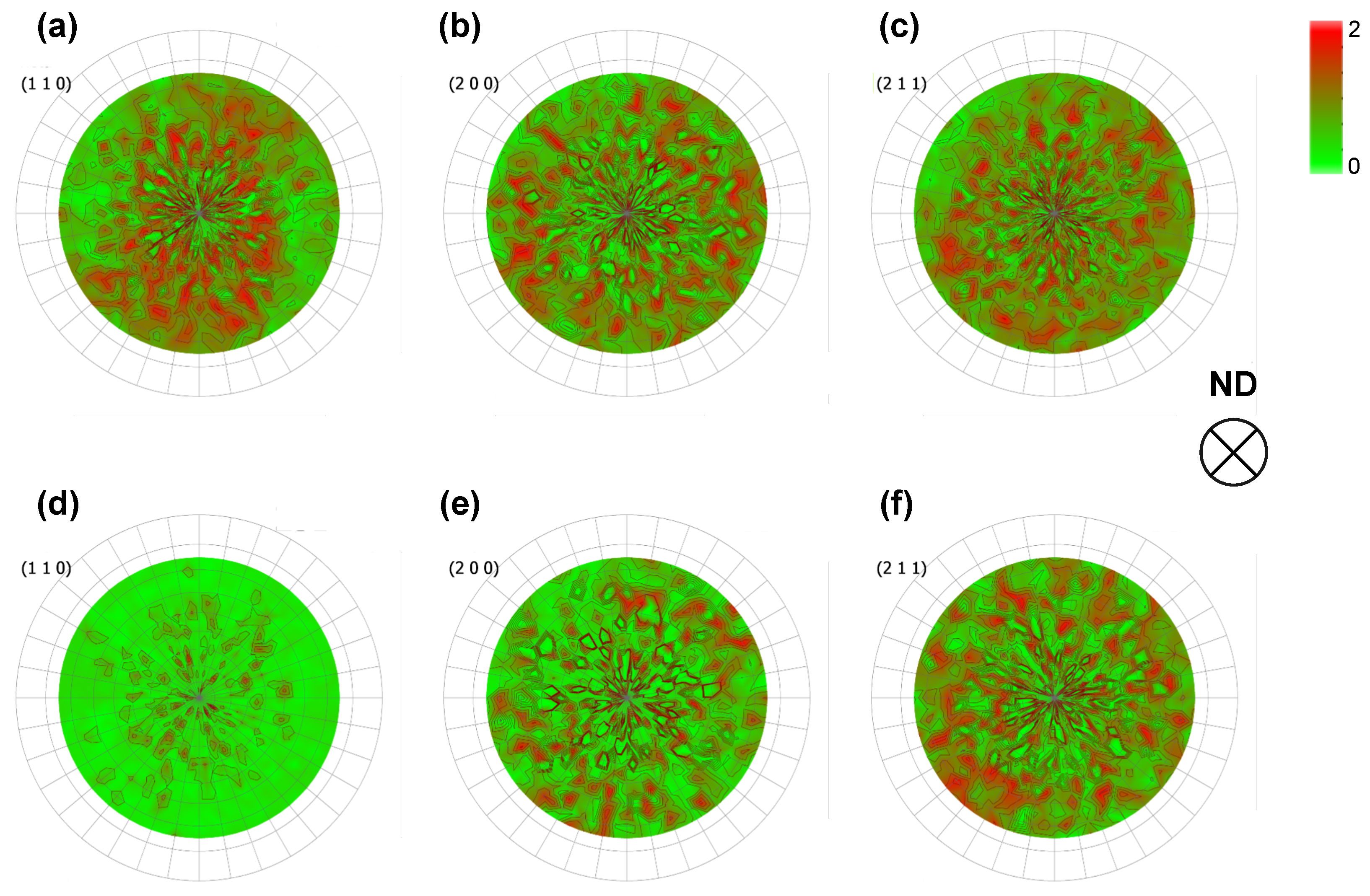
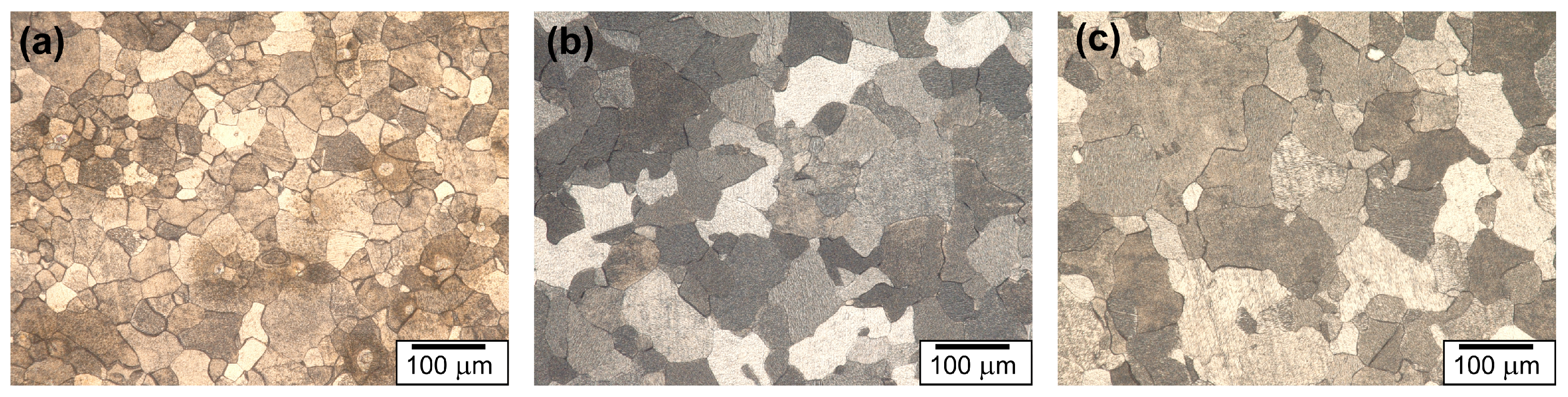
3.1. Microstructure Analysis
3.2. Electrochemical Behavior Analysis
4. Discussion
5. Conclusions
- The corrosion resistance decreases with a reduction in grain size. This conclusion is supported by the lower corrosion resistance of Fe-950 sample than the Fe-1100 sample;
- The recrystallization texture dominates grain size in influencing the corrosion behavior and leads to an improvement in corrosion resistance for a fine-grained sample. This conclusion is supported by the higher corrosion resistance of Fe-700 than Fe-950 sample;
- The corrosion behavior of iron in acidic medium is dominated by the cathodic hydrogen evolution reaction kinetics. This conclusion is supported by the potentiodynamic polarization measurements of the studied samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng-Heng, C.; Preban, A. The effect of ferrite grain size and martensite volume fraction on the tensile properties of dual phase steel. Acta Metall. 1985, 33, 897–903. [Google Scholar] [CrossRef]
- Takaki, S.; Kawasaki, K.; Kimura, Y. Mechanical properties of ultra fine grained steels. J. Mater. Process. Technol. 2001, 117, 359–363. [Google Scholar] [CrossRef]
- Hanamura, T.; Yin, F.; Nagai, K. Ductile-Brittle Transition Temperature of Ultrafine Ferrite/Cementite Microstructure in a Low Carbon Steel Controlled by Effective Grain Size. ISIJ Int. 2004, 44, 610–617. [Google Scholar] [CrossRef]
- Ariza, E.A.; Masoumi, M.; Tschiptschin, A.P. Improvement of tensile mechanical properties in a TRIP-assisted steel by controlling of crystallographic orientation via HSQ&P processes. Mater. Sci. Eng. A 2018, 713, 223–233. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, E.Y.; Woo, W.; Han, S.; Kwak, J. The effect of crystallographic orientation on the micromechanical deformation and failure behaviors of DP980 steel during uniaxial tension. Int. J. Plast. 2013, 45, 85–102. [Google Scholar] [CrossRef]
- Wang, X.; Muñiz-Lerma, J.A.; Attarian Shandiz, M.; Sanchez-Mata, O.; Brochu, M. Crystallographic-orientation-dependent tensile behaviours of stainless steel 316L fabricated by laser powder bed fusion. Mater. Sci. Eng. A 2019, 766, 138395. [Google Scholar] [CrossRef]
- Wang, P.j.; Ma, L.w.; Cheng, X.q.; Li, X.g. Influence of grain refinement on the corrosion behavior of metallic materials: A review. Int. J. Miner. Metall. Mater. 2021, 28, 1112–1126. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yuasa, M.; Rifai, M.; Fujiwara, H. Corrosion Behavior of Severely Deformed Pure and Single-Phase Materials. Mater. Trans. 2019, 60, 1243–1255. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005–075005-13. [Google Scholar] [CrossRef]
- Wang, S.G.; Shen, C.B.; Long, K.; Yang, H.Y.; Wang, F.H.; Zhang, Z.D. Preparation and Electrochemical Corrosion Behavior of Bulk Nanocrystalline Ingot Iron in HCl Acid Solution. J. Phys. Chem. B 2005, 109, 2499–2503. [Google Scholar] [CrossRef]
- Wang, S.G.; Shen, C.B.; Long, K.; Zhang, T.; Wang, F.H.; Zhang, Z.D. The Electrochemical Corrosion of Bulk Nanocrystalline Ingot Iron in Acidic Sulfate Solution. J. Phys. Chem. B 2006, 110, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Jinlong, L.; Hongyun, L. The effects of cold rolling temperature on corrosion resistance of pure iron. Appl. Surf. Sci. 2014, 317, 125–130. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Cheng, X.; Li, X. Effect of grain size and crystallographic orientation on the corrosion behaviors of low alloy steel. J. Alloys Compd. 2021, 857, 158258. [Google Scholar] [CrossRef]
- Fushimi, K.; Azumi, K.; Seo, M. Evaluation of Heterogeneity in Thickness of Passive Films on Pure Iron by Scanning Electrochemical Microscopy. ISIJ Int. 1999, 39, 346–351. [Google Scholar] [CrossRef]
- Schreiber, A.; Schultze, J.; Lohrengel, M.; Kármán, F.; Kálmán, E. Grain dependent electrochemical investigations on pure iron in acetate buffer pH 6.0. Electrochim. Acta 2006, 51, 2625–2630. [Google Scholar] [CrossRef]
- Fushimi, K.; Miyamoto, K.; Konno, H. Anisotropic corrosion of iron in pH 1 sulphuric acid. Electrochim. Acta 2010, 55, 7322–7327. [Google Scholar] [CrossRef]
- Yule, L.C.; Shkirskiy, V.; Aarons, J.; West, G.; Bentley, C.L.; Shollock, B.A.; Unwin, P.R. Nanoscale Active Sites for the Hydrogen Evolution Reaction on Low Carbon Steel. J. Phys. Chem. C 2019, 123, 24146–24155. [Google Scholar] [CrossRef]
- Hutchinson, W.B. Development and control of annealing textures in low-carbon steels. Int. Met. Rev. 1984, 29, 25–42. [Google Scholar] [CrossRef]
- Hölscher, M.; Raabe, D.; Lücke, K. Rolling and recrystallization textures of bcc steels. Steel Res. 1991, 62, 567–575. [Google Scholar] [CrossRef]
- Kestens, L.A.I.; Pirgazi, H. Texture formation in metal alloys with cubic crystal structures. Mater. Sci. Technol. 2016, 32, 1303–1315. [Google Scholar] [CrossRef] [Green Version]
- Young, J.Z.; Smith, S.W.J. The crystal structure of meteoric iron as determined by X-ray analysis. Proc. R. Soc. Lond. Ser. 1926, 112, 630–641. [Google Scholar] [CrossRef]
- Kurdjumow, G.; Sachs, G. Über den Mechanismus der Stahlhärtung. Zeitschrift für Phys. 1930, 64, 325–343. [Google Scholar] [CrossRef]
- Kestens, L.A.I.; Nguyen-Minh, T.; Petrov, R.H. The Role of Parent Phase Topology in Double Young–Kurdjumow–Sachs Variant Selection during Phase Transformation in Low-Carbon Steels. Metals 2022, 12, 939. [Google Scholar] [CrossRef]
- Brent, L.; Adams, J.K. EBSD-Based Microscopy: Resolution of Dislocation Density. Comput. Mater. Contin. 2009, 14, 185–196. [Google Scholar] [CrossRef]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX – Free and Open Source Software Toolbox. In Proceedings of the Texture and Anisotropy of Polycrystals III; Trans Tech Publications Ltd.: Baech, Switzerland, 2010; Volume 160, pp. 63–68. [Google Scholar] [CrossRef]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Grain detection from 2d and 3d EBSD data—Specification of the MTEX algorithm. Ultramicroscopy 2011, 111, 1720–1733. [Google Scholar] [CrossRef]
- Jiang, J.; Britton, T.; Wilkinson, A. Measurement of geometrically necessary dislocation density with high resolution electron backscatter diffraction: Effects of detector binning and step size. Ultramicroscopy 2013, 125, 1–9. [Google Scholar] [CrossRef]
- Konijnenberg, P.; Zaefferer, S.; Raabe, D. Assessment of geometrically necessary dislocation levels derived by 3D EBSD. Acta Mater. 2015, 99, 402–414. [Google Scholar] [CrossRef]
- Bunge, H.J. 2—Orientation of Individual Crystallites. In Texture Analysis in Materials Science; Bunge, H.J., Ed.; Butterworth-Heinemann: Oxford, UK, 1982; pp. 3–41. [Google Scholar] [CrossRef]
- Bai, L.; Conway, B.E. AC Impedance of Faradaic Reactions Involving Electrosorbed Intermediates: Examination of Conditions Leading to Pseudoinductive Behavior Represented in Three-Dimensional Impedance Spectroscopy Diagrams. J. Electrochem. Soc. 1991, 138, 2897–2907. [Google Scholar] [CrossRef]
- Lai, W.Y.; Zhao, W.Z.; Yin, Z.F.; Zhang, J. Electrochemical and XPS studies on corrosion behaviours of AISI 304 and AISI 316 stainless steels under plastic deformation in sulphuric acid solution. Surf. Interface Anal. 2012, 44, 505–512. [Google Scholar] [CrossRef]
- Bellezze, T.; Giuliani, G.; Viceré, A.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 2: Anodic selective dissolution, weight loss and electrochemical impedance spectroscopy tests. Corros. Sci. 2018, 130, 12–21. [Google Scholar] [CrossRef]
- Xu, W.; Han, E.H.; Wang, Z. Effect of tannic acid on corrosion behavior of carbon steel in NaCl solution. J. Mater. Sci. Technol. 2019, 35, 64–75. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Zhao, Y.; Sun, X.; Zhang, H.; Piao, H.G.; Zhang, Y.; Huang, Y. The effect of magnetic field pretreatment on the corrosion behavior of carbon steel in static seawater. RSC Adv. 2020, 10, 2060–2066. [Google Scholar] [CrossRef]
- Schreiber, A.; Rosenkranz, C.; Lohrengel, M. Grain-dependent anodic dissolution of iron. Electrochim. Acta 2007, 52, 7738–7745. [Google Scholar] [CrossRef]
- Schuh, C.A.; Anderson, K.; Orme, C. Rapid assessment of anisotropic surface processes: Experiments on the corrosion of Inconel 600. Surf. Sci. 2003, 544, 183–192. [Google Scholar] [CrossRef]
- Gray, J.; El Dasher, B.; Orme, C. Competitive effects of metal dissolution and passivation modulated by surface structure: An AFM and EBSD study of the corrosion of alloy 22. Surf. Sci. 2006, 600, 2488–2494. [Google Scholar] [CrossRef]
- Jin, H.; Blackwood, D.J.; Wang, Y.; Ng, M.F.; Tan, T.L. First-principles study of surface orientation dependent corrosion of BCC iron. Corros. Sci. 2022, 196, 110029. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Grote, J.P.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Gutić, S.J.; Dobrota, A.S.; Fako, E.; Skorodumova, N.V.; López, N.; Pašti, I.A. Hydrogen Evolution Reaction-From Single Crystal to Single Atom Catalysts. Catalysts 2020, 10, 290. [Google Scholar] [CrossRef] [Green Version]



| Element | Fe | C | N | Si | Mn | P | S | Al | Cr | Cu | Mo | Ni | Sn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | bal. | 0.001 | 0.003 | 0.003 | 0.04 | 0.003 | 0.003 | 0.004 | 0.014 | 0.007 | 0.001 | 0.012 | 0.002 |
| Sample | Average Grain Size (µm) | Grain Boundary Density (µm−1) | HAGB Density (µm−1) | LAGB Density (µm−1) | GND (m−2) |
|---|---|---|---|---|---|
| Fe-700 | 26 | 0.102 | 0.096 | 0.006 | 2.3 ± 0.4 × 1013 |
| Fe-950 | 53 | 0.052 | 0.050 | 0.002 | 7.9 ± 0.2 × 1012 |
| Fe-1100 | 87 | 0.033 | 0.032 | 0.001 | 4.4 ± 0.2 × 1012 |
| Sample | R1 (cm2) | R2 (cm2) | CPE-Y0 (−1cm−2sn × 10−5) | n | R3 (cm2) | L (H·cm2) |
|---|---|---|---|---|---|---|
| Fe-700 | 7 ± 2 | 380 ± 40 | 1.6 ± 0.1 | 0.80 ± 0.01 | 90 ± 30 | 30 ± 10 |
| Fe-950 | 7 ± 1 | 220 ± 50 | 2.3 ± 0.3 | 0.84 ± 0.02 | 60 ± 20 | 10 ± 4 |
| Fe-1100 | 11 ± 3 | 470 ± 40 | 1.7 ± 0.2 | 0.83 ± 0.01 | 130 ± 30 | 50 ± 10 |
| Sample | Ecorr (mV vs. Ag/AgCl) | icorr (µAcm−2) | anodic (mV dec−1) | cathodic (mV dec−1) |
|---|---|---|---|---|
| Fe-700 | −467 ± 7 | 83 ± 5 | 42 ± 5 | −198 ± 8 |
| Fe-950 | −466 ± 4 | 148 ± 4 | 49 ± 4 | −199 ± 4 |
| Fe-1100 | −465 ± 8 | 73 ± 1 | 45 ± 4 | −197 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, S.; Yilmaz, A.; Traka, K.; Sietsma, J.; Gonzalez-Garcia, Y. Role of Grain Size and Recrystallization Texture in the Corrosion Behavior of Pure Iron in Acidic Medium. Metals 2023, 13, 388. https://doi.org/10.3390/met13020388
Kar S, Yilmaz A, Traka K, Sietsma J, Gonzalez-Garcia Y. Role of Grain Size and Recrystallization Texture in the Corrosion Behavior of Pure Iron in Acidic Medium. Metals. 2023; 13(2):388. https://doi.org/10.3390/met13020388
Chicago/Turabian StyleKar, Satyakam, Aytac Yilmaz, Konstantina Traka, Jilt Sietsma, and Yaiza Gonzalez-Garcia. 2023. "Role of Grain Size and Recrystallization Texture in the Corrosion Behavior of Pure Iron in Acidic Medium" Metals 13, no. 2: 388. https://doi.org/10.3390/met13020388
APA StyleKar, S., Yilmaz, A., Traka, K., Sietsma, J., & Gonzalez-Garcia, Y. (2023). Role of Grain Size and Recrystallization Texture in the Corrosion Behavior of Pure Iron in Acidic Medium. Metals, 13(2), 388. https://doi.org/10.3390/met13020388







