The Influences of Heat Treatment on the Microstructure and Mechanical Properties of Rolled Ti2AlNb
Abstract
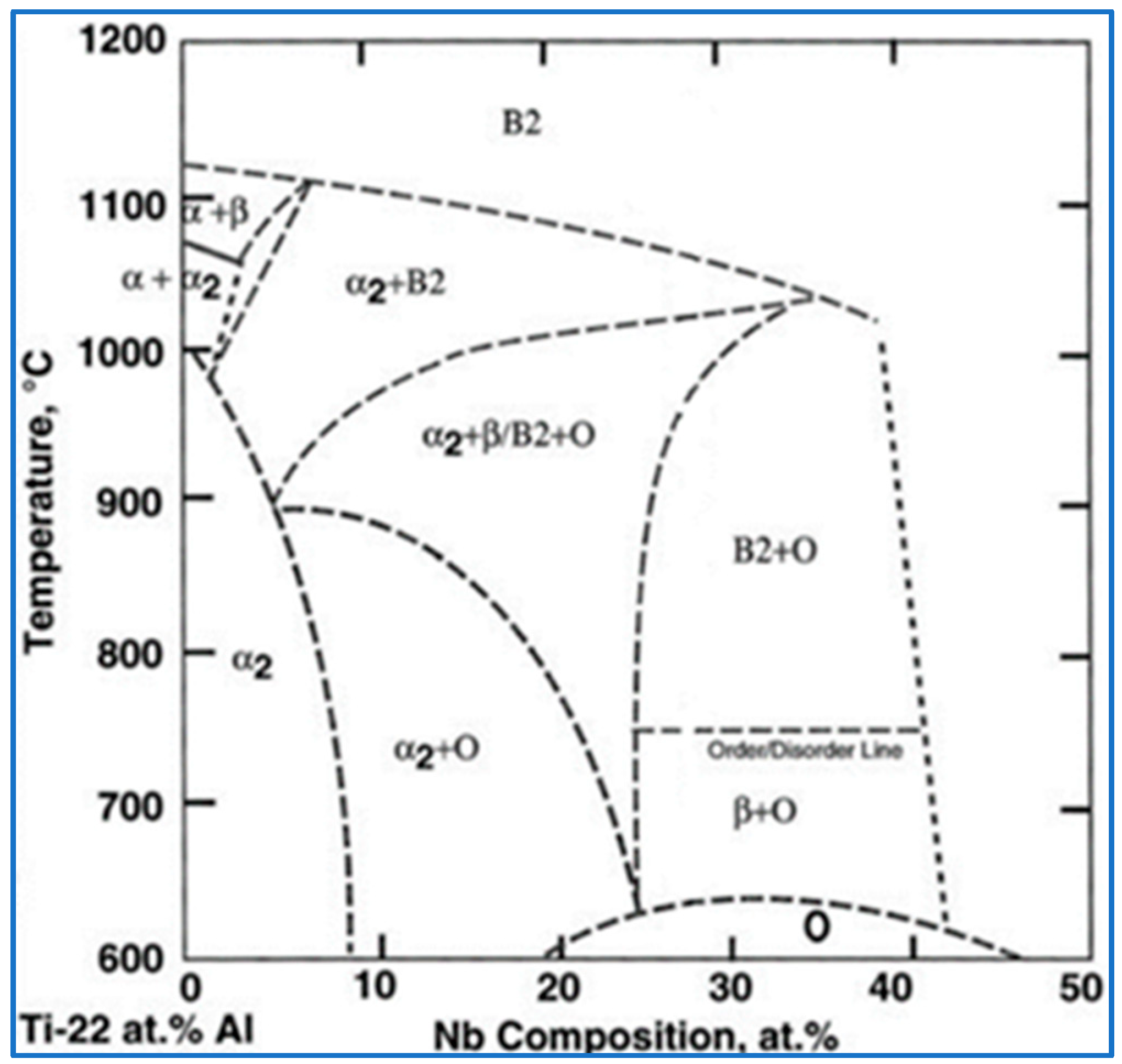
1. Introduction
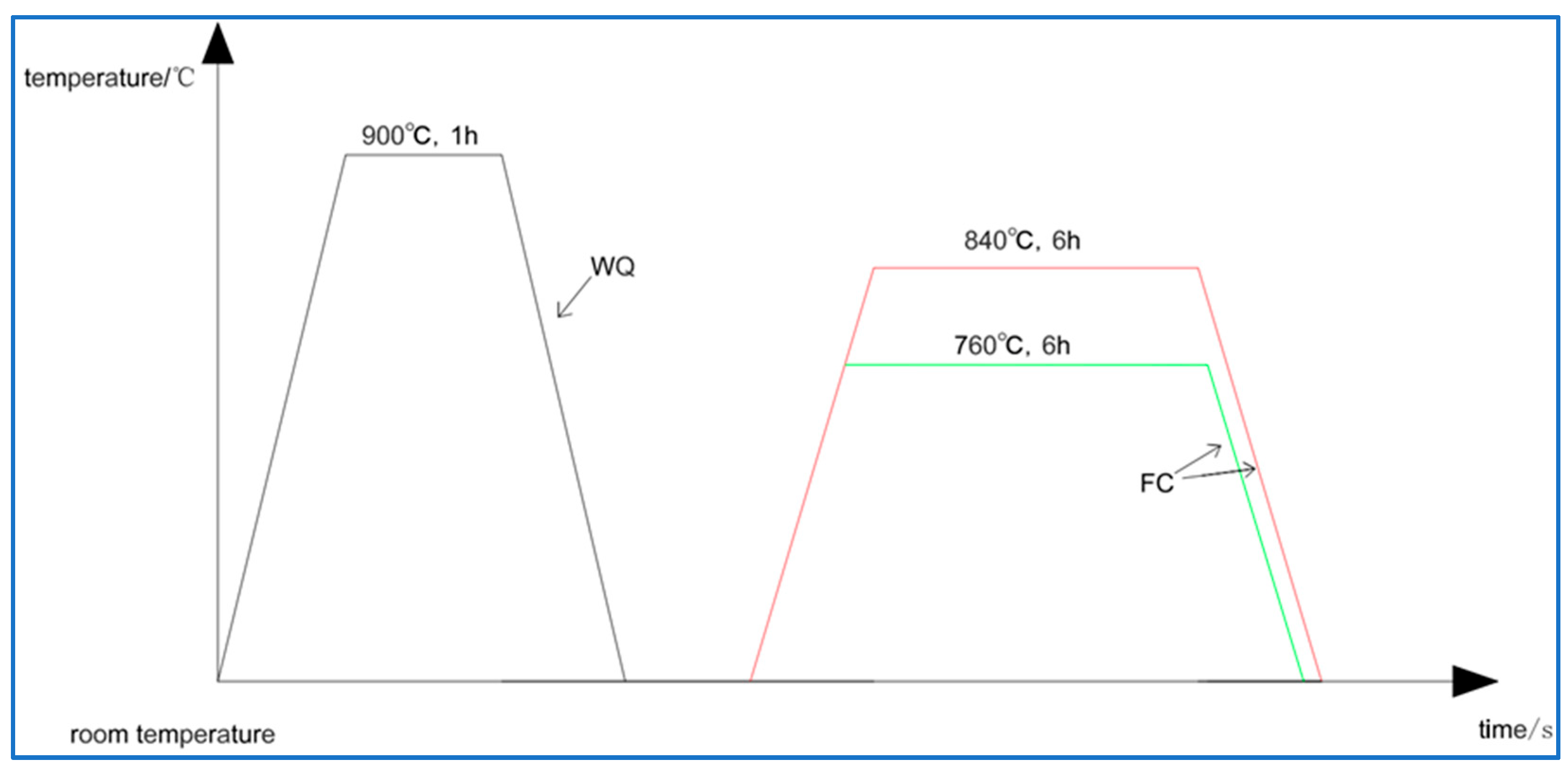
2. Materials and Methods
3. Results and Discussion
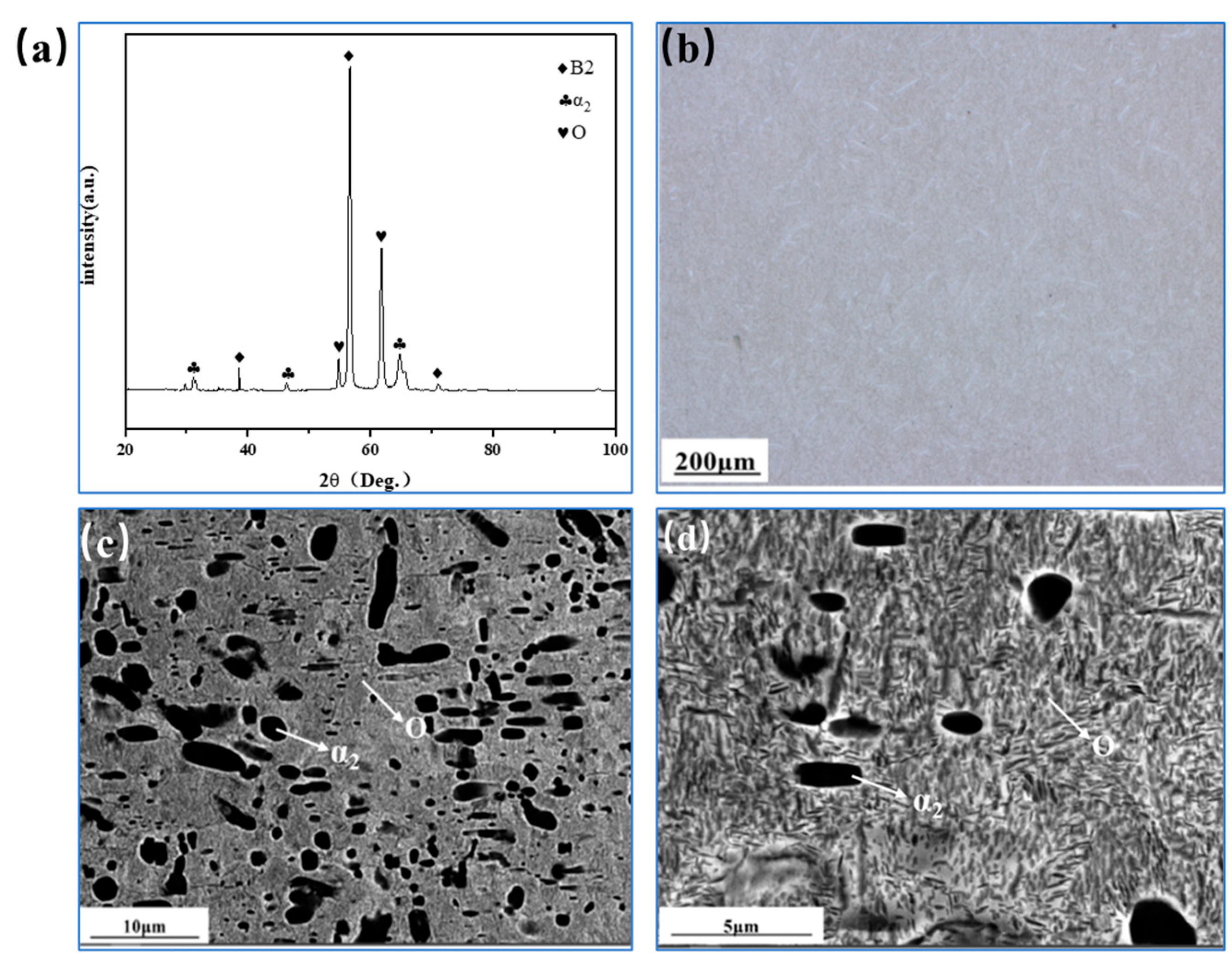
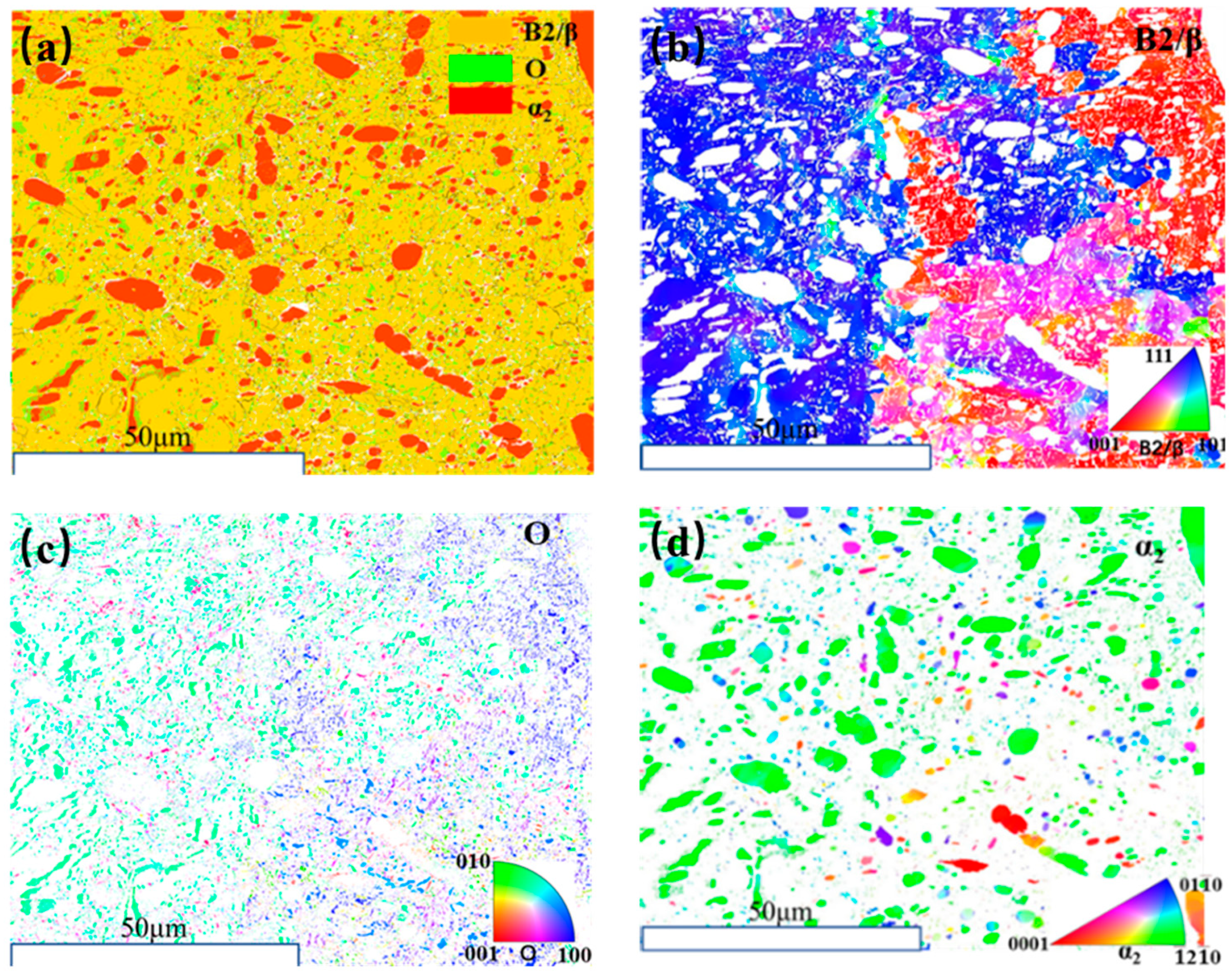
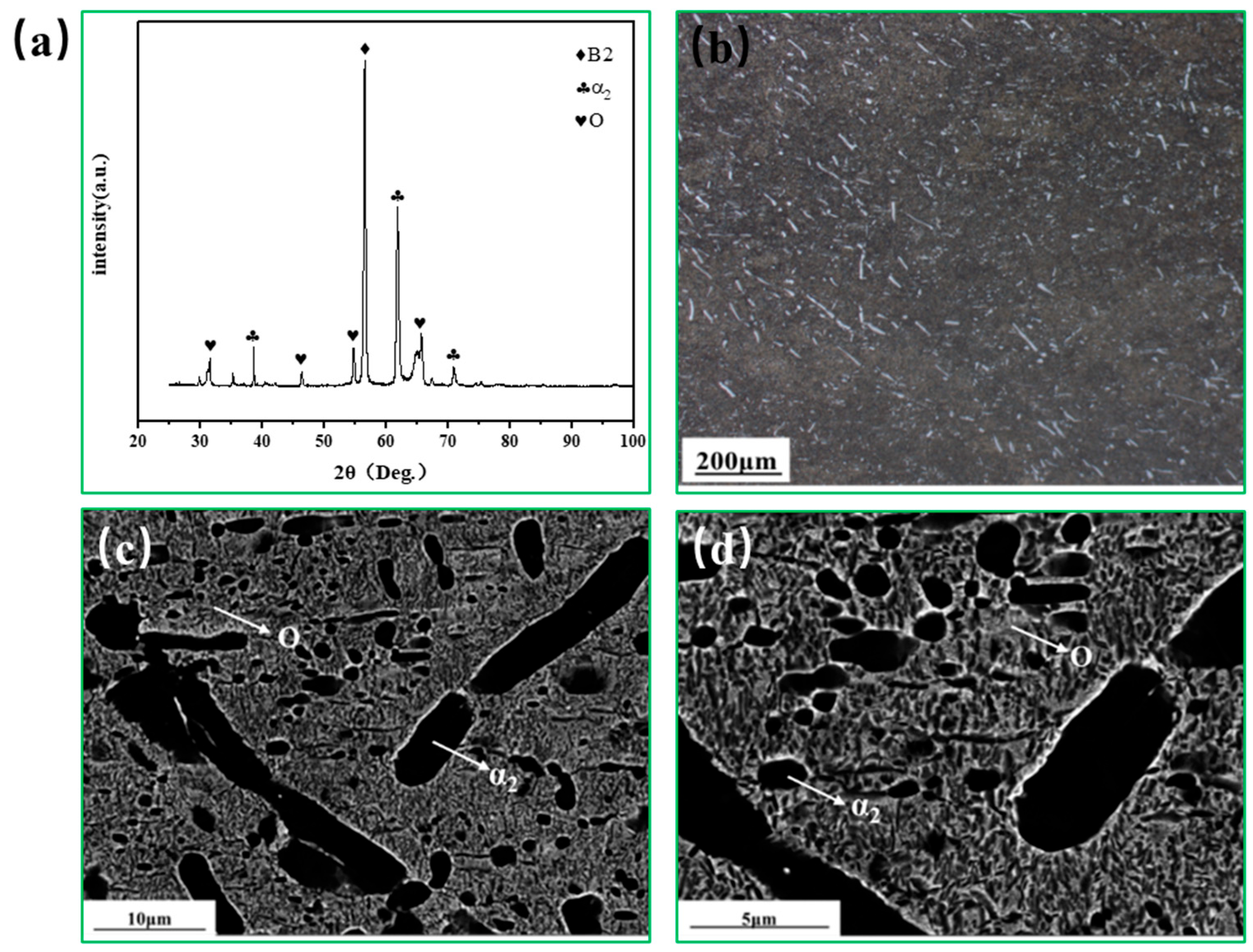
3.1. Microstructure of Rolled Ti2AlNb Alloy
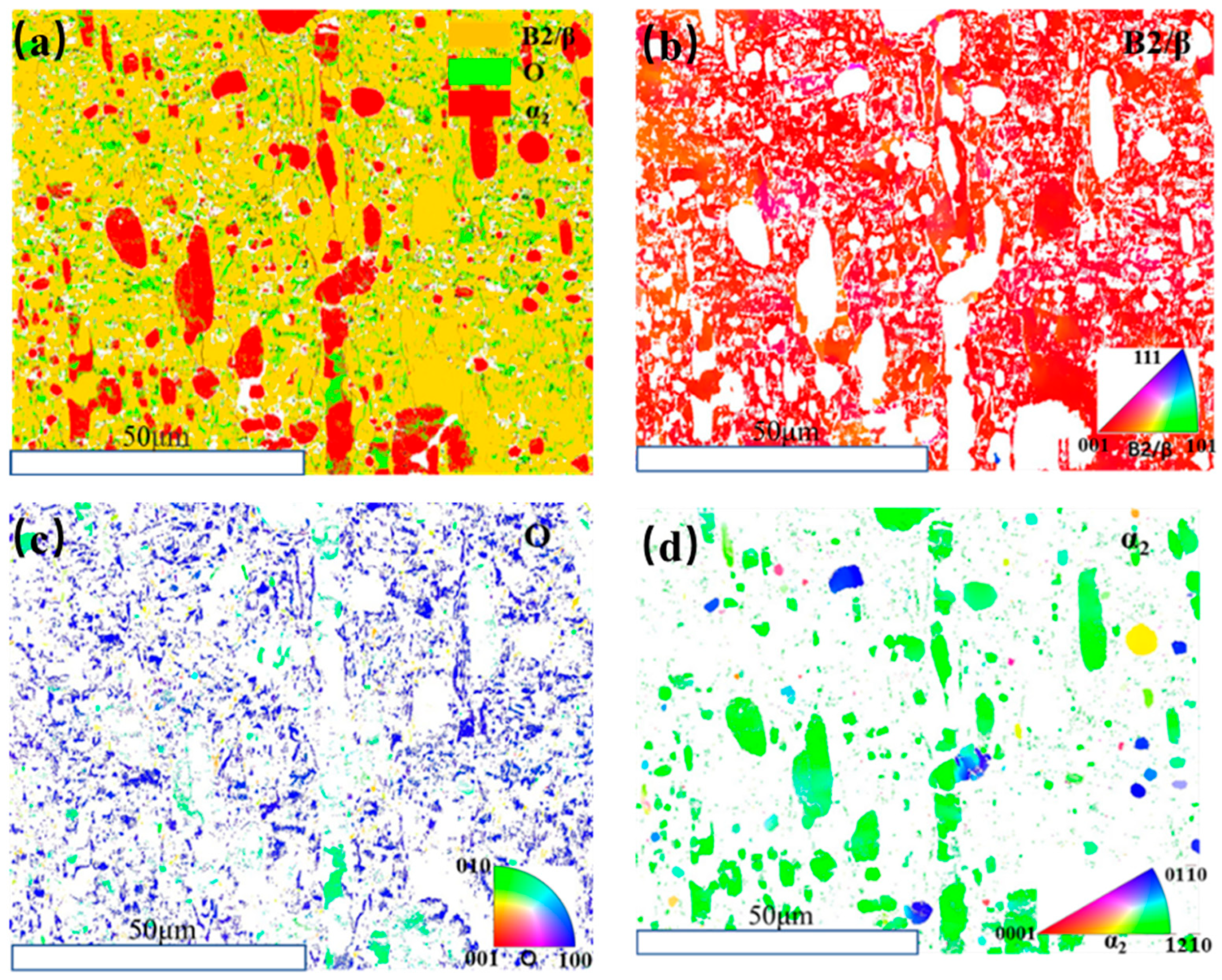
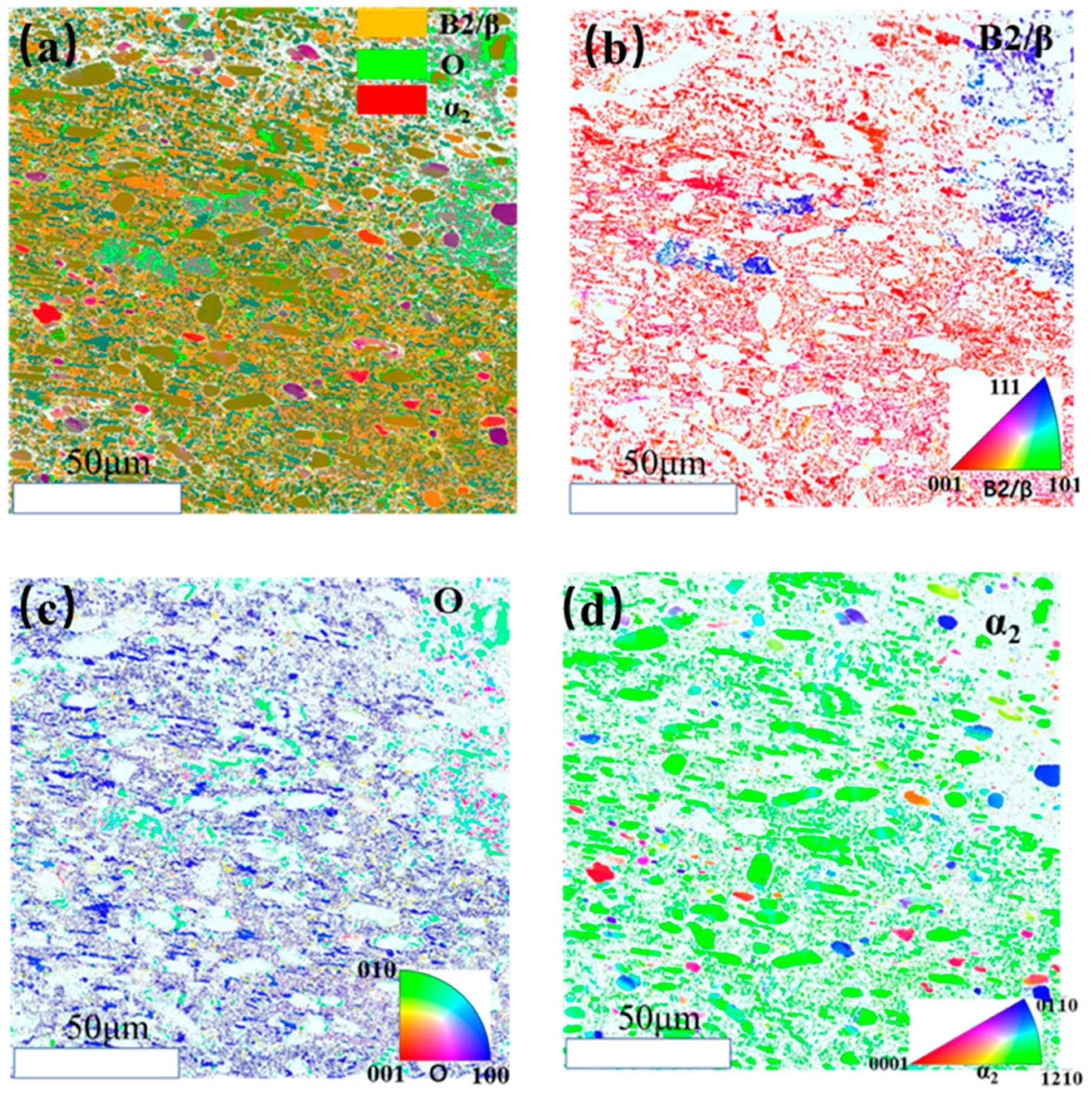
3.2. Microstructure Evolution of Ti2AlNb Alloy after Solution Treatment
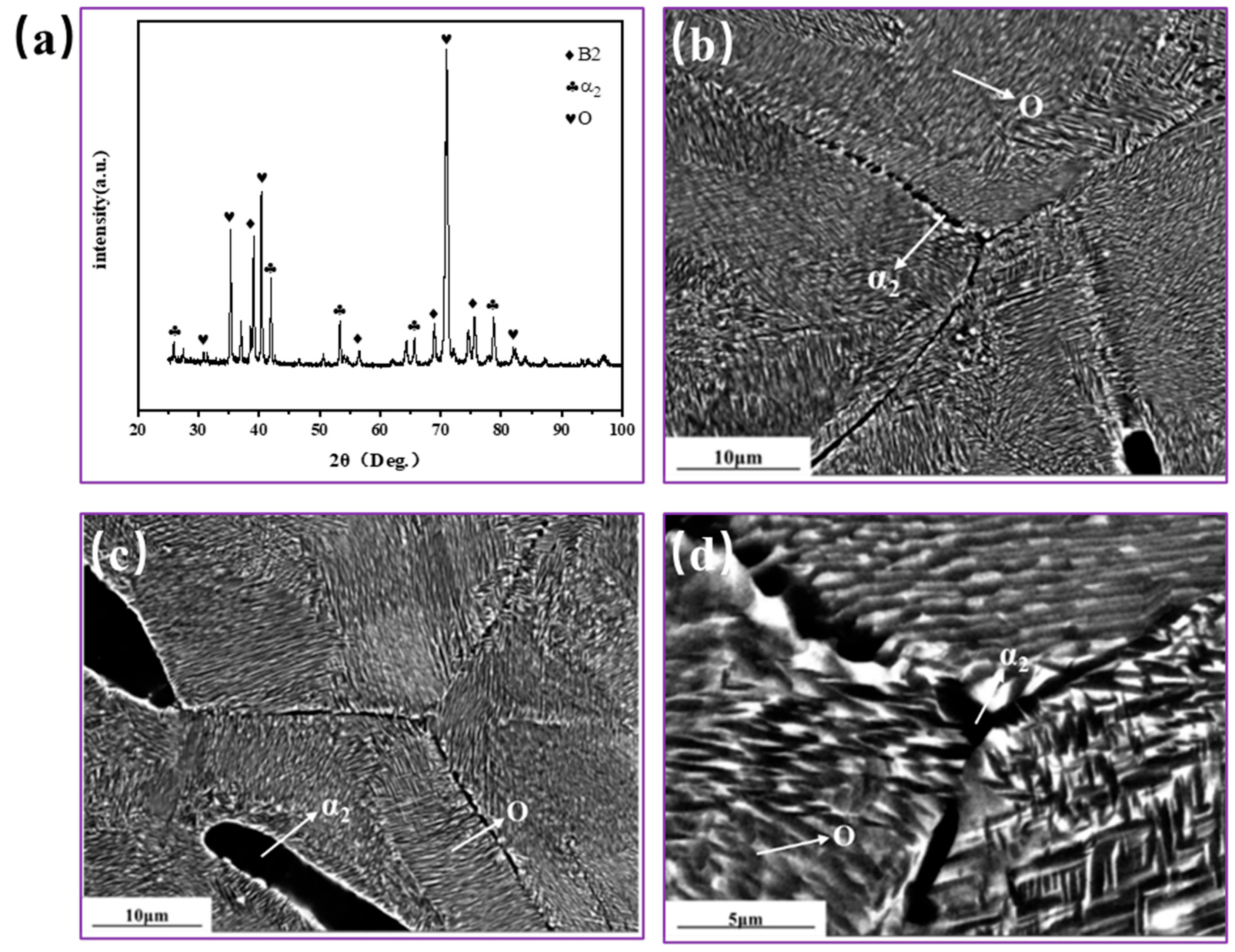
3.3. Microstructure Evolution of Ti2AlNb Alloy after aging Treatment
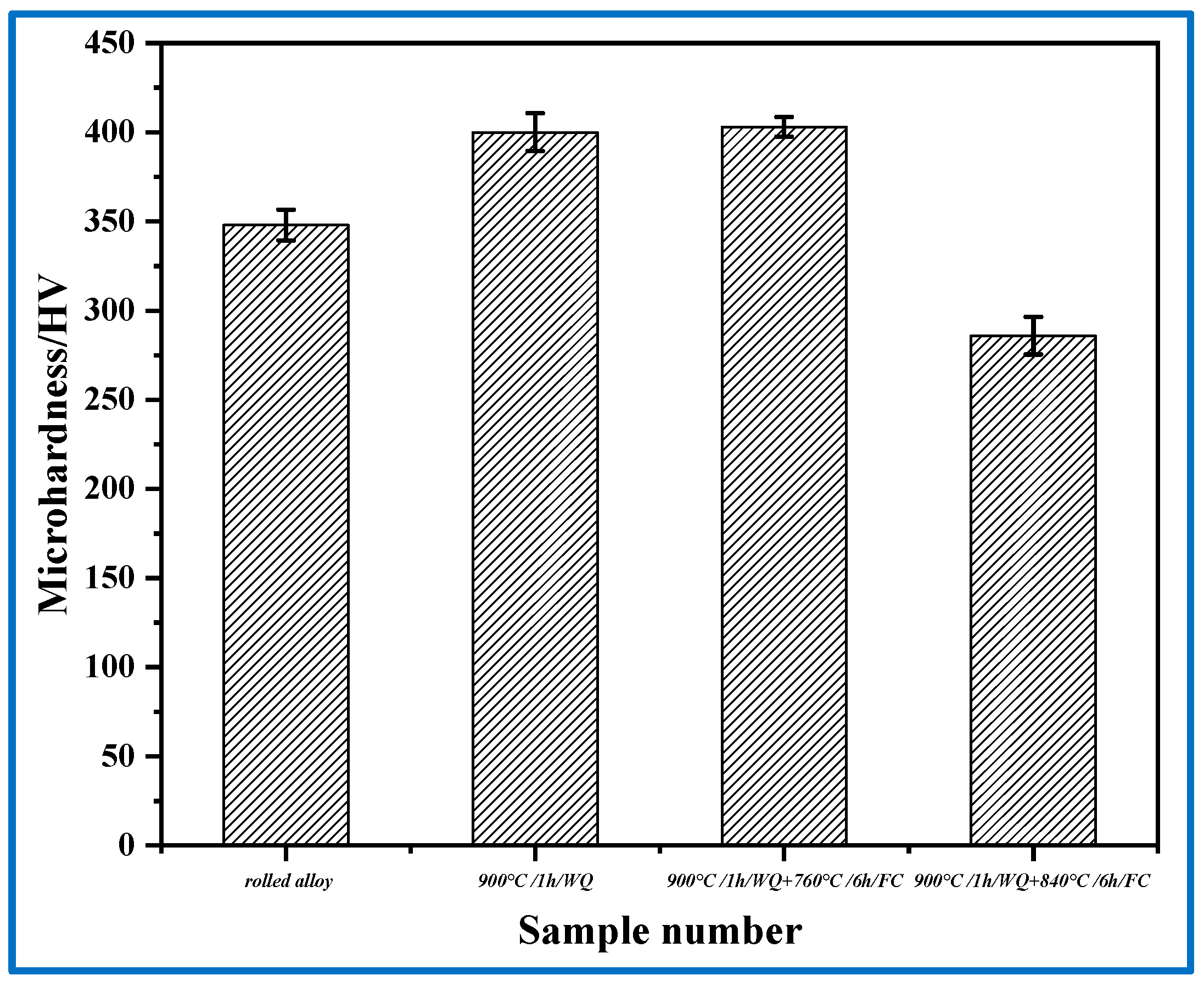
3.4. Hardness of Ti2AlNb Alloy after Heat Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, Q.; Cizek, P.; Wang, J. Enhanced Mechanical Response of an Ultrafine Grained Ti-6Al-4V Alloy Produced through Warm Symmetric and Asymmetric Rolling. Mater. Sci. Eng. A 2016, 650, 404–413. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, S.; Wang, J. Twinning and Sequential Kinking in Lamellar Ti-6Al-4V Alloy. Acta Mater. 2019, 181, 479–490. [Google Scholar] [CrossRef]
- Banerjee, D.; Gogia, A.K.; Nandi, T.K. A New Ordered Orthorhombic Phase in a Ti3Al-Nb Alloy. Acta Metall. 1988, 36, 871–882. [Google Scholar] [CrossRef]
- Katoh, Y.; Vasudevamurthy, G.; Nozawa, T.; Snead, L.L. Properties of zirconium carbide for nuclear fuel applications. J. Nucl. Mater. 2013, 441, 718–742. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yu, L. Microstructures and Tensile Properties of Ti2AlNb and Mo-Modified Ti2AlNb Alloys Fabricated by Hot Isostatic Pressing. Mater. Sci. Eng. A 2020, 776, 139043. [Google Scholar] [CrossRef]
- Mao, Y.; Li, S.; Zhang, J. Microstructure and Tensile Properties of Orthorhombic Ti-Al-Nb-Ta Alloys. Intermetallics 2000, 8, 659–662. [Google Scholar] [CrossRef]
- Kumpfert, J. Intermetallic Alloys Based on Orthorhombic Titanium Aluminide. Adv. Eng. Mater. 2001, 3, 851–864. [Google Scholar] [CrossRef]
- Das, K.; Das, S. Order-Disorder Transformation of the Body Centered Cubic Phase in the Ti-Al-X(X = Ta, Nb, or Mo) System. J. Mater. Sci. 2003, 38, 3995–4002. [Google Scholar] [CrossRef]
- Yang, J.; Cai, Q.; Ma, Z. Effect of W Addition on Phase Transformation and Microstructure of Powder Metallurgic Ti-22Al-25Nb Alloys during Quenching and Furnace Cooling. Chin. J. Aeronaut 2019, 32, 1343–1351. [Google Scholar] [CrossRef]
- Hagiwara, M.; Emura, S.; Araoka, A. Enhanced Mechanical Properties of Orthorhombic Ti2AlNb-Based Intermetallic Alloy. Met. Mater. Int. 2003, 9, 265–272. [Google Scholar] [CrossRef]
- Germann, L.; Banerjee, D.; Gu’edou, J.Y. Effect of Composition on the Mechanical Properties of Newly Developed Ti2AlNb-based Titanium Aluminide. Intermetallics 2005, 13, 920–924. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, B.; Tong, M. Tensile Behavior of Ti-22Al-24Nb-0.5Mo in the Range 25–650 °C. Mater. Sci. Eng. A 2016, 679, 455–464. [Google Scholar] [CrossRef]
- Jiao, X.; Liu, G.; Wang, D. Creep Behavior and Effects of Heat Treatment on Creep Resistance of Ti-22Al-24Nb-0.5Mo Alloy. Mater. Sci. Eng. A 2016, 680, 182–189. [Google Scholar] [CrossRef]
- Yang, J.; Cai, Q.; Liu, Y. Herringbone Structure and Significantly Enhanced Hardness in W-Modified Ti2AlNb Alloys by Spark Plasma Sintering. Met. Mater. Int. 2019, 25, 1000–1007. [Google Scholar] [CrossRef]
- Boehlert, C.J. The Phase Evolution and Microstructural Stability of an Orthorhombic Ti-23Al-27Nb Alloy. J. Phase Equilibria 1999, 20, 101–108. [Google Scholar] [CrossRef]
- Zhan, H.; Kent, D.; Wang, G.; Dargusch, M.S. The dynamic response of a β titanium alloy to high strain rates and elevated temperatures. Mater. Sci. Eng. A 2014, 607, 417–426. [Google Scholar] [CrossRef]
- Lai, M.J.; Li, T.; Raabe, D. ω phase acts as a switch between dislocation channeling and joint twinning-and transformation-induced plasticity in a metastable β titanium alloy. Acta Mater. 2018, 105, 67–77. [Google Scholar] [CrossRef]
- Wojcik, R.; Roessler, R.W.; Zordan, I. Titanium aluminide foil processing. In Advances in the Science and Technology of Titanium Alloy Processing: Proceedings of an International Symposium Sponsored by the TMS Titanium and Shaping and Forming Held at the 125th TMS Annual Meeting and Exhibition, Anaheim, CA, USA, 5–8 February 1996; Weiss, I., Srinivasan, R., Bania, P.J., Eylon, D., Semiatin, S.L., Eds.; TMS: Warrendale, PA, USA, 1997; pp. 293–300. [Google Scholar]
- Boehlert, C.J.; Majumdar, B.S.; Seetharaman, V.; Miracle, D. Part I. The Microstructural Evolution in Ti-Al-Nb O+Bcc Orthorhombic Alloys. Metall. Mater. Trans. 1999, 30, 2305–2323. [Google Scholar] [CrossRef]
- Li, M.; Cai, Q.; Liu, Y.; Ma, Z.; Wang, Z. Microstructure and Mechanical Properties of Ti2AlNb-Based Alloys Synthesized by Spark Plasma Sintering from Pre-Alloyed and Ball-Milled Powder. Adv. Eng. Mater. 2018, 20, 1700659. [Google Scholar] [CrossRef]
- Banerjee, D. The Intermetallic Ti2AlNb. Prog. Mater. Sci. 1997, 42, 135–158. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Li, Z.; Ma, L. Microstructure Evolution and Phase Transformations in Ti-22Al-25Nb Alloys Tailored by Super-Transus Solution Treatment. Vacuum 2019, 161, 209–219. [Google Scholar] [CrossRef]
- Sadi, F.A.; Servant, C. On the B2→O phase transformation in Ti-Al-Nb alloys. Mater. Sci. Eng. A 2003, 346, 19–28. [Google Scholar] [CrossRef]
- Sherby, O.D.; Klundt, R.H.; Miller, A.K. Flow stress, subgrain size, and subgrain stability at elevated temperature. Metall. Trans. A 1977, 8, 83. [Google Scholar] [CrossRef]
- Wang, S.H.; Kao, P.W. The strengthening effect of Ti3Al in high temperature deformation of Al-Ti3Al composites. Acta Mater. 1998, 46, 2675. [Google Scholar] [CrossRef]
- Alabort, E.; Putman, D.; Reed, R.C. Superplasticity in Ti-6Al-4V: Characterisation, modelling and applications. Acta Mater. 2015, 95, 428. [Google Scholar] [CrossRef]
- Nandy, T.K.; Banerjee, D. Deformation mechanisms in the O phase. Intermetallics 2000, 8, 1269. [Google Scholar] [CrossRef]
- Lin, P.; He, Z.B.; Yuan, S.J.; Shen, J. Tensile deformation behavior of Ti-22Al-25Nb alloy at elevated temperatures. Mater. Sci. Eng. A 2012, 556, 617. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, W.D.; Xue, C.; Liang, X.B. Microstructure control and mechanical properties from isothermal forging and heat treatment of Ti-22Al-25Nb (at%) orthorhombic alloy. Intermetallics 2015, 56, 79. [Google Scholar] [CrossRef]
- Chiu, J.M. Effect of fiber coating on the fracture and fatigue resistance of CSC-6/Ti3Al composites. Acta Metall. Mater. 1995, 43, 2581–2587. [Google Scholar] [CrossRef]
- Boet, J. Thermal residual stresses in ceramic matrix composites-I. Axisymmetrical model and finite element analysis. Acta Metall. Mater. 1995, 43, 2241–2253. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, D.; Li, Q.; Zhao, X.; Liang, J.; Zhang, X. Fracture toughness of the bimodal size lamellar O phase microstructures in Ti-22Al-25Nb (at. %) orthorhombic alloy. J. Alloys Compd. 2017, 709, 511–518. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, D.; Li, H.; Ma, P.; Zhang, X. Cleavage fracture of the bimodal size lamellar O phase microstructure of a Ti2AlNb based alloy. J. Alloys Compd. 2019, 799, 267–278. [Google Scholar] [CrossRef]
- Li, Q.; Cai, Y.; Liu, Z.; Ma, Z.; Wang, Y.; Huang, H. Formation of fine B2/β + O structure and enhancement of hardness in the aged Ti2AlNb-based alloys prepared by spark plasma sintering. Metall. Mater. Trans. A 2017, 48, 4365–4371. [Google Scholar] [CrossRef]
- He, R.; Hu, W.; Luo, T.; He, Y.-J.; Lai, Y.-J.; Du, X.-H. Microstructure and mechanical properties of a new Ti2AlNb-based alloy after aging treatment. Rare Met. 2018, 37, 942–951. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, D.; Li, B.; Zhu, Y.; Zheng, X. Microstructural evolution and tensile behavior of Ti2AlNb alloys based α2-phase decomposition. Mater. Sci. Eng. A 2016, 662, 120–128. [Google Scholar] [CrossRef]


| Composition | Ti | Al | Nb | Mo | V | Si | Miscellaneous |
|---|---|---|---|---|---|---|---|
| wt.% | Bal. | 10.80 | 11.38 | 11.79 | 12.79 | 0.04 | 0.12 |
| Sample Number | Solution Condition | Cooling Way | Aging Condition | Cooling Method |
|---|---|---|---|---|
| 1 | − | − | − | − |
| 2 | 900 °C/1 h | WQ | − | − |
| 3 | 900 °C/1 h | WQ | 760 °C/6 h | FC |
| 4 | 900 °C/1 h | WQ | 840 °C/6 h | FC |
| Test Specimen | B2/β (%) | O (%) | α2 (%) | Grain Size (μm) |
|---|---|---|---|---|
| Rolled plate | 67.3 | 16.3 | 16.4 | 0.79 |
| ST900 | 68.3 | 13.4 | 18.4 | 0.89 |
| ST900 + AT760 | 44.2 | 23.9 | 31.9 | 0.73 |
| ST900 + AT840 | 19.5 | 68.9 | 11.6 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, X.; Wei, B. The Influences of Heat Treatment on the Microstructure and Mechanical Properties of Rolled Ti2AlNb. Metals 2023, 13, 886. https://doi.org/10.3390/met13050886
Liu T, Li X, Wei B. The Influences of Heat Treatment on the Microstructure and Mechanical Properties of Rolled Ti2AlNb. Metals. 2023; 13(5):886. https://doi.org/10.3390/met13050886
Chicago/Turabian StyleLiu, Tianze, Xuewen Li, and Boxin Wei. 2023. "The Influences of Heat Treatment on the Microstructure and Mechanical Properties of Rolled Ti2AlNb" Metals 13, no. 5: 886. https://doi.org/10.3390/met13050886
APA StyleLiu, T., Li, X., & Wei, B. (2023). The Influences of Heat Treatment on the Microstructure and Mechanical Properties of Rolled Ti2AlNb. Metals, 13(5), 886. https://doi.org/10.3390/met13050886





