Enhanced Separation of Sulfur and Metals from Polymetallic Sulfur Slag through Recrystallizing Regulation of Sulfur Crystals
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Methods
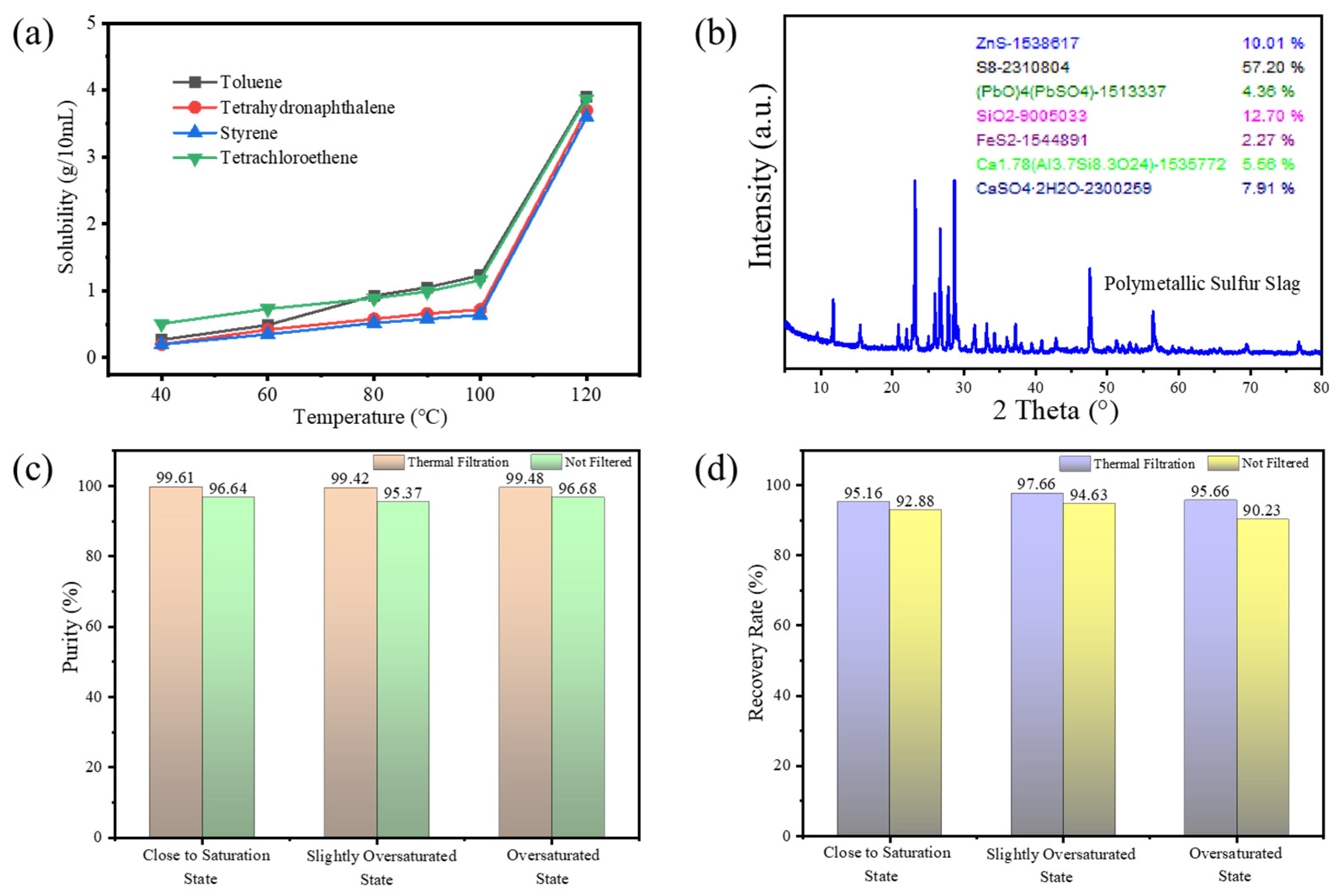
2.2.1. Determination of Solubility
2.2.2. Regulation of the Growth Process of Sulfur Crystals
2.2.3. Recovery of Sulfur from Polymetallic Sulfur slag (PSS) by Organic Solvent Method
2.2.4. CS2 Dissolution Test
2.3. Characterization Methods
3. Results and Discussions
3.1. Separation of Sulfur from Polymetallic Sulfur Slag by Recrystallization
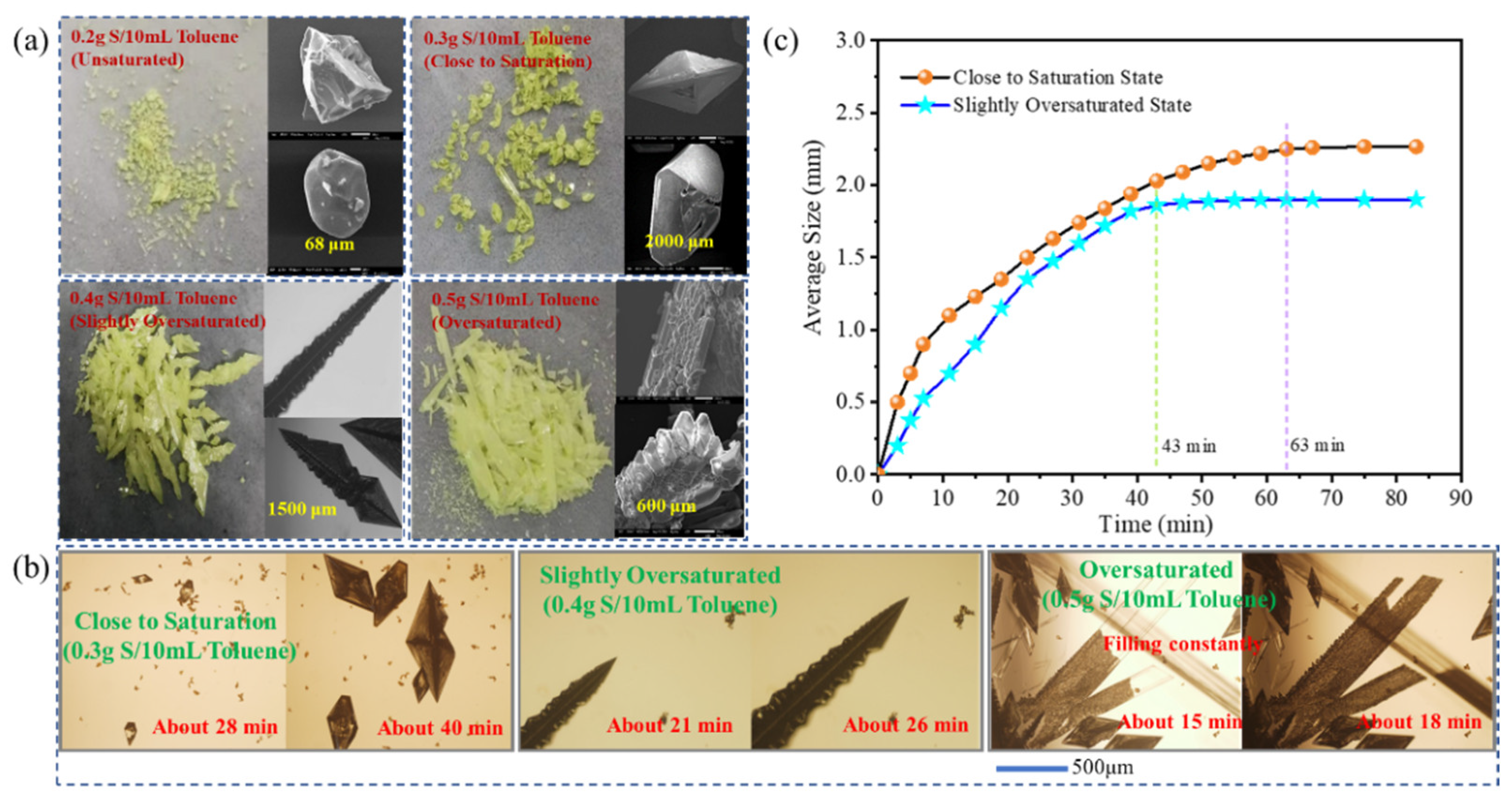
3.2. Crystallization Kinetics and Mechanism of Sulfur in Toluene
3.2.1. Crystallization Kinetics Analysis
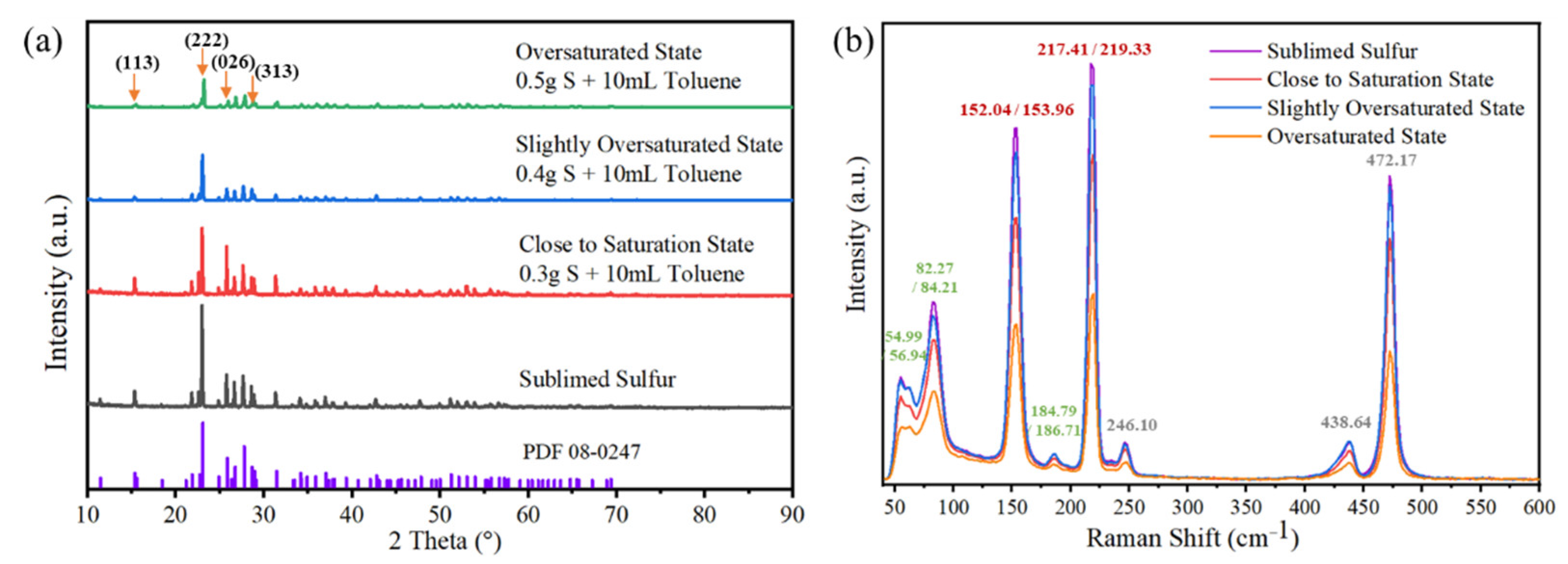
3.2.2. Phase Analysis
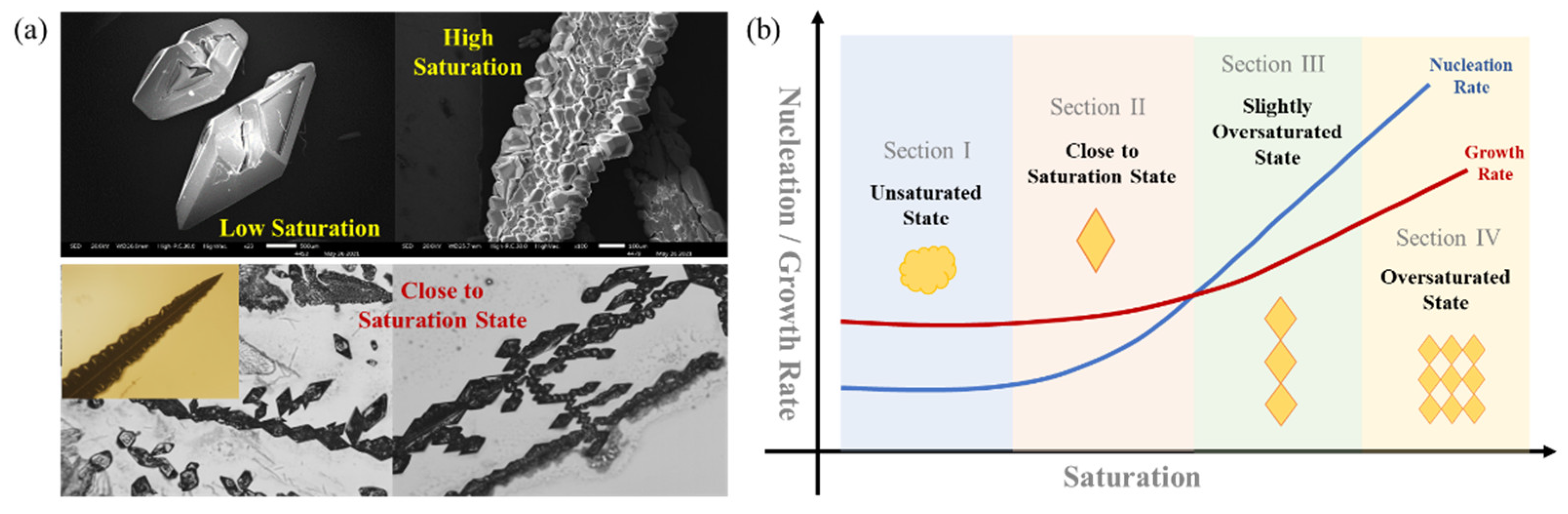
3.2.3. Growth Mechanism of Sulfur Crystals
3.3. Recovery Mechanism of Sulfur in Polymetallic Sulfur Slag
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, H.; Kim, J.; Kim, D.; Nguyen, V.H.; Lee, S.; Han, S.; Lim, J.; Char, K. Aqueous “polysulfide-ene” polymerization for sulfur-rich nanoparticles and their use in heavy metal ion remediation. J. Mater. Chem. A 2018, 6, 23542–23549. [Google Scholar] [CrossRef]
- National Minerals Information Center. Sulfur Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/sulfur-statistics-and-information (accessed on 10 December 2022).
- Fan, Y.Y.; Liu, Y.; Niu, L.P.; Jing, T.L.; Zhang, T.A. Separation and purification of elemental sulfur from sphalerite concentrate direct leaching residue by liquid paraffin. Hydrometallurgy 2019, 186, 162–169. [Google Scholar] [CrossRef]
- Esmaeil, J.; Ahmad, G. Challenges with elemental sulfur removal during the leaching of copper and zinc sulfides, and from the residues: A review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar]
- Li, H.L.; Yao, Z.L.; Wang, M.X.; Wu, S.K.; Ma, W.W.; Wei, W.W.; Li, L.Q. Recovery of elemental sulfur from zinc concentrate direct leaching residue using atmospheric distillation: A pilot-scale experimental study. J. Air Waste Manag. Assoc. 2014, 64, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.Q.; Wang, J.F.; Yang, B.; Yuan, Y.B. Recent advances in controlling the crystallization of two-dimensional perovskites for optoelectronic device. Front. Phys. 2019, 14, 53401. [Google Scholar] [CrossRef]
- Shevchenko, N.; Steinhart, M.; Tomšík, E. Single-step preparation of mono-dispersed sulfur nanoparticles for detention of copper. J. Nanopart. Res. 2019, 21, 246. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Roushani, M.; Kohsai, I.; Hajimirsadeghi, S.S. Novel approach for electrochemical preparation of sulfur nanoparticles. Microchim. Acta 2011, 173, 445–451. [Google Scholar] [CrossRef]
- Wu, K.J.; Tse, E.C.M.; Shang, C.X.; Guo, Z.X. Nucleation and growth in solution synthesis of nanostructures—From fundamentals to advanced applications. Prog. Mater. Sci. 2022, 123, 100821. [Google Scholar] [CrossRef]
- Chen, G.; Chen, C.Y.; Xia, M.Z.; Lei, W.; Wang, F.Y.; Gong, X.D. A study of the solvent effect on the crystal morphology of hexogen by means of molecular dynamics simulations. RSC Adv. 2015, 5, 25581–25589. [Google Scholar] [CrossRef]
- Chen, G.; Xia, M.Z.; Lei, W.; Wang, F.Y.; Gong, X.D. Prediction of crystal morphology of cyclotrimethylene trinitramine in the solvent medium by computer simulation: A case of cyclohexanone solvent. J. Phys. Chem. A 2014, 118, 11471–11478. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Lai, W.P.; Yu, T.; Ma, Y.D.; Kang, Y.; Ge, Z.X. Understanding the growth morphology of explosive crystals in solution: Insights from solvent behavior at the crystal surface. RSC Adv. 2017, 7, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Takiyama, H. Supersaturation operation for quality control of crystalline particles in solution crystallization. Adv. Powder Technol. 2012, 23, 273–278. [Google Scholar] [CrossRef]
- Lovette, M.A.; Doherty, M.F. Predictive modeling of supersaturation-dependent crystal shapes. Cryst. Growth Des. 2012, 12, 656–669. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, K.J. Effect of supersaturation on the morphology of coated surface in coating by solution crystallization. Ind. Eng. Chem. Res. 2011, 50, 3475–3482. [Google Scholar] [CrossRef]
- Park, C.; Park, J.E.; Choi, H.C. Crystallization-induced properties from morphology-controlled organic crystals. Acc. Chem. Res. 2014, 47, 2353–2364. [Google Scholar] [CrossRef] [PubMed]
- Kuvadia, Z.B.; Doherty, M.F. Effect of structurally similar additives on crystal habit of organic molecular crystals at low supersaturation. Cryst. Growth Des. 2013, 13, 1412–1428. [Google Scholar] [CrossRef]
- Salvalaglio, M.; Vetter, T.; Giberti, F.; Mazzotti, M.; Parrinello, M. Uncovering molecular details of urea crystal growth in the presence of additives. J. Am. Chem. Soc. 2012, 134, 17221–17233. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chow, P.S.; Tan, R.B.H. Strong additive-surface interaction leads to the unusual revival of growth at solvent-poisoned faces of dl-alanine crystal. Cryst. Growth Des. 2012, 12, 5555–5560. [Google Scholar] [CrossRef]
- Yang, X.Y.; Qian, G.; Duan, X.Z.; Zhou, X.G. Impurity effect of 1-valine on l-alanine crystal growth. Cryst. Growth Des. 2013, 13, 1295–1300. [Google Scholar] [CrossRef]
- Yang, X.Y.; Qian, G.; Duan, X.Z.; Zhou, X.G. Effect of impurity on the lateral crystal growth of I-alanine: A combined simulation and experimental study. Ind. Eng. Chem. Res. 2012, 51, 14845–14849. [Google Scholar] [CrossRef]
- Sultana, M.; Jensen, K.F. Microfluidic continuous seeded crystallization: Extraction of growth kinetics and impact of impurity on morphology. Cryst. Growth Des. 2012, 12, 6260–6266. [Google Scholar] [CrossRef]
- Wang, R.X.; Han, F.; Chen, B.; Liu, L.M.; Wang, S.Y.; Zhang, H.; Han, Y.; Chen, H.Y. Liquid nanoparticles: Manipulating the nucleation and growth of nanoscale droplets. Angew. Chem. Int. Ed. 2021, 60, 3047–3054. [Google Scholar] [CrossRef]
- Tsukamoto, K. In-situ observation of crystal growth and the mechanism. Prog. Cryst. Growth Charact. 2016, 62, 111–125. [Google Scholar] [CrossRef]
- Donohue, J.; Caron, A.; Goldish, E. Crystal Structure of Rhombohedral Sulphur. Nature 1958, 182, 518. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Sachin; Pramanik, B.K.; Singh, N.; Zizhou, R.; Houshyar, S.; Cole, I.; Yin, H. Fast and Effective Removal of Congo Red by Doped ZnO Nanoparticles. Nanomaterials 2023, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, K.S.; Gorelli, F.A.; Santoro, M.; Yannopoulos, S.N. Elemental sulfur under high hydrostatic pressure. An up-to-date Raman study. High Pressure Res. 2013, 33, 134–140. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Freeman, J.J.; Goffredi, S.K.; Buck, K.R. Raman spectroscopic and laser scanning confocal microscopic analysis of sulfur in living sulfur-precipitating marine bacteria. Chem. Geol. 2001, 180, 3–18. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.L.; Zhu, C.Y.; Dionysiou, D.D.; Zhao, G.C.; Fang, G.D.; Zhou, D.M. New insight into the mechanism of peroxymonosulfate activation by sulfur-containing minerals: Role of sulfur conversion in sulfate radical generation. Water Res. 2018, 35, 208–216. [Google Scholar]
- Oren, A.; Mana, L.; Jehlička, J. Probing single cells of purple sulfur bacteria with Raman spectroscopy: Carotenoids and elemental sulfur. FEMS Microbiol. Lett. 2015, 362, fnv021. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.M.; Jallad, K.N.; Ben-Amotz, D.; Hamers, R.J. Chemical mapping of elemental sulfur on pyrite and arsenopyrite surfaces using near-infrared Raman imaging microscopy. Appl. Surf. Sci. 2001, 178, 105–115. [Google Scholar] [CrossRef]
- Nims, C.; Cron, B.; Wetherington, M.; Macalady, J.; Cosmidis, J. Low frequency Raman Spectroscopy for micron-scale and in vivo characterization of elemental sulfur in microbial samples. Sci. Rep. 2019, 9, 7971. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Gao, Q.; Zhang, J.; Deng, H.; Tian, C.; Lin, Z. Enhanced Separation of Sulfur and Metals from Polymetallic Sulfur Slag through Recrystallizing Regulation of Sulfur Crystals. Metals 2023, 13, 603. https://doi.org/10.3390/met13030603
Chen F, Gao Q, Zhang J, Deng H, Tian C, Lin Z. Enhanced Separation of Sulfur and Metals from Polymetallic Sulfur Slag through Recrystallizing Regulation of Sulfur Crystals. Metals. 2023; 13(3):603. https://doi.org/10.3390/met13030603
Chicago/Turabian StyleChen, Fanyun, Qingshan Gao, Jing Zhang, Hao Deng, Chen Tian, and Zhang Lin. 2023. "Enhanced Separation of Sulfur and Metals from Polymetallic Sulfur Slag through Recrystallizing Regulation of Sulfur Crystals" Metals 13, no. 3: 603. https://doi.org/10.3390/met13030603





