Synergistic Effects of Hydrostatic Pressure and Dissolved Oxygen on the SCC Behavior of Hydrogenated Ti6Al4V Alloy in Deep-Sea Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Cathode Hydrogen Charging
2.3. Microstructural Characterization
2.4. Electrochemical Corrosion Tests
2.5. SSRT Tests
3. Results
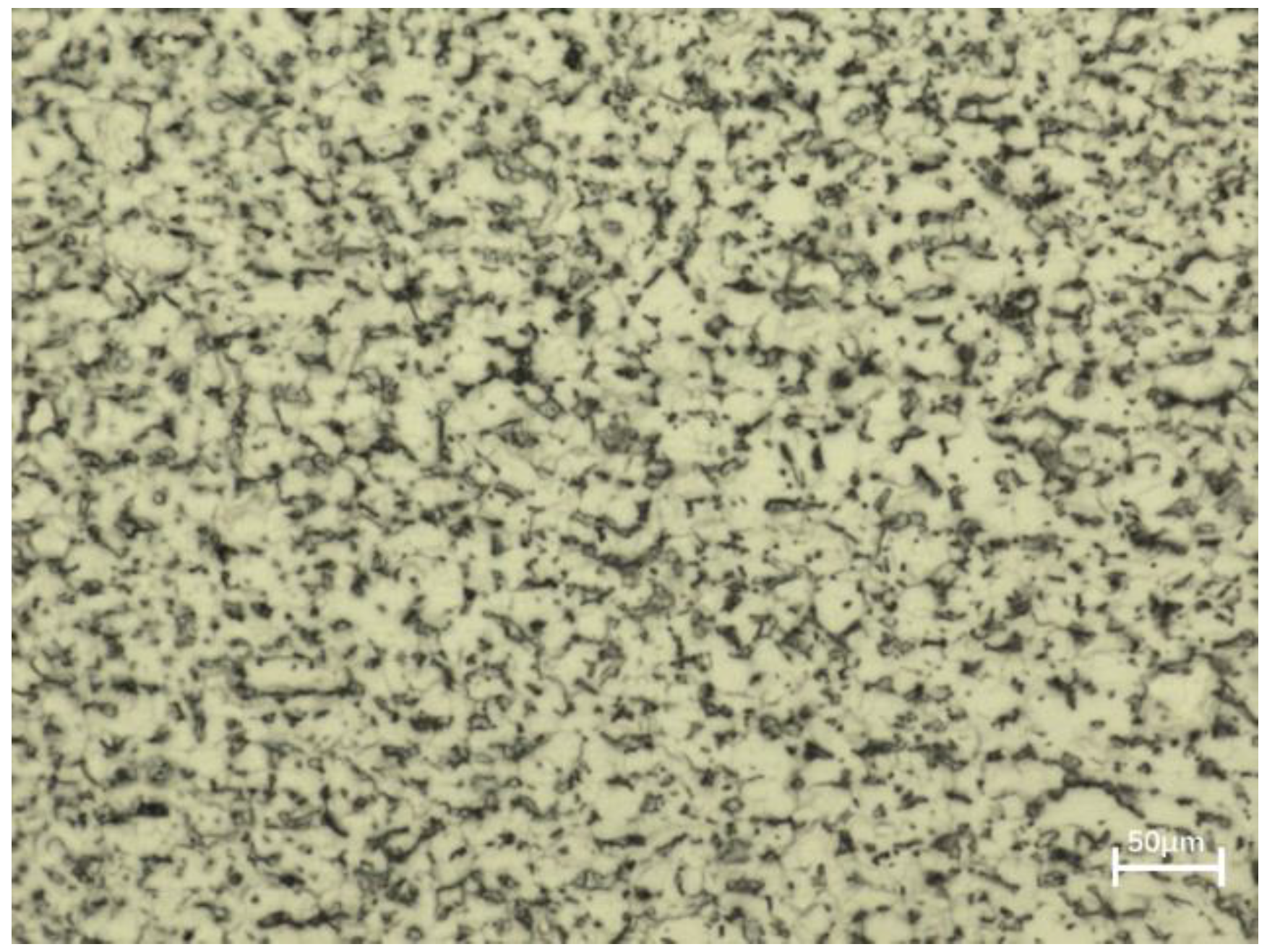
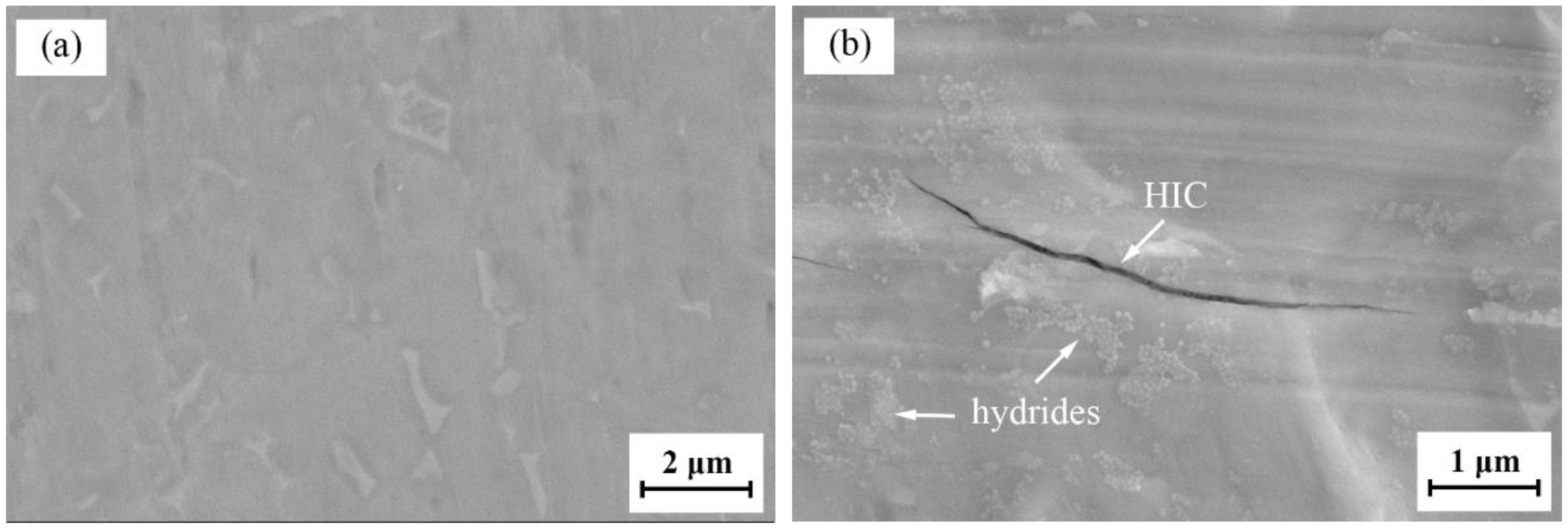
3.1. Microstructure Characterization
3.2. Electrochemical Behavior of Hydrogenated Ti6Al4V Alloy
3.2.1. Influence of Hydrogen Charging on the Corrosion Behavior of Ti6Al4V Alloy
3.2.2. Influence of HP on the Corrosion Behavior of Charged Sample
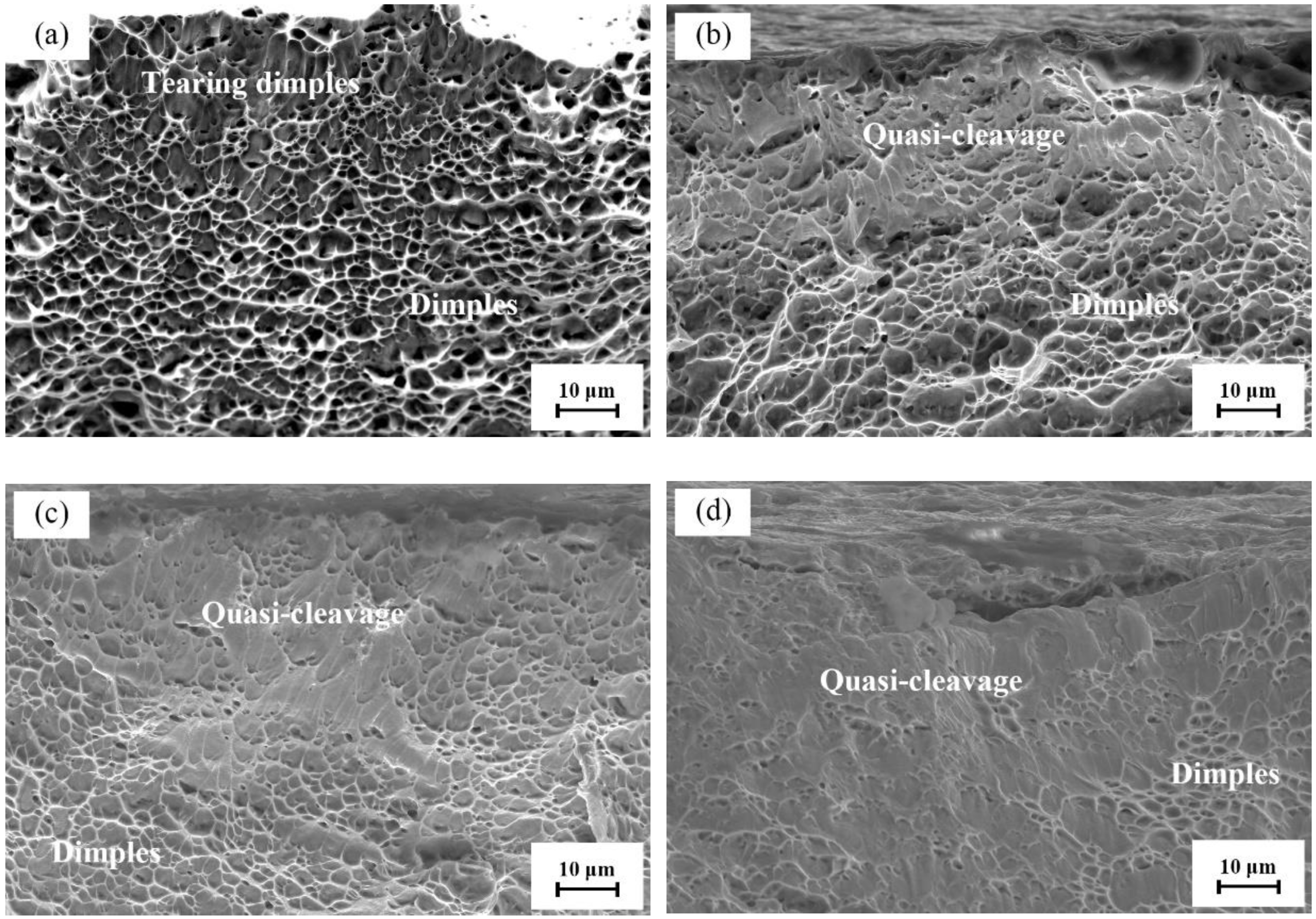
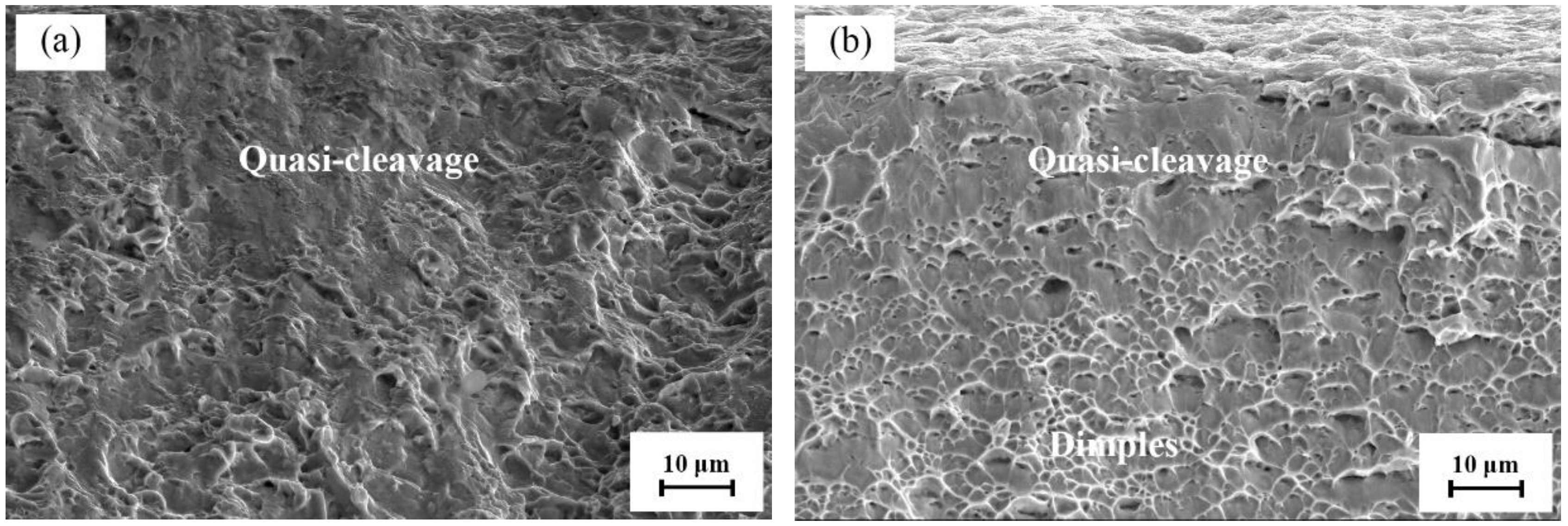
3.3. SCC Behavior of Hydrogenated Ti6Al4V Alloy
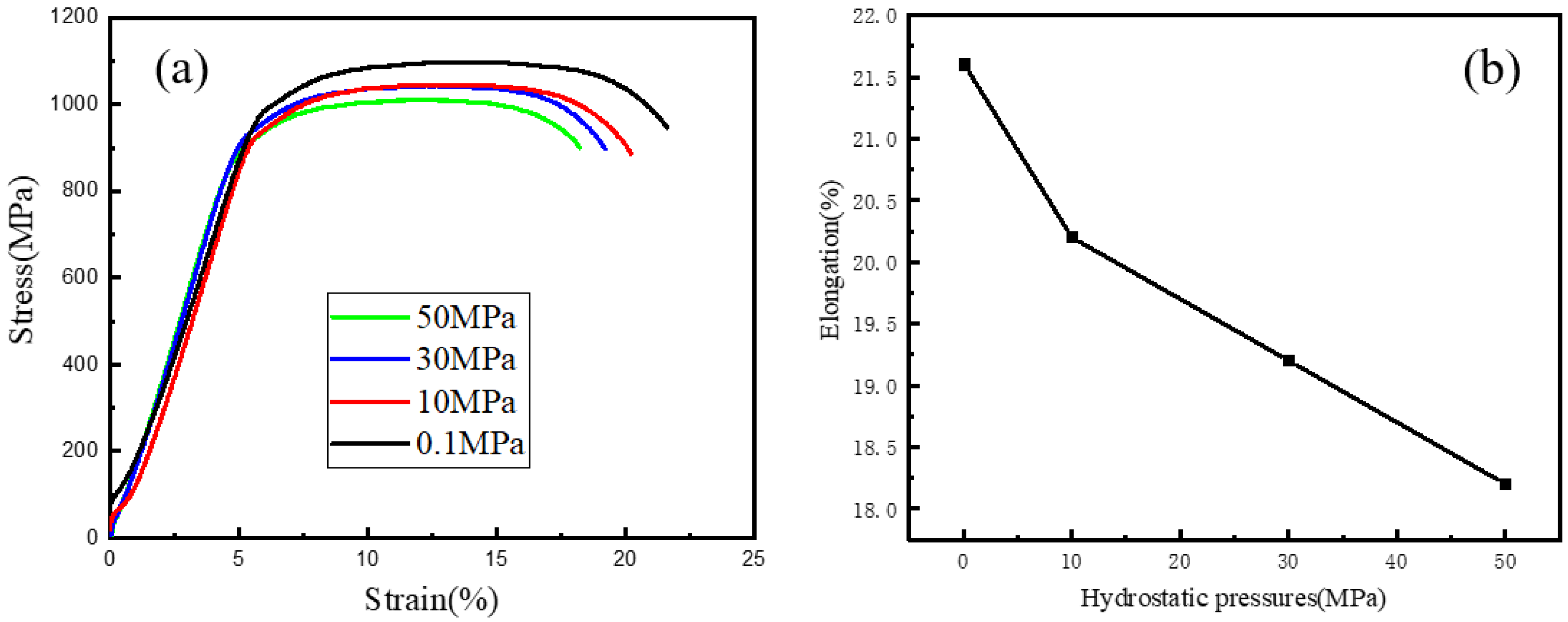
3.3.1. Influence of HP on the SCC Behaivor of Hydrogenated Ti6Al4V Alloy
3.3.2. Influence of DO on the SCC Behavior of Hydrogenated Ti6Al4V Alloy
4. Discussion
5. Conclusions
- Needle-like hydrides, mainly TiH and TiH2, and hydrogen-induced cracks formed on the surface of the Ti6Al4V alloy after electrochemical charging for 1 h. The anodic current density and capacitance arc increased and shrunk, respectively, after hydrogen charging.
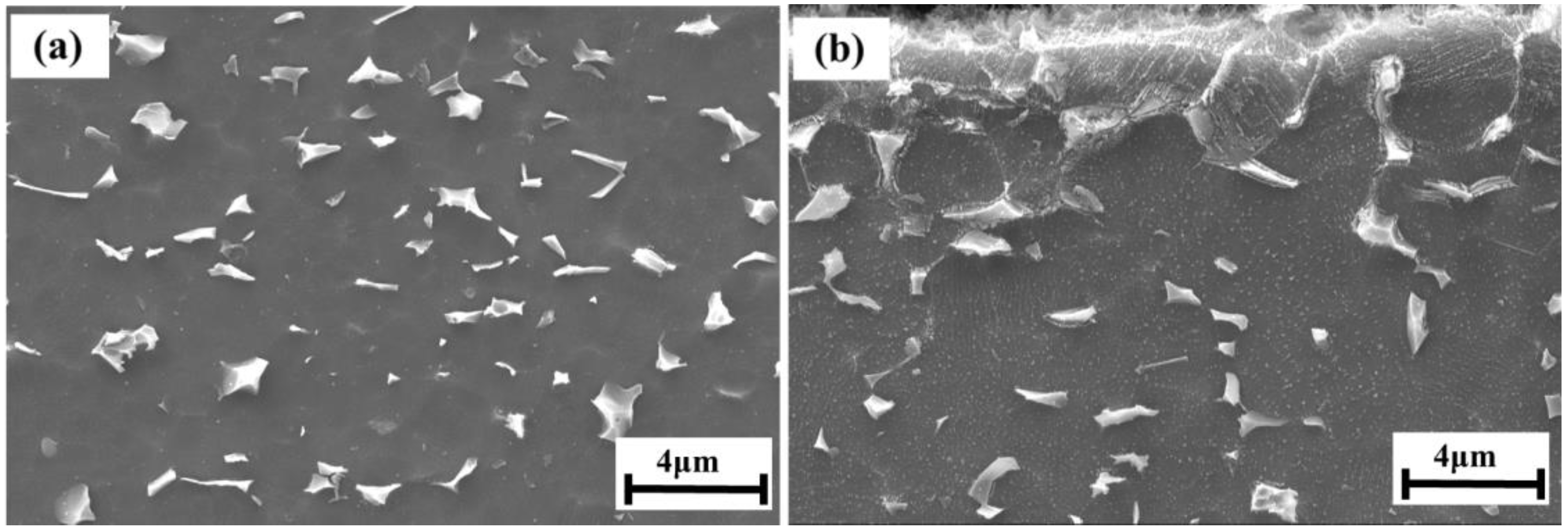
- The SCC of the hydrogen-charged Ti6Al4V alloy in the simulated deep-sea environment was hydrogen embrittlement. The fractography indicated a mixed fracture that was both brittle and ductile. The higher the HPs, the larger the brittle area.
- The SCC susceptibility of the charged Ti6Al4V alloy was enhanced by the higher HPs and/or the lower DO concentrations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Traverso, P.; Canepa, E. A review of studies on corrosion of metals and alloys in deep-sea environment. Ocean Eng. 2014, 87, 10–15. [Google Scholar] [CrossRef]
- Guo, W.M.; Li, W.J.; Chen, G.Z. Corrosion testing in the deep ocean. Equip. Environ. Eng 2006, 3, 10–15. [Google Scholar]
- Liu, R.; Liu, L.; Wang, F.H. The role of hydrostatic pressure on the metal corrosion in simulated deep-sea environments—A review. J. Mater. Sci. Technol. 2022, 112, 230–238. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G.; Arfelli, M.; Mattogno, G. The effect of salt concentration on nickel corrosion behaviour in slightly alkaline solutions at different hydrostatic pressures. Corros. Sci. 1993, 34, 989–1005. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G.; Castello, G. Influence of passive film composition and sea water pressure on resistance to localised corrosion of some stainless steels in sea water. Br. Corros. J. 1995, 30, 283–287. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G.; Gingaud, D.; Castello, G. Effect of Hydrostatic Pressure on Passivating Power of Corrosion Layers Formed on 6061 T6 Aluminium Alloy in Sea Water. Br. Corros. J. 1994, 29, 65–69. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G. Influence of hydrostatic pressure on pitting of aluminium in sea water. Br. Corros. J. 2013, 20, 183–186. [Google Scholar] [CrossRef]
- Mor, E.D.; Beccaria, A.M. Effects of Hydrostatic Pressure on the Corrosion of Copper in Sea Water. Br. Corros. J. 2013, 13, 142–146. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.W.; Liu, L. Degradation in pitting resistance of 316L stainless steel under hydrostatic pressure. Electrochim. Acta 2016, 210, 401–406. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.G.; Shao, Y.W.; Meng, G.Z.; Wang, F.H. A stochastic analysis of the effect of hydrostatic pressure on the pit corrosion of Fe-20Cr alloy. Electrochim. Acta 2009, 54, 3915–3922. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Su, Y.J.; Qiao, L.J. Hydrostatic pressure effects on stress corrosion cracking of X70 pipeline steel in a simulated deep-sea environment. Int. J. Hydrogn Energy 2017, 42, 27446–27457. [Google Scholar] [CrossRef]
- Li, Y.; Song, L.F.; Sun, F.L. Key Factors of Stress Corrosion Cracking of X70 pipeline Steel in Simulated Deep-sea Environment: Role of Localized Strain and Stress. Int. J. Electrochem. Sci. 2018, 13, 10155–10172. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Su, Y.J.; Qiao, L.J. Hydrostatic pressure effects on corrosion behavior of X70 pipeline steel in a simulated deep-sea environment. J. Electroanal. Chem. 2018, 822, 123–133. [Google Scholar] [CrossRef]
- Li, Q.S.; Luo, S.Z.; Xing, X.T.; Yuan, J.; Liu, X.; Wang, J.H.; Hu, W.B. Effect of Deep Sea Pressures on the Corrosion Behavior of X65 Steel in the Artificial Seawater. Acta Metall. Sin. Engl. Lett. 2019, 32, 972–980. [Google Scholar] [CrossRef]
- Shukla, A.K.; Balasubramaniam, R.; Bhargava, S. Properties of passive film formed on CP titanium, Ti–6Al–4V and Ti–13.4Al–29Nb alloys in simulated human body conditions. Intermetallics 2005, 13, 631–637. [Google Scholar] [CrossRef]
- Kolman, D.; Scully, J.R. Understanding the potential and pH dependency of high-strength β-titanium alloy environmental crack initiation. Metall. Mater. Trans. A 1997, 28, 2645–2656. [Google Scholar]
- Krawiec, H.; Vignal, V.; Schwarzenboeck, E.; Banas, J. Role of plastic deformation and microstructure in the micro-electrochemical behaviour of Ti–6Al–4V in sodium chloride solution. Electrochim. Acta 2013, 104, 400–406. [Google Scholar] [CrossRef]
- Dong, J.J.; Fan, L.; Zhang, H.B.; Xu, L.K.; Xue, L.L. Electrochemical Performance of Passive Film Formed on Ti-Al-Nb-Zr Alloy in Simulated Deep Sea Environments. Acta Met. Sin.-Engl. 2020, 33, 595–604. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Zhang, B.; Liu, L.; Wang, F.H. Unveiling the effect of hydrostatic pressure on the passive films of the deformed titanium alloy. Corros. Sci. 2021, 190, 109705. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, S.; Lim, C.V.S.; Zhou, X.; Chen, X.; Hinton, B.R.; Boyer, R.R.; Williams, J.C.; Wu, X. The mechanism of aqueous stress-corrosion cracking of α + β titanium alloys. Corros. Sci. 2017, 125, 29–39. [Google Scholar] [CrossRef]
- Cao, S.; Lim, C.V.S.; Hinton, B.; Wu, X. Effects of microtexture and Ti3Al (α2) precipitates on stress-corrosion cracking properties of a Ti-8Al-1Mo-1V alloy. Corros. Sci. 2017, 116, 22–33. [Google Scholar] [CrossRef]
- Shih, D.; Robertson, I.; Birnbaum, H.K. Hydrogen embrittlement of α titanium: In situ TEM studies. Acta Metall. 1988, 36, 111–124. [Google Scholar] [CrossRef]
- Numakura, H.M.; Metallurgica, K.J.A. Hydride precipitation in titanium. Acta Met. 1984, 32, 1799–1807. [Google Scholar] [CrossRef]
- Dutton, R.; Nuttall, K.; Puls, M.P.; Simpson, L.A. Mechanisms of hydrogen induced delayed cracking in hydride forming materials. Matallurgical Trans. A 1977, 8, 1553–1562. [Google Scholar] [CrossRef]
- Pardee, W.J.; Paton, N.E. Model of sustained load cracking by hydride growth in Ti alloys. Matallurgical Trans. A 1980, 11, 1391–1400. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, D.; Wang, S.; Ma, Y.; Chen, J.; Wang, Y.; Zhou, H. Effect of hydrogen charging on microstructural evolution and corrosion behavior of Ti-4Al-2V-1Mo-1Fe alloy. J. Mater. Sci. Technol. 2021, 60, 168–176. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Liu, L.; Zhang, B.; Wang, F.H. A primary study of the effect of hydrostatic pressure on stress corrosion cracking of Ti-6Al-4V alloy in 3.5% NaCl solution. Corros. Sci. 2020, 165, 108402. [Google Scholar] [CrossRef]
- Conforto, E.; Caillard, D. A fast method for determining favourable orientation relationships and interface planes: Application to titanium–titanium hydrides transformations. Acta Mater. 2007, 55, 785–798. [Google Scholar] [CrossRef]
- Williams, D.P.; Nelson, H.G. Gaseous hydrogen-induced cracking of Ti-5Al-2.5 Sn. Metall. Trans. 1972, 3, 2107–2113. [Google Scholar] [CrossRef]
- Kim, J.; Tasan, C.C. Microstructural and micro-mechanical characterization during hydrogen charging: An in situ scanning electron microscopy study. Int. J. Hydrog. Energ. 2019, 44, 6333–6343. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D. High fugacity hydrogen effects at room temperature in titanium based alloys. J. Alloys Compd. 2005, 404, 613–616. [Google Scholar] [CrossRef]
- Hua, F.; Pasupathi, P.; Mon, K.; Gordon, G.; Shoesmith, D. Modeling the hydrogen-induced cracking of titanium alloys in nuclear waste repository environments. JOM J. Miner. Met. Mater. Soc. 2005, 57, 20–26. [Google Scholar] [CrossRef]
- Gu, J.; Hardie, D. Effect of hydrogen on structure and slow strain rate embrittlement of mill annealed Ti6Al4V. Mater. Sci. Technol. 1996, 12, 802–807. [Google Scholar] [CrossRef]
- Pan, Z.; Wei, Y.; Fu, Y.; Luo, H.; Li, X. Effect of electrochemical hydrogen charging on the mechanical property and corrosion behavior of Ti-3Mo alloy. Corros. Sci. 2022, 200, 110219. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; Ikeda, B.M. The Resistance of Titanium to Pitting, Microbially Induced Corrosion and Corrosion in Unsaturated Conditions; Whiteshell Laboratories, Atomic Energy of Canada Limited: Pinawa, MB, Canada, 1997; pp. 1–47. [Google Scholar]
- Carnot, A.; Frateur, I.; Zanna, S.; Tribollet, B.; Dubois-Brugger, I.; Marcus, P. Corrosion mechanisms of steel concrete moulds in contact with a demoulding agent studied by EIS and XPS. Corros. Sci. 2003, 45, 2513–2524. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Liu, L.; Wang, F.H. Study on the mechanism of hydrostatic pressure promoting electrochemical corrosion of pure iron in 3.5% NaCl solution. Acta Mater. 2021, 203, 116467. [Google Scholar] [CrossRef]
- Sun, H.J.; Liu, L.; Li, Y.; Wang, F.H. Effect of Hydrostatic Pressure on the Corrosion Behavior of a Low Alloy Steel. J. Electrochem. Soc. 2013, 160, C89–C96. [Google Scholar] [CrossRef]
- Beccaria, A.M.F.P.; Mattogno, G. The effect of hydrostatic pressure on the corrosion of nickel in slightly alkaline solutions containing Cl− ions. Corros. Sci. 1989, 29, 403–416. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Zhu, Y.; Yu, M.; Wen, L.; Jin, Y. Synergistic Effects of Hydrostatic Pressure and Dissolved Oxygen on the SCC Behavior of Hydrogenated Ti6Al4V Alloy in Deep-Sea Environment. Metals 2023, 13, 449. https://doi.org/10.3390/met13030449
Huang F, Zhu Y, Yu M, Wen L, Jin Y. Synergistic Effects of Hydrostatic Pressure and Dissolved Oxygen on the SCC Behavior of Hydrogenated Ti6Al4V Alloy in Deep-Sea Environment. Metals. 2023; 13(3):449. https://doi.org/10.3390/met13030449
Chicago/Turabian StyleHuang, Feifei, Yuxiang Zhu, Meng Yu, Lei Wen, and Ying Jin. 2023. "Synergistic Effects of Hydrostatic Pressure and Dissolved Oxygen on the SCC Behavior of Hydrogenated Ti6Al4V Alloy in Deep-Sea Environment" Metals 13, no. 3: 449. https://doi.org/10.3390/met13030449
APA StyleHuang, F., Zhu, Y., Yu, M., Wen, L., & Jin, Y. (2023). Synergistic Effects of Hydrostatic Pressure and Dissolved Oxygen on the SCC Behavior of Hydrogenated Ti6Al4V Alloy in Deep-Sea Environment. Metals, 13(3), 449. https://doi.org/10.3390/met13030449





