High-Entropy Alloy Coatings Deposited by Thermal Spraying: A Review of Strengthening Mechanisms, Performance Assessments and Perspectives on Future Applications
Abstract
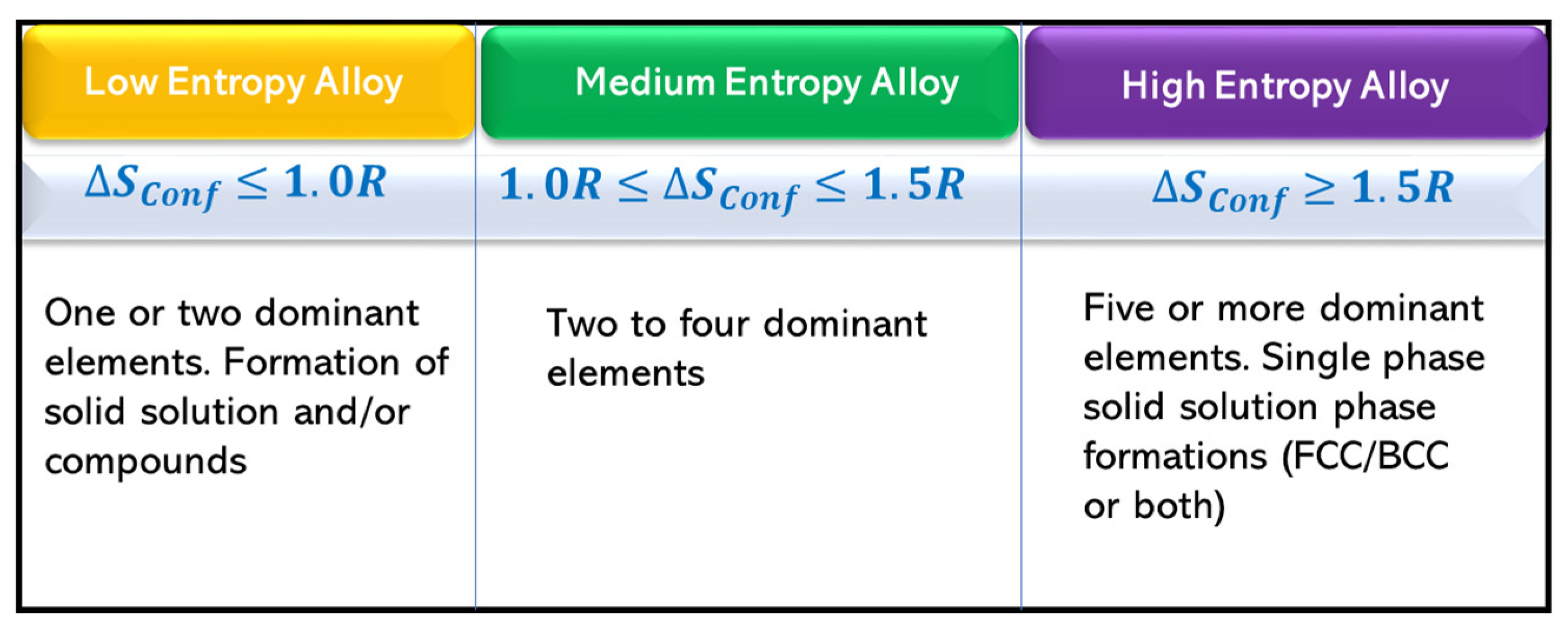
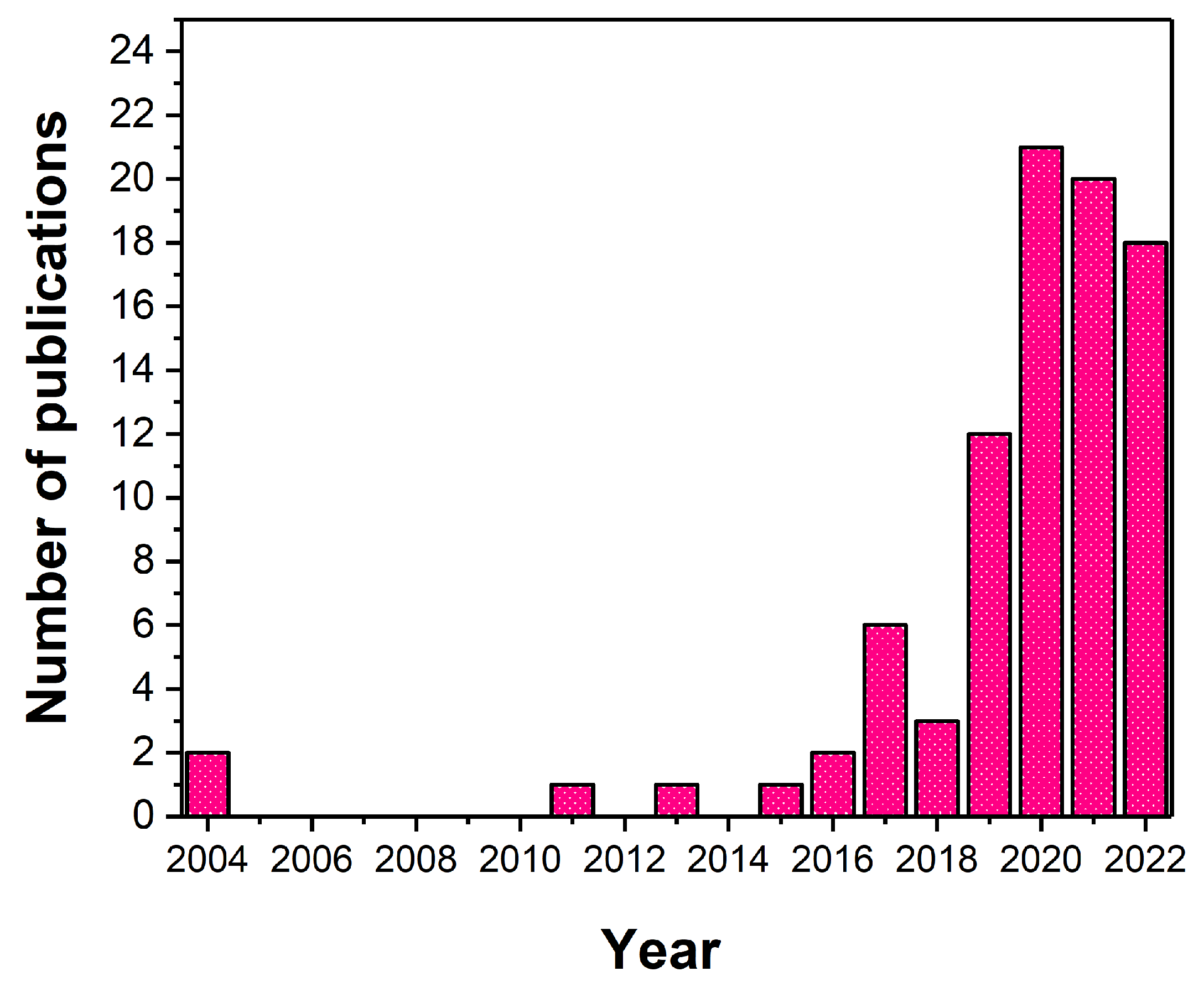
:1. Introduction
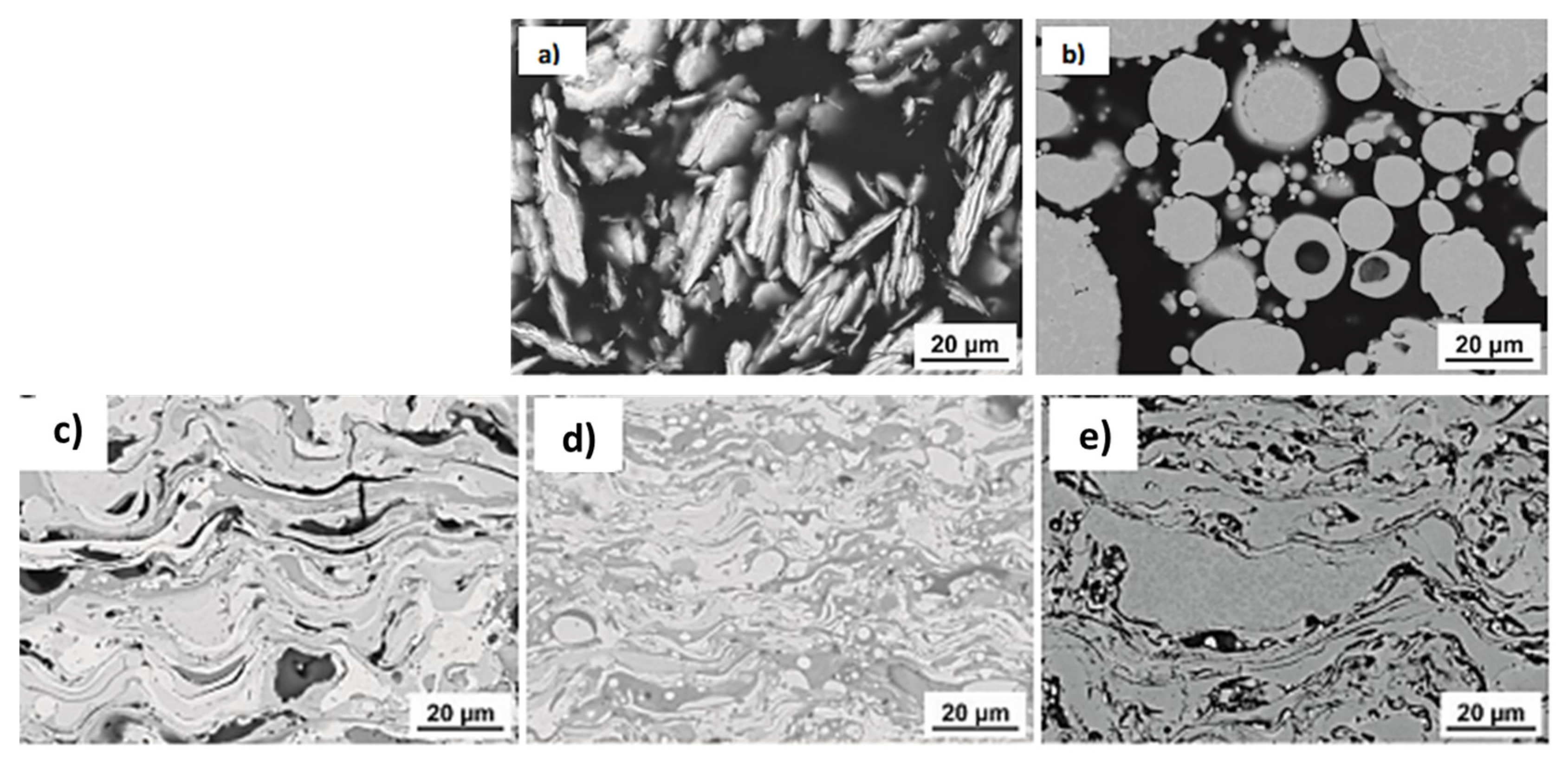
2. Preparation of HEA Feedstock
3. Microstructure and Strengthening Mechanisms of HEA Coatings
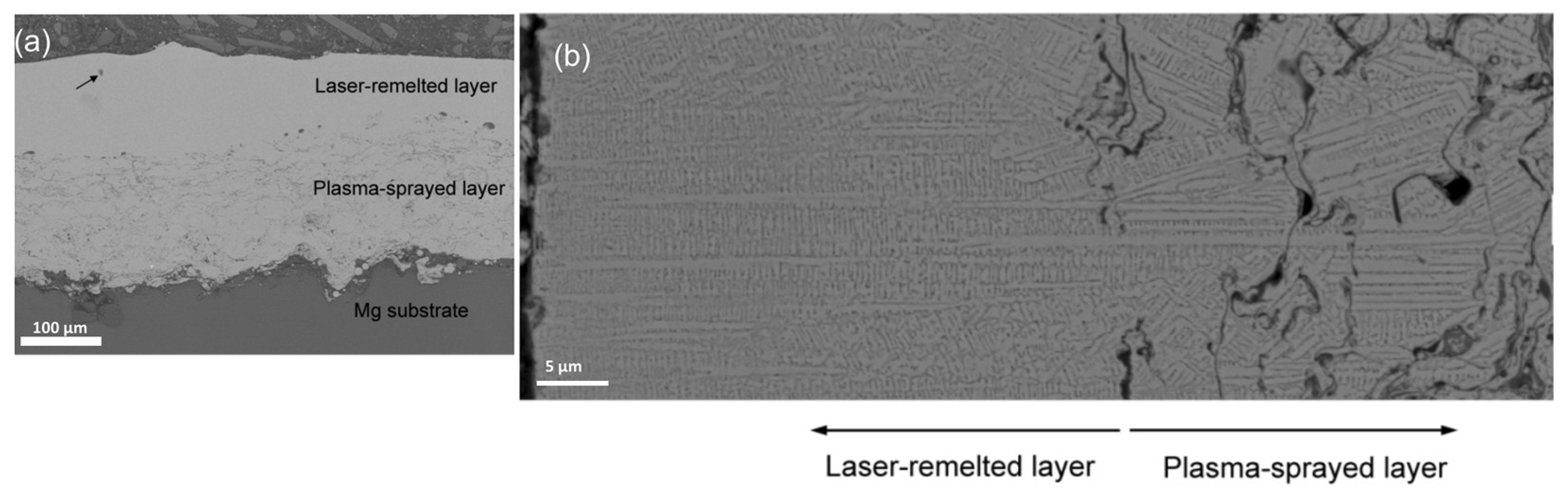
3.1. Microstructure
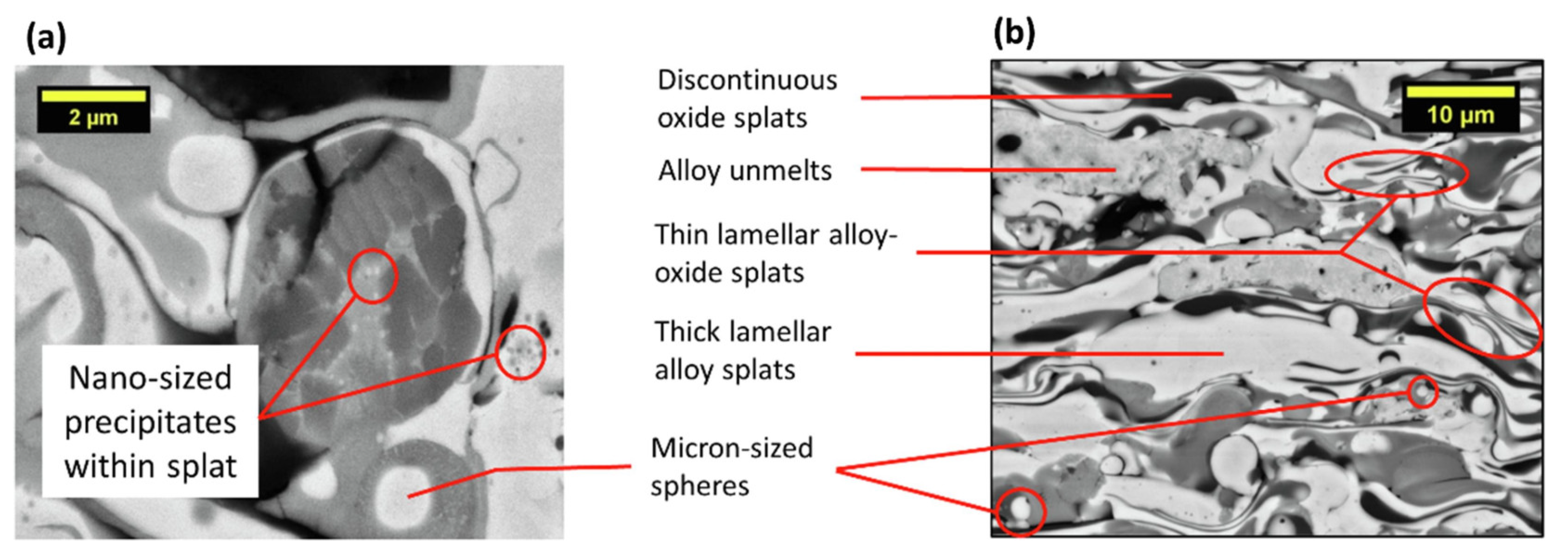
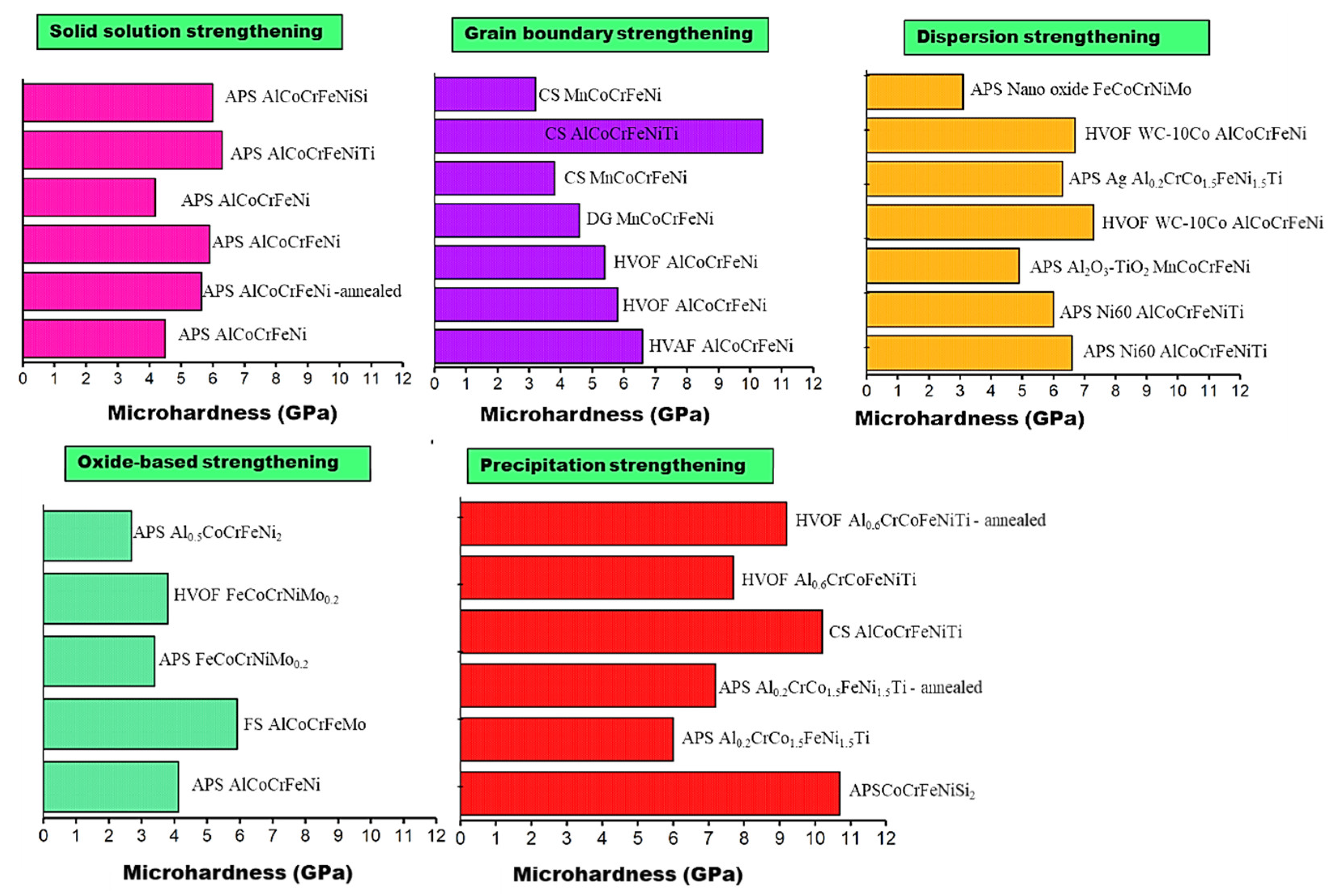
3.2. Strengthening Mechanisms of HEA Coatings
3.2.1. Solid Solution Strengthening
3.2.2. Grain Boundary Strengthening
3.2.3. Oxide-Based Strengthening
3.2.4. Dispersion Strengthening
3.2.5. Precipitation Strengthening
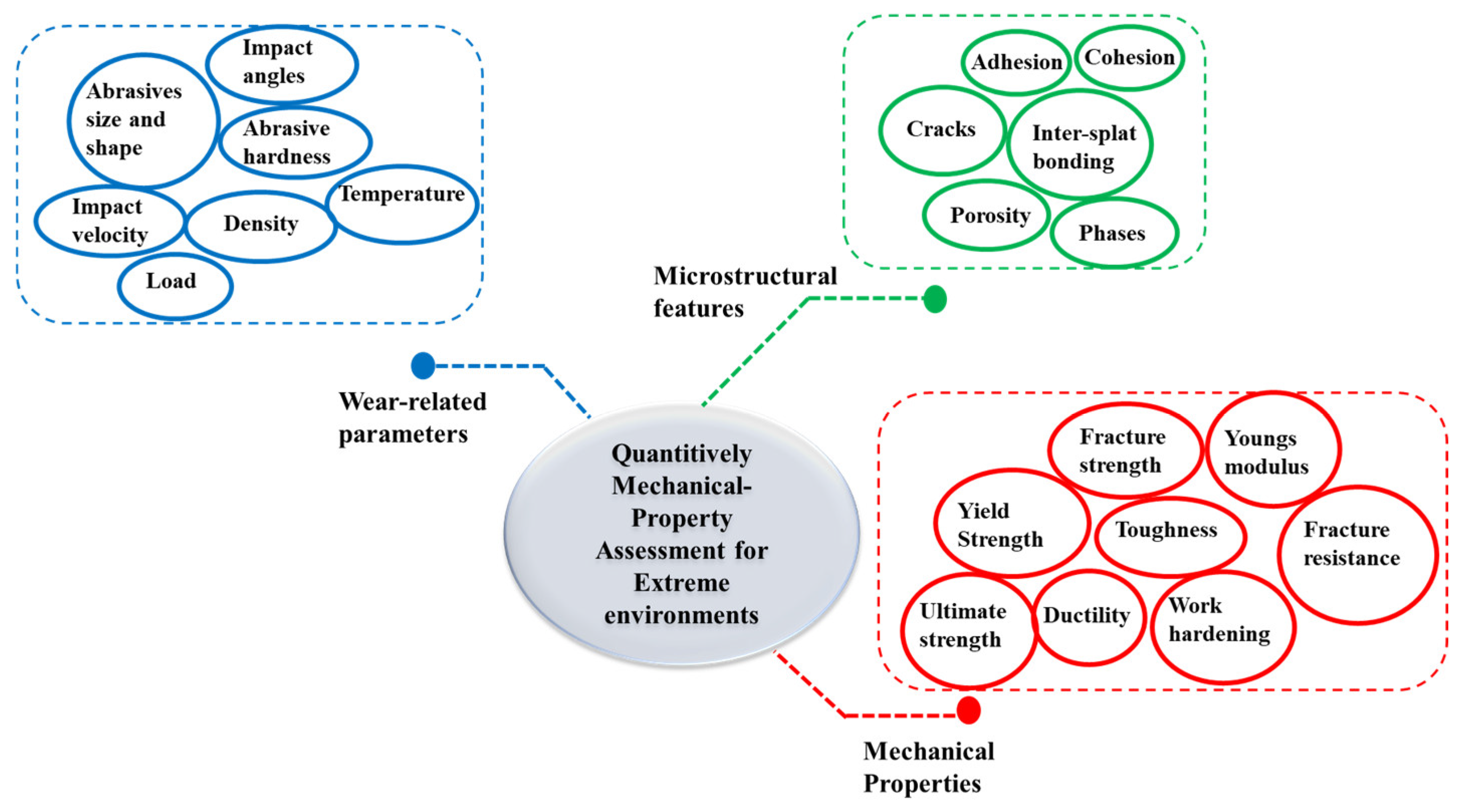
3.3. Quantitative Mechanical Performance and Property Assessment for Extreme Industrial Applications
4. Performance Assessment of HEA Coatings
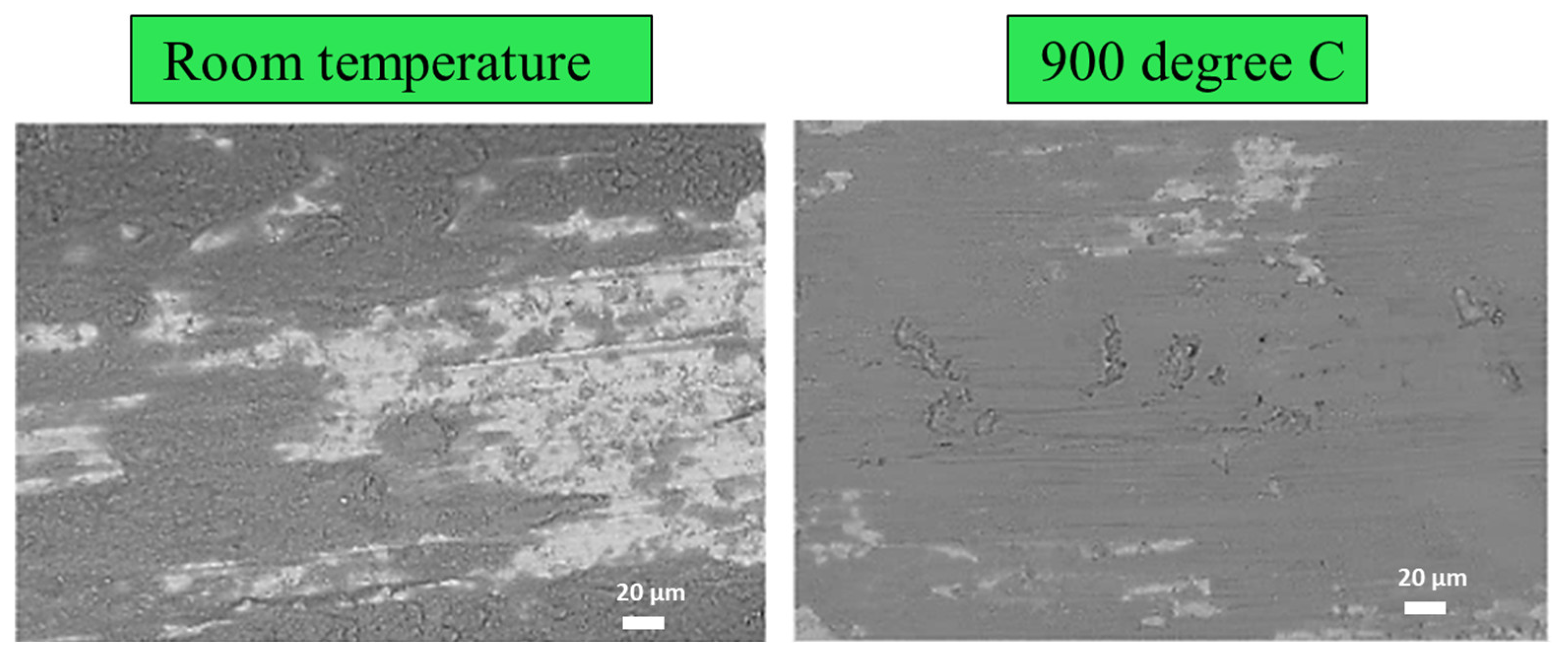
4.1. Wear Behaviour
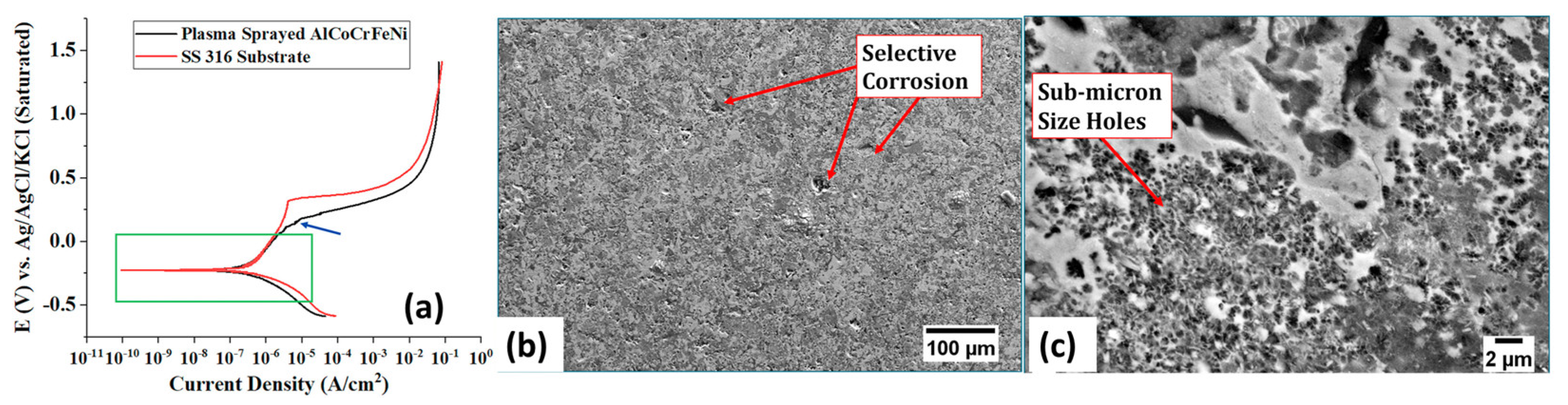
4.2. Corrosion Behaviour
4.3. Oxidation Behaviour
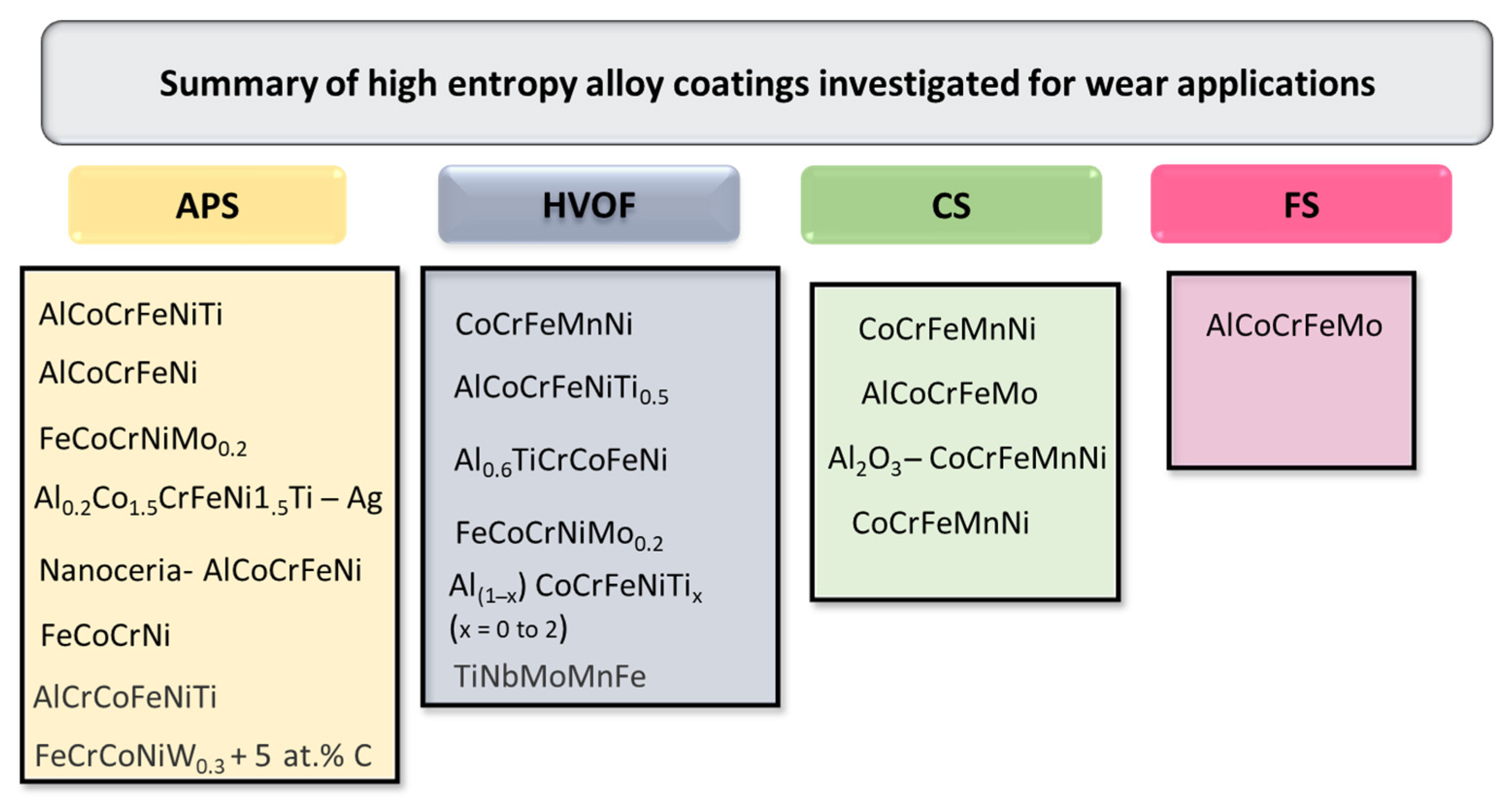
5. Types of HEA Coatings and Their Potential Applications
5.1. Refractory-Based High-Entropy Alloys (RHEAs) for High Temperature Applications
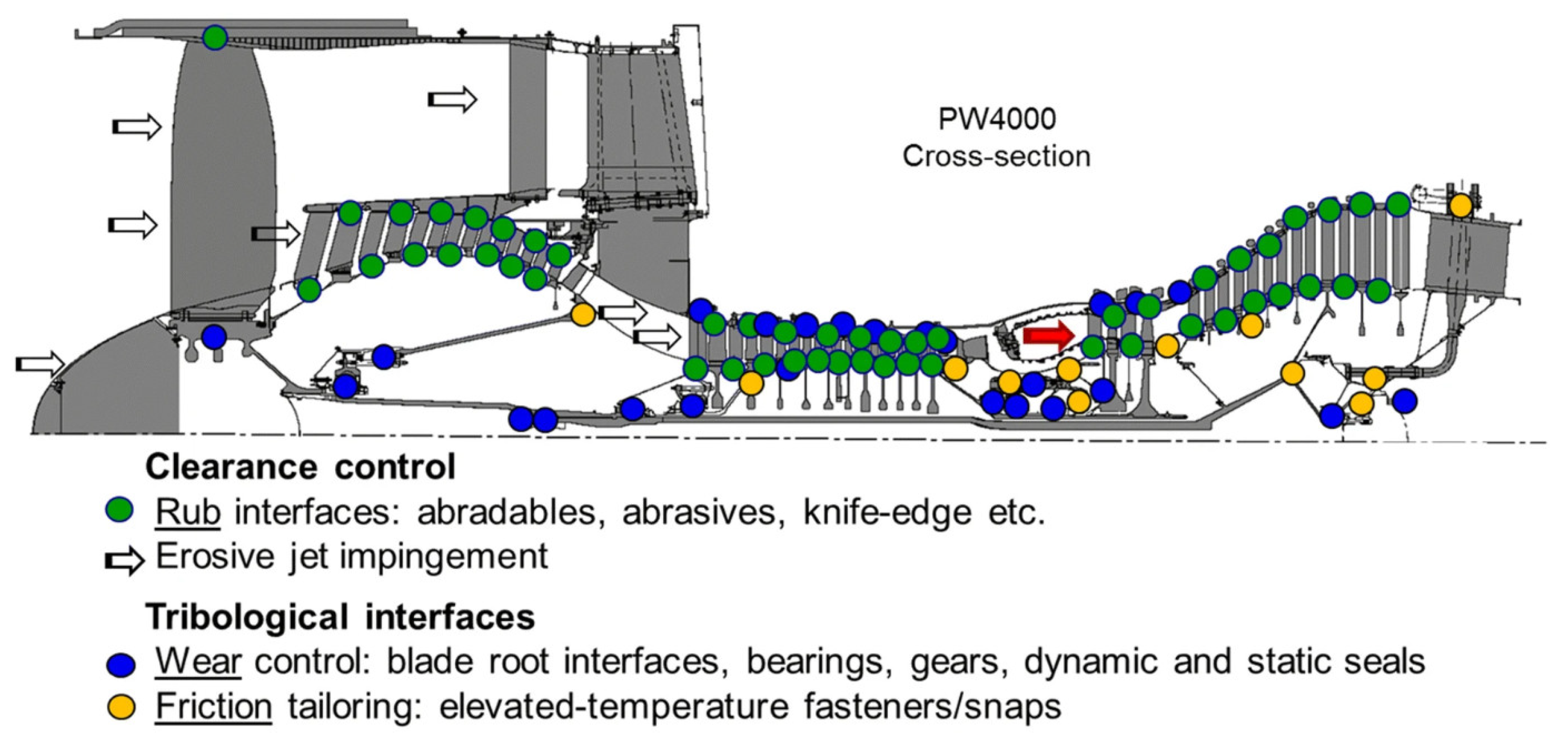
5.2. Transition-Based High-Entropy Alloys for Aerospace Applications
5.3. High-Entropy Carbides
5.4. High-Entropy Oxides
5.5. High-Entropy Alloy Composites
5.6. Perspectives on Other Applications of Thermal-Sprayed HEA Coatings
| High Entropy Alloy Coatings | Feedstock Routes | Substrates | Deposition Routes | Phases | Porosity (%) | Ref. |
|---|---|---|---|---|---|---|
| AlCoCrFeNi | MA | Mild steel | APS | BCC + FCC + oxides | 9.5 ± 2.3 | [20] |
| Gas atomized | APS | BCC and FCC | - | [51] | ||
| Gas atomized | AISI 1045 steel | APS | BCC and B2 | - | [56] | |
| MA | Low carbon steel | APS | BCC + FCC + oxides | - | [57] | |
| MA | Mild steel | HVOF | B2 | - | [61] | |
| MA | Mild steel | HVAF | B2 | - | [61] | |
| Water atomized | 06Cr13Ni5Mo martensitic stainless steel | HVOF | FCC and BCC | - | [62] | |
| MA | SS 316L | APS | Al-rich oxides + AlCrFe oxides + Al-depleted regions | - | [68] | |
| Al0.5CoCrFeNi2 | Gas atomized | SS 304 | APS | FCC | - | [69] |
| AlCoCrFeNiTi | MA | S235 steel | APS | BCC and FCC | - | [14] |
| Gas atomized | S235 steel | APS | BCC and FCC | - | [14] | |
| MA | SS 316 | APS | BCC and oxides | - | [16] | |
| Gas atomized | S235 steel | HVOF | BCC + B2 + A12 | - | [78] | |
| Al0.6CoCrFeNiTi | A572 steel | HVOF | BCC | - | [79] | |
| MnCoCrFeNi | MA | Mild steel | APS | BCC + FCC + oxides | 7.4 ± 1.3 | [20] |
| Gas atomized | SS 316L | Detonation | FCC and oxides | - | [63] | |
| Gas atomized | SS 304 | APS | FCC | - | [152] | |
| FeCoCrNiMo0.2 | Gas atomized | Low carbon steel | APS | FCC and oxides | - | [70] |
| Gas atomized | Low carbon steel | HVOF | FCC and oxides | - | [70] | |
| WC-10Co/AlCoCrFeNi | Water atomized | 06Cr13Ni5Mo martensitic stainless steel | HVOF | FCC + BCC | - | [72] |
| Ni60/AlCoCrFeNiTi | MA _+ gas atomized | SS 316 | APS | BCC and oxides | - | [15] |
| Ag/Al0.2CrCo1.5FeNi1.5Ti | Mechanically blended | Carbon steel | APS | BCC + B2 + FCC + Cr Carbides | - | [69] |
| Al203-13 wt.% TiO2 /MnCoCrFeNi | Gas atomized | Mild steel | APS | FCC and oxides | - | [74] |
| Nano oxides/MoCrCoFeNi | Gas atomized | - | APS | BCC + FCC + oxides | - | [57] |
| AlCoCrFeNiSi | MA | SS 316 | APS | B2 and BCC | - | [58] |
| AlCoCrFeNi | MA | Ni-base super alloy | CS | BCC | 7 | [37] |
| MnCoCrFeNi | Gas atomized | Al 6082 alloy | CS | FCC | - | [38] |
| AlCoCrFeNiTi | MA | Steel | CS | B2 + TiC + σ | 0.50.18 | [47] |
| MnCrFeNi | Gas atomized | Fe52 steel | CS | BCC | - | [36] |
| MnCrFeNi | Gas atomized | Fe52 steel | CS | BCC | - | [36] |
| Ni0.2C0.6Fe0.2CrSi0.2AlTi0.2 | Gas atomized | SS 304 | APS | BCC and Cr3Si | - | [54] |
| NiC0.6Fe0.2CrSiAlTi0.2 | Gas atomized | SS 304 | APS | BCC and Cr3Si | - | [54] |
| Ni0.2C0.6Fe0.2Cr1.5SiAlTi0.2 | Gas atomized | SS 304 | APS | BCC and Cr3Si | - | [54] |
| CrMnFeCo | - | - | APS | FCC | 2.9 | [42] |
| NiCo0.6Fe0.2Cr1.5SiAlTi0.2 | Arc melting + mechanical milling | - | APS | BCC | 1–5 | [107] |
| Al0.6TiCrFeCoNi | Gas atomized | - | HVOF | BCC | 1.68 | [79] |
| Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 | Arc melting + mechanical milling | HVOF APS | BCC BCC | 2.8 ± 0.5 4.3 ± 0.5 | [29] [29] |
6. Conclusions
- HEA coating fabrication: Although several thermal-sprayed HEA coatings have been reported, there is a need to investigate HEA compositions utilizing high-velocity air fuel (HVAF) and cold spraying (CS) deposition techniques. Researchers can develop HEA coatings using these methods and study their microstructure and phase evolution, along with post-treatment methods for specific service conditions;
- Quantitative mechanical properties for extreme environments: While the previous studies of HEA coatings have focused primarily on micro-hardness, the coatings’ reliability under various loading conditions also depends on other mechanical properties such as yield strength, ultimate strength, ductility, toughness, strain hardening, and elastic modulus. However, these properties have not been adequately explored to correlate them with mechanical damage due to wear;
- Parametric optimization: The parameters for thermal spraying should be optimized to produce high-quality HEA coatings that exhibit desirable mechanical properties such as strength and toughness. Researchers should explore various processing techniques and optimize parameters such as feedstock feed rate, spray distance, gas flow rates, etc, to enhance the coatings’ reliability. One area of research could be using machine learning algorithms to optimize the process parameters for the thermal spraying of HEA coatings;
- Unraveling different HEA classifications through thermal spraying: To identify their potential for industrial applications, researchers should explore different HEA systems. One such example is refractory-based high-entropy alloys and high-entropy oxides for bond and top coatings for thermal barrier coatings in high-temperature applications;
- Investigation of microstructure and phase evolution: A comprehensive understanding of the microstructure and phase evolution of HEA coatings is essential for the development of advanced coatings with optimal properties. Researchers can undertake a detailed investigation of the microstructure and phase evolution of HEA coatings at different stages of the coating’s life cycle, including during the spraying process, post-treatment, and under service conditions. By analyzing the evolution of the coating’s microstructure and phases over time, researchers can gain insights into the underlying mechanisms governing the coating’s behavior and identify opportunities for improving the coating’s performance;
- Evaluation of wear properties: HEA coatings have demonstrated potential for wear applications. However, further research is required to understand their ex-situ mechanism for tribological interfaces and under extreme environmental conditions;
- Evaluation of corrosion properties: HEA coatings exhibit good corrosion resistance in seawater and sodium chloride electrolyte conditions. Nonetheless, studies on corrosion properties under different electrolyte conditions, such as HCl and H2SO4, are limited.
Authors Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Gorsse, S.; Couzinié, J.-P.; Miracle, D.B. From high-entropy alloys to complex concentrated alloys. C. R. Phys. 2018, 19, 721–736. [Google Scholar] [CrossRef]
- Miracle, D.B. Critical Assessment 14: High entropy alloys and their development as structural materials. Mater. Sci. Technol. 2015, 31, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Murty, B.S.; Yeh, J.-W.; Ranganathan, S. High-Entropy Alloys; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Wang, J.; Zhang, Z.; Dai, H.; Fujiwara, H.; Chen, X.; Ameyama, K. Enhanced corrosion resistance of CoCrFeMnNi high entropy alloy using heterogeneous structure design. Corros. Sci. 2022, 209, 110761. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Tejero-Martin, D.; Rad, M.R.; McDonald, A.; Hussain, T. Beyond traditional coatings: A review on thermal-sprayed functional and smart coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, X.; Gong, Y.; Tian, Y.; McDonald, A.; Li, H. In-situ SEM observations of ultrasonic cavitation erosion behavior of HVOF-sprayed coatings. Ultrason. Sonochem. 2020, 60, 104760. [Google Scholar] [CrossRef] [PubMed]
- Galedari, S.A.; Mahdavi, A.; Azarmi, F.; Huang, Y.; McDonald, A. A comprehensive review of corrosion resistance of thermally-sprayed and thermally-diffused protective coatings on steel structures. J. Therm. Spray Technol. 2019, 28, 645–677. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal spray high-entropy alloy coatings: A review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A review on high entropy alloys coatings: Fabrication processes and property assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Kohrt, C.; Lampke, T. Processing of AlCoCrFeNiTi high entropy alloy by atmospheric plasma spraying. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Chemnitz, Germany, 16–17 March 2017; p. 012015. [Google Scholar] [CrossRef]
- Tian, L.; Feng, Z.; Xiong, W. Microstructure, microhardness, and wear resistance of AlCoCrFeNiTi/Ni60 coating by plasma spraying. Coatings 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.-H.; Xiong, W.; Liu, C.; Lu, S.; Fu, M. Microstructure and wear behavior of atmospheric plasma-sprayed AlCoCrFeNiTi high-entropy alloy coating. J. Mater. Eng. Perform. 2016, 25, 5513–5521. [Google Scholar] [CrossRef]
- Zhu, B.; Alavi, S.; Cheng, C.; Sun, H.; Zhao, H.; Kim, K.S.; Mostaghimi, J.; Zou, Y. Fast and High-Throughput Synthesis of Medium-and High-Entropy Alloys Using Radio Frequency Inductively Coupled Plasma. Adv. Eng. Mater. 2021, 23, 2001116. [Google Scholar] [CrossRef]
- Tu, R.; Li, N.; Li, Q.; Zhang, S.; Zhang, L.; Goto, T. Microstructure and mechanical properties of B4C–HfB2–SiC ternary eutectic composites prepared by arc melting. J. Eur. Ceram. Soc. 2016, 36, 959–966. [Google Scholar] [CrossRef]
- Cedillos-Barraza, O.; Manara, D.; Boboridis, K.; Watkins, T.; Grasso, S.; Jayaseelan, D.D.; Konings, R.J.; Reece, M.J.; Lee, W.E. Investigating the highest melting temperature materials: A laser melting study of the TaC-HfC system. Sci. Rep. 2016, 6, 37962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, A.S.M.; Berndt, C.C.; Sesso, M.L.; Anupam, A.; Praveen, S.; Kottada, R.S.; Murty, B. Plasma-sprayed high entropy alloys: Microstructure and properties of AlCoCrFeNi and MnCoCrFeNi. Metall. Mater. Trans. A 2015, 46, 791–800. [Google Scholar] [CrossRef]
- Davis, J. Handbook of Thermal Spray Technology, 1st ed.; ASM International, Materials Park: Geauga, OH, USA, 2004; Volume 6994, pp. 1–14. [Google Scholar]
- Sharma, A. High Entropy Alloy Coatings and Technology. Coatings 2021, 11, 372. [Google Scholar] [CrossRef]
- Smith, R.W.; Knight, R. Thermal spraying I: Powder consolidation—From coating to forming. JOM 1995, 47, 32–39. [Google Scholar] [CrossRef]
- Bengtsson, P.; Johannesson, T. Characterization of microstructural defects in plasma-sprayed thermal barrier coatings. J. Therm. Spray Technol. 1995, 4, 245–251. [Google Scholar] [CrossRef]
- Huang, R.; Fukanuma, H. Study of the influence of particle velocity on adhesive strength of cold spray deposits. J. Therm. Spray Technol. 2012, 21, 541–549. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Alfano, M.; Pagnotta, L.; Taurino, A.; Zekonyte, J.; Wood, R. On the early stage isothermal oxidation of APS CoNiCrAlY coatings. J. Mater. Eng. Perform. 2012, 21, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Planche, M.; Liao, H.; Coddet, C. Oxidation control in atmospheric plasma spraying coating. Surf. Coat. Technol. 2007, 202, 69–76. [Google Scholar] [CrossRef]
- Wei, Q.; Yin, Z.; Li, H. Oxidation control in plasma spraying NiCrCoAlY coating. Appl. Surf. Sci. 2012, 258, 5094–5099. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Yeh, J.-W.; Yeh, A.-C.; Shimoda, K. On the study of thermal-sprayed Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 HEA overlay coating. Surf. Coat. Technol. 2017, 316, 71–74. [Google Scholar] [CrossRef]
- Alcala, J.; Gaudette, F.; Suresh, S.; Sampath, S. Instrumented spherical micro-indentation of plasma-sprayed coatings. Mater. Sci. Eng. A 2001, 316, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Neiser, R.; Smith, M.; Dykhuizen, R. Oxidation in wire HVOF-sprayed steel. J. Therm. Spray Technol. 1998, 7, 537–545. [Google Scholar] [CrossRef]
- Mauer, G.; Vaßen, R.; Stöver, D. Plasma and particle temperature measurements in thermal spray: Approaches and applications. J. Therm. Spray Technol. 2011, 20, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Anupam, A.; Kottada, R.S.; Kashyap, S.; Meghwal, A.; Murty, B.; Berndt, C.; Ang, A. Understanding the microstructural evolution of high entropy alloy coatings manufactured by atmospheric plasma spray processing. Appl. Surf. Sci. 2020, 505, 144117. [Google Scholar] [CrossRef]
- Shi, P.; Yu, Y.; Xiong, N.; Liu, M.; Qiao, Z.; Yi, G.; Yao, Q.; Zhao, G.; Xie, E.; Wang, Q. Microstructure and tribological behavior of a novel atmospheric plasma sprayed AlCoCrFeNi high entropy alloy matrix self-lubricating composite coatings. Tribol. Int. 2020, 151, 106470. [Google Scholar] [CrossRef]
- Lehtonen, J.; Koivuluoto, H.; Ge, Y.; Juselius, A.; Hannula, S.-P. Cold gas spraying of a high-entropy CrFeNiMn equiatomic alloy. Coatings 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Anupam, A.; Kumar, S.; Chavan, N.M.; Murty, B.S.; Kottada, R.S. First report on cold-sprayed AlCoCrFeNi high-entropy alloy and its isothermal oxidation. J. Mater. Res. 2019, 34, 796–806. [Google Scholar] [CrossRef]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn high entropy alloy (HEA) coating via cold spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Matthews, S. Shrouded plasma spray of Ni–20Cr coatings utilizing internal shroud film cooling. Surf. Coat. Technol. 2014, 249, 56–74. [Google Scholar] [CrossRef]
- Morks, M.; Berndt, C. Corrosion and oxidation properties of NiCr coatings sprayed in presence of gas shroud system. Appl. Surf. Sci. 2010, 256, 4322–4327. [Google Scholar] [CrossRef]
- Salhi, Z.; Klein, D.; Gougeon, P.; Coddet, C. Development of coating by thermal plasma spraying under very low-pressure condition <1 mbar. Vacuum 2005, 77, 145–150. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Zhang, Y.; Yu, Y. Phase evolution and solidification cracking sensibility in laser remelting treatment of the plasma-sprayed CrMnFeCoNi high entropy alloy coating. Mater. Des. 2019, 182, 108040. [Google Scholar] [CrossRef]
- Nair, R.B.; Ngan, S.; McDonald, A. Dry abrasive wear and solid particle erosion assessments of high entropy alloy coatings fabricated by cold spraying. Mater. Today. Commun. 2023, 34, 105527. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Schulz, C.; Hall, C.; Murty, B.S.; Kottada, R.S.; Vijay, R.; Munroe, P.; Berndt, C.C.; Ang, A.S.M. Tribological and corrosion performance of an atmospheric plasma sprayed AlCoCr0.5Ni high-entropy alloy coating. Wear 2022, 506, 204443. [Google Scholar] [CrossRef]
- Pal, S.; Nair, R.B.; McDonald, A. Toward understanding the microstructure and electrical resistivity of thermal-sprayed high-entropy alloy coatings. J. Mater. Sci. 2022, 57, 20928–20944. [Google Scholar] [CrossRef]
- Xing, B.; Zuo, X.; Li, Q.; Jin, B.; Zhang, N.; Yin, S. Influence of Microstructure Evolution on the Electrochemical Corrosion Behavior of (CoCrFeNi)94Ti1.5Al4.5 High Entropy Alloy Coatings. J. Therm. Spray Technol. 2022, 31, 1375–1385. [Google Scholar] [CrossRef]
- Yurkova, A.; Hushchyk, D.; Minitsky, A. Synthesis of High-Entropy AlNiCoFeCrTi Coating by Cold Spraying. Powder Metall. Met. Ceram. 2021, 59, 681–694. [Google Scholar] [CrossRef]
- Ghadami, F.; Davoudabadi, M.A.; Ghadami, S. Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique. Coatings 2022, 12, 372. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Tung, C.-C.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. On the elemental effect of AlCoCrCuFeNi high-entropy alloy system. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Chen, J.-H.; Stadler, S.; Chen, S.-H. Properties of atomized AlCoCrFeNi high-entropy alloy powders and their phase-adjustable coatings prepared via plasma spray process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.; Meng, G. Microstructure of laser re-melted AlCoCrCuFeNi high entropy alloy coatings produced by plasma spraying. Entropy 2013, 15, 2833–2845. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, C.; Yeh, J.; Ke, S. The microstructure and strengthening mechanism of thermal spray coating NixCo0.6Fe0.2CrySizAlTi0.2 high-entropy alloys. Mater. Chem. Phys. 2011, 126, 880–885. [Google Scholar] [CrossRef]
- Thirathipviwat, P.; Sato, S.; Song, G.; Bednarcik, J.; Nielsch, K.; Jung, J.; Han, J. A role of atomic size misfit in lattice distortion and solid solution strengthening of TiNbHfTaZr high entropy alloy system. Scr. Mater. 2022, 210, 114470. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Yan, S.; Yu, G.; Chen, J.; He, J.; Yin, F. Microstructure evolution and mechanical properties of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings under post-annealing. J. Alloys Compd. 2021, 872, 159607. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, L.; Xu, L.; Prashanth, K.; Zhang, N.; Ma, X.; Jia, Y.; Xu, Y.; Jia, Y.; Wang, G. Frictional wear and corrosion behavior of AlCoCrFeNi high-entropy alloy coatings synthesized by atmospheric plasma spraying. Entropy 2020, 22, 740. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Fu, M.; Xiong, W. Microstructural Evolution of AlCoCrFeNiSi High-Entropy Alloy Powder during Mechanical Alloying and Its Coating Performance. Materials 2018, 11, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, R.B.; Perumal, G.; McDonald, A. Effect of Microstructure on Wear and Corrosion Performance of Thermally-Sprayed AlCoCrFeMo High Entropy Alloy Coatings. Adv. Eng. Mater. 2022, 24, 2101713. [Google Scholar] [CrossRef]
- Rojas, D.F.; Li, H.; Orhan, O.K.; Shao, C.; Hogan, J.D.; Ponga, M. Mechanical and microstructural properties of a CoCrFe0.75NiMo0.3Nb0.125 high-entropy alloy additively manufactured via cold-spray. J. Alloys Compd. 2022, 893, 162309. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Rymer, L.-M.; Lampke, T.; Björklund, S.; Joshi, S. Microstructure and Corrosion Properties of AlCoCrFeNi High-Entropy Alloy Coatings Prepared by HVAF and HVOF. In Proceedings of the Thermal Spray 2021: Proceedings from the International Thermal Spray Conference (ITSC), Quebec City, QC, Canada, 24–28 May 2021; pp. 416–421. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Cheng, J.; Zhu, S. Ultrasonic cavitation erosion behaviors of high-velocity oxygen-fuel (HVOF) sprayed AlCoCrFeNi high-entropy alloy coating in different solutions. Surf. Coat. Technol. 2021, 409, 126899. [Google Scholar] [CrossRef]
- Liao, W.-B.; Wu, Z.-X.; Lu, W.; He, M.; Wang, T.; Guo, Z.; Huang, J. Microstructures and mechanical properties of CoCrFeNiMn high-entropy alloy coatings by detonation spraying. Intermetallics 2021, 132, 107138. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Wu, Q.; Li, J.; Wang, J.; Liu, C. Phase separation of metastable CoCrFeNi high entropy alloy at intermediate temperatures. Scr. Mater. 2017, 126, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Rogachev, A.; Vadchenko, S.; Kochetov, N.; Rouvimov, S.; Kovalev, D.Y.; Shchukin, A.; Moskovskikh, D.; Nepapushev, A.; Mukasyan, A. Structure and properties of equiatomic CoCrFeNiMn alloy fabricated by high-energy ball milling and spark plasma sintering. J. Alloys Compd. 2019, 805, 1237–1245. [Google Scholar] [CrossRef]
- Mahdavi, A.; Pourasghar, A.; Chen, Z.; McDonald, A. Particle–Substrate Transient Thermal Evolution During Cold Spray Deposition Process: A Hybrid Heat Conduction Analysis. J. Therm. Spray Technol. 2020, 29, 1609–1627. [Google Scholar] [CrossRef]
- Lee, Y.T.R.; Ashrafizadeh, H.; Fisher, G.; McDonald, A. Effect of type of reinforcing particles on the deposition efficiency and wear resistance of low-pressure cold-sprayed metal matrix composite coatings. Surf. Coat. Technol. 2017, 324, 190–200. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Luzin, V.; Schulz, C.; Hall, C.; Murty, B.S.; Kottada, R.S.; Berndt, C.C.; Ang, A.S.M. Multiscale mechanical performance and corrosion behaviour of plasma sprayed AlCoCrFeNi high-entropy alloy coatings. J. Alloys Compd. 2021, 854, 157140. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Yan, C.; Zhang, X.; Xiong, D. Microstructure and Tribological Properties of Plasma-Sprayed Al0.2Co1.5CrFeNi1.5 Ti-Ag Composite Coating from 25 to 750 °C. J. Mater. Eng. Perform. 2020, 29, 1640–1649. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Liu, B.; Guo, W.; Xu, L. Microstructure and Wear Behavior of FeCoCrNiMo0.2 High Entropy Coatings Prepared by Air Plasma Spray and the High Velocity Oxy-Fuel Spray Processes. Coatings 2017, 7, 151. [Google Scholar] [CrossRef] [Green Version]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Cheng, J.; Zhu, S. Effect of WC-10Co on cavitation erosion behaviors of AlCoCrFeNi coatings prepared by HVOF spraying. Ceram. Int. 2021, 47, 15121–15128. [Google Scholar] [CrossRef]
- Wu, H.; Huang, S.; Zhu, H.; Xie, Z. Strengthening FeCrNiCu high entropy alloys via combining V additions with in-situ TiC particles. Scr. Mater. 2021, 195, 113724. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Zhang, B.; Yu, Y.; Wang, Z.; Zhang, X.; Lu, B. Microstructure and Properties of Al2O3-13wt.%TiO2-Reinforced CoCrFeMnNi High-Entropy Alloy Composite Coatings Prepared by Plasma Spraying. J. Therm. Spray Technol. 2021, 30, 772–786. [Google Scholar] [CrossRef]
- Dieter, G.E.; Bacon, D.J. Mechanical Metallurgy; McGraw-Hill: New York, NY, USA, 1976; Volume 3. [Google Scholar]
- Deng, K.-K.; Wang, X.-J.; Wang, C.-J.; Shi, J.-Y.; Hu, X.-S.; Wu, K. Effects of bimodal size SiC particles on the microstructure evolution and fracture mechanism of AZ91 matrix at room temperature. Mater. Sci. Eng. A 2012, 553, 74–79. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, N.; Guan, S.; Zhang, Y.; Li, D. Microstructure and properties of laser re-melting FeCoCrNiAl0.5Six high-entropy alloy coatings. Surf. Coat. Technol. 2018, 349, 867–873. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Microstructure and wear resistance of AlCoCrFeNiTi high-entropy alloy coatings produced by HVOF. Coatings 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Wear behavior of HVOF-sprayed Al0.6TiCrFeCoNi high entropy alloy coatings at different temperatures. Surf. Coat. Technol. 2019, 358, 215–222. [Google Scholar] [CrossRef]
- Munday, G.; Hogan, J.; McDonald, A. On the microstructure-dependency of mechanical properties and failure of low-pressure cold-sprayed tungsten carbide-nickel metal matrix composite coatings. Surf. Coat. Technol. 2020, 396, 125947. [Google Scholar] [CrossRef]
- Zhuang, W.Z.; Halford, G.R. Investigation of residual stress relaxation under cyclic load. Int. J. Fatigue 2001, 23, 31–37. [Google Scholar] [CrossRef]
- McGrann, R.; Greving, D.; Shadley, J.; Rybicki, E.F.; Kruecke, T.; Bodger, B. The effect of coating residual stress on the fatigue life of thermal spray-coated steel and aluminum. Surf. Coat. Technol. 1998, 108, 59–64. [Google Scholar] [CrossRef]
- Oka, Y.; Ohnogi, H.; Hosokawa, T.; Matsumura, M. The impact angle dependence of erosion damage caused by solid particle impact. Wear 1997, 203, 573–579. [Google Scholar] [CrossRef]
- Oka, Y.; Mihara, S.; Yoshida, T. Impact-angle dependence and estimation of erosion damage to ceramic materials caused by solid particle impact. Wear 2009, 267, 129–135. [Google Scholar] [CrossRef]
- Grewal, H.S.; Nair, R.B.; Arora, H.S. Complex concentrated alloy bimodal composite claddings with enhanced cavitation erosion resistance. Surf. Coat. Technol. 2020, 392, 125751. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Grewal, H.S. Slurry erosion–corrosion of bimodal complex concentrated alloy composite cladding. Adv. Eng. Mater. 2020, 22, 2000626. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Boyana, A.V.; Saiteja, P.; Grewal, H.S. Tribological behavior of microwave synthesized high entropy alloy claddings. Wear 2019, 436, 203028. [Google Scholar] [CrossRef]
- Sexsmith, M.; Troczynski, T. Peel adhesion test for thermal spray coatings. J. Therm. Spray Technol. 1994, 3, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Amada, S.; Hirose, T. Influence of grit blasting pre-treatment on the adhesion strength of plasma sprayed coatings: Fractal analysis of roughness. Surf. Coat. Technol. 1998, 102, 132–137. [Google Scholar] [CrossRef]
- Bolis, C.; Berthe, L.; Boustie, M.; Arrigoni, M.; Barradas, S.; Jeandin, M. Physical approach to adhesion testing using laser-driven shock waves. J. Phys. D Appl. Phys. 2007, 40, 3155. [Google Scholar] [CrossRef]
- Barradas, S.; Molins, R.; Jeandin, M.; Arrigoni, M.; Boustie, M.; Bolis, C.; Berthe, L.; Ducos, M. Application of laser shock adhesion testing to the study of the interlamellar strength and coating–substrate adhesion in cold-sprayed copper coating of aluminum. Surf. Coat. Technol. 2005, 197, 18–27. [Google Scholar] [CrossRef]
- Beydon, R.; Bernhart, G.; Segui, Y. Measurement of metallic coatings adhesion to fibre reinforced plastic materials. Surf. Coat. Technol. 2000, 126, 39–47. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-temperature wear behaviour of AlCoCrFeNiTi0.5 coatings produced by HVOF. Surf. Coat. Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Patel, P.; Alidokht, S.A.; Sharifi, N.; Roy, A.; Harrington, K.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Microstructural and Tribological Behavior of Thermal Spray CrMnFeCoNi High Entropy Alloy Coatings. J. Therm. Spray Technol. 2022, 31, 1285–1301. [Google Scholar] [CrossRef]
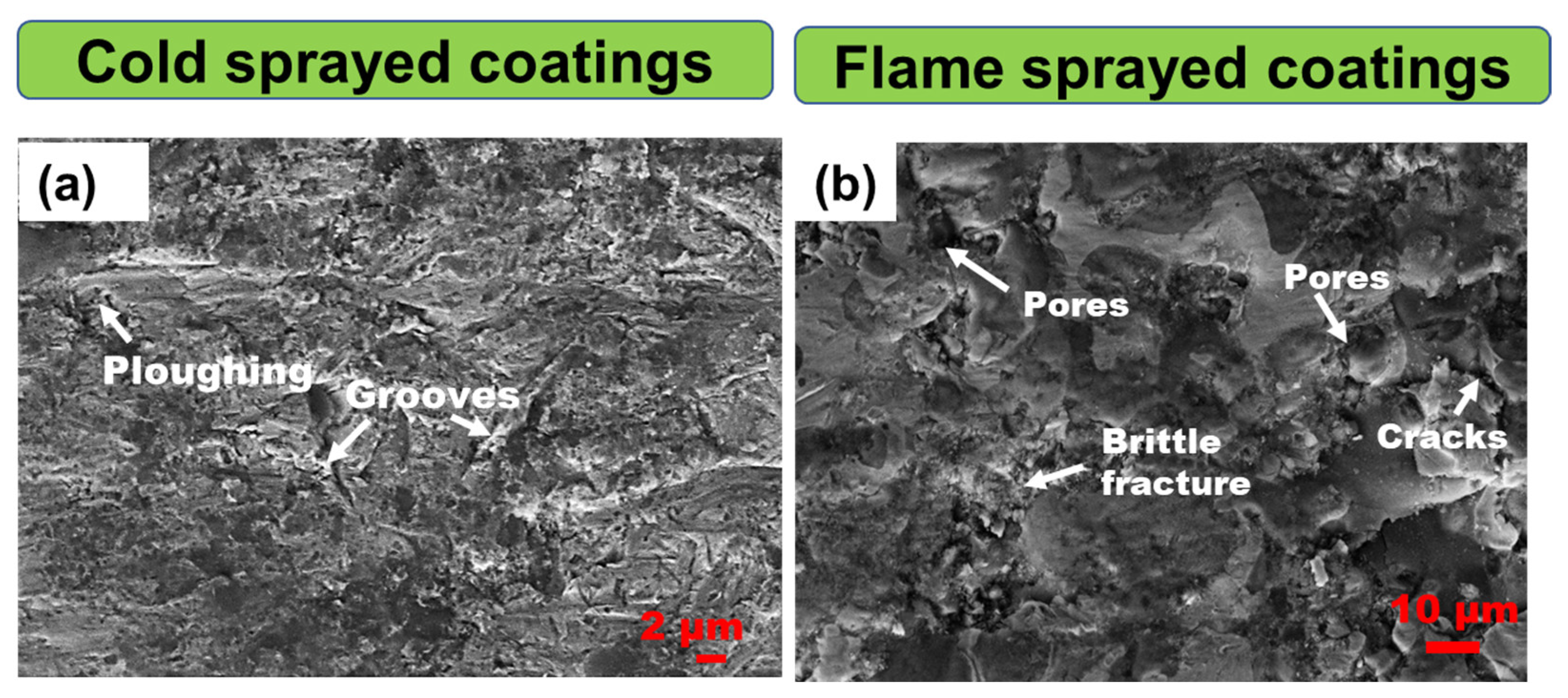
- Supekar, R.; Nair, R.B.; McDonald, A.; Stoyanov, P. Sliding wear behavior of high entropy alloy coatings deposited through cold spraying and flame spraying: A comparative assessment. Wear 2023, 516, 204596. [Google Scholar] [CrossRef]
- Silvello, A.; Cavaliere, P.; Yin, S.; Lupoi, R.; Garcia Cano, I.; Dosta, S. Microstructural, Mechanical and Wear Behavior of HVOF and Cold-Sprayed High-Entropy Alloys (HEAs) Coatings. J. Therm. Spray Technol. 2022, 31, 1184–1206. [Google Scholar] [CrossRef]
- Nair, R.; Arora, H.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H. Exceptionally high cavitation erosion and corrosion resistance of a high entropy alloy. Ultrason. Sonochem. 2018, 41, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Qi, W.; Xie, L.; Yang, X.; Li, J.; Zhang, Y. Microstructure and corrosion behavior of (CoCrFeNi)95Nb5 high-entropy alloy coating fabricated by plasma spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallimanalan, A.; Kumaresh Babu, S.P.; Muthukumaran, S.; Murali, M.; Gaurav, V.; Mahendran, R. Corrosion behaviour of thermally sprayed Mo added AlCoCrNi high entropy alloy coating. Mater. Today Proc. 2020, 27, 2398–2400. [Google Scholar] [CrossRef]
- Liu, S.; Peng, Y.; Zhang, Y.; Wang, Y.; Fan, W.; Wang, A.; Zhang, W.; Tan, Y.; Ma, Q.; Lan, Y. Effect of Nanostructure on Wear and Corrosion Behavior of HVAF-Sprayed Eutectic High-Entropy Alloy Coatings. J. Therm. Spray Technol. 2022, 31, 1252–1262. [Google Scholar] [CrossRef]
- Fan, Q.; Chen, C.; Fan, C.; Liu, Z.; Cai, X.; Lin, S.; Yang, C. AlCoCrFeNi high-entropy alloy coatings prepared by gas tungsten arc cladding: Microstructure, mechanical and corrosion properties. Intermetallics 2021, 138, 107337. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Microstructure-corrosion property correlation in electrodeposited AlCrFeCoNiCu high entropy alloys-graphene oxide composite coatings. Thin Solid Films 2019, 686, 137434. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Liu, C.-G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, Y.; Dai, W.; Xu, Q.; Li, W.; Liu, Y. Corrosion and slurry erosion wear performances of coaxial direct laser deposited CoCrFeNiCu1-xMox high-entropy coatings by modulating the second-phase precipitation. Mater. Des. 2021, 212, 110277. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Araki, H.; Watanabe, M.; Kuroda, S.; Yeh, A.-C.; Yeh, J.-W. A Study of NiCo0.6Fe0.2CrxSiAlTiyHigh-Entropy Alloys for Applications as a High-Temperature Protective Coating and a Bond Coat in Thermal Barrier Coating Systems. J. Electrochem. Soc. 2018, 165, C524–C531. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Yang, Y.-C.; Chen, C.-Y.; Yeh, J.-W. Thermal sprayed high-entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 coating with improved mechanical properties and oxidation resistance. Intermetallics 2017, 89, 105–110. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Qu, L.; Yang, X.; Song, B.; Lupoi, R.; Yin, S. Solid-state cold spraying of FeCoCrNiMn high-entropy alloy: An insight into microstructure evolution and oxidation behavior at 700–900 °C. J. Mater. Sci. Technol. 2021, 68, 172–183. [Google Scholar] [CrossRef]
- Senkov, O.; Wilks, G.; Miracle, D.; Chuang, C.; Liaw, P. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, S.; Tang, H.; Zhang, A. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Gorr, B.; Azim, M.; Christ, H.-J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloys Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Gorr, B.; Müller, F.; Azim, M.; Christ, H.-J.; Müller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High-temperature oxidation behavior of refractory high-entropy alloys: Effect of alloy composition. Oxid. Met. 2017, 88, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.; Gorr, B.; Christ, H.-J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the oxidation mechanism of refractory high entropy alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb20Mo20Cr20Ti20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Chen, H.; Kauffmann, A.; Schweiger, R.; Szabó, D.V.; Heilmaier, M. Development of Oxidation Resistant Refractory High Entropy Alloys for High Temperature Applications: Recent Results and Development Strategy. In Proceedings of the TMS Annual Meeting & Exhibition, Phoenix, AZ, USA, 11–15 March 2018; pp. 647–659. [Google Scholar]
- Gorr, B.; Azim, M.; Christ, H.-J.; Chen, H.; Szabo, D.V.; Kauffmann, A.; Heilmaier, M. Microstructure evolution in a new refractory high-entropy alloy W-Mo-Cr-Ti-Al. Metall. Mater. Trans. A 2016, 47, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, P.; Harrington, K.M.; Frye, A. Insights into the Tribological Characteristic of Cu-Based Coatings Under Extreme Contact Conditions. JOM 2020, 72, 2191–2197. [Google Scholar] [CrossRef]
- Chupp, R.E.; Hendricks, R.C.; Lattime, S.B.; Steinetz, B.M. Sealing in Turbomachinery. J. Propuls. Power 2006, 22, 313–349. [Google Scholar] [CrossRef]
- Lim, K.R.; Lee, K.S.; Lee, J.S.; Kim, J.Y.; Chang, H.J.; Na, Y.S. Dual-phase high-entropy alloys for high-temperature structural applications. J. Alloys Compd. 2017, 728, 1235–1238. [Google Scholar] [CrossRef]
- Grewal, H.S.; Sanjiv, R.M.; Arora, H.S.; Kumar, R.; Ayyagari, A.; Mukherjee, S.; Singh, H. Activation Energy and High Temperature Oxidation Behavior of Multi-Principal Element Alloy. Adv. Eng. Mater. 2017, 19, 1700182. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Zhang, F.; Niu, B.; Lei, L.; Wang, W. High-entropy carbide: A novel class of multicomponent ceramics. Ceram. Int. 2018, 44, 22014–22018. [Google Scholar] [CrossRef]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, A.; Jia, J.; Meng, J.; Su, B. Phase evolution and properties of (VNbTaMoW) C high entropy carbide prepared by reaction synthesis. J. Eur. Ceram. Soc. 2020, 40, 2746–2751. [Google Scholar] [CrossRef]
- Wang, Y.; Csanádi, T.; Zhang, H.; Dusza, J.; Reece, M.J.; Zhang, R.Z. Enhanced Hardness in High-Entropy Carbides through Atomic Randomness. Adv. Theory Simul. 2020, 3, 2000111. [Google Scholar] [CrossRef]
- Musicó, B.L.; Gilbert, D.; Ward, T.Z.; Page, K.; George, E.; Yan, J.; Mandrus, D.; Keppens, V. The emergent field of high entropy oxides: Design, prospects, challenges, and opportunities for tailoring material properties. APL Mater. 2020, 8, 040912. [Google Scholar] [CrossRef] [Green Version]
- Witte, R.; Sarkar, A.; Kruk, R.; Eggert, B.; Brand, R.A.; Wende, H.; Hahn, H. High-entropy oxides: An emerging prospect for magnetic rare-earth transition metal perovskites. Phys. Rev. Mater. 2019, 3, 034406. [Google Scholar] [CrossRef] [Green Version]
- Mao, A.; Xiang, H.-Z.; Zhang, Z.-G.; Kuramoto, K.; Zhang, H.; Jia, Y. A new class of spinel high-entropy oxides with controllable magnetic properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Li, Z.; Lu, Y.; Velasco, L.; Bhattacharya, S.S.; Brezesinski, T.; Hahn, H.; Breitung, B. High entropy oxides as anode material for Li-ion battery applications: A practical approach. Electrochem. Commun. 2019, 100, 121–125. [Google Scholar] [CrossRef]
- Albedwawi, S.H.; AlJaberi, A.; Haidemenopoulos, G.N.; Polychronopoulou, K. High entropy oxides-exploring a paradigm of promising catalysts: A review. Mater. Des. 2021, 202, 109534. [Google Scholar] [CrossRef]
- Zhou, S.; Pu, Y.; Zhang, Q.; Shi, R.; Guo, X.; Wang, W.; Ji, J.; Wei, T.; Ouyang, T. Microstructure and dielectric properties of high entropy Ba(Zr0.2Ti0.2Sn0.2Hf0.2Me0.2)O3 perovskite oxides. Ceram. Int. 2020, 46, 7430–7437. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef] [Green Version]
- Dehaghani, S.T.; Dolatabadi, A.; McDonald, A. Thermally sprayed metal matrix composite coatings as heating systems. Appl. Therm. Eng. 2021, 196, 117321. [Google Scholar] [CrossRef]
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Riva, S.; Tudball, A.; Mehraban, S.; Lavery, N.P.; Brown, S.G.R.; Yusenko, K.V. A novel High-Entropy Alloy-based composite material. J. Alloys Compd. 2018, 730, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Yu, Y.; Zhang, B.; Zhang, Z.; Yan, X.; Wang, Z. Microstructure and wear behaviour of in-situ TiN-Al2O3 reinforced CoCrFeNiMn high-entropy alloys composite coatings fabricated by plasma cladding. Mater. Lett. 2020, 272, 127870. [Google Scholar] [CrossRef]
- Fan, Q.C.; Li, B.S.; Zhang, Y. The microstructure and properties of (FeCrNiCo)AlxCuy high-entropy alloys and their TiC-reinforced composites. Mater. Sci. Eng. A 2014, 598, 244–250. [Google Scholar] [CrossRef]
- Colombini, E.; Lassinantti Gualtieri, M.; Rosa, R.; Tarterini, F.; Zadra, M.; Casagrande, A.; Veronesi, P. SPS-assisted Synthesis of SICp reinforced high entropy alloys: Reactivity of SIC and effects of pre-mechanical alloying and post-annealing treatment. Powder Metall. 2018, 61, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, X.; Guo, K.; Wang, H.; Cai, B.; Chang, F.; Hong, C.; Dai, P. Microstructure and properties of Ti(C, N)–TiB2–FeCoCrNiAl high-entropy alloys composite cermets. Mater. Sci. Eng. A 2019, 767, 138427. [Google Scholar] [CrossRef]
- Karthik, G.M.; Panikar, S.; Ram, G.D.J.; Kottada, R.S. Additive manufacturing of an aluminum matrix composite reinforced with nanocrystalline high-entropy alloy particles. Mater. Sci. Eng. A 2017, 679, 193–203. [Google Scholar] [CrossRef]
- Amar, A.; Li, J.; Xiang, S.; Liu, X.; Zhou, Y.; Le, G.; Wang, X.; Qu, F.; Ma, S.; Dong, W. Additive manufacturing of high-strength CrMnFeCoNi-based High Entropy Alloys with TiC addition. Intermetallics 2019, 109, 162–166. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Nagase, T.; Iijima, Y.; Matsugaki, A.; Ameyama, K.; Nakano, T. Design and fabrication of Ti–Zr-Hf-Cr-Mo and Ti–Zr-Hf-Co-Cr-Mo high-entropy alloys as metallic biomaterials. Mater. Sci. Eng. C 2020, 107, 110322. [Google Scholar] [CrossRef]
- Castro, D.; Jaeger, P.; Baptista, A.C.; Oliveira, J.P. An overview of high-entropy alloys as biomaterials. Metals 2021, 11, 648. [Google Scholar] [CrossRef]
- Gurel, S.; Nazarahari, A.; Canadinc, D.; Cabuk, H.; Bal, B. Assessment of biocompatibility of novel TiTaHf-based high entropy alloys for utility in orthopedic implants. Mater. Chem. Phys. 2021, 266, 124573. [Google Scholar] [CrossRef]
- Perumal, G.; Grewal, H.S.; Pole, M.; Reddy, L.V.K.; Mukherjee, S.; Singh, H.; Manivasagam, G.; Arora, H.S. Enhanced biocorrosion resistance and cellular response of a dual-phase high entropy alloy through reduced elemental heterogeneity. ACS Appl. Bio Mater. 2020, 3, 1233–1244. [Google Scholar] [CrossRef]
- Shittu, J.; Pole, M.; Cockerill, I.; Sadeghilaridjani, M.; Reddy, L.V.K.; Manivasagam, G.; Singh, H.; Grewal, H.S.; Arora, H.S.; Mukherjee, S. Biocompatible High Entropy Alloys with Excellent Degradation Resistance in a Simulated Physiological Environment. ACS Appl. Bio Mater. 2020, 3, 8890–8900. [Google Scholar] [CrossRef]
- Cusentino, M.; Wood, M.; Dingreville, R. Compositional and structural origins of radiation damage mitigation in high-entropy alloys. J. Appl. Phys. 2020, 128, 125904. [Google Scholar] [CrossRef]
- Patel, D.; Richardson, M.D.; Jim, B.; Akhmadaliev, S.; Goodall, R.; Gandy, A.S. Radiation damage tolerance of a novel metastable refractory high entropy alloy V2.5Cr1.2WMoCo0.04. J. Nucl. Mater. 2020, 531, 152005. [Google Scholar] [CrossRef]
- Guo, L.; Gu, J.; Gong, X.; Ni, S.; Song, M. CALPHAD aided design of high entropy alloy to achieve high strength via precipitate strengthening. Sci. China Mater. 2020, 63, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.-K.; Tan, H.; Wu, Y.-Q.; Chen, J.; Zhang, C. Microstructure and wear behavior of FeCoNiCrMn high entropy alloy coating deposited by plasma spraying. Surf. Coat. Technol. 2020, 385, 125430. [Google Scholar] [CrossRef]
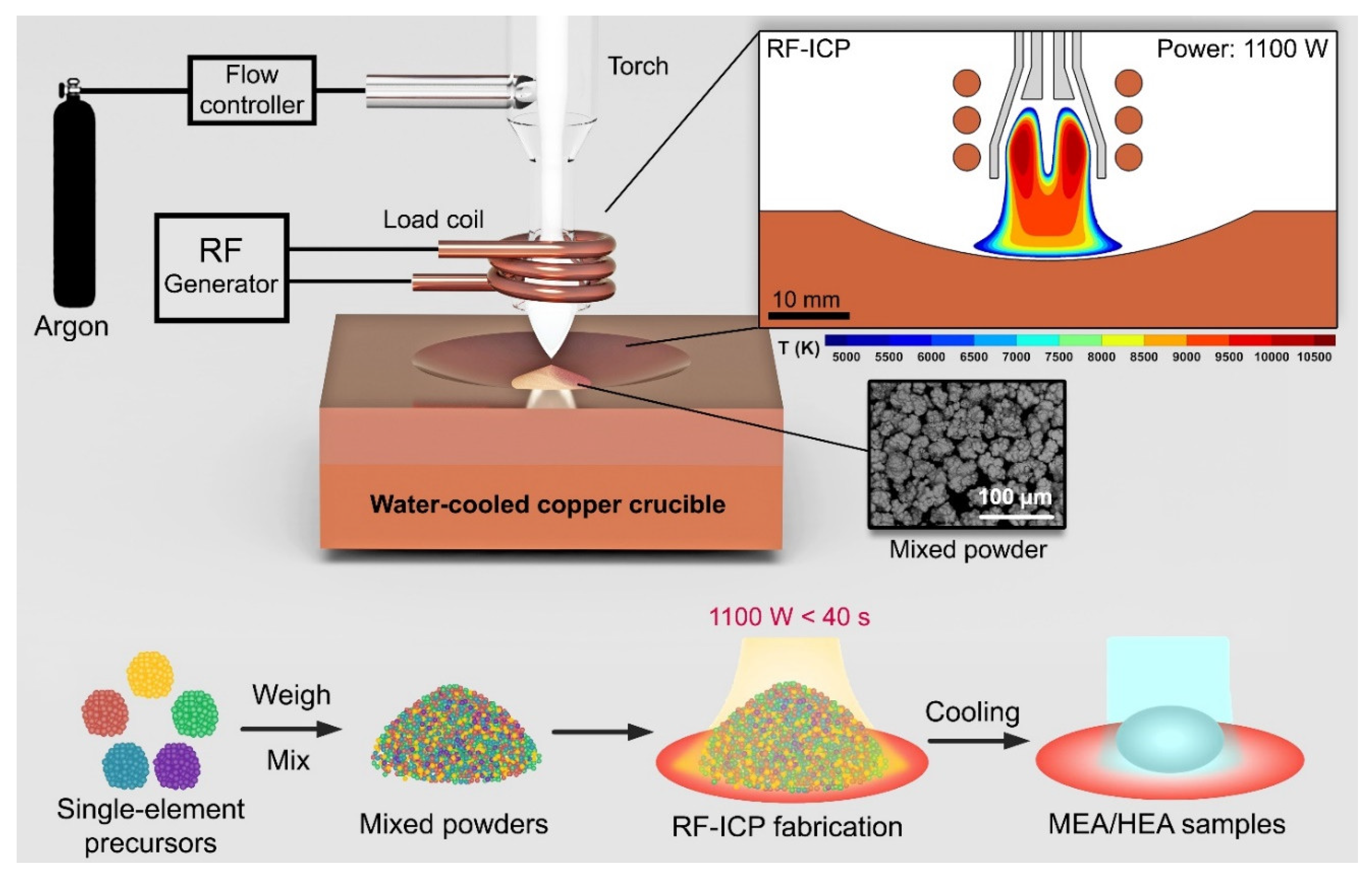
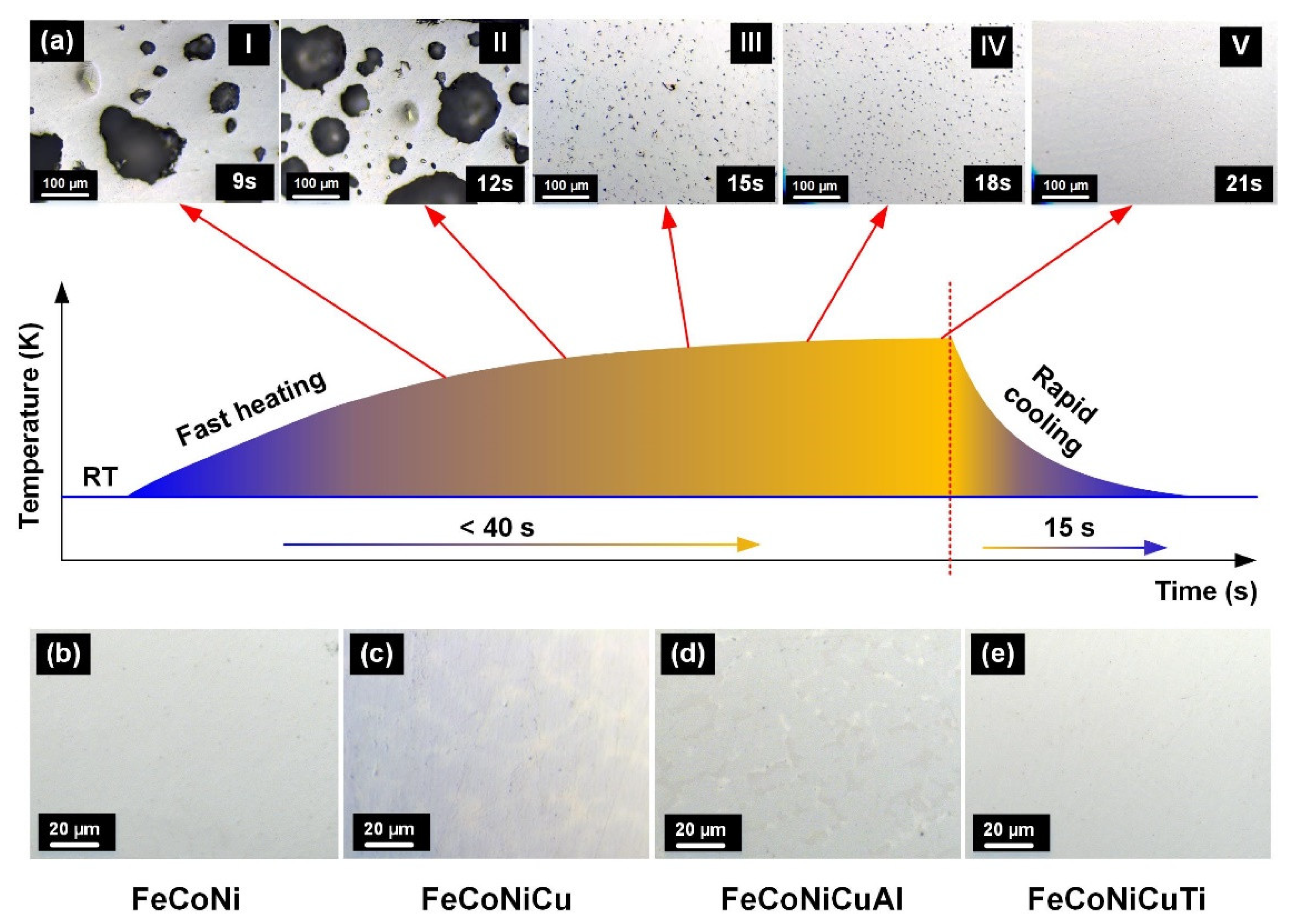




| Suppliers | Type of Feedstocks |
|---|---|
| F.J. Broadmann & Co.,LLC, Harvey, LA, USA | Mechanically alloyed |
| Vilory Advanced Materials Technology Ltd. Jiansgu, China | Gas atomized |
| Eutectic powders, Edmonton, AB, Canada | Gas atomized |
| Stanford Advanced Materials, Lake Forest, CA, USA | Gas atomized |
| Metal Powder Emergence Ltd., London, UK | Gas atomized |
| Kinaltek, Villawood, NSW, Australia | Gas atomized |
| Jiangsu Weilali New Material Technology Co., Ltd., Xuzhou, Jiangsu, China | Gas atomized |
| Beijing Yanbang New Material Technology Co., Ltd. Xuzhou, Jiangsu, China | Gas atomized |
| ABM Nano INC, Missouri City, Texas, USA | Mechanically alloyed |
| Sandvik Osprey LTD, Neath SA11 1NJ, UK | Gas atomized |
| Elements | Al | Co | Cr | Fe | Ni | Ti | Si | Mn | Nb | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Atomic size radius (nm) | 0.143 | 0.125 | 0.128 | 0.127 | 0.125 | 0.146 | 0.111 | 0.126 | 0.246 | 0.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhaskaran Nair, R.; Supekar, R.; Morteza Javid, S.; Wang, W.; Zou, Y.; McDonald, A.; Mostaghimi, J.; Stoyanov, P. High-Entropy Alloy Coatings Deposited by Thermal Spraying: A Review of Strengthening Mechanisms, Performance Assessments and Perspectives on Future Applications. Metals 2023, 13, 579. https://doi.org/10.3390/met13030579
Bhaskaran Nair R, Supekar R, Morteza Javid S, Wang W, Zou Y, McDonald A, Mostaghimi J, Stoyanov P. High-Entropy Alloy Coatings Deposited by Thermal Spraying: A Review of Strengthening Mechanisms, Performance Assessments and Perspectives on Future Applications. Metals. 2023; 13(3):579. https://doi.org/10.3390/met13030579
Chicago/Turabian StyleBhaskaran Nair, Rakesh, Raunak Supekar, Seyyed Morteza Javid, Wandong Wang, Yu Zou, André McDonald, Javad Mostaghimi, and Pantcho Stoyanov. 2023. "High-Entropy Alloy Coatings Deposited by Thermal Spraying: A Review of Strengthening Mechanisms, Performance Assessments and Perspectives on Future Applications" Metals 13, no. 3: 579. https://doi.org/10.3390/met13030579





