EIS Study of Doped High-Entropy Alloy
Abstract
:1. Introduction
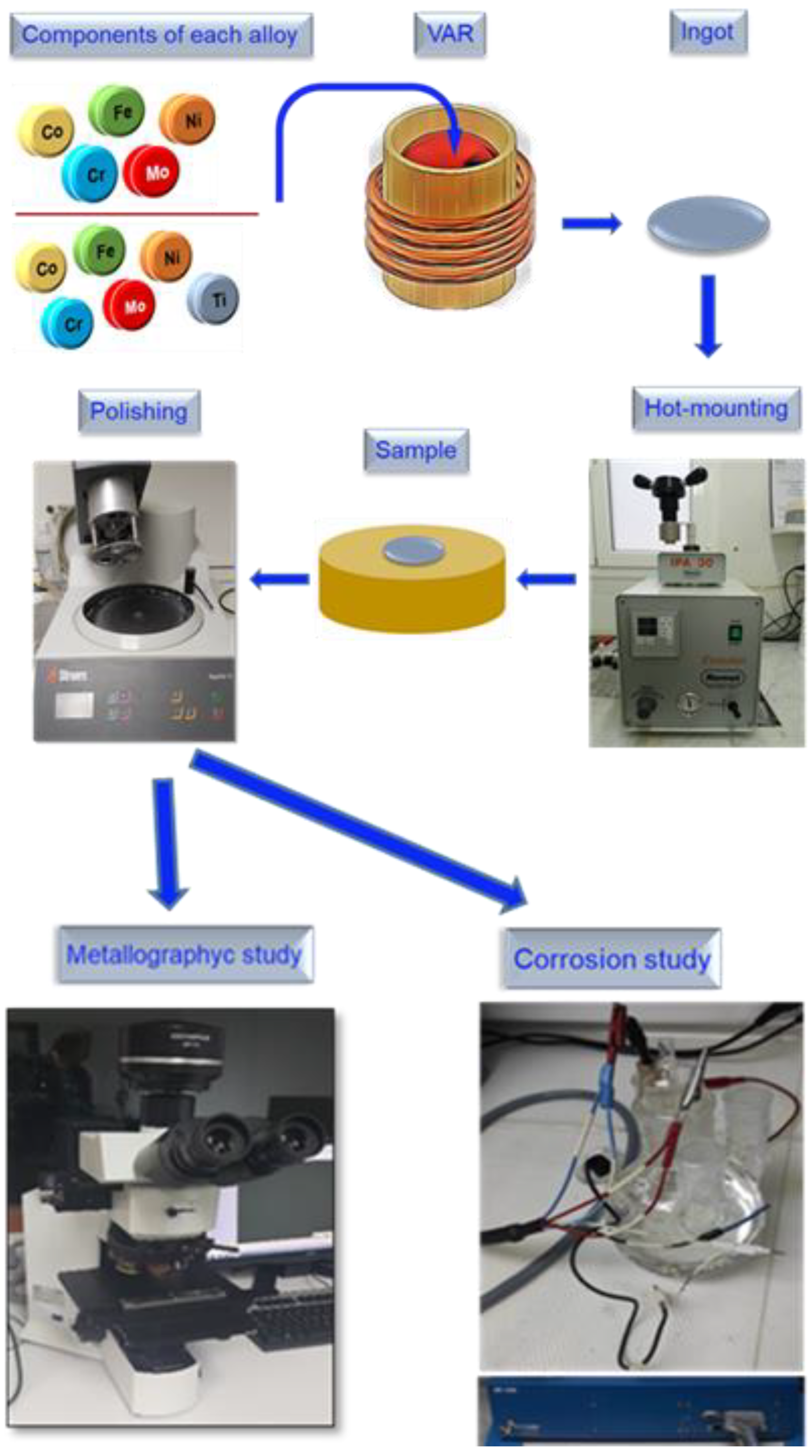
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructural Characterization
2.3. Electrochemical Measurements
2.3.1. Electrodes and Electrolyte
2.3.2. Potentiodynamic Polarization
2.3.3. Potentiostatic Electrochemical Impedance Spectroscopy (PEIS)
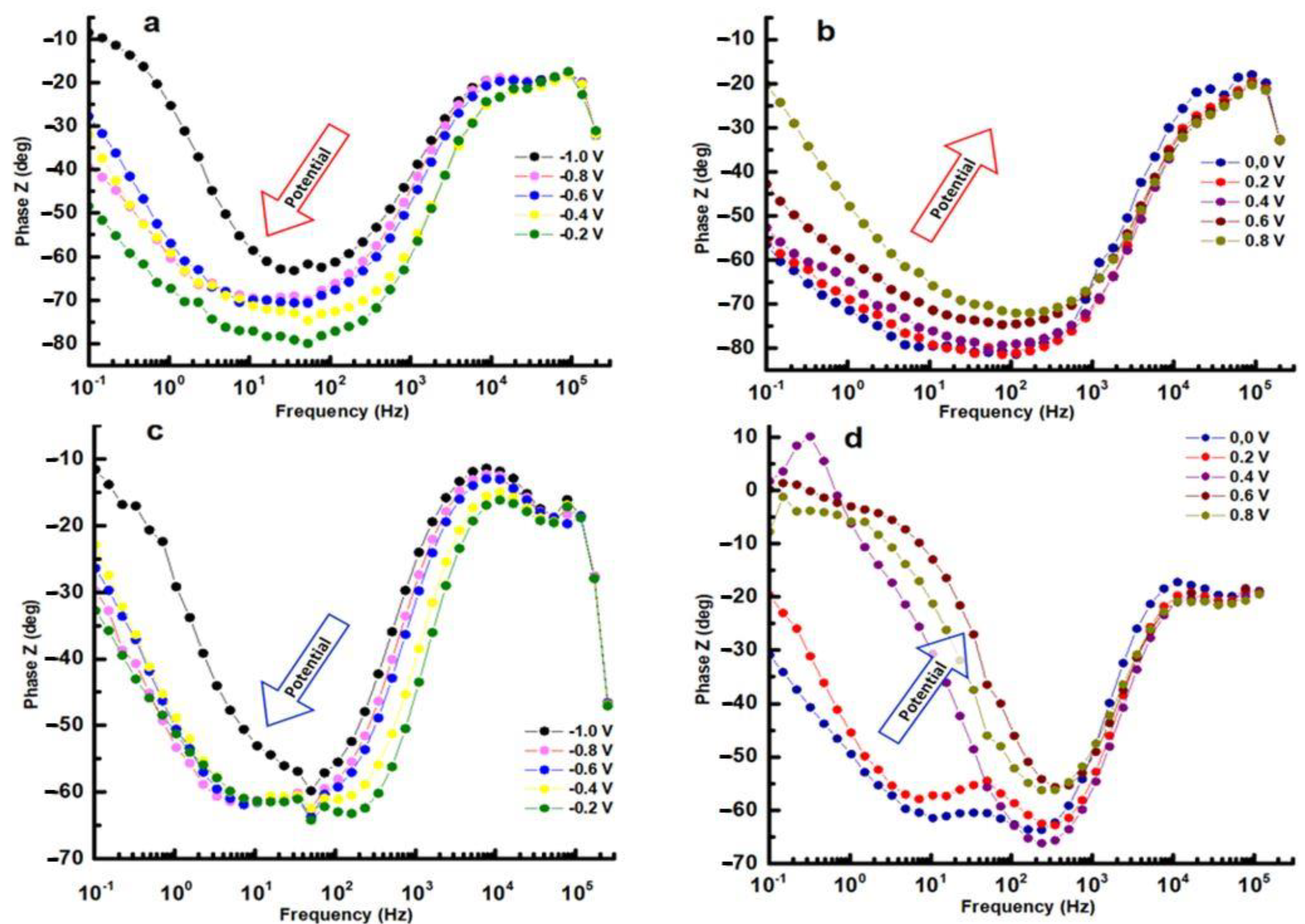
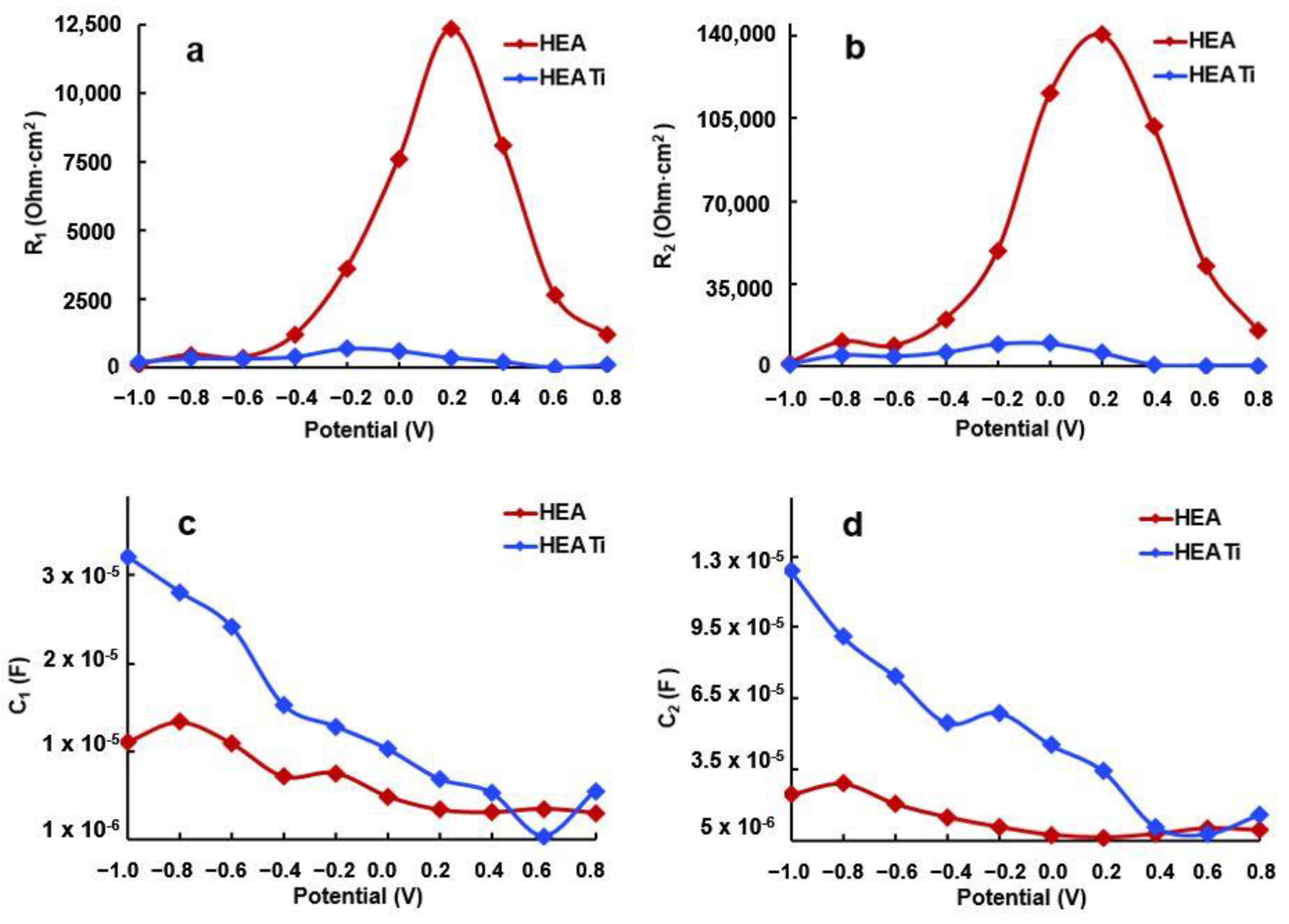
3. Results and Discussions
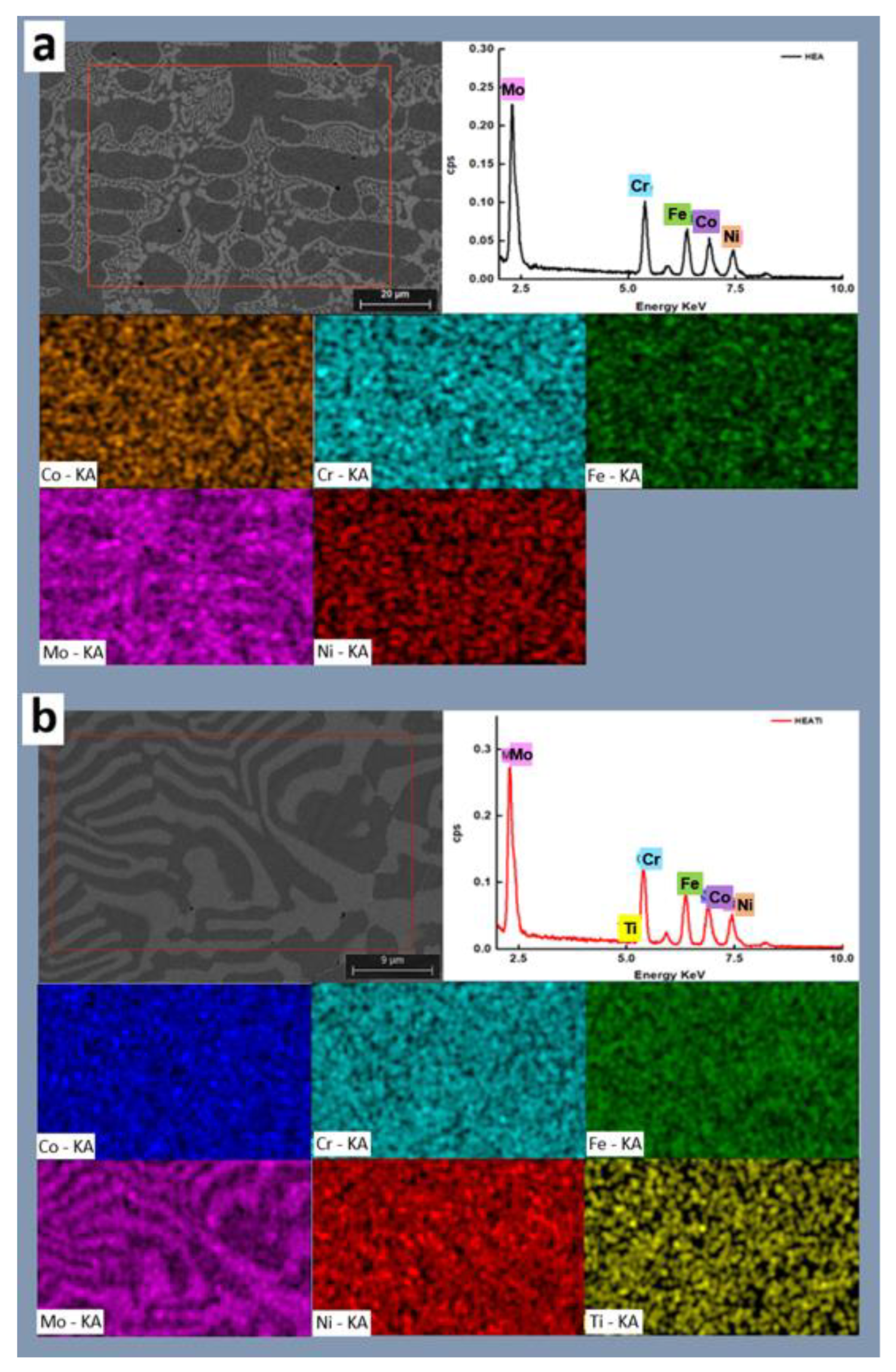
3.1. Microstructural Characterization
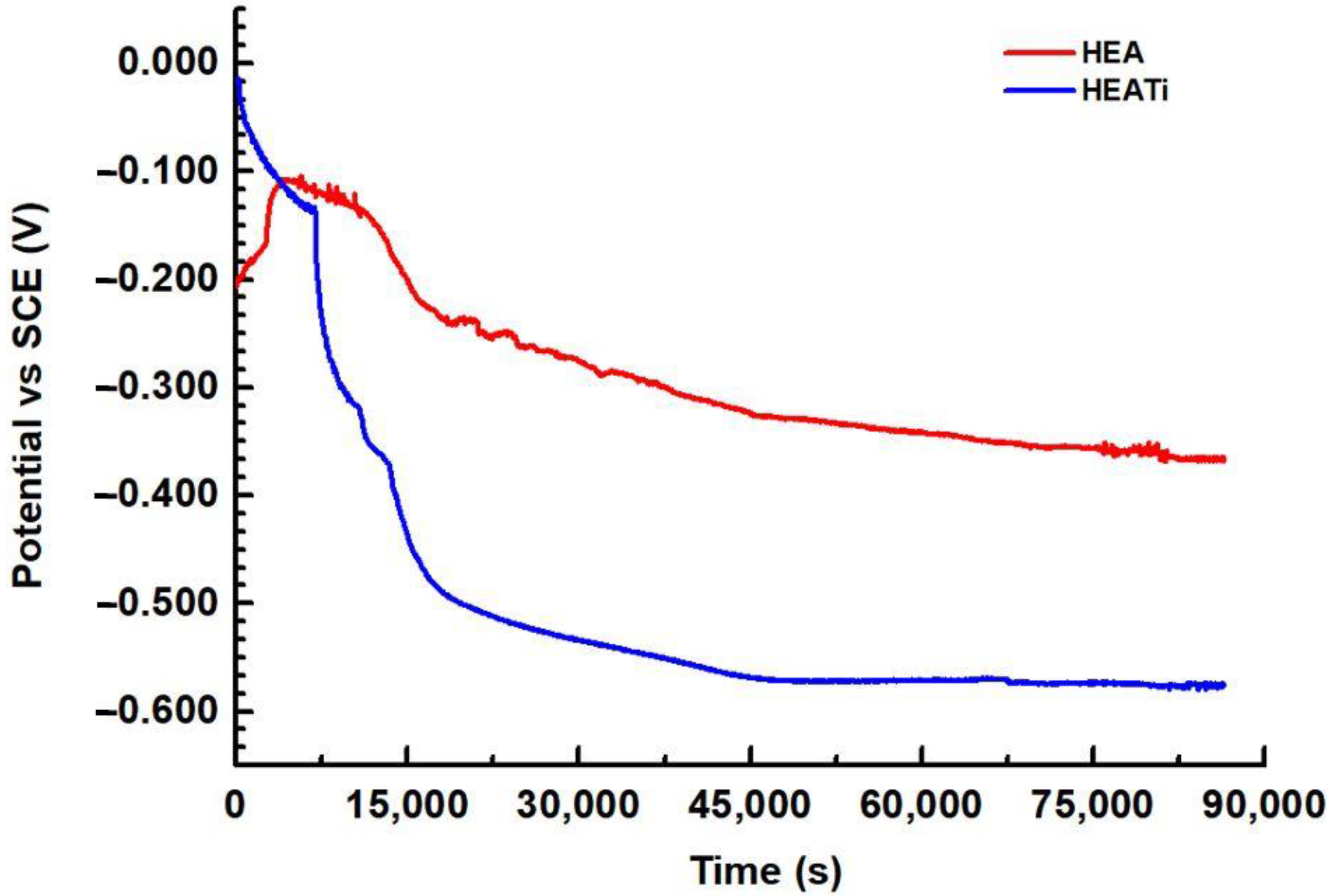
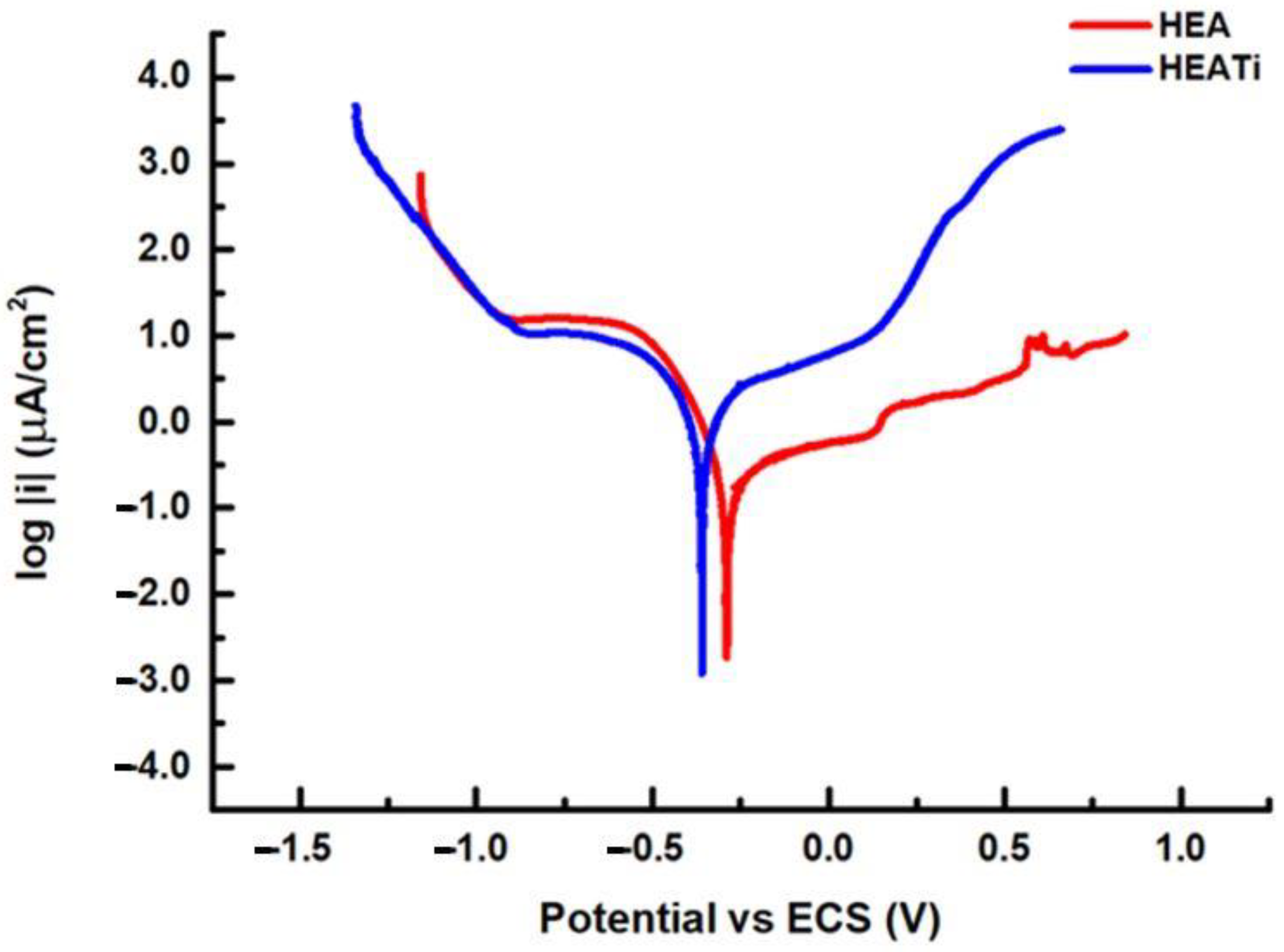
3.2. Electrochemical Measurements
- R is equal to R1 plus R2;
- T is the product of τ1 and τ2;
- X is equal to τ1 plus τ2 plus C1 times R2;
- Y is equal to τ2 times R1;
- τ1 represents the time constant of the porous layer in seconds;
- τ2 represents the time constant of the compact layer in seconds.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Bodunrin, M.O.; Oke, S.R. Processing, alloy composition and phase transition effect on the mechanical and corrosion properties of high entropy alloys: A review. J. Mater. Res. Technol. 2016, 5, 384–393. [Google Scholar] [CrossRef]
- Dada, M.; Popoola, P.; Adeosun, S.; Mathe, N. High Entropy Alloys for Aerospace Applications. Aerodynamics. IntechOpen. 2021, 1–16. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Zhang, Y.; Wu, Z.; Zhang, Z.; Zhao, F.; Huot, J.; Grobivć Novaković, J.; Novaković, N. Recent progress on the development of high entropy alloys (HEAs) for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]
- Fu, M.; Ma, X.; Zhao, K.; Li, X.; Su, D. High-entropy materials for energy-related applications. iScience 2021, 24, 102177. [Google Scholar] [CrossRef]
- Rios, M.L.; Baldevenites, V.L.; Voiculescu, I.; Rosca, J.M. AlCoCrFeNi High Entropy Alloys as Possible Nuclear Materials. Microsc. Microanal. 2020, 26, 406–407. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Ahmad, M.; Chen, Y.; Shuang, S.; Javed, M.S.; Yang, Y.; Zhang, K. High entropy alloys as electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103405. [Google Scholar] [CrossRef]
- Castro, D.; Jaeger, P.; Baptista, A.C.; Oliveira, J.P. An overview of high-entropy alloys as biomaterials. Metals 2021, 11, 648. [Google Scholar] [CrossRef]
- López Ríos, M.; Socorro Perdomo, P.P.; Voiculescu, I.; Geanta, V.; Crăciun, V.; Boerasu, I.; Mirza Rosca, J.C. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Socorro-Perdomo, P.; Florido-Suarez, N.; Voiculescu, I.; Mirza-Rosca, J. Biocompatibility of New High-Entropy Alloys with Non-Cytotoxic Elements. Microsc. Microanal. 2021, 27, 1772–1774. [Google Scholar] [CrossRef]
- Feng, J.; Tang, Y.; Liu, J.; Zhang, P.; Liu, C.; Wang, L. Bio-high entropy alloys: Progress, challenges, and opportunities. Front. Bioeng. Biotechnol. 2022, 10, 1–26. [Google Scholar] [CrossRef]
- Cui, G.; Han, B.; Yang, Y.; Wang, Y.; Chunyang, H. Microstructure and tribological property of CoCrFeMoNi High entropy alloy treated by ion sulfurization. J. Mater. Res. Technol. 2020, 9, 2598–2609. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, J.; Zhang, G.H.; Fan, X.H.; Zhang, L. Effect of temperature on the passive film structure and corrosion performance of CoCrFeMoNi high-entropy alloy. Corros. Sci. 2022, 208, 110661. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, F.; Yang, F.; Yang, Y.; Liang, P. Novel laser cladding FeCoNiCrNb0.5Mox high-entropy alloy coatings with excellent corrosion resistance. Mater. Lett. 2023, 335, 133714. [Google Scholar] [CrossRef]
- Zhang, J.; Tse, K.; Wong, M.; Zhang, Y.; Zhu, J. A brief review of co-doping. Front. Phys. 2016, 11, 117405. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Chen, B.; Ma, W.; Jing, Q.; Zhang, X.; Ma, M.; Wang, Q.; Liu, R. Effect of Ti addition on the mechanical properties and microstructure of novel Al-rich low-density multi-principal-element alloys. J. Alloys Compd. 2022, 891, 162028. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Wu, G.; Mao, X. Titanium microalloying of steel: A review of its effects on processing, microstructure and mechanical properties. Int. J. Miner. Metall. Mater. 2022, 29, 645–661. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.H.; Wu, H. tian Effects of chromium on the microstructure and hot ductility of Nb-microalloyed steel. Int. J. Miner. Metall. Mater. 2021, 28, 1011–1021. [Google Scholar] [CrossRef]
- Lin, Y.J.; Huang, C.S.; Tsai, P.C.; Hsiao, Y.L.; Chen, C.Y.; Jou, J.H. Minor Copper-Doped Aluminum Alloy Enabling Long-Lifetime Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 55898–55904. [Google Scholar] [CrossRef]
- Jing, J.; Gu, X.; Zhang, S.; Sun, J.; Chen, Y.; Sun, T. Doping of aluminum (Al) into copper sulfide (CuS) nanocrystals enhanced their solar spectral selectivity. CrystEngComm 2019, 21, 4969–4975. [Google Scholar] [CrossRef]
- Missaghian, P. Oxidation of magnesium doped Al-surfaces. KTH Skolan for Teknikvetenskap 2020, 1, 1–33. [Google Scholar]
- Meroueh, L.; Eagar, T.W.; Hart, D.P. Effects of Mg and Si Doping on Hydrogen Generation via Reduction of Aluminum Alloys in Water. ACS Appl. Energy Mater. 2020, 3, 1860–1868. [Google Scholar] [CrossRef]
- Gunen, A.; Keddam, M.; Sunbul, S.E.; Icin, K.; Doleker, K.M.; Gok, M.S.; Dal, S.; Erdogan, A. Powder-pack boronizing of CoCrFeNiAl0.5Nb0.5 HEA: Modeling of kinetics, microstructural, mechanical, and tribological characterizations. J. Alloys Compd. 2022, 929, 167310. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Chen, Y.; Dong, J.; Yin, S.; Hua, K.; Wang, H. Characterization of the microstructure and self-lubrication properties of a AlCoCrFeNi2.1 eutectic high-entropy alloy with powder-pack boronizing. Mater. Charact. 2022, 191, 112118. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yang, H.J.; Guo, R.P.; Wang, X.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Tribological behavior of boronized Al0.1CoCrFeNi high-entropy alloys under dry and lubricated conditions. Wear 2020, 460–461, 203452. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, M.; Yang, H.; Qiao, J.; Wu, Y. Surface strengthening in Al0.25CoCrFeNi high-entropy alloy by boronizing. Mater. Lett. 2019, 238, 258–260. [Google Scholar] [CrossRef]
- Qi, W.; Wang, W.; Yang, X.; Zhang, G.; Ye, W.; Su, Y.; Li, Y.; Chen, S. Effects of Al and Ti co-doping on the strength-ductility- corrosion resistance of CoCrFeNi-AlTi high-entropy alloys. J. Alloys Compd. 2022, 925, 166751. [Google Scholar] [CrossRef]
- Kurt, B.; Günen, A.; Kanca, Y.; Koç, V.; Gök, M.S.; Kırar, E.; Askerov, K. Properties and Tribologic Behavior of Titanium Carbide Coatings on AISI D2 Steel Deposited by Thermoreactive Diffusion. JOM 2018, 70, 2650–2659. [Google Scholar] [CrossRef]
- Günen, A.; Soylu, B.; Karakaş, Ö. Titanium carbide coating to improve surface characteristic, wear and corrosion resistance of spheroidal graphite cast irons. Surf. Coatings Technol. 2022, 437, 128280. [Google Scholar] [CrossRef]
- Mareci, D.; Ungureanu, G.; Aelenei, N.; Chelariu, R.; Rosca, J.C.M. Eis diagnosis of some dental alloys in artificial saliva. Environ. Eng. Manag. J. 2007, 6, 313–317. [Google Scholar] [CrossRef]
- Socorro-Perdomo, P.P.; Florido-Suárez, N.R.; Mirza-Rosca, J.C.; Saceleanu, M.V. EIS Characterization of Ti Alloys in Relation to Alloying Additions of Ta. Materials 2022, 15, 476. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, P.P.S.; Suárez, N.R.F.; Verdú-Vázquez, A.; Rosca, J.C.M. Comparative EIS study of titanium-based materials in high corrosive environments. Int. J. Surf. Sci. Eng. 2021, 15, 152–164. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- ASTM E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA,, 2017.
- Material, C.; Databases, P. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic. Annu. B. ASTM Stand. 2004, 94, 1–12. [Google Scholar]
- ASTM-Standards Standard practice for calculation of corrosion rates and related information from electrochemical measurements. Astm G 102-89 1999, 89, 1–7.
- ISO 16773-1-4:2016; Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens. International Organization for Standardization: Geneva, Switzerland, 2016.
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Brito-Garcia, S.; Mirza-Rosca, J.; Geanta, V.; Voiculescu, I. Mechanical and Corrosion Behavior of Zr-Doped High-Entropy Alloy from CoCrFeMoNi System. Materials 2023, 16, 1832. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Sun, Z.; Li, J.; Li, L.; Song, X.; Wen, X.; Xie, L.; Yang, X. Effect of Mo and aging temperature on corrosion behavior of (CoCrFeNi)100−xMox high-entropy alloys. J. Alloys Compd. 2020, 812, 152139. [Google Scholar] [CrossRef]
- Pourbaix, M.; Zhang, H.; Pourbaix, A. Presentation of an Atlas of chemical and electrochemical equilibria in the presence of a gaseous phase. Mater. Sci. Forum 1997, 251–254, 143–148. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Zhang, L. Effect of high temperature on the corrosion behavior and passive film composition of 316 L stainless steel in high H2S-containing environments. Corros. Sci. 2020, 174, 108844. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, Z.; Lu, M. Combined effect of pH and H2S on the structure of passive film formed on type 316L stainless steel. Appl. Surf. Sci. 2018, 458, 686–699. [Google Scholar] [CrossRef]
- Scully, R.; Silverman, D.C.; Kendig, M. Electrochemical Impedance: Analysis and Interpretation; ASTM: Philadelphia, PA, USA, 1993. [Google Scholar] [CrossRef]
- Lutton, K.; Han, J.; Ha, H.M.; Sur, D.; Romanovskaia, E.; Scully, J.R. Passivation of Ni-Cr and Ni-Cr-Mo Alloys in Low and High pH Sulfate Solutions. J. Electrochem. Soc. 2023, 170, 021507. [Google Scholar] [CrossRef]
- Boukamp, B.A. A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.-H.; Fan, X.-H.; Jin, J.; Zhang, L.; Du, Y.-X. Corrosion behavior and surface characterization of an equiatomic CoCrFeMoNi high-entropy alloy under various pH conditions. J. Alloys Compd. 2022, 900, 163432. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito-Garcia, S.J.; Mirza-Rosca, J.C.; Jimenez-Marcos, C.; Voiculescu, I. EIS Study of Doped High-Entropy Alloy. Metals 2023, 13, 883. https://doi.org/10.3390/met13050883
Brito-Garcia SJ, Mirza-Rosca JC, Jimenez-Marcos C, Voiculescu I. EIS Study of Doped High-Entropy Alloy. Metals. 2023; 13(5):883. https://doi.org/10.3390/met13050883
Chicago/Turabian StyleBrito-Garcia, Santiago Jose, Julia Claudia Mirza-Rosca, Cristina Jimenez-Marcos, and Ionelia Voiculescu. 2023. "EIS Study of Doped High-Entropy Alloy" Metals 13, no. 5: 883. https://doi.org/10.3390/met13050883







