Evolution Behavior of the Surface Oxide Film of Al Alloy Scraps in the Melt
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
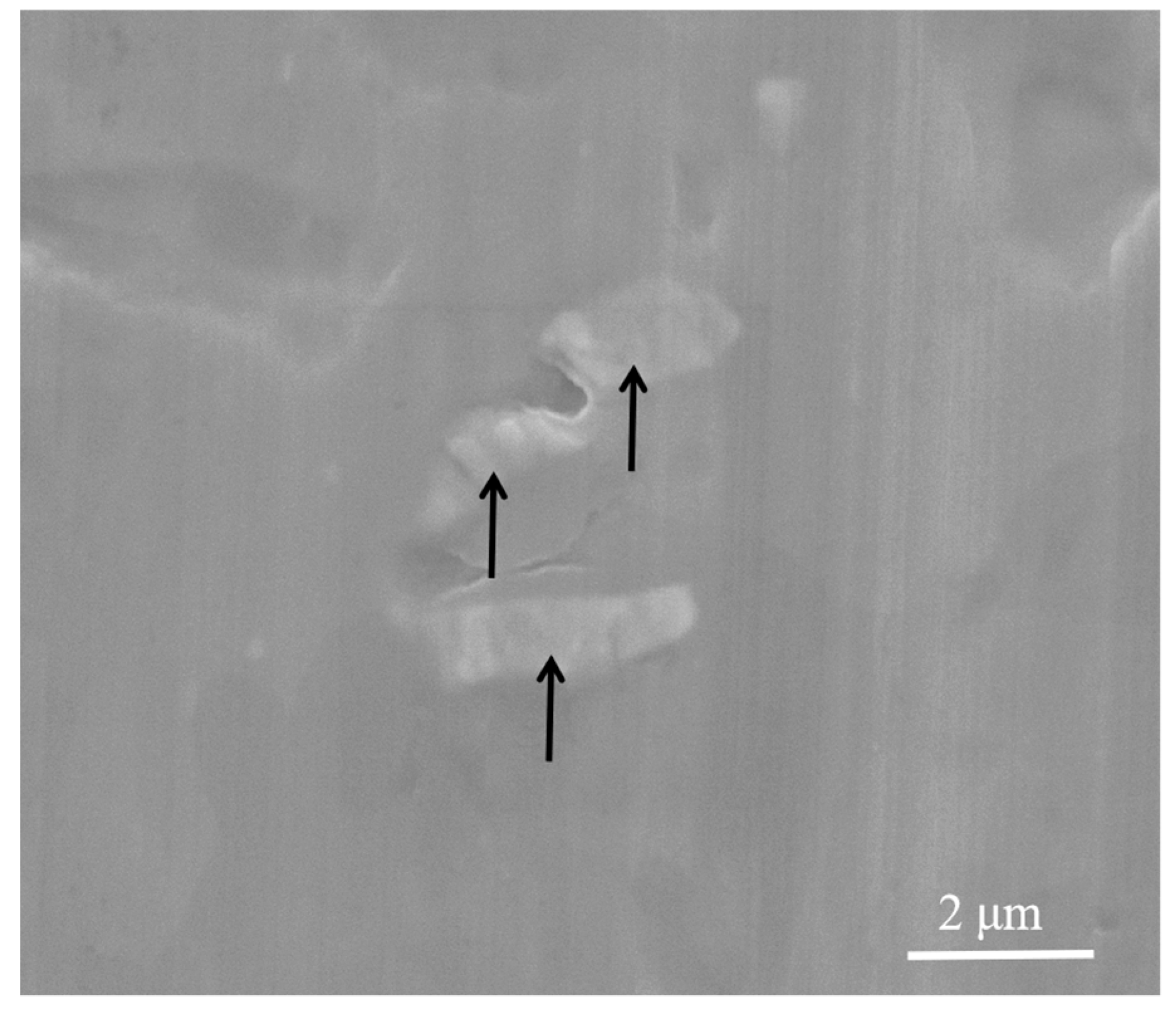
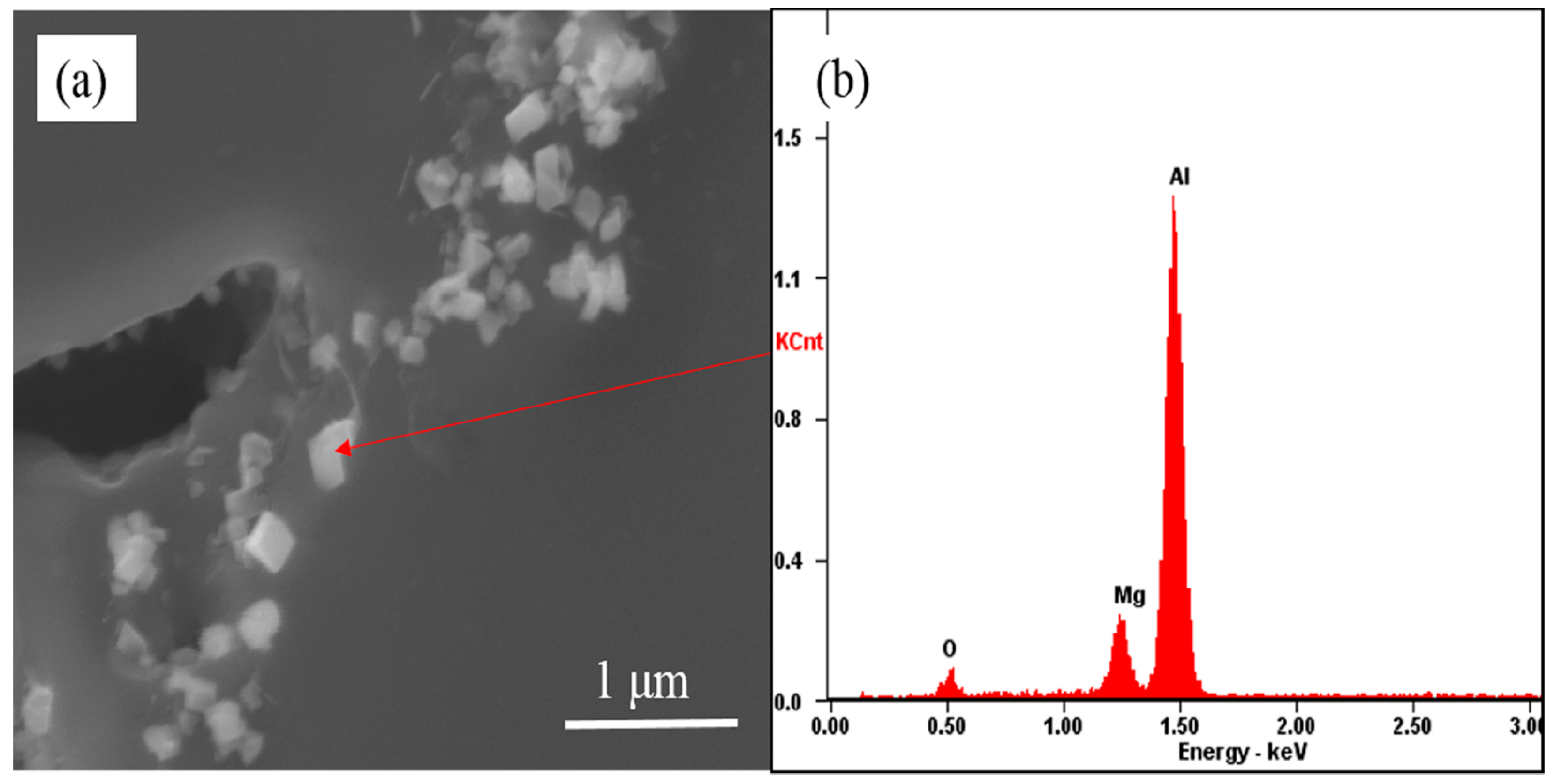
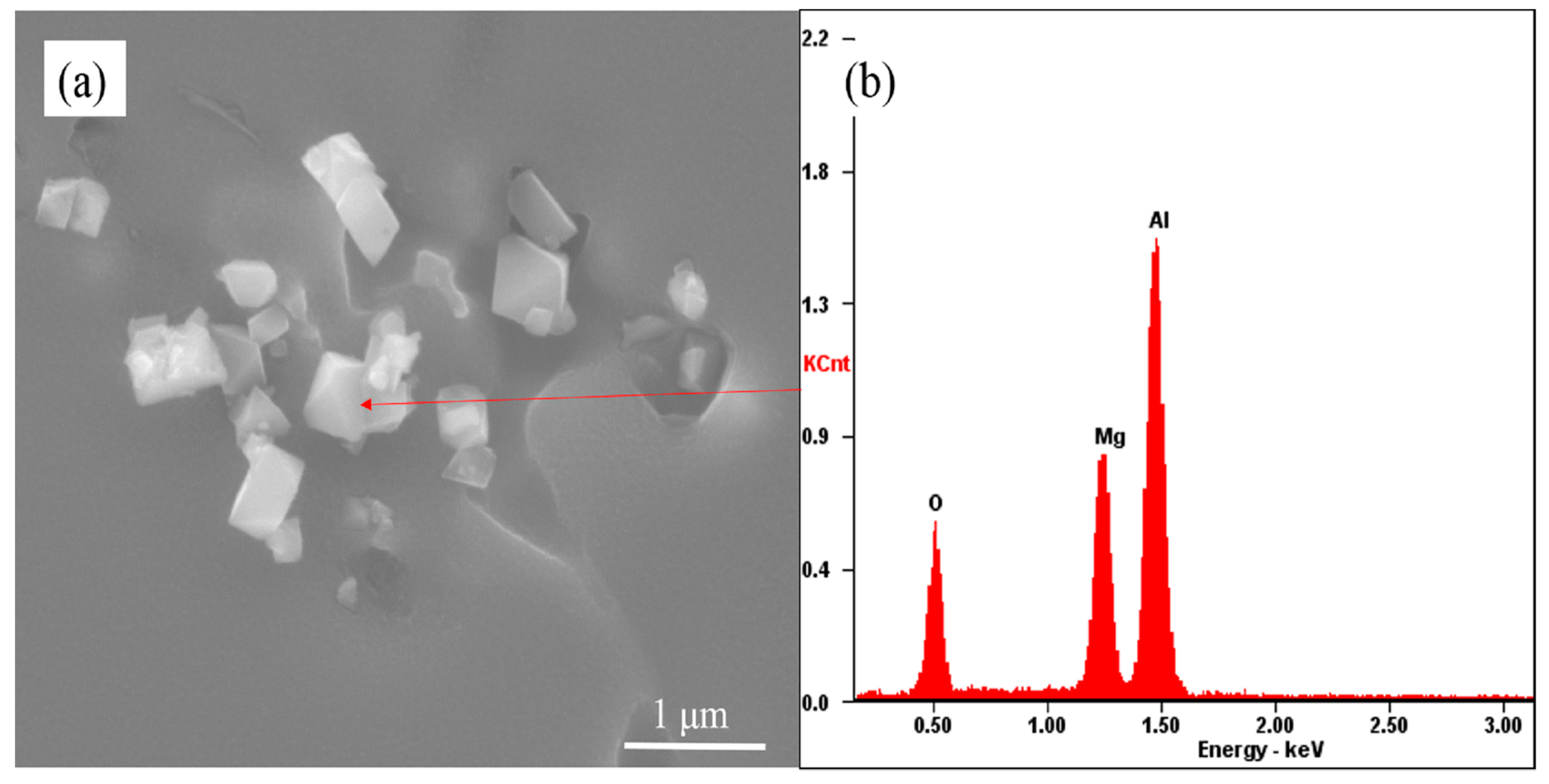
3.1. The Evolution Behavior of the Surface Oxide Film of 1235 Alloy Foils in the Melt
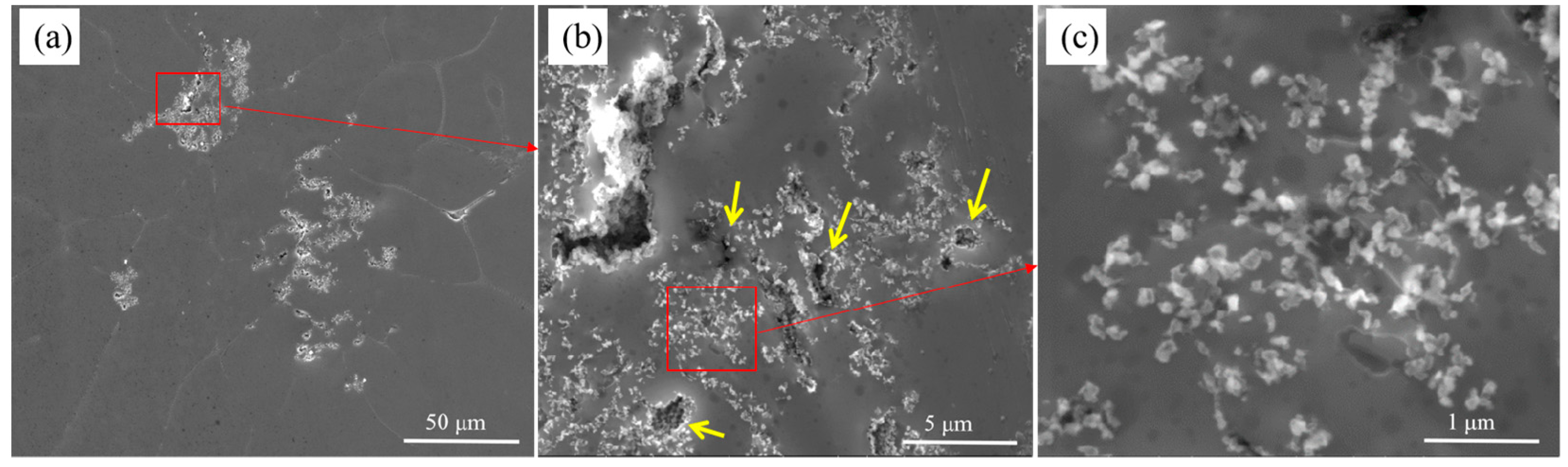
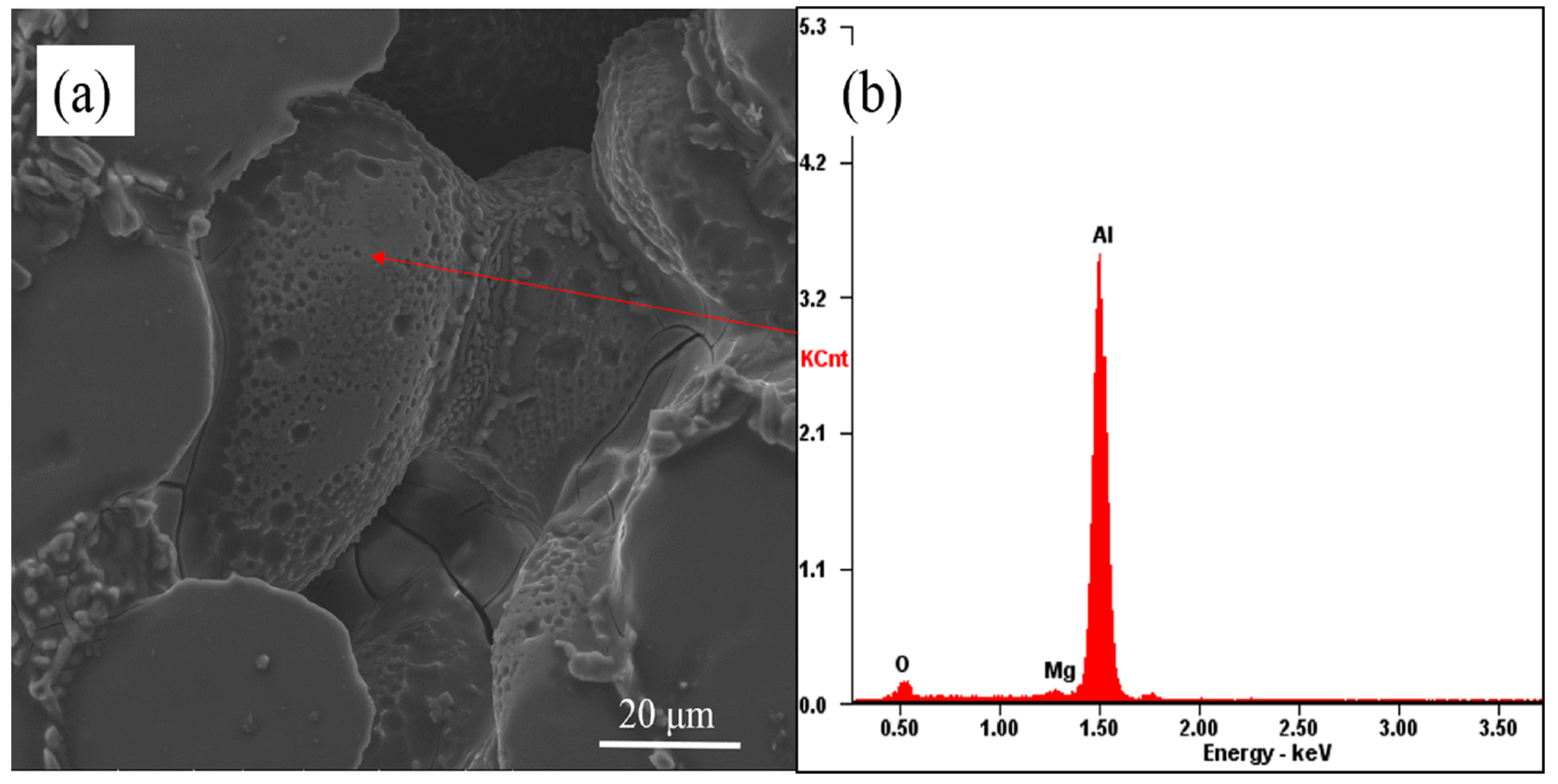
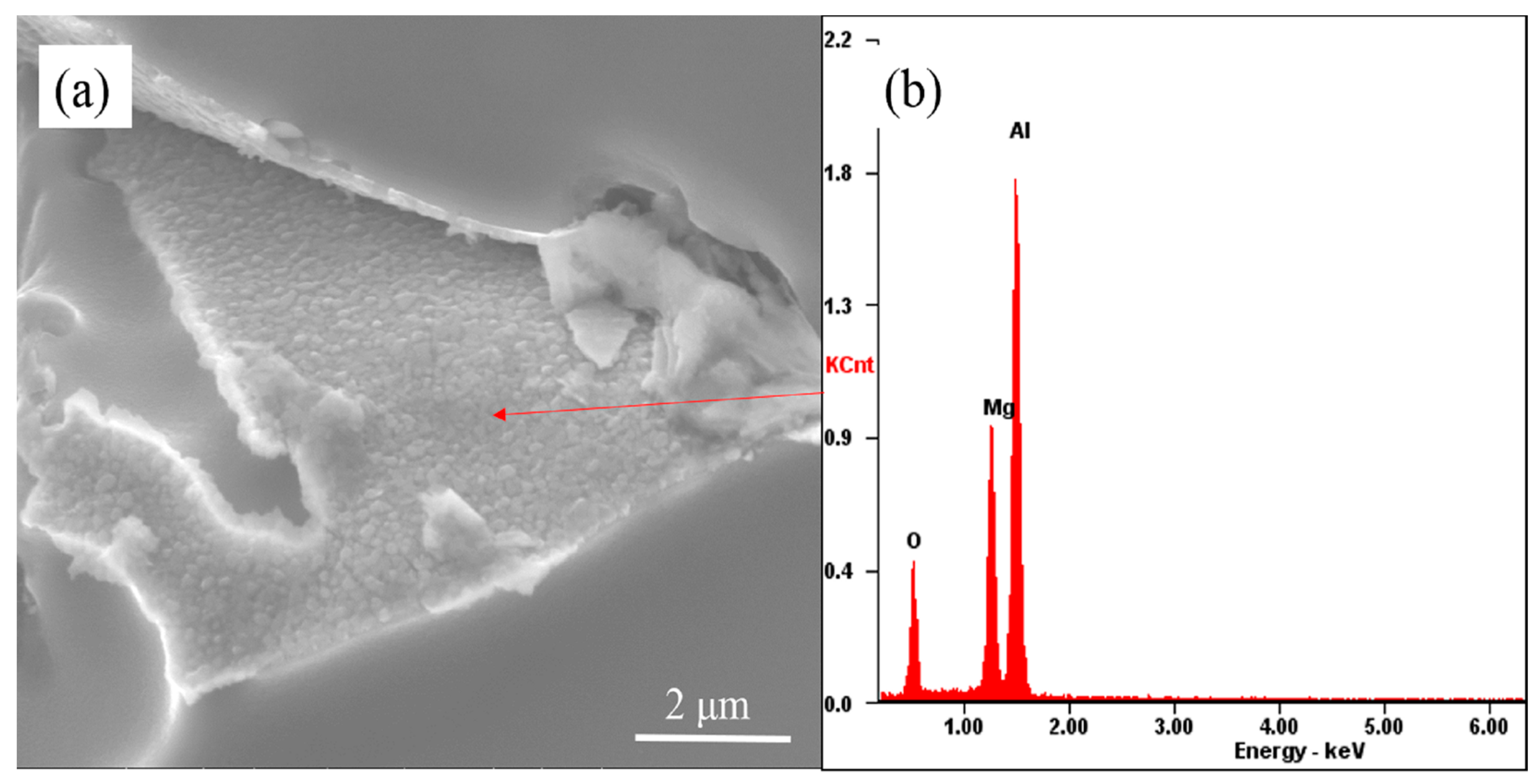
3.2. The Evolution Behavior of the Surface Oxide Film of A356 Alloy Scraps in the Melt
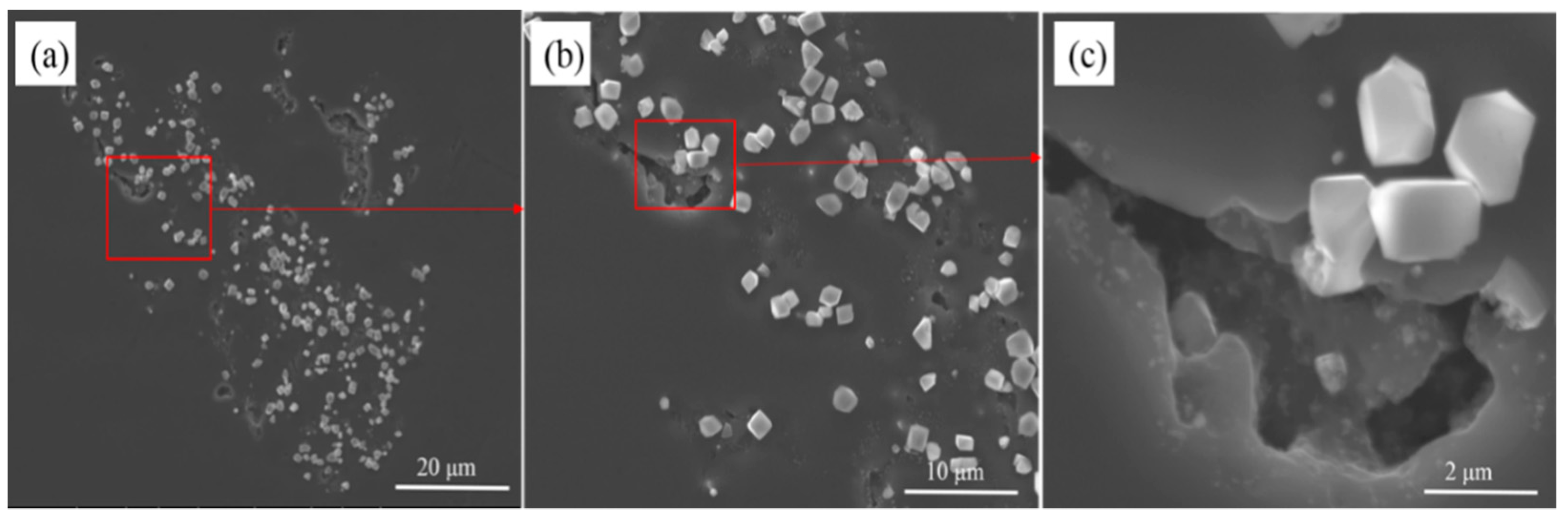
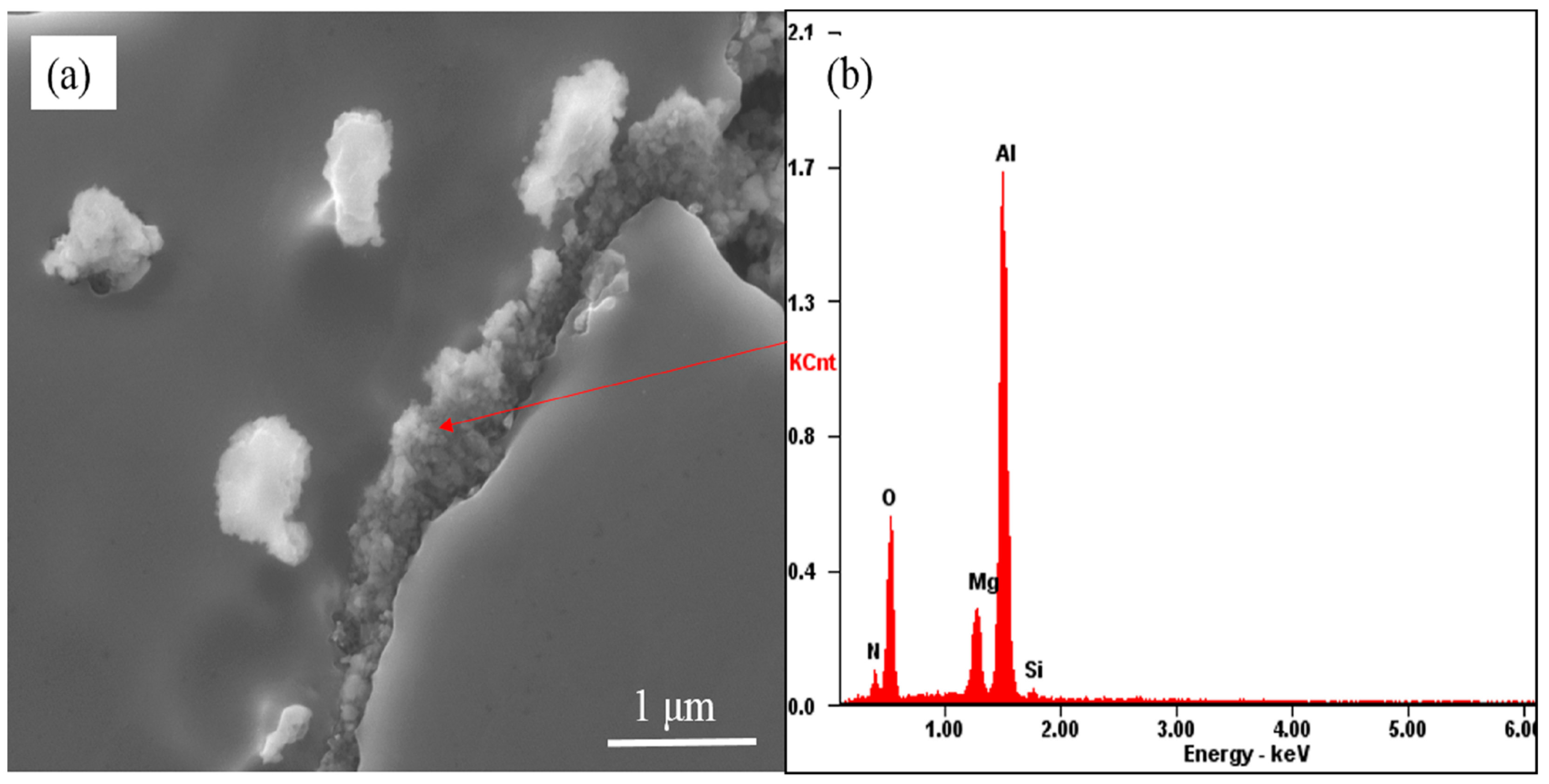
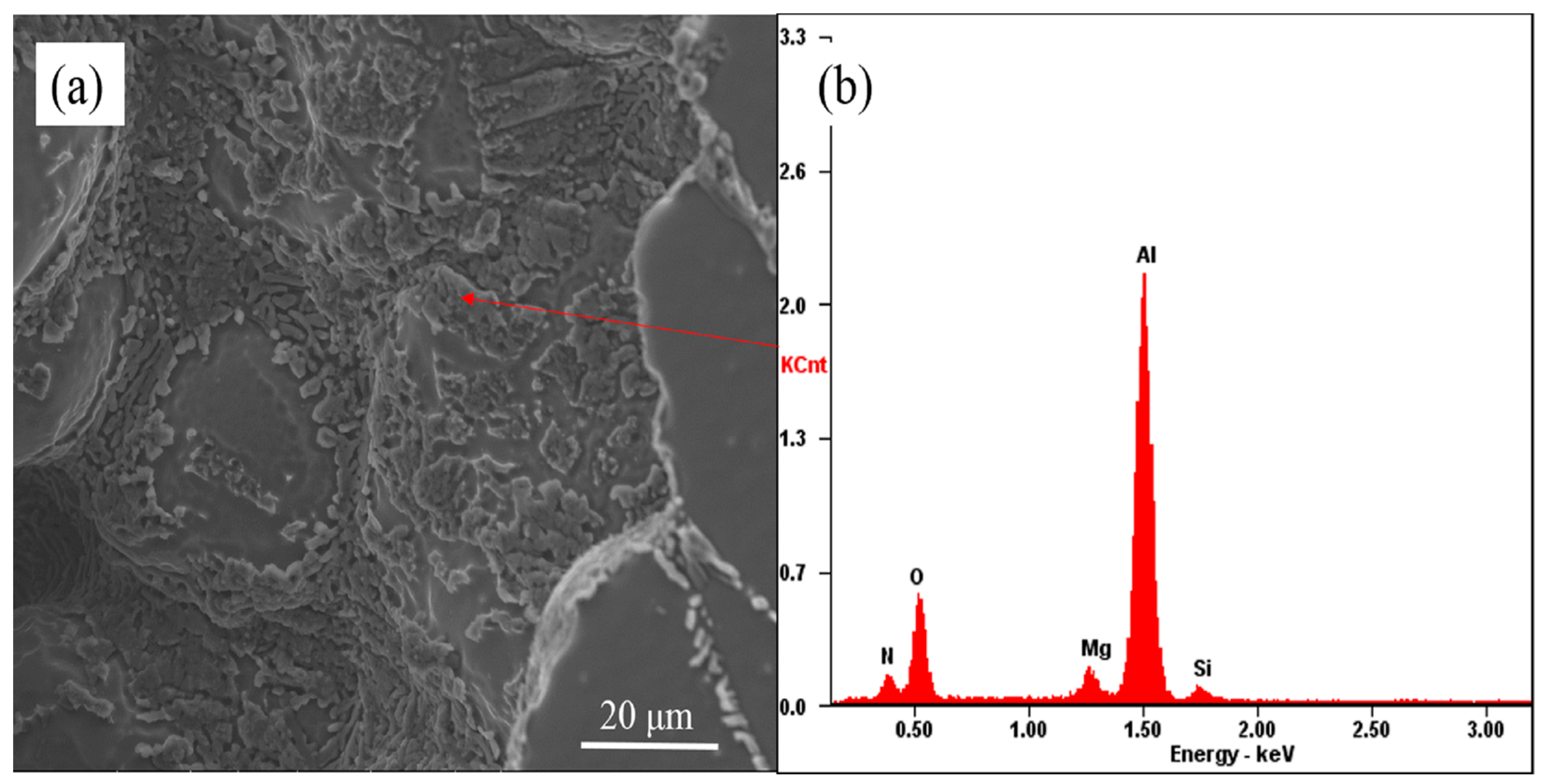
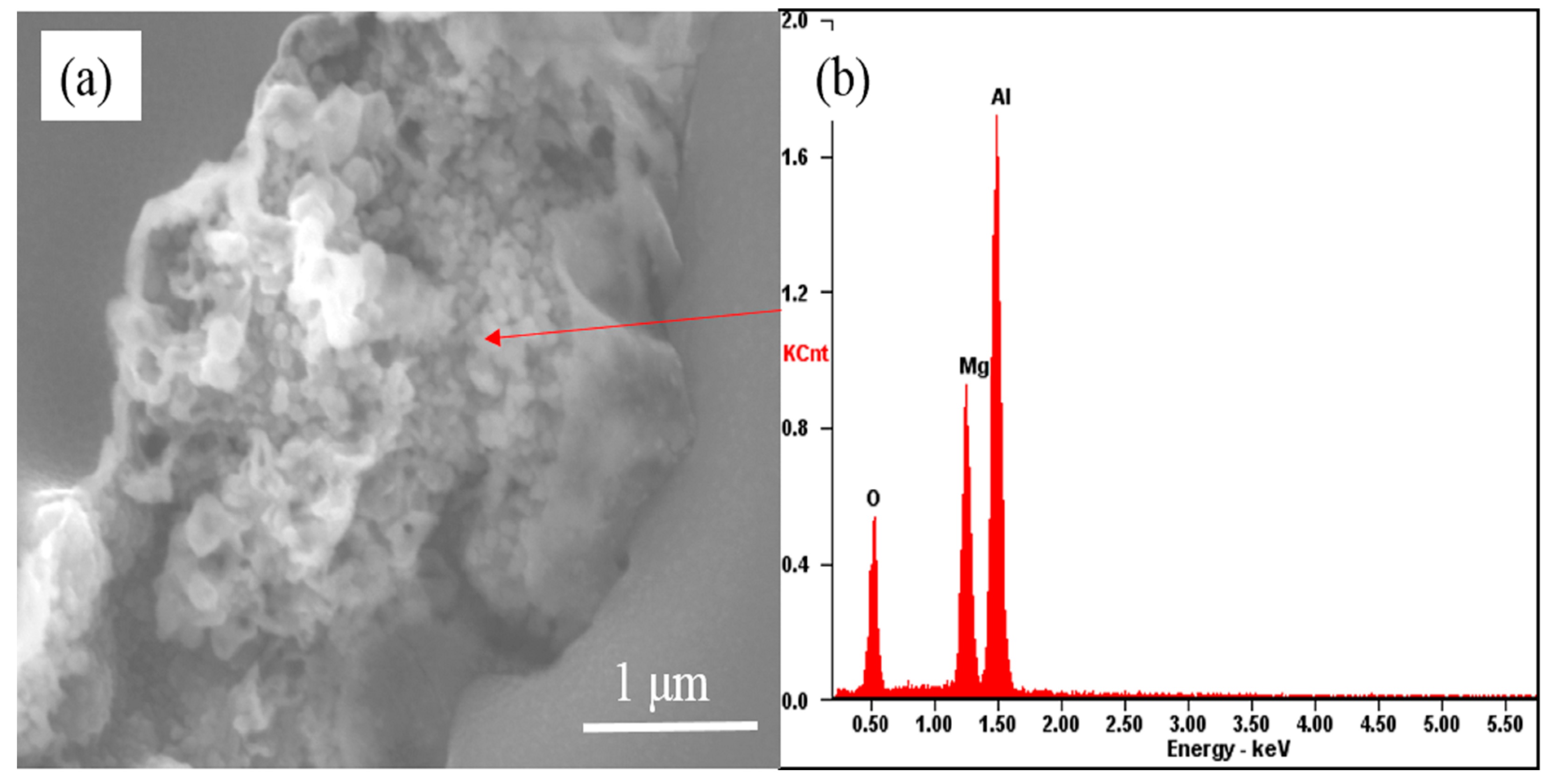
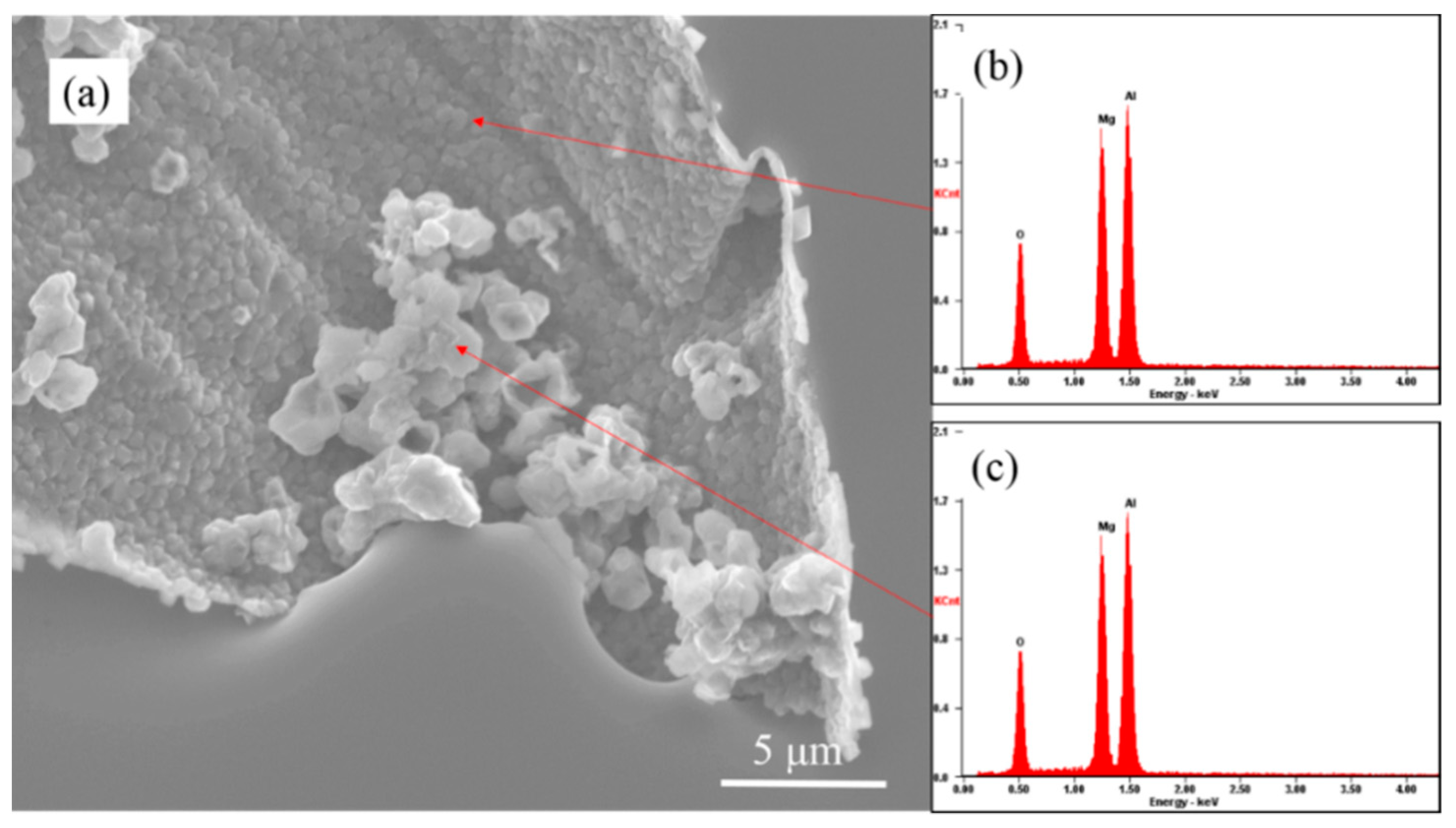
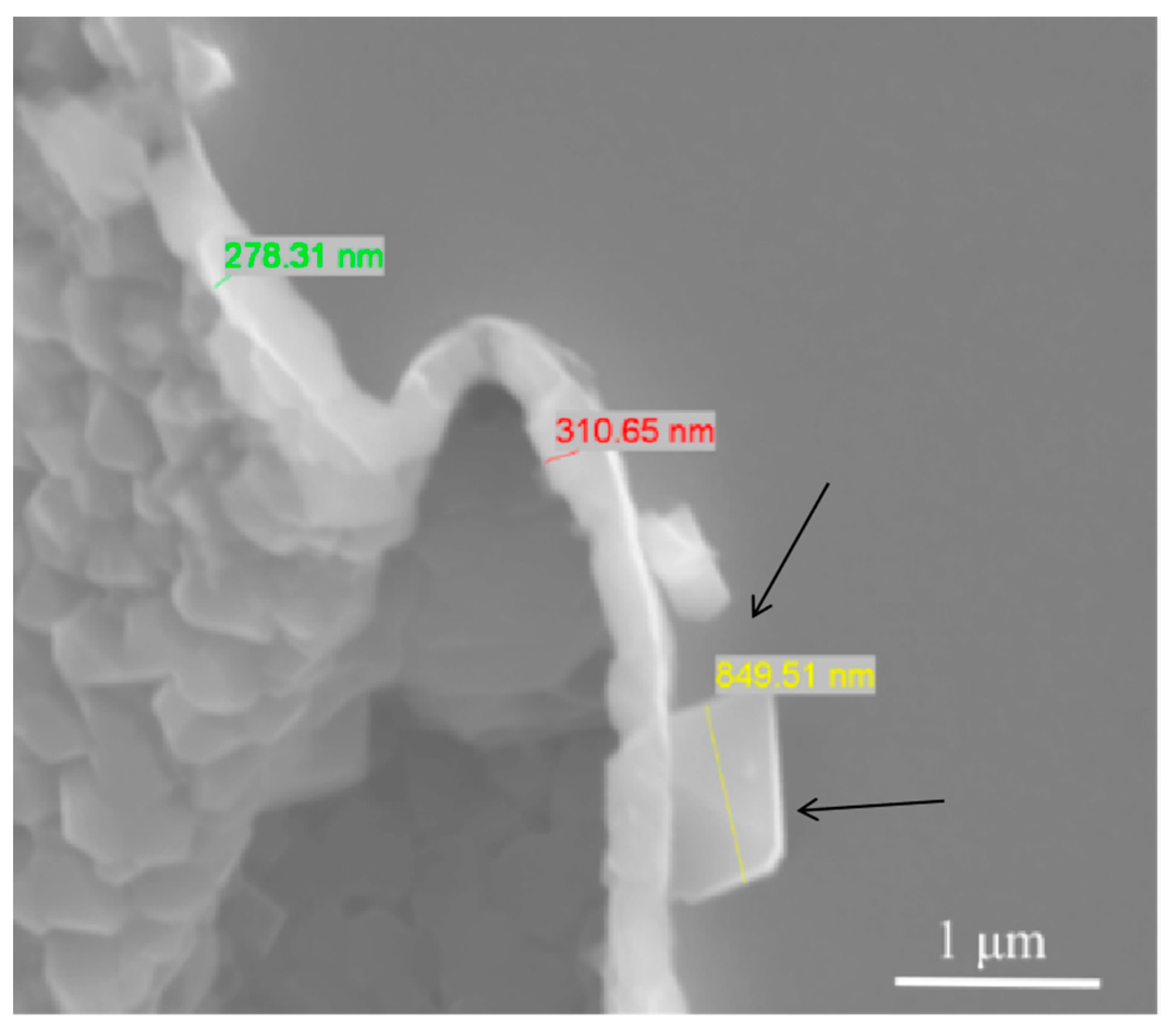
3.3. The Evolution Behavior of the Surface Oxide Film of 5083 Alloy Scraps in Melt
4. Conclusions
- When 1235 alloy foil was held in the commercial pure aluminum melt, the surface oxide films were broken into fine oxide film in 15 min, and then transformed into cuboid shape oxide in 30 min. When the aluminum alloy melt with 1235 alloy foil was treated, the appropriate holding time was helpful to break the oxide film in the melt.
- The surface oxide film of A356 scrap was broken from a large film shape to a small flake shape, and then into a cuboid shape in the commercial pure aluminum melt. While in the A356 alloy melt, the oxide film changed from large to minor and covered the secondary dendrite of the A356 alloy.
- When the 5083 alloy scraps were held in the commercial pure aluminum melt, the surface oxide film transformed to cuboid-like oxides. When the scrap was charged into and held in Al-4.5Mg alloy melt, the oxide film became thicker and more difficult to break.
- If the alloying element magnesium is added before purification treatment or the oxide film in the melt is not removed thoroughly, the presence of alloying element magnesium will make the oxide film thicker and in the form of a thick sheet shape.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.P.; Ji, G.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.W.; Vleugels, J.; Humbeeck, J.V.; Kruth, J.P. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta Mater. 2017, 129, 183–193. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, D.; Ma, C.; Xi, L.; Zhang, H. Microstructure characteristics and its formation mechanism of selective laser melting SiC reinforced Al-based composites. Vacuum 2019, 160, 189–196. [Google Scholar] [CrossRef]
- Wilms, M.B.; Rittinghaus, S.K.; Goßling, M.; Gokce, B. Additive manufacturing of oxide-dispersion strengthened alloys: Materials, synthesis and manufacturing. Prog. Mater. Sci. 2023, 133, 101049. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xia, M.; Arumuganathar, S. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing. Acta Mater. 2009, 57, 4891–4901. [Google Scholar] [CrossRef]
- Bonner, S.J. A Microstructure and Kinetic Study of Molten Aluminum Oxidation in Relation to Dross Formation. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2015. [Google Scholar]
- Impey, S.A.; Stephenson, D.J.; Nicholls, J.R. Mechanism of scale growth on liquid aluminum. Mater. Sci. Technol. 1988, 4, 1126–1132. [Google Scholar] [CrossRef]
- Aryafar, M.; Raiszadeh, R.; Shalbafzadeh, A. Healing of double oxide film defects in A356 aluminum melt. J. Mater. Sci. 2010, 45, 3041–3051. [Google Scholar] [CrossRef]
- Akbarifar, M.; Divandari, M.; Boutorabi SM, A.; Ha, S.H.; Yoon, Y.O.; Kim, S.K. Short-Time Oxidation of Al-Mg in Dynamic Conditions. Oxid. Met. 2020, 94, 409–429. [Google Scholar] [CrossRef]
- Campbell, J. The consolidation of metals: The origin of biofilms. J. Mater. Sci. 2016, 51, 96–106. [Google Scholar] [CrossRef]
- Bakhtiarani, F.N.; Ramin, R. Healing of Double-Oxide Film Defects in Commercial Purity Aluminum Melt. Metall. Mater. Trans. B 2011, 42, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpour, A.; Raiszadeh, R.; Doostmohammadi, H. Effect of stirring on behaviour of double oxide film defects in A356 aluminium melt. Int. J. Cast Met. Res. 2014, 4, 221–229. [Google Scholar] [CrossRef]
- Amirinejad, S.; Raiszadeh, R.; Doostmohammadi, H. Study of double oxide film defect behaviour in liquid Al-Mg alloys. Cast Metals 2013, 26, 330–338. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.T.; Fan, Z. Oxidation of Aluminum Alloy Melts and Inoculation by Oxide Particles. Trans. Indian Inst. Met. 2012, 65, 653–661. [Google Scholar] [CrossRef]
- Li, C.; Li, J.G.; Mao, Y.Z.; Ji, J.C. Mechanism to remove oxide inclusions from molten aluminum by solid fluxes refining method. China Foundry 2017, 14, 233–243. [Google Scholar] [CrossRef]
- Li, C.; Gökelma, M.; Dang, T.; Huang, J.; Huang, C.; Li, J.; Friedrich, B. Assessment of Melt Cleanliness of Secondary 5000 Aluminum Alloy Via Non-metallic Inclusions Characterization. Metall. Mater. Trans. B 2023, 1–15. [Google Scholar] [CrossRef]
- Keles, O.; Dundar, M. Aluminum foil: Its typical quality problems and their causes. J. Mater. Process. Technol. 2007, 186, 125–137. [Google Scholar] [CrossRef]
- Kang, J.X.; Fu, G.S. The Behavior of Inclusions and Hydrogen in Molten Aluminum. Chin. J. Nonferrous Met. 1995, 5, 5–8. [Google Scholar]
- Jia, Y.C. The Study on Hydrogen and Inclusion in Aluminum Melt. Master’s. Thesis, Northeastern University, Shenyang, China, 2010. [Google Scholar]
- Snijders, P.C.; Jeurgens, L.; Sloof, W.G. Structural ordering of ultra-thin, amorphous aluminium-oxide films. Surf. Sci. 2005, 589, 98–105. [Google Scholar] [CrossRef]
- Dignam, M.J.; Fawcett, W.R.; Bohni, H. The kinetics and Mechanism of Oxidation of Superpurity Aluminum in Dry Oxygen I. Apparatus Description and the Growth of “Amorphous” Oxide. J. Electrochem. Soc. 1966, 113, 656–662. [Google Scholar] [CrossRef]
- Preston, G.D.; Bircumshaw, L.L. Studies on the oxidation of metals-Part IV. The oxide film on aluminum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1936, 22, 654–665. [Google Scholar] [CrossRef]
- Yang, B.C.; Chen, S.F.; Song, H.W.; Zhang, S.H.; Chang, H.P.; Xu, S.W.; Zhu, Z.H.; Li, C.H. Effects of microstructure coarsening and casting pores on the tensile and fatigue properties of cast A356-T6 aluminum alloy: A comparative investigation. Mater. Sci. Eng. A 2022, 857, 144106. [Google Scholar] [CrossRef]
- Mahmoud, A.E. The Behaviour of Bifilm Defects in Cast Al-7Si-Mg Alloy. PLoS ONE 2016, 11, e0160633. [Google Scholar]
- Jorge, A.S.; Espinosa, D.C.R. High-Temperature Oxidation of Al-Mg Alloys. Oxid. Met. 2000, 53, 361–373. [Google Scholar]
- Li, H.T.; Wang, Y.; Fan, Z. Mechanisms of enhanced heterogeneous nucleation during solidification in binary Al–Mg alloys. Acta Mater. 2012, 60, 1528–1537. [Google Scholar] [CrossRef] [Green Version]
- Field, D.J.; Scamans, G.M.; Butler, E.P. The high temperature oxidation of Al-4.2 Wt Pct Mg alloy. Metall. Trans. A 1987, 18, 463–472. [Google Scholar] [CrossRef]
- Bakhtiarani, F.N.; Raiszadeh, R. The behaviour of double oxide film defects in Al-4.5 wt.% Mg melt. J. Mater. Sci. 2011, 46, 1305–1315. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Liu, Z.; Li, J. Evolution Behavior of the Surface Oxide Film of Al Alloy Scraps in the Melt. Metals 2023, 13, 510. https://doi.org/10.3390/met13030510
Huang C, Liu Z, Li J. Evolution Behavior of the Surface Oxide Film of Al Alloy Scraps in the Melt. Metals. 2023; 13(3):510. https://doi.org/10.3390/met13030510
Chicago/Turabian StyleHuang, Chunfa, Zhiguo Liu, and Jianguo Li. 2023. "Evolution Behavior of the Surface Oxide Film of Al Alloy Scraps in the Melt" Metals 13, no. 3: 510. https://doi.org/10.3390/met13030510





