Kinetics of Martensite Decomposition and Microstructure Stability of Ti-6246 during Rapid Heating to Service Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
2.2. Heat Treatment Schedules
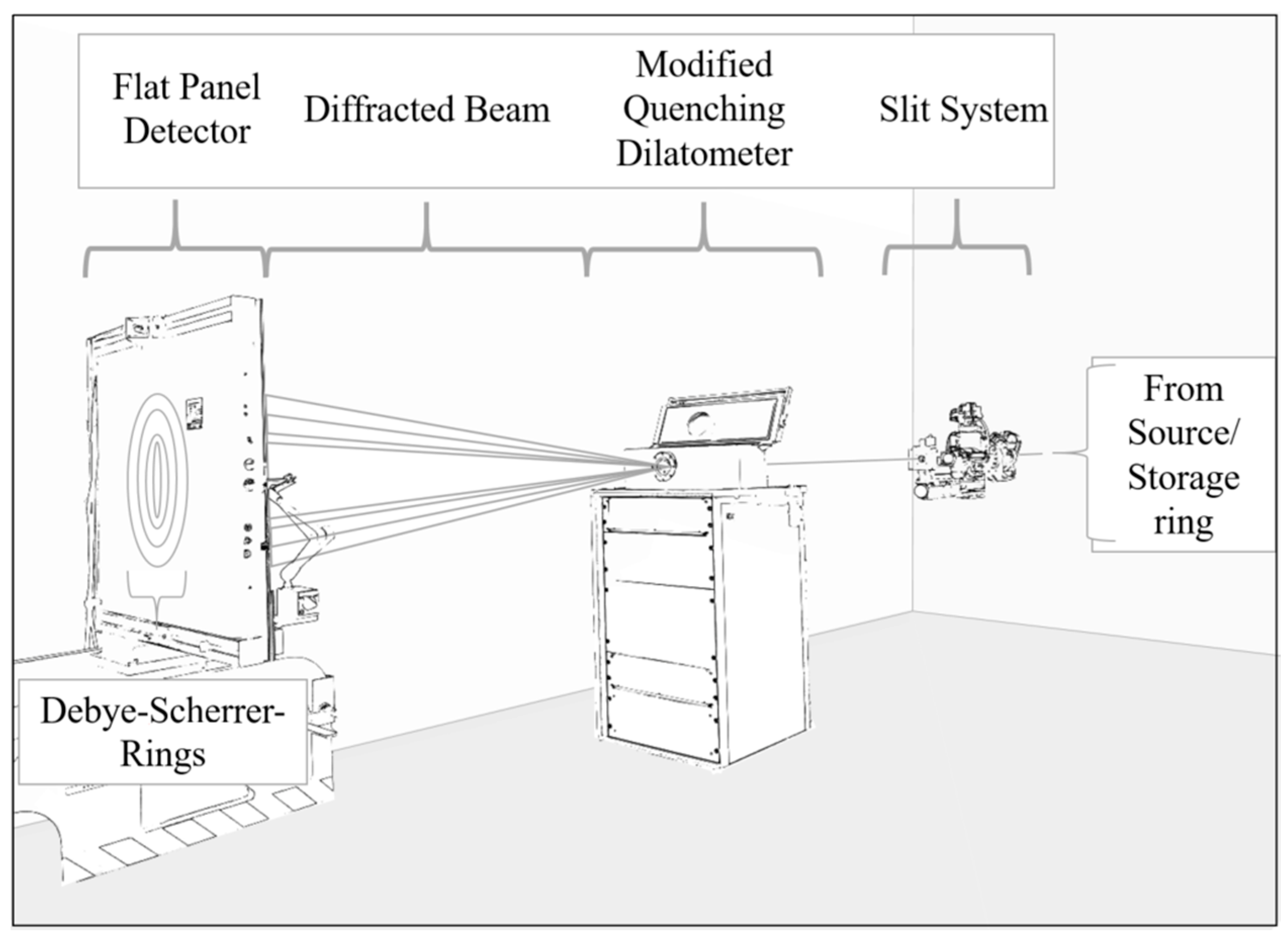
2.3. In Situ Data Acquisition
2.4. In Situ Data Post-Processing and Analyses
2.5. Characterization of Microstructural Properties
3. Results and Discussion
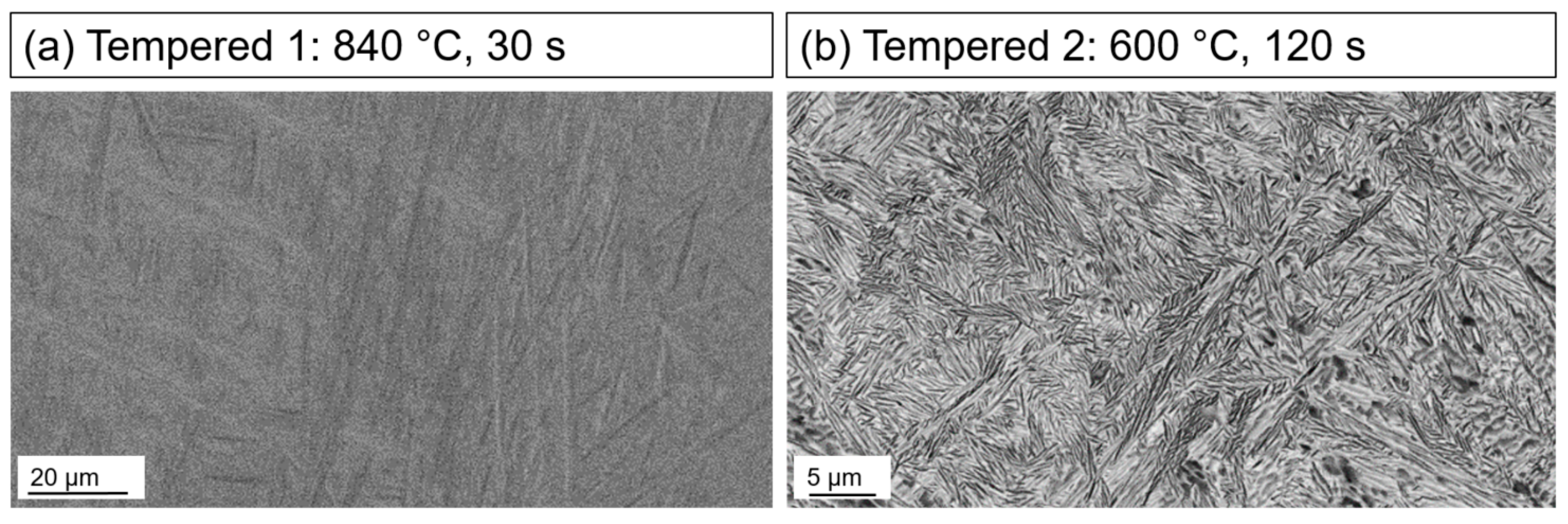
3.1. Ex Situ Microstructural Characterization
3.2. In Situ Characterization
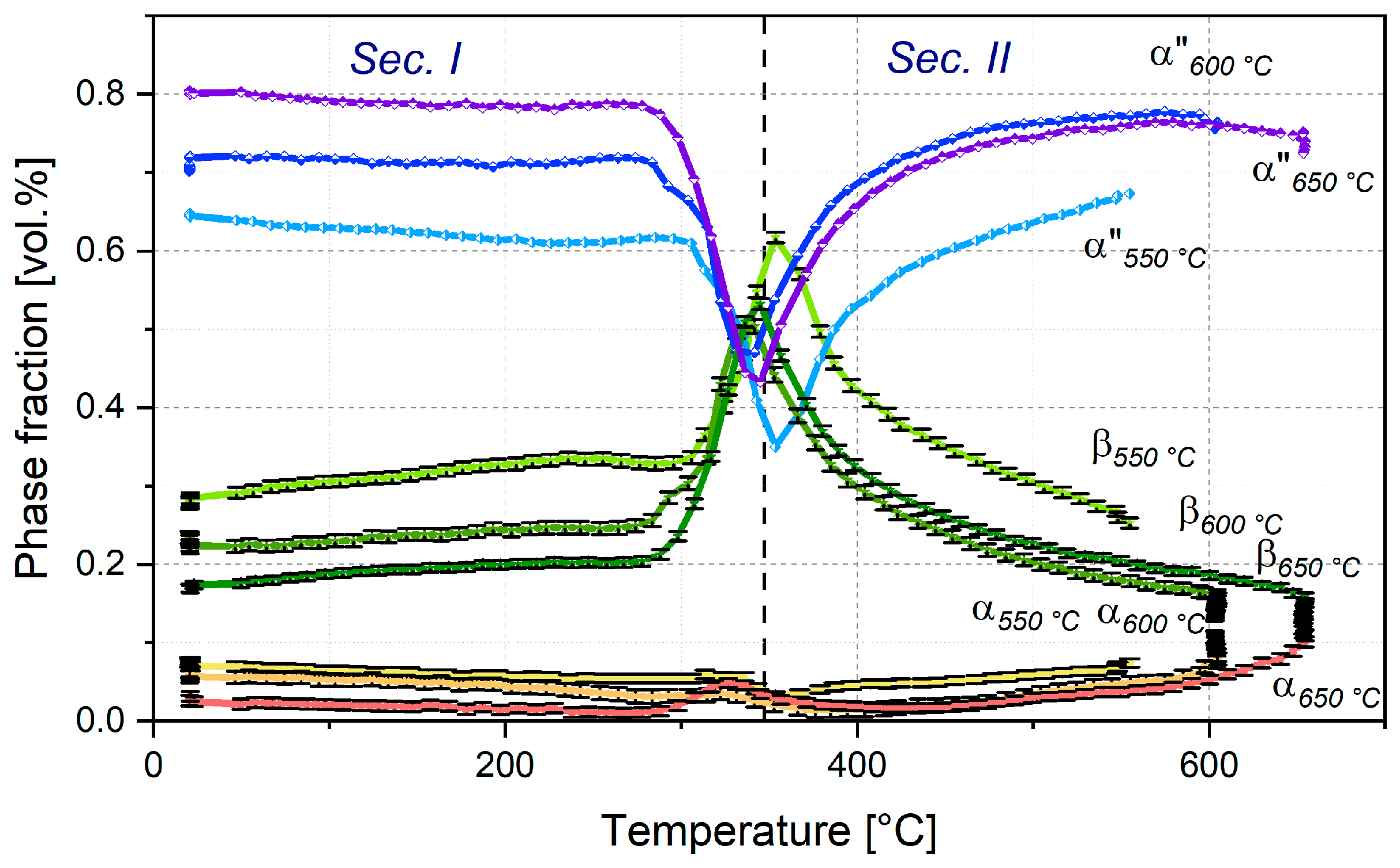
3.2.1. Evolution of Phase Fractions during Continuous Reheating to Service Temperature and Martensite Start Temperature
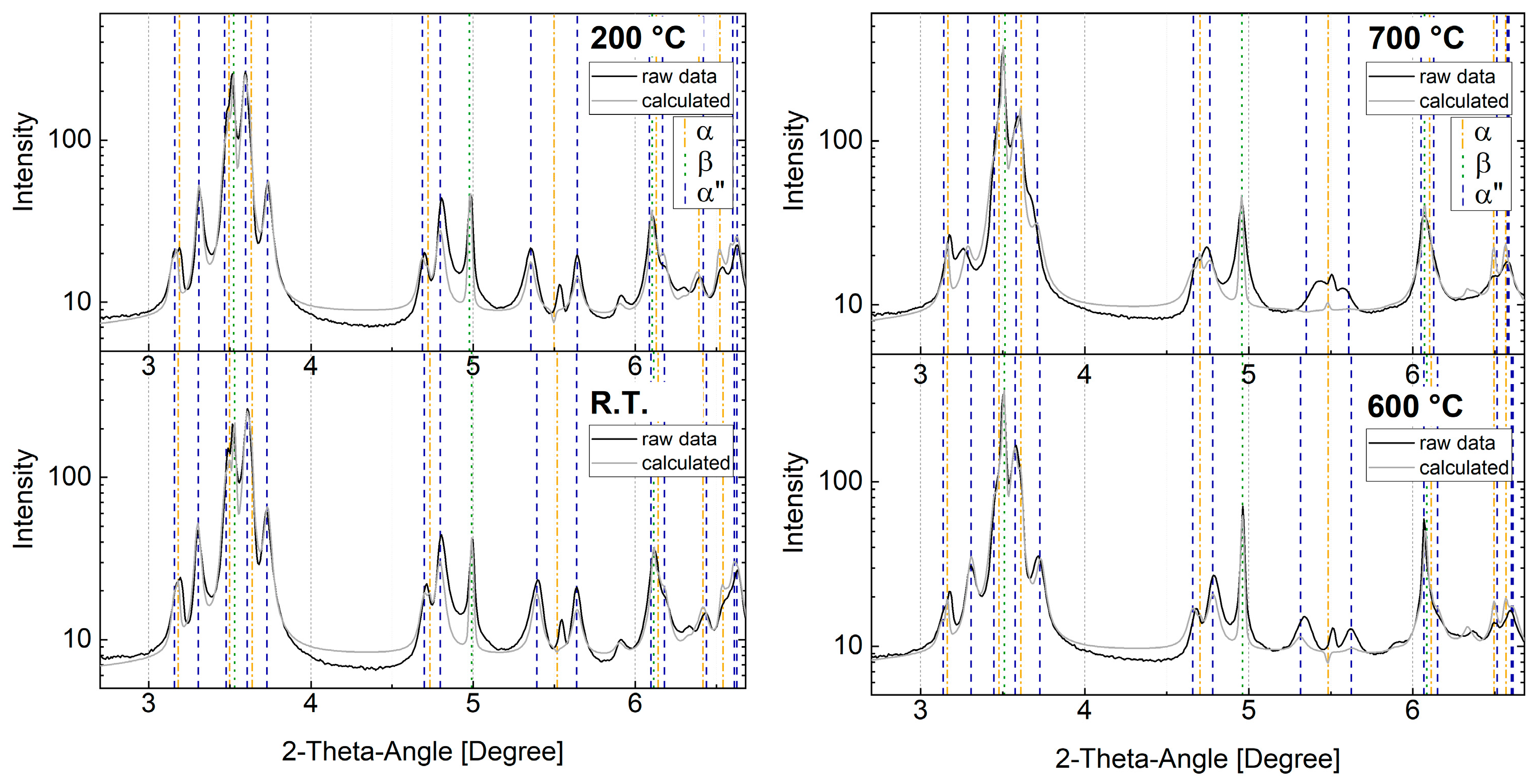
3.2.2. Challenges Regarding Phase Analysis of Ternary α + β + α″ Microstructure
- i.
- The superposition of interference lines of α″-martensite and α-phase. The hexagonal α-phase, especially, has no single distinct diffraction line as every diffraction line is surrounded by at least two martensite diffraction lines (see Figure 9). This effect is further amplified as the heat treatment progresses. Peak broadening takes place most likely due to an increase in lattice strains/dislocation density caused by high cooling rates. Additionally, martensite diffraction lines gradually approach the α-diffraction lines. This may lead to overestimated α″-phase fractions and underestimated α-phase fractions as highlighted in Figure 8;
- ii.
- A second challenge is the grain coarsening of the parent β-phase, which was found during the evaluation of the Debye–Scherrer rings as well as shown by the SEM images. It affects the peak intensity ratios due to the low grain statistics in the measurement volume and subsequently causes erroneous phase fraction calculation by Rietveld analysis and any other peak fit routines. Two strategies can be applied to tackle this problem: (i) separately evaluate the areas with all three phases as well as the areas where the β-phase can be excluded, and (ii) keep the analyses with all three phases and interpret the results with consideration of the possible and, in this context, reasonable developments. Although strategy one seems to be the more promising solution, on several examinations, no better results could be achieved since the deviations became too large when the results of the evaluation ranges were combined.
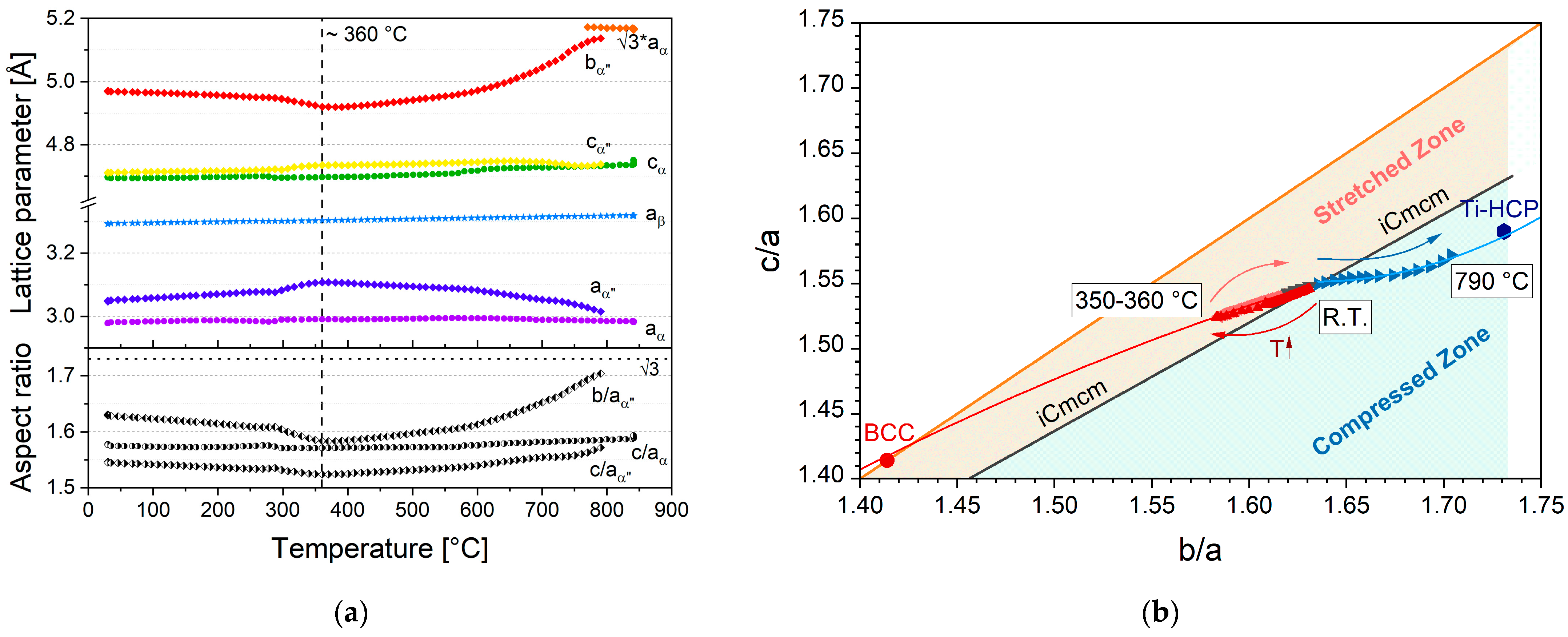
3.2.3. Evolution of Lattice Parameters and α″-Martensite Transformation Path
4. Conclusions
- i.
- The data analysis via Rietveld refinement of heat-treated Ti-6246 with its ternary α-β-α″ microstructure is a challenging endeavor and may be misleading since, especially with the presence of the orthorhombic α″-phase, a high degree of overlapping diffraction lines meets a high degree of freedom due to the large number of refinement parameters;
- ii.
- Sequential refinements should therefore be critically reviewed and brought into context. In the present study, the calculation of phase fractions was found to be less reliable due to the superposition of diffraction line and grain coarsening. Therefore, the evolution of the lattice parameters was used in order to analyze the ongoing phase transformations. The calculated 2θ-line positions were critically reviewed and found to be reliable;
- iii.
- The microstructure of Ti-6246 was characterized after each process step of the heat treatments. The H + Q (homogenized at 970 °C and quenched) microstructure mainly consisted of α″-martensite needles and residual β-Ti and α-Ti;
- iv.
- The reheating to three different temperatures in the range of service temperatures with a holding time of 600 s had different effects. With increasing temperature, the martensite content in the final sample state decreased. The microstructure of the 650 °C sample showed distinct α- and β-grains, which were not observable for both states reheated to lower temperatures (550 °C and 600 °C);
- v.
- Based on the H + Q microstructure, the phase transformation path of the phases α, β and α″-martensite was characterized during continuous heating up to 550–650 °C and 840 °C. It shows a two-tier nature. In the lower temperature segment (until approx. 370 °C), α″ transforms into β. With further heating, α″ gradually transforms into α and is completely decomposed at 790 °C;
- vi.
- With the help of the crystal-structure-based model recently proposed by Demakov et al. [16], the α″-martensite transformation pathway could have been graphically reprocessed, highlighting the transformation trends in a very focused manner. It allows the identification of the location of α″-martensite on the b.c.c.-Cmcm-h.c.p. transformation path without extensive crystallographic background.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- John, R.; Buchanan, D.; Jha, S.; Larsen, J. Stability of shot-peen residual stresses in an α + β titanium alloy. Scr. Mater. 2009, 61, 343–346. [Google Scholar] [CrossRef]
- Yang, S. HCF Property Improvement through Microstructure Optimization and Shot Peening in (α + β) Ti Alloys. Ph.D. Thesis, Technical University of Clausthal, Clausthal-Zellerfeld, Germany, 2014. [Google Scholar]
- Güler, E.; Güler, M.; Uğur, G.; Uğur, Ş. A first-principles study for the elastic and mechanical properties of Ti64, Ti6242 and Ti6246 alloys. Eur. Phys. J. B 2021, 94, 2021. [Google Scholar] [CrossRef]
- Sauer, C.; Lütjering, G. Processing, Microstructure and Properties of Ti-6246. In Titanium ’99: Science and Technology, Proceedings of the 9th World Conference on Titanium, Saint-Petersburg, Saint-Petersburg, Russia, 7–11 June 1999; Goryn, I.V., Ushkov, S.S., Eds.; Central Research Institute of Structural Materials: Saint Petersburg, Russia, 2000; Volume 1, pp. 390–397. [Google Scholar]
- Armendia, M.; Osborne, P.; Garay, A.; Belloso, J.; Turner, S.; Arrazola, P.-J. Influence of Heat Treatment on the Machinability of Titanium Alloys. Mater. Manuf. Process. 2012, 27, 457–461. [Google Scholar] [CrossRef]
- Terlinde, G.; Witulski, T.; Fischer, G. Thermomechanical Treatment of Titanium alloys—Forging of Titanium. In Titanium and Titanium Alloys; Leyens, C., Peter, M., Eds.; WILEY-VCH: Weinheim, Germany, 2003; pp. 289–304. [Google Scholar]
- Kapoor, K.; Ravi, P.; Naragani, D.; Park, J.-S.; Almer, J.; Sangid, M. Strain rate sensitivity, microstructure variations, and stress-assisted phase transformation investigation on the mechanical behavior of dual-phase titanium alloys. Mater. Charact. 2020, 166, 110410. [Google Scholar] [CrossRef]
- Birosca, S.; Buffiere, J.; Garcia-Pastor, F.; Karadge, M.; Babout, L.; Preuss, M. Three-dimensional characterization of fatigue cracks in Ti-6246 using X-ray tomography and electron backscatter diffraction. Acta Mater. 2009, 57, 5834–5847. [Google Scholar] [CrossRef]
- Titanium Engineers. Titanium 6Al-2Sn-4Zr-6Mo Ti 6246 (UNS R56260). Texas. 2016. Available online: http://www.titaniumengineers.com/uploads/1/7/9/5/17957627/6246datasheet.pdf (accessed on 16 July 2019).
- Young, M.; Levine, E.; Margolin, H. The aging behavior of orthorhombic martensite in Ti-6-2-4-6. Metall. Trans. 1974, 5, 1891–1898. [Google Scholar] [CrossRef]
- Stella, P.; Giovanetti, I.; Masi, G.; Leoni, M.; Molinari, A. Microstructure and microhardness of heat-treated Ti-6Al-2Sn-4Zr-6Mo alloy. J. Alloys Compd. 2013, 567, 134–140. [Google Scholar] [CrossRef]
- Pederson, R.; Niklasson, F.; Skystedt, F.; Warren, R. Microstructure and mechanical properties of friction- and electron-beam welded Ti–6Al–4V and Ti–6Al–2Sn–4Zr–6Mo. Mater. Sci. Eng. A 2012, 552, 555–565. [Google Scholar] [CrossRef]
- Bönisch, M. Revealing how martensite in Ti-alloys expands and decomposes. Sci. Highlights-Struct. Mater. 2018, 135–136. [Google Scholar] [CrossRef]
- Bönisch, M.; Panigrahi, A.; Calin, M.; Stoica, M.; Waitz, T.; Zehetbauer, M.; Skrotzki, W.; Eckert, J. Diffusive and Diffusionless Transformations of Martensitic Ti-Nb Alloys. In Proceedings of the International Conference on Martensitic Transformations ICOMAT 2017, Chicago, IL, USA, 9–14 July 2017. [Google Scholar] [CrossRef]
- Bönisch, M.; Panigrahi, A.; Stoica, M.; Calin, M.; Ahrens, E.; Zehetbauer, M.; Skrotzki, W.; Eckert, J. Giant thermal expansion and alpha-precipitation pathways in Ti-alloys. Nat. Commun. 2017, 8, 1429. [Google Scholar] [CrossRef] [Green Version]
- Demakov, S.; Kylosova, I.; Stepanov, S.; Bönisch, M. A general model for the crystal structure of orthorhombic martensite in Ti alloys. Acta Crystallogr. 2021, 77, 749–762. [Google Scholar] [CrossRef]
- Davis, R.; Flower, H.; West, D. The decomposition of Ti-Mo martensites by nucleation and growth and spinodal mechanisms. Acta Metall. 1979, 27, 1041–1052. [Google Scholar] [CrossRef]
- Motyka, M. Martensite Formation and Decomposition during Traditional and AM Processing of Two-Phase Titanium Alloys–An Overview. Metals 2021, 11, 481. [Google Scholar] [CrossRef]
- Mur, F.G.; Rodríguez, D.; Planell, J. Influence of tempering temperature and time on the α′-Ti-6Al-4V martensite. J. Alloys Compd. 1996, 243, 287–289. [Google Scholar]
- Barriobero-Vila, P.; Oliveira, V.B.; Schwarz, S.; Buslaps, T.; Requena, G. Tracking the α martensite decomposition during continuous heating of a Ti-6Al-6V-2Sn alloy. Acta Mater. 2017, 135, 132–143. [Google Scholar] [CrossRef]
- Salvador, C.; Lopes, E.; Ospina, C.; Caram, R. Orthorhombic martensite formation upon aging in a Ti-30Nb-4Sn alloy. Mater. Chem. Phys. 2016, 183, 238–246. [Google Scholar] [CrossRef]
- Carrozza, A.; Aversa, A.; Fino, P.; Lombardi, M. A study on the microstructure and mechanical properties of the Ti-6Al-2Sn-4Zr-6Mo alloy produced via Laser Powder Bed Fusion. J. Alloys Compd. 2021, 870, 159329. [Google Scholar] [CrossRef]
- Mai, P.; Bormann, T.; Sonntag, R.; Kretzer, J.; Gibmeier, J. Short-Term Heat Treatment of Ti6Al4V ELI as Implant Material. Materials 2020, 13, 4948. [Google Scholar] [CrossRef]
- Esin, V.A.; Mallick, R.; Dadé, M.; Denand, B.; Delfosse, J.; Sallot, P. Combined synchrotron X-ray diffraction, dilatometry and electrical resistivity in situ study of phase transformations in a Ti2AlNb alloy. Mater. Charact. 2020, 169, 110654. [Google Scholar] [CrossRef]
- Lin, S.; Borggren, U.; Stark, A.; Borgenstam, A.; Mu, W.; Hedström, P. In-Situ High-Energy X-ray Diffraction Study of Austenite Decomposition During Rapid Cooling and Isothermal Holding in Two HSLA Steels. Metall. Mater. Trans. A 2021, 52, 1812–1825. [Google Scholar] [CrossRef]
- Toby, B.; Dreele, R.V. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Ji, X.; Gutierrez-Urrutia, I.; Emura, S.; Liu, T.; Hara, T.; Min, X.; Ping, D.; Tsuchiya, K. Twinning behavior of orthorhombic-α” martensite in a Ti-7.5Mo alloy. Sci. Technol. Adv. Mater. 2019, 20, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaschel, F.R.; Vijayaraghavan, R.K.; Shmeliov, A.; McCarthy, E.K.; Canavan, M.; McNally, P.J.; Dowling, D.P.; Nicolosi, V.; Celikin, M. Mechanism of stress relaxation and phase transformation in additively manufactured Ti-6Al-4V via in situ high temperature XRD and TEM analyses. Acta Mater. 2020, 188, 720–732. [Google Scholar] [CrossRef]
- Siemers, C. Researchgate. September 2015. Available online: https://www.researchgate.net/post/Whats-the-difference-in-the-crystal-lattice-between-alpha-and-alpha-primemartensite-in-titanium-alloys (accessed on 18 August 2022).
- Bönisch, M. Researchgate. June 2018. Available online: https://www.researchgate.net/post/Whats-the-difference-in-the-crystal-lattice-between-alpha-and-alpha-primemartensite-in-titanium-alloys (accessed on 18 August 2022).
- The strengthening molybdenum and aluminum equivalents of alloying elemts in titanium. Titanium’99: Science and Technology. 1999, Volume 1–2, pp. 56–60. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiBleDh8K39AhWxNOwKHcHQCsUQFnoECAkQAQ&url=https%3A%2F%2Fcdn.ymaws.com%2Ftitanium.org%2Fresource%2Fresmgr%2FZZ-WTCP1999-VOL1%2F1999_Vol.1-2-The_Strengtheni.pdf&usg=AOvVaw1UyTxaSFWEauhtGa4U_e-3 (accessed on 10 August 2022).
- Barriobero-Vila, P.; Requena, G.; Warchomicka, F.; Stark, A.; Schell, N.; Buslaps, T. Phase transformation kinetics during continuous heating of a beta-quenched Ti–10V–2Fe–3Al alloy. J. Mater. Sci. 2015, 50, 1412–1426. [Google Scholar] [CrossRef]





| Phase | Crystal Group | Cell Length [Å] | ||
|---|---|---|---|---|
| a | b | c | ||
| α (hcp) | P63/mmc | 2.9707 | 4.6880 | |
| β (bcc) | Im-3m | 3.2877 | ||
| α″ (orthorhombic) | Cmcm | 3.0387 | 4.9731 | 4.7176 |
| Designation | Temperature [°C] | Duration [s] | Cooling Rate [°C/s] |
|---|---|---|---|
| H+Q | 970 | 600 | Max. −1581 |
| T | 550-650 | 600 | Max. −1096 |
| T1 | 840 | 30 | Max. −274 |
| T2 | 600 | 120 | Max. −204 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otte, A.-L.; Mai, P.T.; Stark, A.; Hoelzel, M.; Hofmann, M.; Gibmeier, J. Kinetics of Martensite Decomposition and Microstructure Stability of Ti-6246 during Rapid Heating to Service Temperatures. Metals 2023, 13, 484. https://doi.org/10.3390/met13030484
Otte A-L, Mai PT, Stark A, Hoelzel M, Hofmann M, Gibmeier J. Kinetics of Martensite Decomposition and Microstructure Stability of Ti-6246 during Rapid Heating to Service Temperatures. Metals. 2023; 13(3):484. https://doi.org/10.3390/met13030484
Chicago/Turabian StyleOtte, Anna-Lena, Phuong Thao Mai, Andreas Stark, Markus Hoelzel, Michael Hofmann, and Jens Gibmeier. 2023. "Kinetics of Martensite Decomposition and Microstructure Stability of Ti-6246 during Rapid Heating to Service Temperatures" Metals 13, no. 3: 484. https://doi.org/10.3390/met13030484





