Erosion–Corrosion Behavior of 90/10 and 70/30 Copper–Nickel Tubes in 1 wt% NaCl Solution
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. In Situ Electrochemical Monitoring during Erosion–Corrosion Test
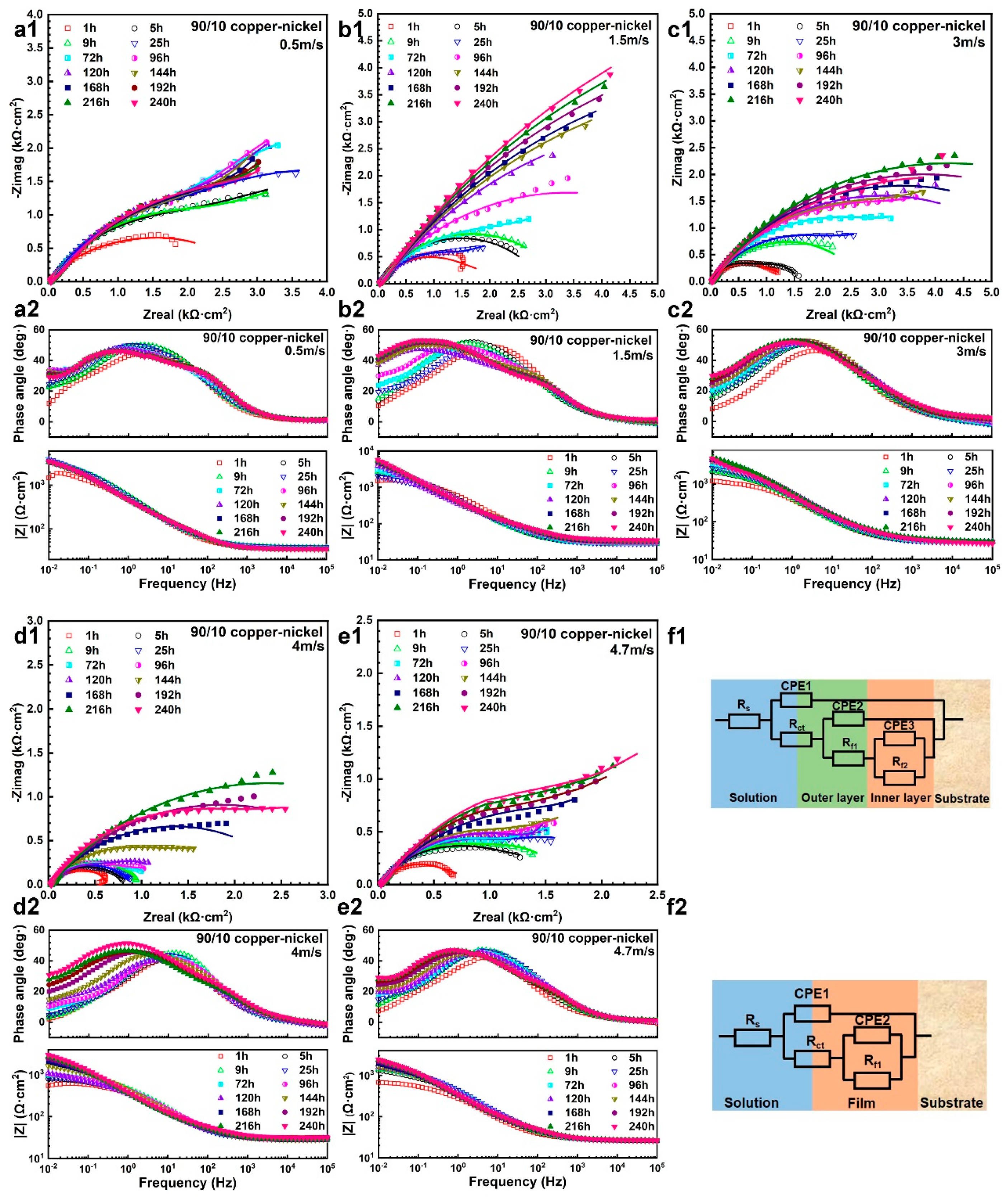
3.1.1. Electrochemical Impedance Spectrum
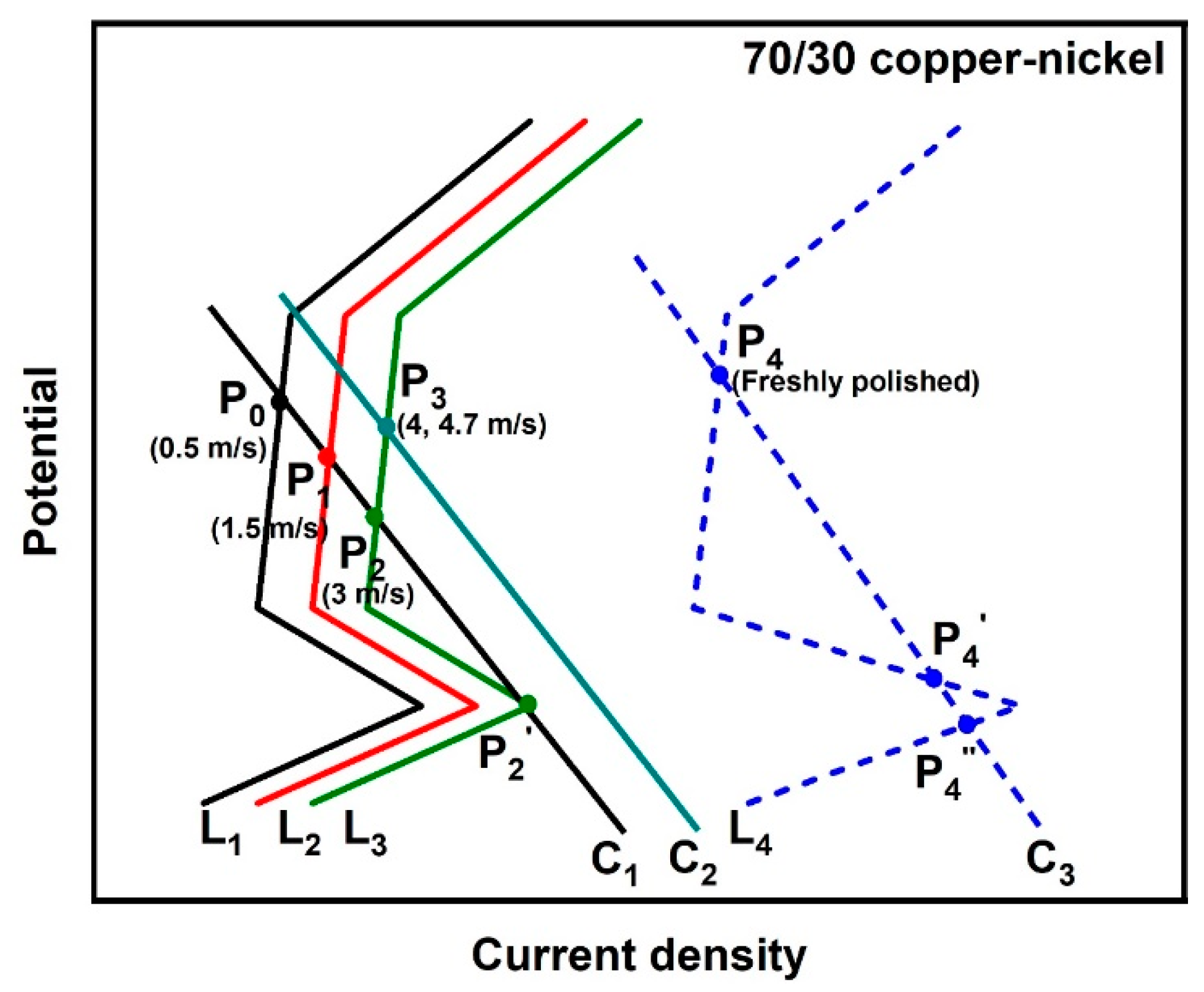
3.1.2. Potentiodynamic Polarization Curves
3.2. Ex Situ Characterization of Corrosion Product Film after Erosion–Corrosion Test
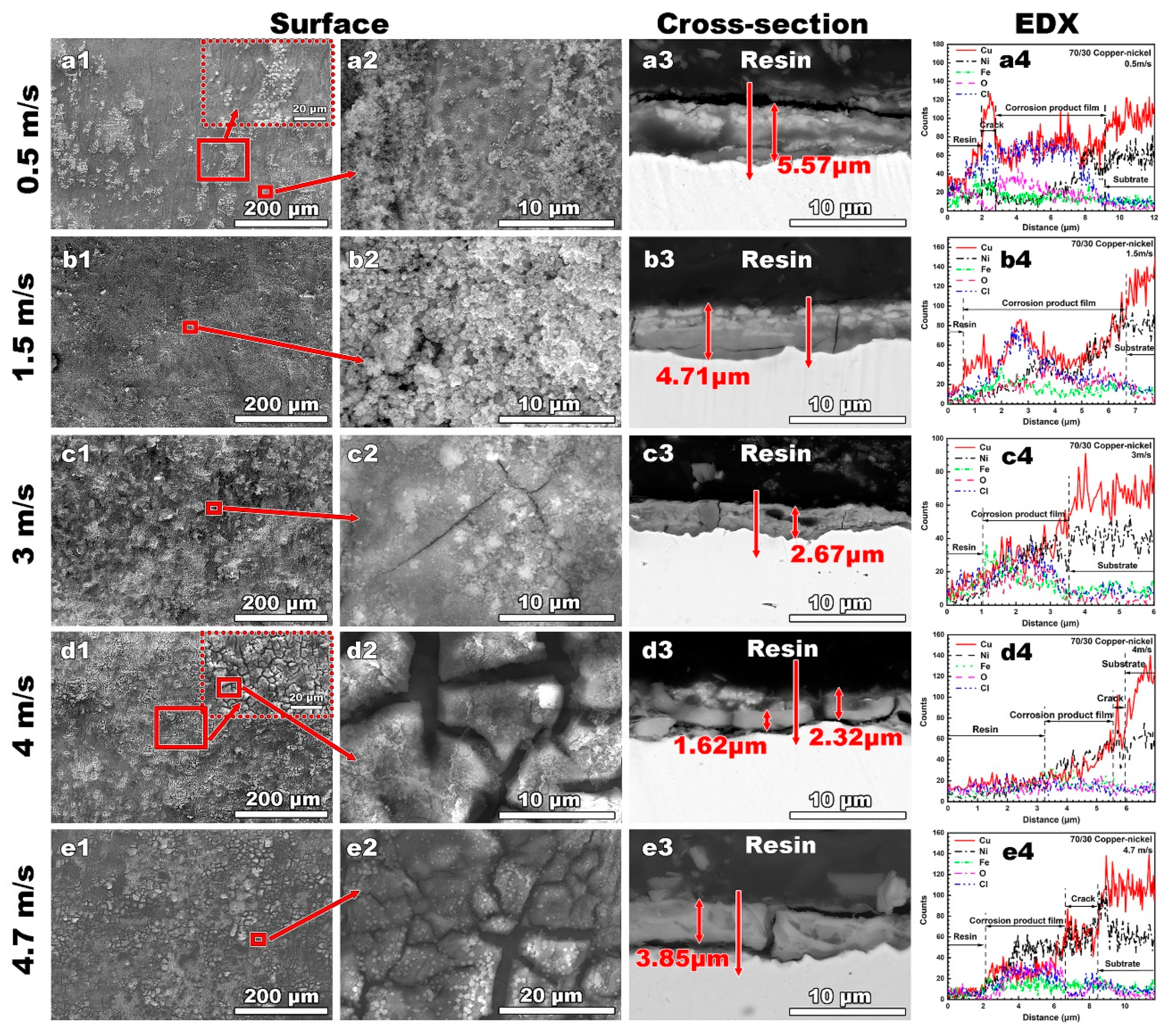
3.2.1. SEM and EDS Results
3.2.2. XPS Results
4. Discussion
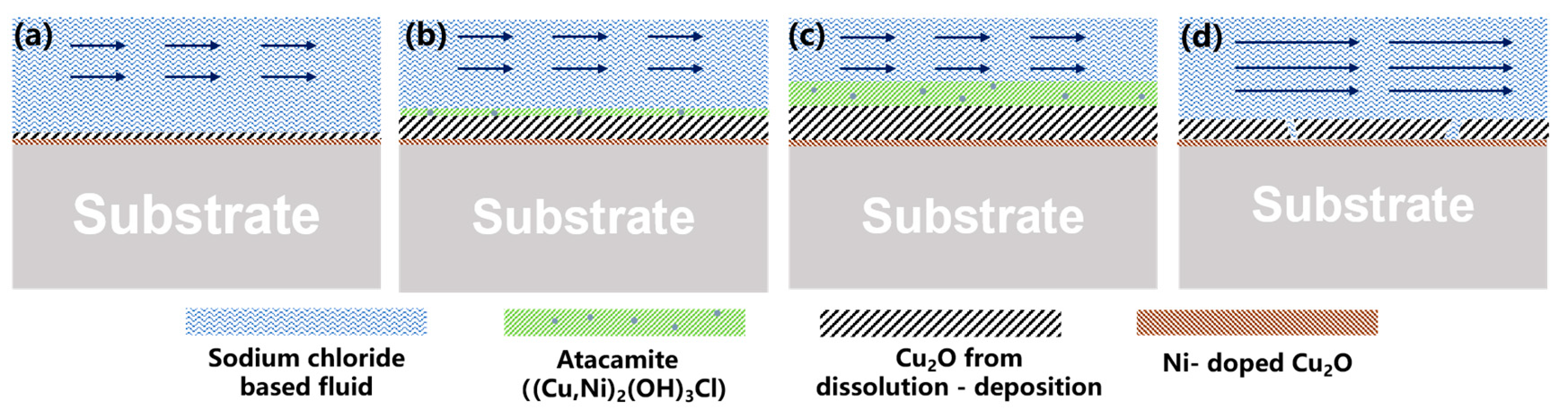
4.1. Erosion–Corrosion Behavior of the 90/10 Copper–Nickel Tube in Sodium Chloride-Based Fluid
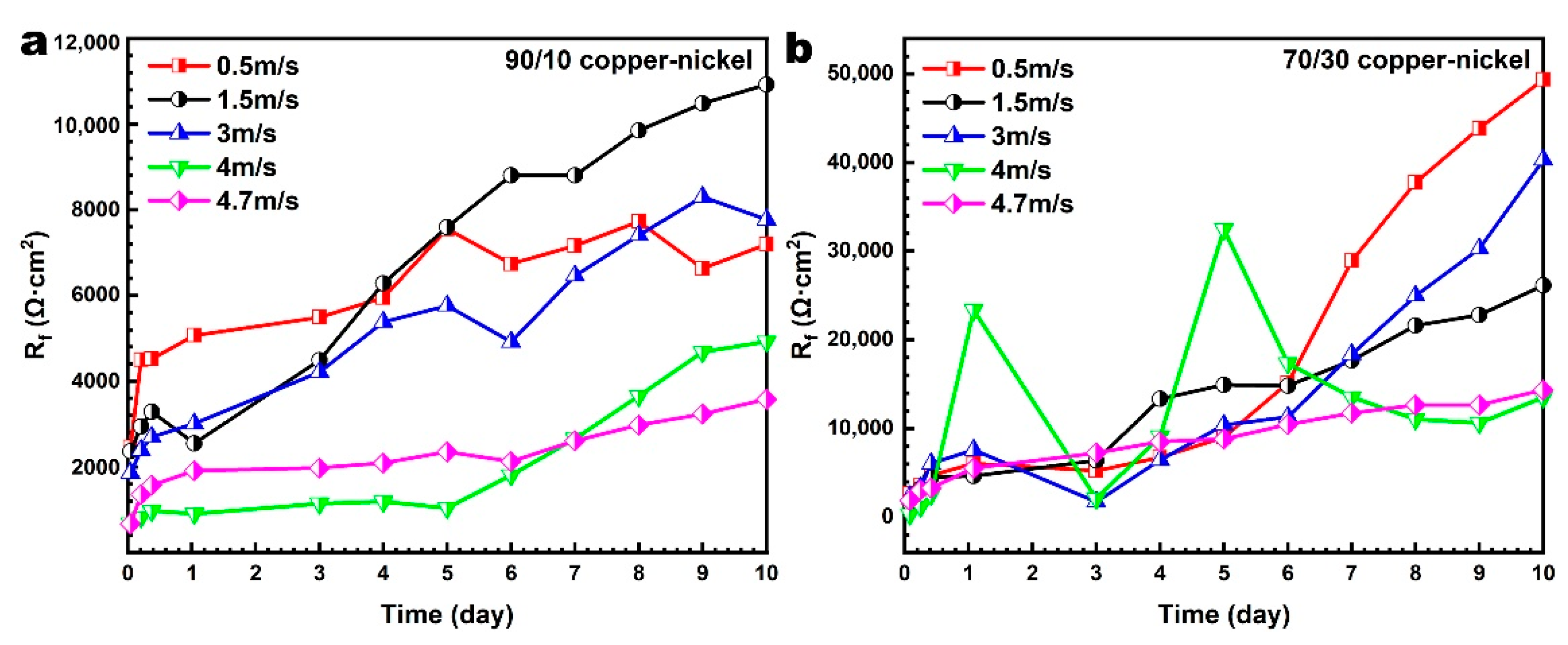
4.1.1. Effect of Corrosion Product Film on Corrosion Behavior
4.1.2. Effect of Flow Velocity on Corrosion Product Film
4.2. Erosion–Corrosion Behavior of the 70/30 Copper–Nickel Tube in Sodium Chloride-Based Fluid
4.2.1. Effect of Corrosion Product Film on Corrosion Behavior
4.2.2. Effect of Flow Velocity on Corrosion Product Film
4.3. Comparison of Erosion–Corrosion Behavior for the 90/10 and 70/30 Copper–Nickel Tubes
5. Conclusions
- (1)
- The corrosion product film on copper–nickel samples shows different corrosion resistance at different flow velocities. It is found that the corrosion product film formed at 1.5 m/s for the 90/10 copper–nickel tube and at 0.5 m/s for the 70/30 copper–nickel tube shows the best corrosion resistance compared with that formed at other flow velocities. A critical flow velocity phenomenon is found in both 90/10 and 70/30 copper–nickel samples. Based on whether there is a continuous corrosion product film as the judgment criterion, the “critical flow velocity” of the 90/10 copper–nickel alloy is between 3 m/s and 4 m/s, and the “critical flow velocity” of the 70/30 copper–nickel alloy is beyond 4.7 m/s;
- (2)
- There is an atypical passivation region in the anodic polarization curve of the 90/10 copper–nickel alloy because the corrosion product film is not a typical passive film. By comparison of the polarization curves of the freshly polished 90/10 copper–nickel samples with those of the samples after the 10-day erosion–corrosion test, we can see that the presence of corrosion product film mainly inhibits the cathodic process, i.e., the oxygen reduction reaction (ORR) on the sample surface. The cathodic polarization curves will intersect the anodic polarization curves in different regions with the presence or absence of a corrosion product film on the substrate;
- (3)
- The 70/30 copper–nickel alloy samples after the 10-day erosion–corrosion test show spontaneous passivation behavior, and typical passivation regions are recorded in their anodic curves, indicating that the corrosion product film on the 70/30 copper–nickel alloy is more similar to the passive film compared to that on the 90/10 copper–nickel alloy. By comparison of the polarization curves of the freshly polished 70/30 samples with those of the samples after the 10-day erosion–corrosion test, we can see that the presence of corrosion product film inhibits both anodic and cathodic processes of the 70/30 copper–nickel alloy, but at different flow velocities and with different surface films formed, the cathodic curve intersects the anodic curve in different regions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francis, R. The Corrosion of Copper and Its Alloys: A Practical Guide for Engineers; Nace International: Houston, TX, USA, 2010. [Google Scholar]
- Schleich, W. Application of Copper-Nickel Alloy UNS C70600 for Seawater Service. In Proceedings of the Corrosion/2005 Annual Conference and Exhibition, Queensland, Australia, 20–23 November 2005; Volume 5222, pp. 1–14. [Google Scholar]
- Wood, R.J.K.; Hutton, S.P.; Schiffrin, D.J. Mass transfer effects of non-cavitating seawater on the corrosion of Cu and 70Cu-30Ni. Corros. Sci. 1990, 30, 1177–1201. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Cui, Y.; Li, Y.; Wang, F. Influence of hydrostatic pressure on the corrosion behavior of 90/10 copper-nickel alloy tube under alternating dry and wet condition. Corros. Sci. 2019, 146, 202–212. [Google Scholar] [CrossRef]
- Ma, A.L.; Jiang, S.L.; Zheng, Y.G.; Ke, W. Corrosion product film formed on the 90/10 copper-nickel tube in natural seawater: Composition/structure and formation mechanism. Corros. Sci. 2015, 91, 245–261. [Google Scholar] [CrossRef]
- Ijsseling, F.P. The Application of The Polarization Resistance Method to The Study of The Corrosion Behavior of CuNil0Fe in Sea-Water. Corros. Sci. 1974, 14, 97–110. [Google Scholar] [CrossRef]
- Abraham, G.J.; Kain, V.; Dey, G.K. MIC failure of cupronickel condenser tube in fresh water application. Eng. Fail. Anal. 2009, 16, 934–943. [Google Scholar] [CrossRef]
- Chandra, K.; Mahanti, A.; Singh, A.P.; Kain, V.; Gujar, H.G. Microbiologically influenced corrosion of 70/30 cupronickel tubes of a heat-exchanger. Eng. Fail. Anal. 2019, 105, 1328–1339. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Sinha, S.K.; Gujar, H.G. Metallurgical investigation of a heat-exchanger tube of 70/30 cupronickel failed by fretting corrosion. Eng. Fail. Anal. 2020, 116, 104756. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Yang, F.; Yao, Z.M.; Ke, W. On the critical flow velocity for erosion-corrosion of Cu-Ni alloy BFe30-1-1 in artificial sea water. Z. Metallkd. 2000, 91, 323–328. [Google Scholar]
- Zheng, Z.B.; Zheng, Y.G.; Zhou, X.; He, S.Y.; Sun, W.H.; Wang, J.Q. Determination of the critical flow velocities for erosion–corrosion of passive materials under impingement by NaCl solution containing sand. Corros. Sci. 2014, 88, 187–196. [Google Scholar] [CrossRef]
- Wood, R.J.K. Erosion-corrosion interactions and their effect on marine and offshore materials. Wear 2006, 261, 1012–1023. [Google Scholar] [CrossRef]
- Efird, K.D. Effect of fluid dynamics on the corrosion of copper-based alloys in sea water. Corrosion 1977, 33, 3–8. [Google Scholar] [CrossRef]
- Henrikson, S.; Knutsson, L. Corrosion Tests in Baltic Sea Water on Heat Exchahger Tubes of Various Metallic Materials. Br. Corros. J. 1975, 10, 128–135. [Google Scholar] [CrossRef]
- Gilbert, P.T. Corrosion Resisting Properties of 90/10 Copper-Nickel-Iron Alloy with Particular Reference to Offshore Oil and Gas Applications. Br. Corros. J. 1979, 14, 20–25. [Google Scholar] [CrossRef]
- Glover, T.J. Copper–Nickel Alloy for the Construction of Ship and Boat Hulls. Br. Corros. J. 1982, 17, 155–158. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Stokes, K.; Walsh, F.C. Electrochemical corrosion behaviour of 90-10 Cu-Ni alloy in chloride-based electrolytes. J. Appl. Electrochem. 2004, 34, 659–669. [Google Scholar] [CrossRef]
- Bianchi, G.; Fiori, G.; Longhi, P.; Mazza, F. Horse Shoe Corrosion of Copper Alloys in Flowing Sea Water: Mechanism, and Possibility of Cathodic Protection of Condenser Tubes in Power Stations. Corrosoin 1978, 34, 369–406. [Google Scholar] [CrossRef]
- Nesic, S.; Bienkowski, J.; Bremhorst, K.; Yang, K.-S. Testing for Erosion-Corrosion Under Disturbed Flow Conditions Using a Rotating Cylinder with a Stepped Surface. Corrosion 2000, 56, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Kang, H.; Chen, Z.; Guo, E.; Zeng, Y.; Wang, W.; Wang, T. Electrochemical corrosion mechanisms of nickel-aluminium bronze with different nickel contents using the rotating disc electrode. Corros. Sci. 2019, 157, 438–449. [Google Scholar] [CrossRef]
- Sasaki, K.; Burstein, G.T. Erosion-corrosion of stainless steel under impingement by a fluid jet. Corros. Sci. 2007, 49, 92–102. [Google Scholar] [CrossRef]
- Wu, L.; Ma, A.; Zhang, L.; Zheng, Y. Intergranular erosion corrosion of pure copper tube in flowing NaCl solution. Corros. Sci. 2022, 201, 110304. [Google Scholar] [CrossRef]
- Ekerenam, O.O.; Ma, A.-L.; Zheng, Y.-G.; He, S.-Y.; Okafor, P.C. Evolution of the Corrosion Product Film and Its Effect on the Erosion–Corrosion Behavior of Two Commercial 90Cu–10Ni Tubes in Seawater. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 1148–1170. [Google Scholar] [CrossRef]
- Li, L.; Qiao, Y.X.; Zhang, L.M.; Ma, A.L.; Ma, R.Y.; Zheng, Y.G. Understanding the Corrosion Behavior of Nickel-Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion. Materials 2023, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.D.; Syrett, B.C.; Wing, S.S. The corrosion of copper-nickel alloys 706 and 715 in flowing sea water. I—Effect of oxygen. Corrosion 1978, 55, 297–311. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Mansfeld, F. Electrochemical impedance spectroscopy (EIS) as a new tool for investigating methods of corrosion protection. Electrochim. Acta 1990, 35, 1533. [Google Scholar] [CrossRef]
- O’Keeffe, M. Infrared optical properties of cuprous oxide. J. Chem. Phys. 1963, 39, 1789–1793. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Huang, H.; Bu, F. Correlations between the inhibition performances and the inhibitor structures of some azoles on the galvanic corrosion of copper coupled with silver in artificial seawater. Corros. Sci. 2020, 165, 108413. [Google Scholar] [CrossRef]
- Brownlie, F.; Hodgkiess, T.; Fanicchia, F. Erosion-corrosion behaviour of CoCrFeNiMo0.85 and Al0.5CoCrFeNi complex concentrated alloys produced by laser metal deposition. Sruf. Coat. Technol. 2021, 423, 127634. [Google Scholar]
- Popić, J.P.; Dražić, D.M. Electrochemistry of active chromium. Electrochim. Acta 2004, 49, 4877–4891. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Zheng, Y.G.; Okafor, P.C.; Ke, W. Electrochemical behaviour of high nitrogen bearing stainless steel in acidic chloride solution: Effects of oxygen, acid concentration and surface roughness. Electrochim. Acta 2009, 54, 2298–2304. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Liu, C.B.; Zheng, Y.G. The effect of fluoride ions on the corrosion behavior of pure titanium in 0.05M sulfuric acid. Electrochim. Acta 2014, 135, 526–535. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, T.; Ma, Z.; Zhou, H.; Cui, L.; Yu, K.Y. Comparison of cracking behavior of nanocrystalline Cu film on substrates of different plastic deformation mechanisms. Mater. Today Commun. 2022, 31, 103289. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, L.; Ma, A.; Zhang, L.; Cao, S.; Hu, Q.; Hu, L.; Zheng, Y. Study on anisotropic oxide formation rate in the initial corrosion stage of 90Cu-10Ni alloy in alkaline NaCl solution by experiments and first-principles calculation. Corros. Sci. 2022, 209, 110768. [Google Scholar] [CrossRef]
- Chen, T.Y.; Moccari, A.A.; Macdonald, D.D. Development of controlled hydrodynamic techniques for corrosion testing. Corrosion 1992, 48, 239–255. [Google Scholar] [CrossRef]
- Pandey, R.K. Failure analysis of refinery tubes of overhead condenser. Eng. Fail. Anal. 2006, 13, 739–746. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Crousier, J. Dealloying of Cu-Ni alloys in natural sea water. Br. Corros. J. 1989, 24, 49–52. [Google Scholar] [CrossRef]
- North, R.F.; Pryor, M.J. The influence of corrosion product structure on the corrosion rate of Cu-Ni alloys. Corros. Sci. 1970, 10, 297–311. [Google Scholar] [CrossRef]







| Materials | Ni | Fe | Mn | C | Pb | S | P | Cu |
|---|---|---|---|---|---|---|---|---|
| 90/10 copper–nickel tube | 10.72 | 1.68 | 0.76 | 0.0024 | 0.002 | 0.0025 | 0.003 | Bal. |
| 70/30 copper–nickel tube | 30.4 | 0.75 | 0.88 | 0.04 | 0.001 | 0.002 | 0.004 | Bal. |
| Velocity (m/s) | Time (h) | Rs (Ω·cm2) | Rct (Ω·cm2) | CPE1 × 10−4 (F·cm−2) | n1 | CPE2 × 10−4 (F·cm−2) | n2 | Rf1 (Ω·cm2) | Rf2 (Ω·cm2) | CPE3 × 10−4 (F·cm−2) | n3 | Σχ2 × 10−3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 36.87 | 139.8 | 2.82 | 0.75 | 3.43 | 0.57 | 1427 | 1035 | 19.01 | 0.72 | 0.160 |
| 5 | 36.64 | 147.2 | 3.53 | 0.68 | 0.84 | 0.73 | 2188 | 2312 | 16.44 | 0.69 | 0.511 | |
| 9 | 37.68 | 134.3 | 2.80 | 0.72 | 1.48 | 0.70 | 2112 | 2406 | 14.61 | 0.64 | 0.402 | |
| 25 | 37.60 | 141.8 | 2.56 | 0.72 | 2.34 | 0.71 | 2605 | 2464 | 18.65 | 0.81 | 0.331 | |
| 72 | 35.76 | 142.4 | 2.08 | 0.75 | 3.54 | 0.72 | 2001 | 3490 | 13.62 | 0.76 | 0.774 | |
| 96 | 35.00 | 215.9 | 2.51 | 0.72 | 3.19 | 0.75 | 1944 | 4005 | 14.87 | 0.73 | 0.711 | |
| 120 | 34.76 | 146.8 | 1.66 | 0.78 | 4.21 | 0.71 | 2303 | 5357 | 17.24 | 0.71 | 0.612 | |
| 144 | 34.66 | 142.4 | 1.59 | 0.78 | 4.91 | 0.66 | 2554 | 4182 | 15.74 | 0.65 | 0.579 | |
| 168 | 34.61 | 117.3 | 1.30 | 0.81 | 5.48 | 0.64 | 2823 | 4336 | 13.72 | 0.58 | 0.501 | |
| 192 | 34.26 | 143.5 | 1.55 | 0.78 | 5.40 | 0.65 | 2977 | 4748 | 13.02 | 0.52 | 0.383 | |
| 216 | 33.61 | 145.1 | 1.57 | 0.78 | 5.23 | 0.66 | 2509 | 4123 | 10.89 | 0.56 | 0.468 | |
| 240 | 34.33 | 135.4 | 1.43 | 0.78 | 5.25 | 0.63 | 2528 | 4667 | 12.61 | 0.59 | 0.529 | |
| 1.5 | 1 | 28.55 | 107.3 | 3.79 | 0.68 | 0.95 | 0.90 | 1359 | 1291 | 24.61 | 0.58 | 1.553 |
| 5 | 27.82 | 105.1 | 3.09 | 0.73 | 1.32 | 0.80 | 1148 | 1747 | 6.52 | 0.58 | 0.252 | |
| 9 | 27.78 | 135.6 | 3.78 | 0.71 | 1.99 | 0.75 | 1701 | 1631 | 10.26 | 0.54 | 0.114 | |
| 25 | 28.07 | 111.5 | 3.89 | 0.69 | 3.02 | 0.56 | 1322 | 1464 | 13.93 | 0.58 | 0.193 | |
| 72 | 28.92 | 153.7 | 2.49 | 0.73 | 3.11 | 0.76 | 1488 | 3934 | 13.85 | 0.51 | 0.232 | |
| 96 | 29.43 | 111.9 | 2.02 | 0.74 | 3.75 | 0.69 | 1750 | 4781 | 5.95 | 0.54 | 0.411 | |
| 120 | 31.03 | 116.3 | 1.94 | 0.76 | 6.59 | 0.68 | 1086 | 6510 | 6.00 | 0.74 | 0.197 | |
| 144 | 31.88 | 105.0 | 1.44 | 0.78 | 5.22 | 0.67 | 1481 | 7320 | 4.21 | 0.73 | 0.702 | |
| 168 | 32.62 | 80.2 | 1.12 | 0.81 | 5.60 | 0.67 | 1603 | 7200 | 4.05 | 0.78 | 0.891 | |
| 192 | 33.23 | 138.2 | 2.25 | 0.72 | 4.35 | 0.74 | 1682 | 8164 | 4.87 | 0.76 | 0.667 | |
| 216 | 33.81 | 137.3 | 2.41 | 0.71 | 4.26 | 0.75 | 1894 | 8586 | 4.82 | 0.79 | 0.741 | |
| 240 | 34.77 | 101.1 | 1.83 | 0.73 | 4.85 | 0.71 | 2092 | 8829 | 4.37 | 0.83 | 0.965 | |
| 3 | 1 | 28.77 | 15.79 | 6.05 | 0.66 | 3.22 | 0.66 | 1856 | 0.240 | |||
| 5 | 28.71 | 10.75 | 0.51 | 0.90 | 6.30 | 0.63 | 2392 | 0.506 | ||||
| 9 | 28.59 | 12.62 | 0.57 | 0.90 | 6.77 | 0.62 | 2696 | 0.557 | ||||
| 25 | 28.43 | 84.64 | 3.68 | 0.70 | 2.81 | 0.58 | 3010 | 0.682 | ||||
| 72 | 28.63 | 42.70 | 1.96 | 0.75 | 3.75 | 0.59 | 4213 | 0.472 | ||||
| 96 | 29.94 | 53.27 | 2.32 | 0.71 | 3.24 | 0.58 | 5382 | 0.483 | ||||
| 120 | 30.04 | 43.20 | 1.91 | 0.71 | 3.46 | 0.59 | 5762 | 0.488 | ||||
| 144 | 29.80 | 52.47 | 2.89 | 0.69 | 2.29 | 0.62 | 4918 | 1.020 | ||||
| 168 | 29.41 | 31.09 | 2.55 | 0.67 | 3.06 | 0.61 | 6462 | 0.553 | ||||
| 192 | 29.66 | 45.22 | 3.39 | 0.63 | 2.30 | 0.63 | 7407 | 0.596 | ||||
| 216 | 29.81 | 49.65 | 3.37 | 0.62 | 2.32 | 0.64 | 8299 | 0.633 | ||||
| 240 | 26.51 | 52.31 | 5.12 | 0.58 | 1.89 | 0.66 | 7768 | 1.510 | ||||
| 4 | 1 | 31.38 | 27.25 | 4.94 | 0.68 | 3.03 | 0.08 | 680 | 1.200 | |||
| 5 | 29.9 | 16.45 | 0.99 | 0.85 | 6.19 | 0.51 | 823 | 0.179 | ||||
| 9 | 30.04 | 25.28 | 0.90 | 0.88 | 4.81 | 0.51 | 975 | 0.1.94 | ||||
| 25 | 30.26 | 30.12 | 1.06 | 0.87 | 5.45 | 0.53 | 857 | 0.316 | ||||
| 72 | 29.31 | 23.40 | 0.56 | 0.90 | 5.65 | 0.56 | 1000 | 0.923 | ||||
| 96 | 29.04 | 26.36 | 0.44 | 0.66 | 1.18 | 0.58 | 1188 | 0.684 | ||||
| 120 | 29.08 | 23.84 | 1.85 | 0.77 | 7.73 | 0.51 | 1042 | 1.870 | ||||
| 144 | 27.04 | 25.50 | 1.73 | 0.75 | 5.70 | 0.52 | 1807 | 0.744 | ||||
| 168 | 27.93 | 23.03 | 0.66 | 0.87 | 8.22 | 0.57 | 2658 | 0.501 | ||||
| 192 | 28.04 | 27.38 | 0.82 | 0.84 | 8.89 | 0.57 | 3655 | 0.340 | ||||
| 216 | 28.48 | 30.20 | 0.93 | 0.82 | 9.12 | 0.57 | 4680 | 0.309 | ||||
| 240 | 22.42 | 39.25 | 2.49 | 0.71 | 7.81 | 0.58 | 4922 | 1.200 | ||||
| 4.7 | 1 | 26.49 | 156.8 | 9.11 | 0.64 | 0.12 | 1.00 | 512 | 1.14 | |||
| 5 | 26.2 | 122.6 | 7.73 | 0.62 | 0.65 | 1.00 | 1231 | 1.16 | ||||
| 9 | 25.85 | 122.3 | 3.44 | 0.71 | 4.69 | 0.52 | 1449 | 0.906 | ||||
| 25 | 25.86 | 142.0 | 4.66 | 0.67 | 3.88 | 0.56 | 1771 | 0.736 | ||||
| 72 | 25.74 | 151.7 | 4.99 | 0.66 | 3.35 | 0.53 | 1573 | 1.705 | ||||
| 96 | 25.94 | 153.9 | 7.14 | 0.62 | 2.12 | 0.34 | 1937 | 1.18 | ||||
| 120 | 25.74 | 179.4 | 7.15 | 0.61 | 2.35 | 0.18 | 2172 | 1.14 | ||||
| 144 | 26.11 | 119.0 | 6.78 | 0.62 | 3.77 | 0.52 | 2007 | 1.19 | ||||
| 168 | 25.84 | 127.1 | 7.66 | 0.60 | 3.04 | 0.58 | 2482 | 0.968 | ||||
| 192 | 26.42 | 128.9 | 6.87 | 0.61 | 3.55 | 0.62 | 2849 | 0.910 | ||||
| 216 | 26.79 | 130.0 | 5.50 | 0.64 | 4.75 | 0.61 | 3104 | 1.10 | ||||
| 240 | 25.64 | 137.3 | 6.18 | 0.61 | 3.80 | 0.62 | 3440 | 1.07 |
| Velocity (m/s) | Time (h) | Rs (Ω·cm2) | Rct (Ω·cm2) | CPE1 × 10−4 (F·cm−2) | n1 | CPE2 × 10−4 (F·cm−2) | n2 | Rf1 (Ω·cm2) | Rf2 (Ω·cm2) | CPE3 × 10−4 (F·cm−2) | n3 | Σχ2 × 10−3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 2 | 34.09 | 149.6 | 0.99 | 0.88 | 1.23 | 0.65 | 1411 | 1239 | 5.93 | 0.73 | 0.405 |
| 6 | 33.89 | 207.5 | 1.23 | 0.83 | 0.14 | 0.51 | 1167 | 2364 | 3.92 | 0.62 | 0.272 | |
| 10 | 34.06 | 176.3 | 0.92 | 0.87 | 0.67 | 0.51 | 1328 | 3390 | 3.04 | 0.55 | 1.88 | |
| 26 | 34.08 | 178.8 | 0.51 | 0.92 | 0.64 | 0.78 | 1081 | 4972 | 1.54 | 0.53 | 1.151 | |
| 72 | 31.94 | 189 | 0.89 | 0.83 | 0.56 | 0.64 | 3169 | 2072 | 8.66 | 0.78 | 0.761 | |
| 96 | 31.46 | 128.7 | 0.71 | 0.84 | 0.55 | 0.66 | 3661 | 3072 | 6.89 | 0.66 | 0.683 | |
| 120 | 31.42 | 133.4 | 0.74 | 0.82 | 0.22 | 0.72 | 3518 | 5479 | 2.09 | 0.56 | 0.915 | |
| 144 | 32.18 | 177.2 | 0.55 | 0.84 | 0.21 | 0.79 | 3637 | 11,510 | 0.79 | 0.59 | 1.001 | |
| 168 | 28.82 | 110.1 | 0.39 | 0.84 | 0.26 | 0.78 | 6404 | 22,500 | 0.69 | 0.57 | 1.068 | |
| 192 | 32.86 | 162.9 | 0.53 | 0.8 | 0.14 | 0.77 | 7862 | 29,870 | 0.27 | 0.55 | 0.195 | |
| 216 | 32.64 | 125.3 | 0.42 | 0.81 | 0.19 | 0.75 | 8367 | 35,490 | 0.23 | 0.63 | 0.272 | |
| 240 | 33.13 | 165.6 | 0.47 | 0.79 | 0.13 | 0.77 | 8520 | 40,830 | 0.27 | 0.52 | 0.681 | |
| 1.5 | 2 | 27.05 | 40.65 | 1.48 | 0.81 | 0.63 | 0.5 | 986 | 624 | 7.33 | 0.88 | 0.268 |
| 6 | 27.28 | 56.83 | 1.14 | 0.81 | 0.35 | 0.7 | 1622 | 1608 | 11.63 | 0.67 | 0.106 | |
| 10 | 27.14 | 52.17 | 1.39 | 0.78 | 0.32 | 0.66 | 2460 | 2032 | 9.03 | 0.63 | 0.065 | |
| 26 | 27.07 | 50.99 | 0.62 | 0.86 | 0.47 | 0.81 | 744 | 3882 | 3.68 | 0.5 | 0.754 | |
| 72 | 27.96 | 54.22 | 0.53 | 0.83 | 0.24 | 0.8 | 1157 | 5208 | 1.47 | 0.55 | 0.976 | |
| 96 | 28 | 48.26 | 0.49 | 0.83 | 0.15 | 0.81 | 3150 | 8186 | 1.31 | 0.54 | 0.485 | |
| 120 | 31.19 | 52.08 | 0.5 | 0.91 | 7.87 | 0.58 | 2224 | 12,690 | 1.22 | 0.6 | 0.731 | |
| 144 | 28.55 | 56.22 | 0.24 | 0.9 | 0.19 | 0.87 | 1254 | 13,550 | 0.92 | 0.57 | 2.4 | |
| 168 | 28.53 | 57.58 | 0.29 | 0.87 | 0.17 | 0.84 | 1649 | 16,010 | 0.7 | 0.52 | 1.135 | |
| 192 | 28.26 | 51.82 | 0.28 | 0.86 | 0.15 | 0.86 | 1986 | 19,640 | 0.71 | 0.52 | 1.952 | |
| 216 | 27.67 | 47.31 | 0.22 | 0.88 | 0.15 | 0.87 | 1445 | 21,360 | 0.64 | 0.53 | 1.943 | |
| 240 | 27.81 | 52.73 | 0.21 | 0.89 | 0.17 | 0.85 | 2083 | 24,050 | 0.66 | 0.53 | 2.635 | |
| 3 | 2 | 27.88 | 82.92 | 1.11 | 0.86 | 3.33 | 0.54 | 1499 | 0.448 | |||
| 6 | 27.97 | 73.61 | 0.75 | 0.86 | 1.98 | 0.53 | 3372 | 0.515 | ||||
| 10 | 28.03 | 82.32 | 0.34 | 0.94 | 2.23 | 0.55 | 6038 | 0.918 | ||||
| 26 | 28.68 | 66.51 | 0.31 | 0.93 | 1.2 | 0.59 | 6487 | 1.5 | ||||
| 72 | 28.01 | 76.52 | 0.46 | 0.95 | 2.89 | 0.57 | 1220 | 2.83 | ||||
| 96 | 28.74 | 65.88 | 0.25 | 0.97 | 1.58 | 0.55 | 6434 | 0.704 | ||||
| 120 | 31.67 | 74.43 | 0.32 | 0.91 | 1.14 | 0.63 | 10,390 | 1.933 | ||||
| 144 | 34.3 | 24.75 | 0.14 | 0.97 | 1.49 | 0.76 | 11,310 | 1.08 | ||||
| 168 | 38.71 | 29.44 | 0.11 | 1 | 1.53 | 0.77 | 18,370 | 4.092 | ||||
| 192 | 40.67 | 37.28 | 0.12 | 0.97 | 1.54 | 0.77 | 24,970 | 4.951 | ||||
| 216 | 37.42 | 56.16 | 0.46 | 0.78 | 1.22 | 0.79 | 30,280 | 1.753 | ||||
| 240 | 37.92 | 66.86 | 0.44 | 0.75 | 1.27 | 0.79 | 40,300 | 2.994 | ||||
| 4 | 2 | 29.03 | 26.92 | 1.28 | 0.87 | 4.17 | 0.62 | 312 | 0.971 | |||
| 6 | 28.66 | 36.17 | 0.61 | 0.89 | 2.66 | 0.54 | 1069 | 1.102 | ||||
| 10 | 28.75 | 34.55 | 0.5 | 0.87 | 1.81 | 0.52 | 2221 | 0.813 | ||||
| 26 | 29.76 | 25.97 | 0.33 | 0.86 | 0.3 | 0.54 | 23,360 | 1.714 | ||||
| 72 | 28.6 | 22.89 | 0.4 | 0.94 | 2.56 | 0.53 | 2083 | 0.355 | ||||
| 96 | 28.59 | 25.24 | 0.33 | 0.88 | 0.63 | 0.53 | 9110 | 0.873 | ||||
| 120 | 28.18 | 35.18 | 0.24 | 0.9 | 0.3 | 0.54 | 32,460 | 0.573 | ||||
| 144 | 27.44 | 48.29 | 0.24 | 0.92 | 0.39 | 0.59 | 17,360 | 2.121 | ||||
| 168 | 26.66 | 34.65 | 0.13 | 0.98 | 0.46 | 0.69 | 13,520 | 1.03 | ||||
| 192 | 25.97 | 23.9 | 0.25 | 0.92 | 0.39 | 0.62 | 11,050 | 0.721 | ||||
| 216 | 25.69 | 24.68 | 0.3 | 0.9 | 0.35 | 0.59 | 10,630 | 0.762 | ||||
| 240 | 22.08 | 34.81 | 0.25 | 0.93 | 0.58 | 0.52 | 13,510 | 1.433 | ||||
| 4.7 | 2 | 26.3 | 30.25 | 3.3 | 0.73 | 3.62 | 0.23 | 1838 | 0.766 | |||
| 6 | 26.35 | 32.18 | 1.33 | 0.79 | 2.05 | 0.51 | 3037 | 1.404 | ||||
| 10 | 25.83 | 32.73 | 1.06 | 0.79 | 1.29 | 0.58 | 3276 | 1.68 | ||||
| 26 | 25.87 | 31.74 | 0.47 | 0.88 | 1.35 | 0.56 | 5482 | 1.488 | ||||
| 72 | 25.58 | 31.77 | 0.55 | 0.86 | 0.96 | 0.52 | 6575 | 0.713 | ||||
| 96 | 25.75 | 31.51 | 0.5 | 0.87 | 0.89 | 0.53 | 7812 | 1.15 | ||||
| 120 | 25.68 | 36.19 | 1.54 | 0.75 | 1.74 | 0.57 | 9404 | 1.152 | ||||
| 144 | 25.32 | 37.64 | 0.49 | 0.86 | 1.04 | 0.53 | 10,400 | 0.799 | ||||
| 168 | 24.98 | 37.6 | 0.52 | 0.84 | 0.6 | 0.54 | 10,510 | 1.312 | ||||
| 192 | 25.8 | 31.13 | 0.49 | 0.85 | 0.54 | 0.52 | 10,940 | 0.893 | ||||
| 216 | 25.72 | 32.24 | 0.44 | 0.87 | 0.56 | 0.54 | 12,040 | 0.182 | ||||
| 240 | 25.42 | 34.28 | 0.46 | 0.86 | 0.48 | 0.54 | 14,320 | 0.572 |
| Sample Status | Flow Velocity (m/s) | Ecorr (mV vs. SCE) | Icorr (μA·cm−2) |
|---|---|---|---|
| Freshly polished 90/10 copper–nickel samples | 0.5 | −160.5 | 18.16 |
| 1.5 | −171.5 | 43.53 | |
| 3 | −169.3 | 61.47 | |
| 4 | −180.8 | 84.13 | |
| 4.7 | −184.3 | 90.93 | |
| 90/10 copper–nickel samples after the 10-day erosion–corrosion test | 0.5 | −429.0 | 3.85 |
| 1.5 | −438.9 | 2.39 | |
| 3 | −465.8 | 8.54 | |
| 4 | −282.5 | 11.16 | |
| 4.7 | −280.3 | 13.44 | |
| Freshly polished 70/30 copper–nickel samples | 0.5 | −173.0 | 16.57 |
| −386.4 | |||
| −416.4 | |||
| 1.5 | −180.3 | 28.99 | |
| −364.4 | |||
| −421.4 | |||
| 3 | −206.2 | 47.02 | |
| −373.0 | |||
| −408.7 | |||
| 4 | −203.0 | 51.37 | |
| −332.6 | |||
| −405.6 | |||
| 4.7 | −209.9 | 52.86 | |
| −334.8 | |||
| −364.4 | |||
| 70/30 copper–nickel samples after the 10-day erosion–corrosion test | 0.5 | 263.0 | 1.60 |
| 1.5 | −303.2 | 3.56 | |
| 3 | −342.3 | 4.33 | |
| −361.7 | |||
| 4 | −255.8 | 6.62 | |
| 4.7 | −251.5 | 8.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Ma, A.; Zhang, L.; Li, G.; Hu, L.; Wang, Z.; Zheng, Y. Erosion–Corrosion Behavior of 90/10 and 70/30 Copper–Nickel Tubes in 1 wt% NaCl Solution. Metals 2023, 13, 401. https://doi.org/10.3390/met13020401
Wu L, Ma A, Zhang L, Li G, Hu L, Wang Z, Zheng Y. Erosion–Corrosion Behavior of 90/10 and 70/30 Copper–Nickel Tubes in 1 wt% NaCl Solution. Metals. 2023; 13(2):401. https://doi.org/10.3390/met13020401
Chicago/Turabian StyleWu, Lei, Aili Ma, Lianmin Zhang, Guangming Li, Lingyue Hu, Zhengbin Wang, and Yugui Zheng. 2023. "Erosion–Corrosion Behavior of 90/10 and 70/30 Copper–Nickel Tubes in 1 wt% NaCl Solution" Metals 13, no. 2: 401. https://doi.org/10.3390/met13020401
APA StyleWu, L., Ma, A., Zhang, L., Li, G., Hu, L., Wang, Z., & Zheng, Y. (2023). Erosion–Corrosion Behavior of 90/10 and 70/30 Copper–Nickel Tubes in 1 wt% NaCl Solution. Metals, 13(2), 401. https://doi.org/10.3390/met13020401







