Effect of Glue, Thiourea, and Chloride on the Electrochemical Reduction in CuSO4–H2SO4 Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrolyte Solutions
2.2. Electrochemical Measurements
2.3. Massive Deposits Obtention
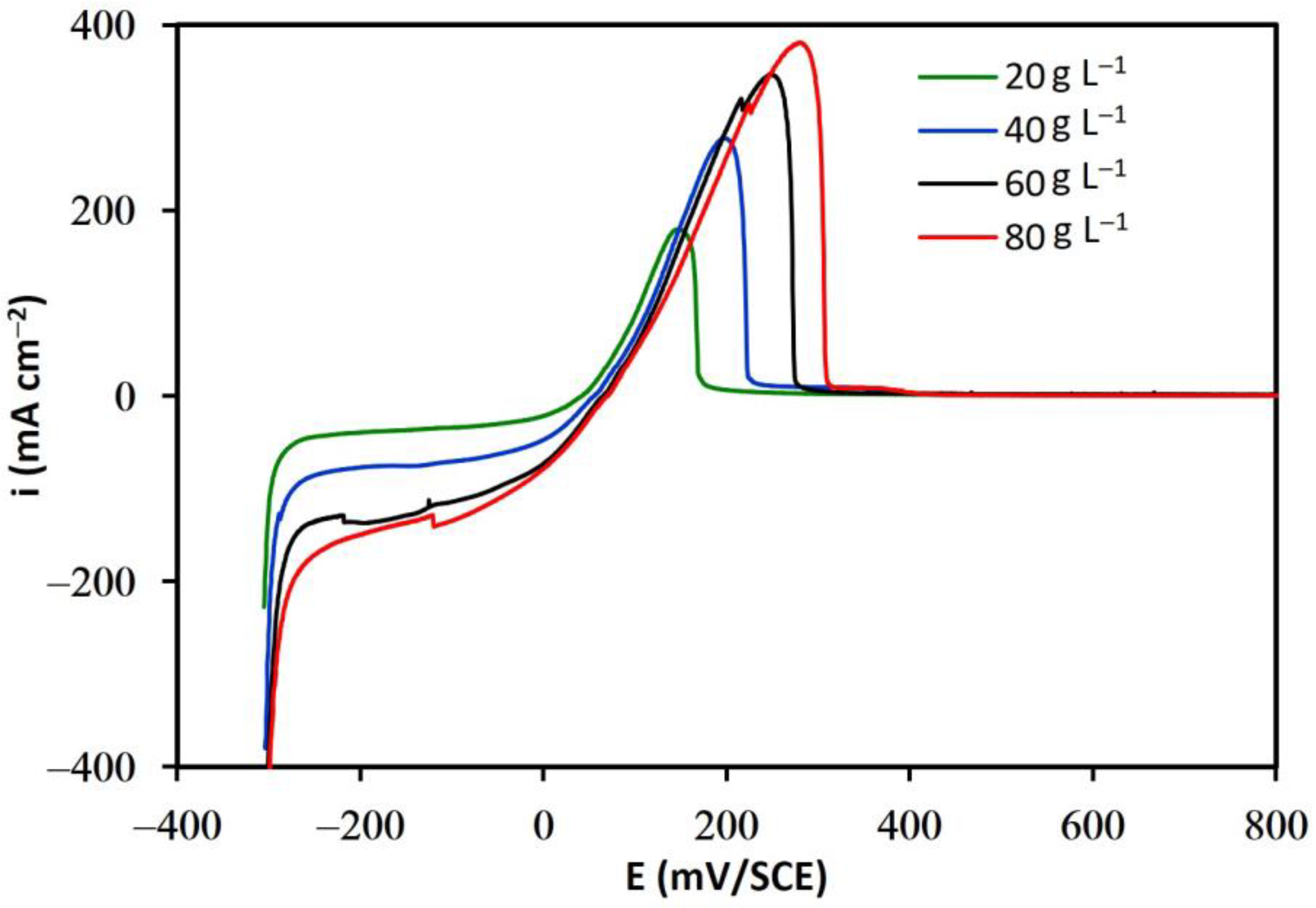
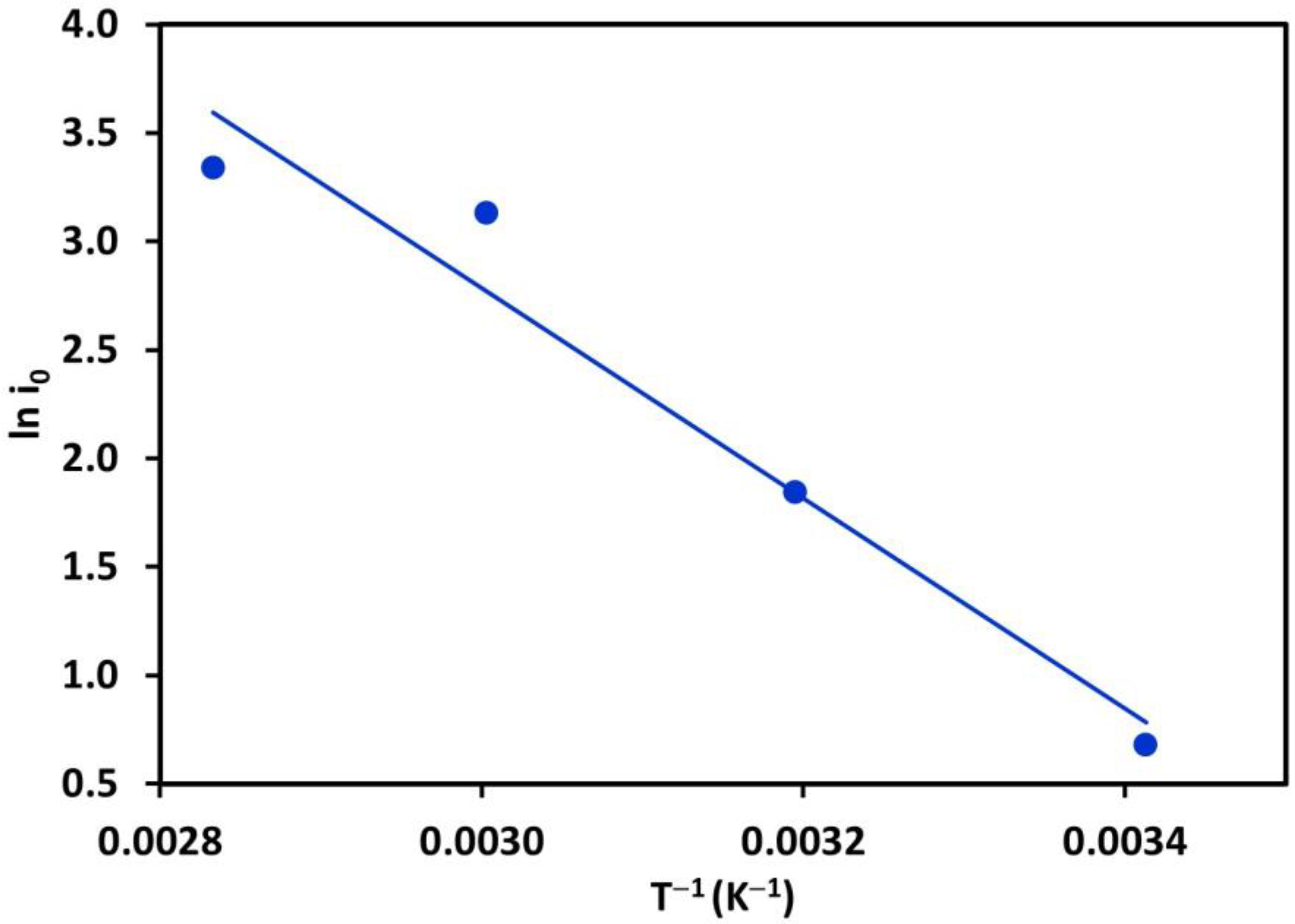
3. Results and Discussions
3.1. Determination of Electrochemical Kinetics Parameters, and
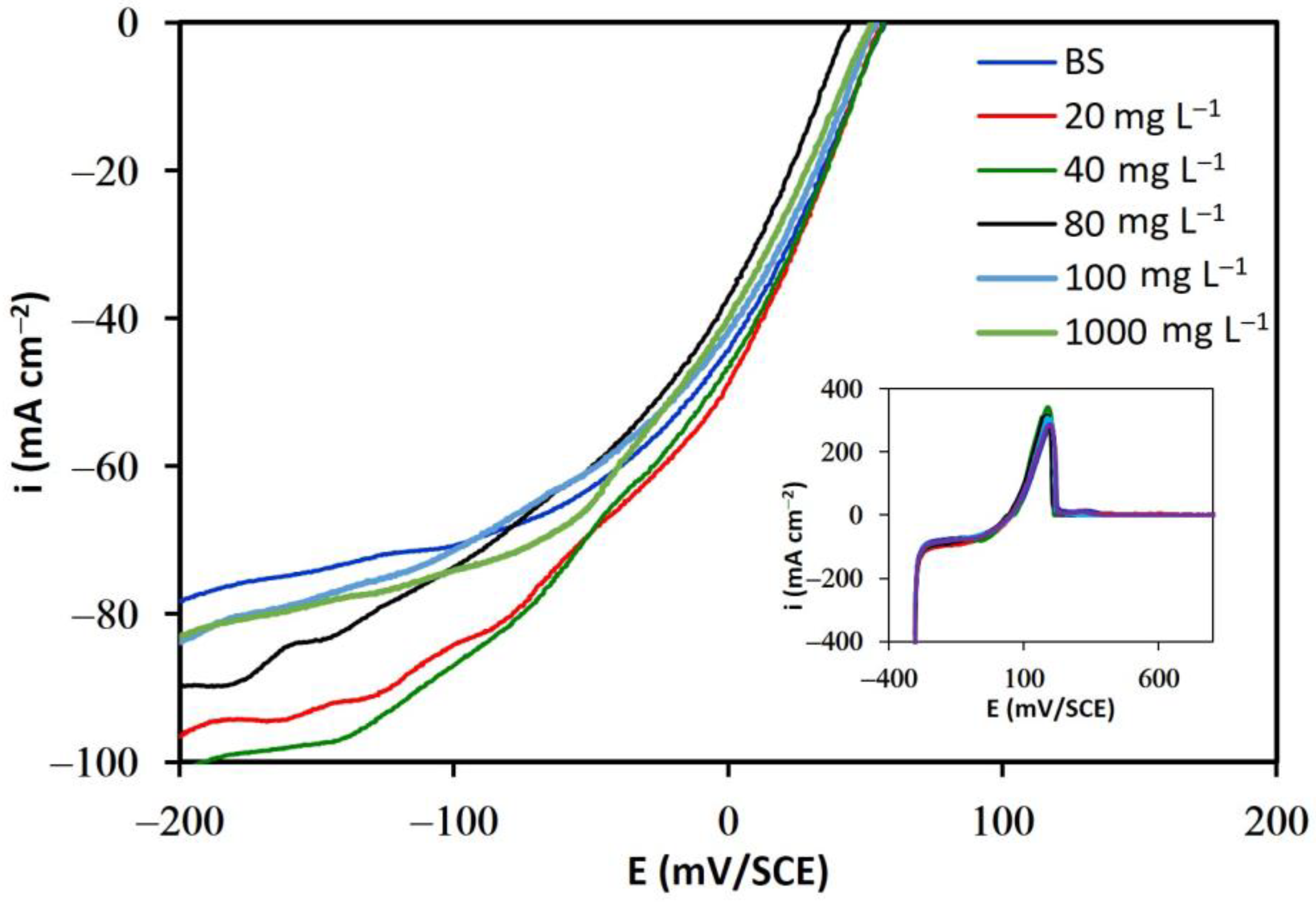
3.1.1. Additive-Free Base Solutions
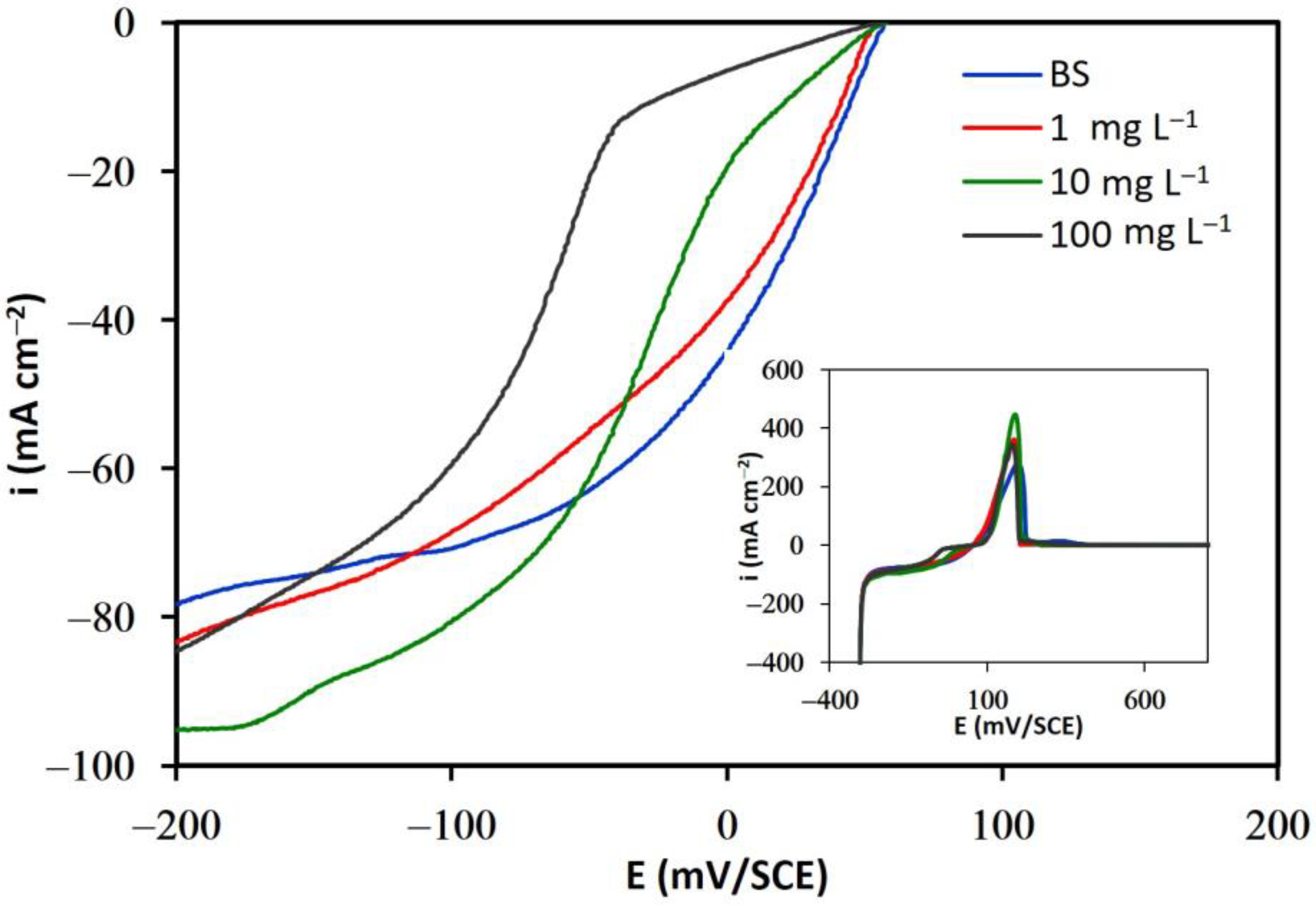
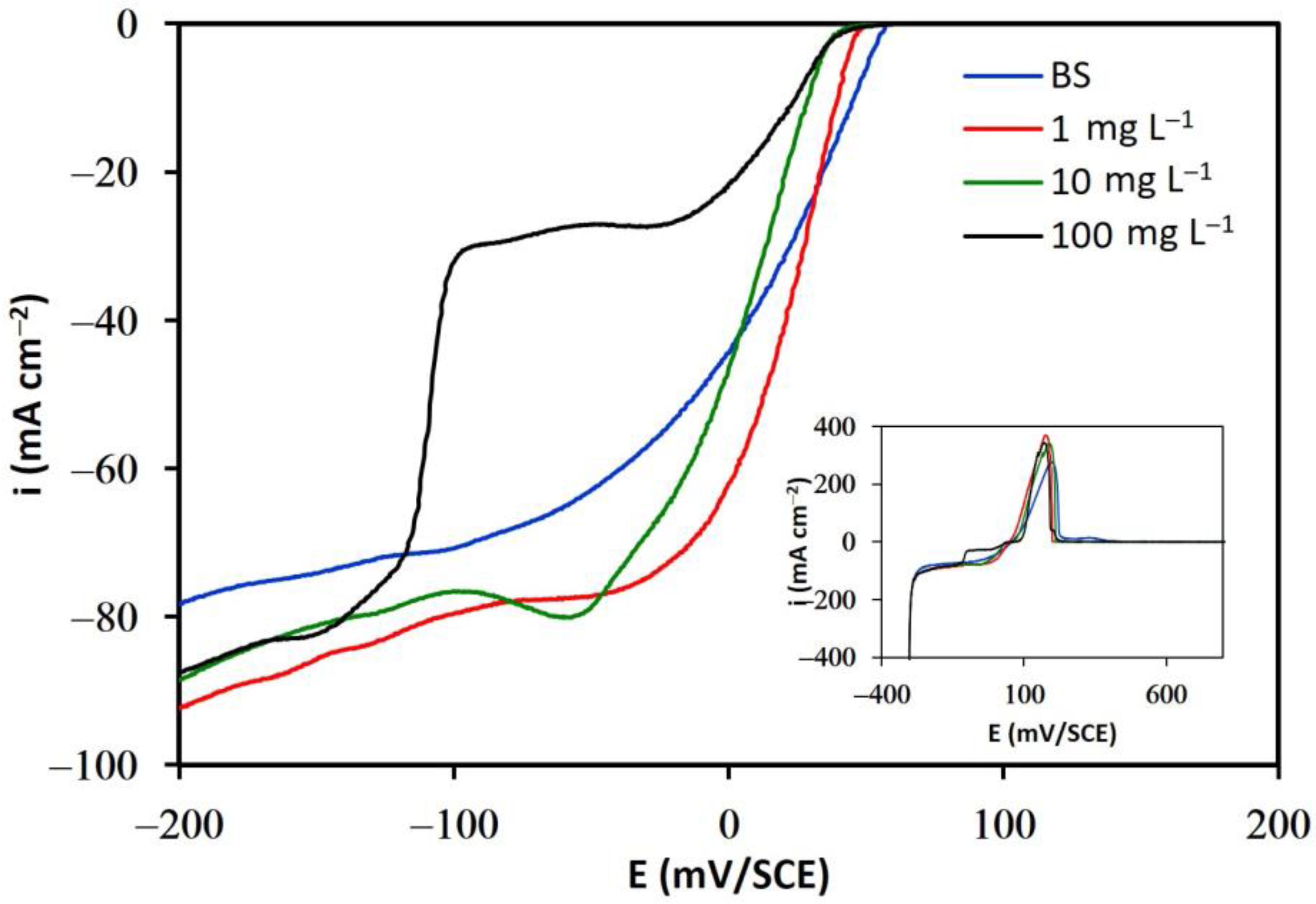
3.1.2. Solutions in the Presence of Additives
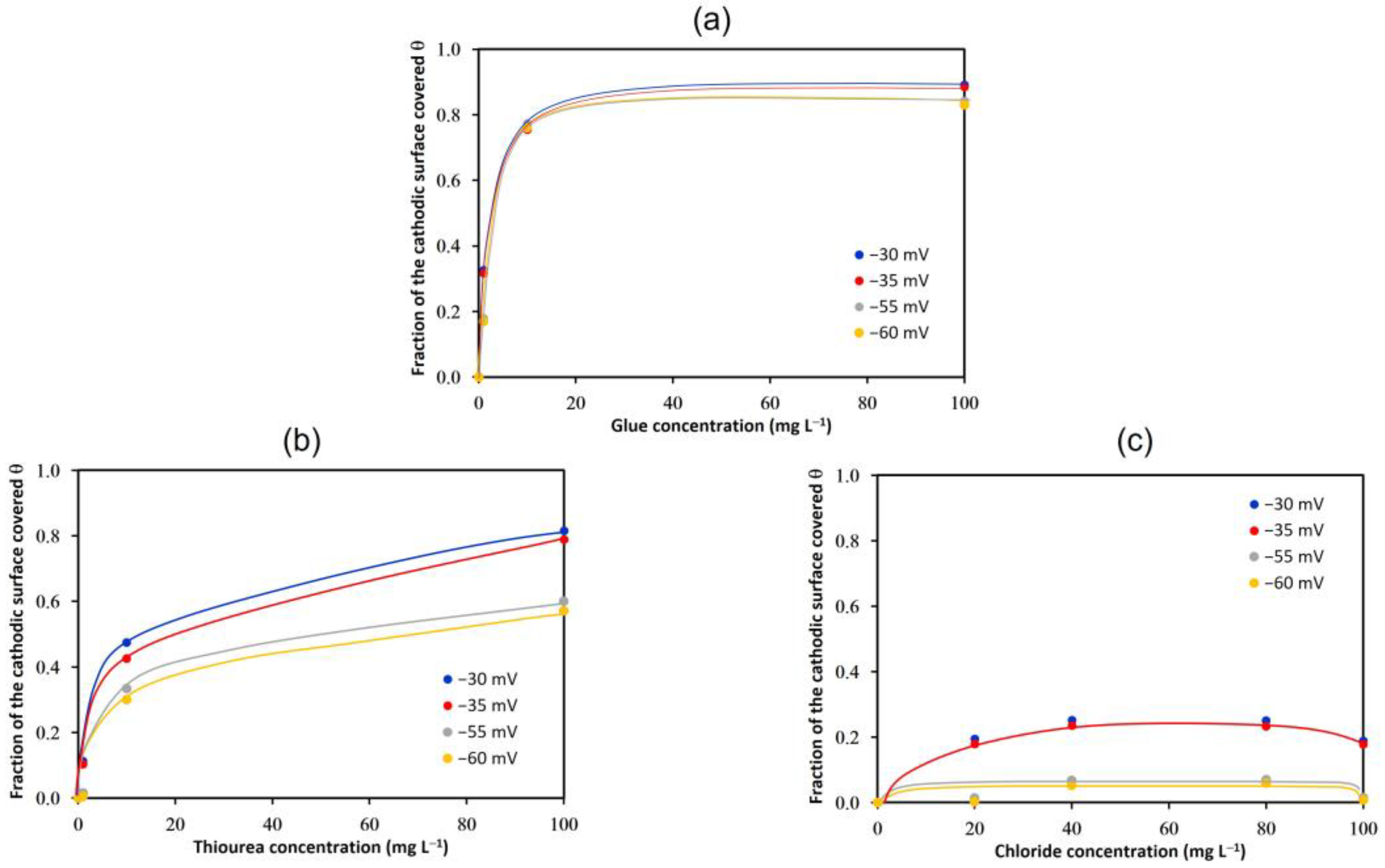
3.2. Adsorption Isotherms
3.3. Deposits Characterization
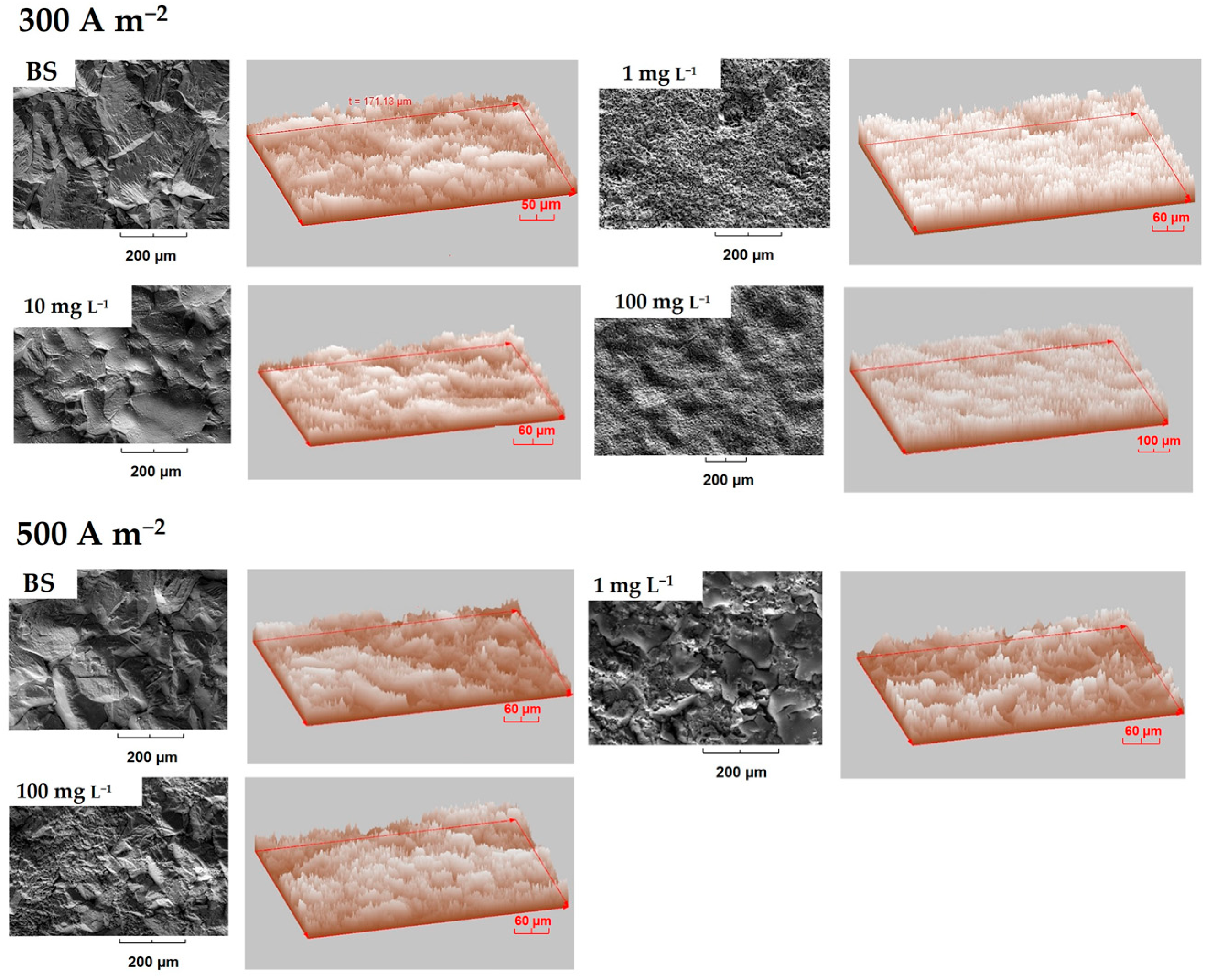
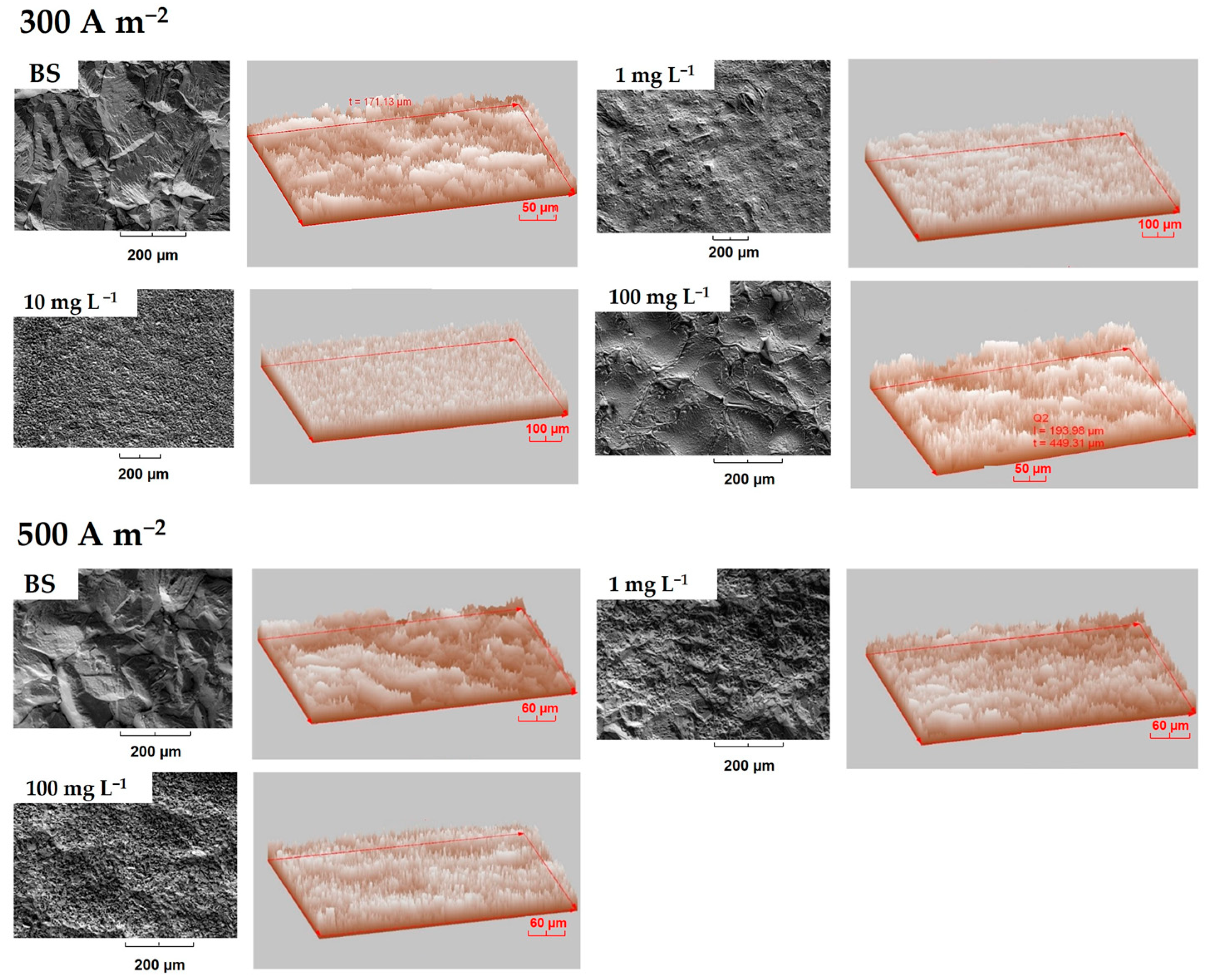
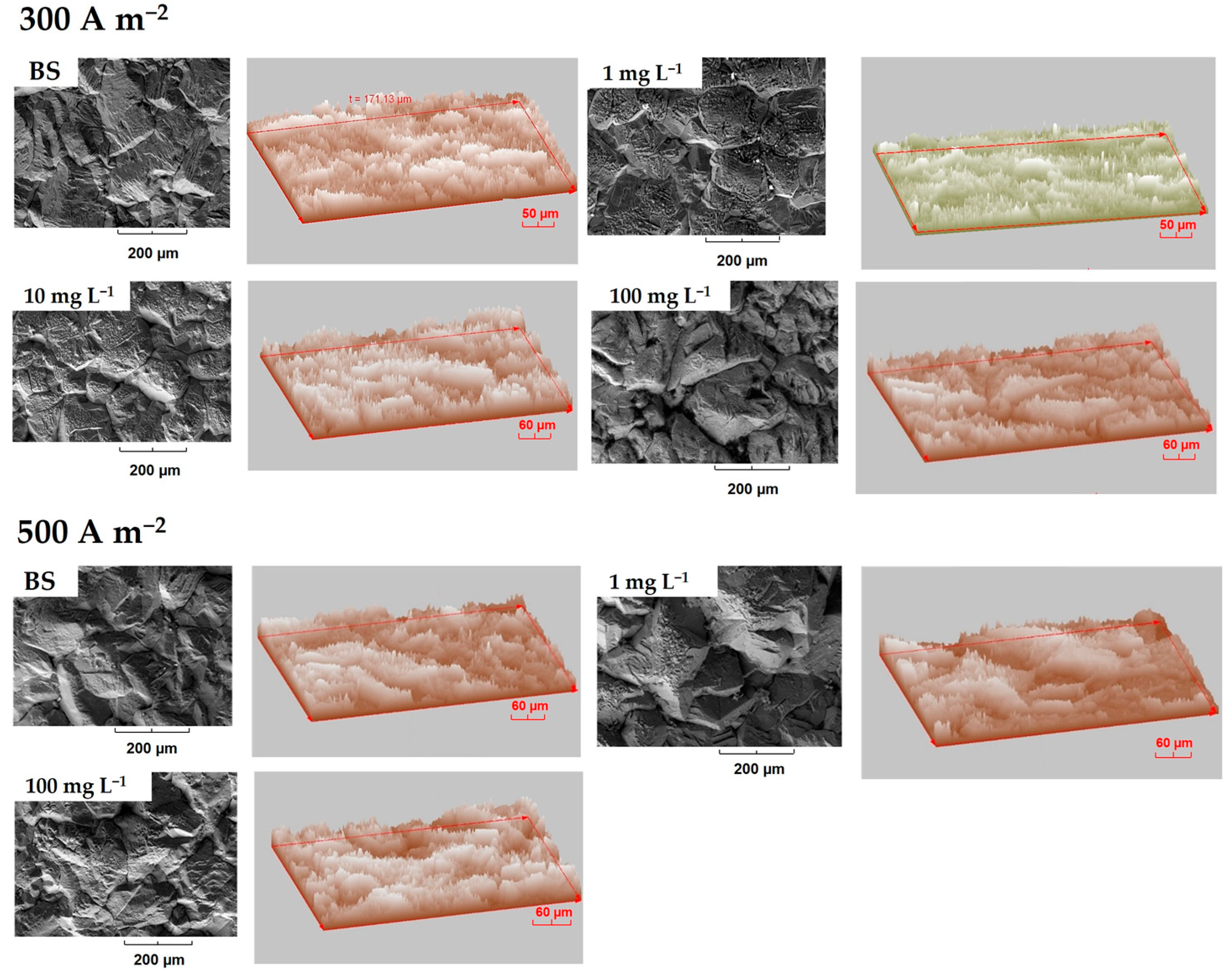
3.3.1. Deposits Surface Roughness
3.3.2. Effect of Additives on Deposit Texture Coefficient
4. Conclusions
- The results of linear voltammetry indicate that the addition of glue and thiourea lead to a decrease in the exchange current density, , and an increase in the cathodic charge transfer coefficient, . In contrast, the addition of chloride ions did not substantially modify these kinetic parameters.
- It was determined that the partial order of the Cu2+/Cu reduction reaction concerning the concentration of glue and thiourea results in negative values, inhibiting the reduction kinetics.
- It was also determined that organic additives are adsorbed on the cathodic surface, blocking the active sites available for the cupric ions reduction process.
- The fraction of the cathodic surface covered increased significantly with the concentration of glue and thiourea, and the Temkin adsorption model was verified in both cases. In contrast, the addition of chloride did not significantly modify the fraction of the cathodic surface covered, nor was it possible to correlate any adsorption model in the presence of this ion.
- In the same sense, the inhibitory effect of the organic additives on the morphology and texture of the massive deposits obtained for two levels of current density compared to the base solution was verified.
- The levelling effect of the additives was dependent on both the concentration and current density. It was found that the presence of chloride has no effect on the morphological characteristics of the deposit.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pourgharibshahi, M.; Khorasani, S.M.J.; Yaghoobi, N.; Lambert, P. A Descriptive Model for Twin-Textured Growth and Nodulation of Copper Cathodes. Electrocatalysis 2023, 14, 138–147. [Google Scholar] [CrossRef]
- Fabbri, L.; Giurlani, W.; Mencherini, G.; De Luca, A.; Passaponti, M.; Piciollo, E.; Fontanesi, C.; Caneschi, A.; Innocenti, M. Optimisation of Thiourea Concentration in a Decorative Copper Plating Acid Bath Based on Methanesulfonic Electrolyte. Coatings 2022, 12, 376. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yim, S.-B.; Hwang, Y.-J.; Lee, K.-H. Effect of Thiourea on the Copper Electrodeposition. J. Korean Inst. Surf. Eng. 2010, 43, 289–296. [Google Scholar] [CrossRef]
- Soares, D.M.; Wasle, S.; Weil, K.G.; Doblhofer, K. Copper Ion Reduction Catalyzed by Chloride Ions. J. Electroanal. Chem. 2002, 532, 353–358. [Google Scholar] [CrossRef]
- Chibwe, C.; Tadie, M. An Experimental Review of the Physicochemical Properties of Copper Electrowinning Electrolytes. Min. Metall. Explor. 2021, 38, 1225–1237. [Google Scholar] [CrossRef]
- Coetzee, C.; Tadie, M.; Dorfling, C. Evaluating the Effect of Molecular Properties of Polyacrylamide Reagents on Deposit Growth in Copper Electrowinning. Hydrometallurgy 2020, 195, 105407. [Google Scholar] [CrossRef]
- Guo, J.; Li, B.; Wei, Y.; Wang, H. Effect of Thiourea on Copper Cathode from Simulated Leachate of Copper and Cadmium Residues by Cyclone Electrowinning Technology. J. Sustain. Metall. 2021, 7, 1876–1885. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharitonov, D.S.; Makarova, I.V.; Wrzesińska, A.; Zharskii, I.M.; Kurilo, I.I. Effect of Thiourea on Electrocrystallization of Cu–Sn Alloys from Sulphate Electrolytes. Surf. Coat. Technol. 2020, 399, 126137. [Google Scholar] [CrossRef]
- Gabrielli, C.; Moçotéguy, P.; Perrot, H.; Wiart, R. Mechanism of Copper Deposition in a Sulphate Bath Containing Chlorides. J. Electroanal. Chem. 2004, 572, 367–375. [Google Scholar] [CrossRef]
- Sun, M.; O’Keefe, T.J. The Effect of Additives on the Nucleation and Growth of Copper onto Stainless Steel Cathodes. Metall. Trans. B 1992, 23, 591–599. [Google Scholar] [CrossRef]
- Hölzle, M.H.; Apsel, C.W.; Will, T.; Kolb, D.M. Copper Deposition onto Au(111) in the Presence of Thiourea. J. Electrochem. Soc. 1995, 142, 3741–3749. [Google Scholar] [CrossRef]
- Aragón, J.; Camus, J. Efecto de La Concentración de Ión Cloruro En La Estructura de Electrodepósitos de Cobre. Rev. Latinoam. Metal. Mater. 2011, 11, 128–133. [Google Scholar]
- Kang, M.S.; Kim, S.K.; Kim, K.; Kim, J.J. The Influence of Thiourea on Copper Electrodeposition: Adsorbate Identification and Effect on Electrochemical Nucleation. Thin Solid Film. 2008, 516, 3761–3766. [Google Scholar] [CrossRef]
- Quinet, M.; Lallemand, F.; Ricq, L.; Hihn, J.-Y.; Delobelle, P.; Arnould, C.; Mekhalif, Z. Influence of Organic Additives on the Initial Stages of Copper Electrodeposition on Polycrystalline Platinum. Electrochim. Acta 2009, 54, 1529–1536. [Google Scholar] [CrossRef]
- Shao, W.; Pattanaik, G.; Zangari, G. Influence of Chloride Anions on the Mechanism of Copper Electrodeposition from Acidic Sulfate Electrolytes. J. Electrochem. Soc. 2007, 154, D201–D207. [Google Scholar] [CrossRef]
- Nagy, Z.; Blaudeau, J.P.; Hung, N.C.; Curtiss, L.A.; Zurawski, D.J. Chloride Ion Catalysis of the Copper Deposition Reaction. J. Electrochem. Soc. 1995, 142, L87–L89. [Google Scholar] [CrossRef]
- Tantavichet, N.; Pritzker, M.D. Effect of Plating Mode, Thiourea and Chloride on the Morphology of Copper Deposits Produced in Acidic Sulphate Solutions. Electrochim. Acta 2005, 50, 1849–1861. [Google Scholar] [CrossRef]
- Tantavichet, N.; Damronglerd, S.; Chailapakul, O. Influence of the Interaction between Chloride and Thiourea on Copper Electrodeposition. Electrochim. Acta 2009, 55, 240–249. [Google Scholar] [CrossRef]
- Veilleux, B.; Lafront, A.M.; Ghali, E. Computerized Scaled Cells to Study the Effect of Additive Ratios and Concentrations on Nodulation during Copper Electrorefining. J. Appl. Electrochem. 2001, 31, 1017–1024. [Google Scholar] [CrossRef]
- Zeng, T.W.; Yen, S.C. Effects of Additives in an Electrodeposition Bath on the Surface Morphologic Evolution of Electrodeposited Copper. Int. J. Electrochem. Sci. 2021, 16, 210245. [Google Scholar] [CrossRef]
- Collet, T.; Hallemans, N.; Wouters, B.; Ramharter, K.; Lataire, J.; Pintelon, R.; Hubin, A. An Operando ORP-EIS Study of the Copper Reduction Reaction Supported by Thiourea and Chlorides as Electrorefining Additives. Electrochim. Acta 2021, 389, 138762. [Google Scholar] [CrossRef]
- Bockris, J.O.; Enyo, M. Mechanism of Electrodeposition and Dissolution Processes of Copper in Aqueous Solutions. Trans. Faraday Soc. 1962, 58, 1187–1202. [Google Scholar] [CrossRef]
- Szymaszek, A.; Biernat, J.; Pajdowski, L. Polarographic Studies on the Effect of Thiourea on Deposition of Copper in the Presence of 2M H2SO4. Electrochim. Acta 1977, 22, 359–364. [Google Scholar] [CrossRef]
- Collet, T.; Wouters, B.; Hallemans, N.; Ramharter, K.; Lataire, J.; Hubin, A. The Time-Varying Effect of Thiourea on the Copper Electroplating Process with Industrial Copper Concentrations. Electrochim. Acta 2023, 437, 141412. [Google Scholar] [CrossRef]
- Ren, P.; An, M.; Yang, P.; Zhang, J. Unveiling the Synergistic Inhibition of Cl− Copper Plating: Pivotal Roles of Adsorption and Desorption. J. Electroanal. Chem. 2021, 898, 115624. [Google Scholar] [CrossRef]
- Collet, T.; Wouters, B.; Eeltink, S.; Schmidt, P.; Ramharter, K.; Hubin, A. An Ex Situ and Operando Analysis of Thiourea Consumption and Activity during a Simulated Copper Electrorefining Process. J. Electroanal. Chem. 2022, 920, 116581. [Google Scholar] [CrossRef]
- Ahmadi, A.; Sheibani, S.; Mokmeli, M.; Khorasani, S.M.J.; Yaghoobi, N.S. Factors Affecting the Cathode Edge Nodulation in Copper Electrorefining Process. Int. J. Eng. Trans. C Asp. 2022, 35, 2370–2376. [Google Scholar] [CrossRef]
- Mori, K.; Yamakawa, Y.; Oue, S.; Taninouchi, Y.K.; Nakano, H. Effect of Impurity Ions and Additives in Solution of Copper Electrorefining on the Passivation Behavior of Low-Grade Copper Anode. Mater. Trans. 2023, 64, 242–251. [Google Scholar] [CrossRef]
- Hinatsu, J.T.; Foulkes, F.R. Electrochemical Kinetic Parameters for the Cathodic Deposition of Copper from Dilute Aqueous Acid Sulfate Solutions. Can. J. Chem. Eng. 1991, 69, 571–577. [Google Scholar] [CrossRef]
- Cifuentes, L.; Simpson, J. Temperature Dependence of the Cathodic and Anodic Kinetics in a Copper Electrowinning Cell Based on Reactive Electrodialysis. Chem. Eng. Sci. 2005, 60, 4915–4923. [Google Scholar] [CrossRef]
- Milora, C.J.; Henrickson, J.F.; Hahn, W.C. Diffusion Coefficients and Kinetic Parameters in Copper Sulfate Electrolytes and in Copper Fluoborate Electrolytes Containing Organic Addition Agents. J. Electrochem. Soc. 1973, 120, 488. [Google Scholar] [CrossRef]
- Pearson, I.M.; Schrader, G.F. The Ac Impedance at Cu Electrodes and the Electrodeposition of Cu. Electrochim. Acta 1968, 13, 2021–2028. [Google Scholar] [CrossRef]
- Sahu, S.; Rani Sahoo, P.; Patel, S.; Mishra, B.K. Oxidation of Thiourea and Substituted Thioureas: A Review. J. Sulfur Chem. 2011, 32, 171–197. [Google Scholar] [CrossRef]
- Ratajczak, H.M.; Pajdowski, L.; Ostern, M. Polarographic Studies on Aqueous Copper(II) Solutions with Thiourea-II. Electrochim. Acta 1975, 20, 431–434. [Google Scholar] [CrossRef]
- Doona, C.J.; Stanbury, D.M. Equilibrium and Redox Kinetics of Copper(II)-Thiourea Complexes. Inorg. Chem. 1996, 35, 3210–3216. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, A.; Heerman, L. Influence of Thiourea on the Nucleation of Copper on Polycrystalline Platinum. J. Appl. Electrochem. 1999, 29, 585–591. [Google Scholar] [CrossRef]
- Jin, S.; Ghali, E. Effect of Thiourea on the Copper Cathode Polarization Behavior in Acidic Copper Sulfate at 65 °C. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2001, 32, 887–893. [Google Scholar] [CrossRef]
- Stanković, Z.D.; Vuković, M. The Influence of Thiourea on Kinetic Parameters on the Cathodic and Anodic Reaction at Different Metals in H2SO4 Solution. Electrochim. Acta 1996, 41, 2529–2535. [Google Scholar] [CrossRef]
- Blechta, V.K.; Wang, Z.Z.; Krueger, D.W. Glue Analysis and Behavior in Copper Electrolyte. Metall. Trans. B 1993, 24, 277–287. [Google Scholar] [CrossRef]
- Bockris, J.O.; Reddy, A.K.N.; Gamboa-Aldeco, M. Modern Electrochemistry 2A, 2nd ed.; Kluwer Academic Publishiers: London, UK, 2002; Volume 2A. [Google Scholar]
- Arafat, Y.; Sultana, S.T.; Dutta, I.; Panat, R. Effect of Additives on the Microstructure of Electroplated Tin Films. J. Electrochem. Soc. 2018, 165, D816–D824. [Google Scholar] [CrossRef]
- Horányi, G.; Vértes, G. Study of the Adsorption of Chloride Ions in the Course of Electrosorption of Copper on Platinized Platinum Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1973, 45, 295–299. [Google Scholar] [CrossRef]
- Winand, R. Electrocristallisation. Théorie et Applications. J. Phys. IV JP 1994, 4, 55–73. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Gómez, P.P.; Cano, E. The Isotherm Slope. A Criterion for Studying the Adsorption Mechanism of Benzotriazole on Copper in Sulphuric Acid. Rev. Metal. 2005, 41, 98–106. [Google Scholar] [CrossRef]
- Budevski, E.; Staikov, G.; Lorenz, W.J. Electrochemical Phase Formation and Growth: An Introduction to the Initial Stages of Metal Deposition; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 28, ISBN 3527282734. [Google Scholar]
- Markov, I. Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy, 2nd ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2003. [Google Scholar]
- Milchev, A. Electrocrystallization: Fundamentals of Nucleation and Growth; Kluwer Academic Publishiers: London, UK, 2002. [Google Scholar]


| Additive | Concentration mg L−1 |
mA cm−2 | |
|---|---|---|---|
| Base solution | 0 | 22.9 | 0.19 |
| Glue | 1 | 15.7 | 0.26 |
| 10 | 4.79 | 0.32 | |
| 100 | 1.96 | 0.38 | |
| Thiourea | 1 | 20.9 | 0.25 |
| 10 | 7.74 | 0.48 | |
| 100 | 2.05 | 0.61 | |
| Chloride | 20 | 18.2 | 0.27 |
| 40 | 16.4 | 0.28 | |
| 80 | 16.4 | 0.29 | |
| 100 | 18.9 | 0.26 | |
| 1000 | 15.3 | 0.29 |
| Additive | B | f | R2 |
|---|---|---|---|
| Glue | 21.7 | 8.14 | 0.91 |
| Thiourea | 2.14 | 6.55 | 1.00 |
| Solution | Additive Concentration (mg L−1) | Average Relative Height | Standard Deviation | Kurtosis | Asimmetry |
|---|---|---|---|---|---|
| Base Solution | - | 88.6 | 50.5 | 0.60 | 0.90 |
| Glue | 1 | 113.9 | 64.2 | −0.60 | 0.40 |
| 10 | 79.4 | 50.0 | 0.80 | 1.00 | |
| 100 | 102.8 | 62.8 | −0.20 | 0.70 | |
| Thiourea | 1 | 98.0 | 56.6 | −0.02 | 0.59 |
| 10 | 94.0 | 57.8 | −0.38 | 0.41 | |
| 100 | 101.8 | 48.6 | 0.86 | 0.96 | |
| Chloride | 1 | 71.5 | 44.6 | 0.80 | 0.90 |
| 10 | 76.7 | 52.1 | 0.90 | 1.00 | |
| 100 | 85.44 | 47.3 | 1.22 | 1.05 |
| Solution | Additive Concentration (mg L−1) | Average Relative Height | Standard Deviation | Kurtosis | Asimmetry |
|---|---|---|---|---|---|
| Base Solution | - | 83.6 | 50.8 | 0.61 | 0.85 |
| Glue | 1 | 86.1 | 59.8 | −0.02 | 0.76 |
| 100 | 88.5 | 44.9 | 0.39 | 0.74 | |
| Thiourea | 1 | 83.8 | 51.7 | 0.27 | 0.77 |
| 100 | 94.0 | 51.0 | 0.02 | 0.52 | |
| Chloride | 1 | 52.9 | 41.5 | 1.47 | 1.19 |
| 100 | 84.5 | 46.9 | 0.74 | 0.91 |
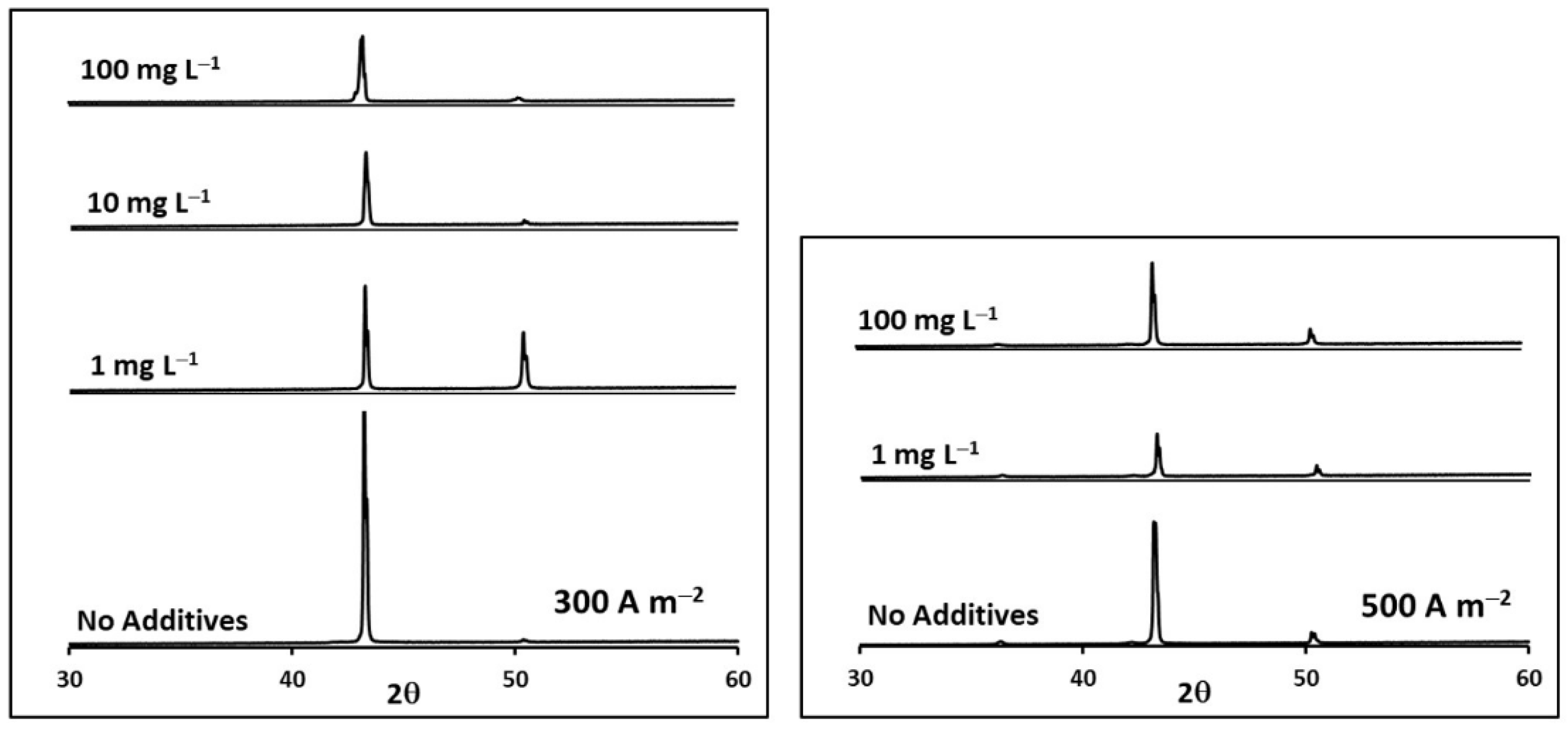
| i (A m−2) | Glue Concentration(mg L−1) | Relative Intensity P(111)/P(200) | TC(111) | TC(200) |
|---|---|---|---|---|
| 300 | 0 | 0.97 | 0.94 | 0.06 |
| 1 | 0.64 | 0.45 | 0.55 | |
| 10 | 0.89 | 0.79 | 0.21 | |
| 100 | 0.90 | 0.81 | 0.19 | |
| 500 | 0 | 0.90 | 0.80 | 0.20 |
| 1 | 0.76 | 0.59 | 0.41 | |
| 100 | 0.81 | 0.67 | 0.33 |
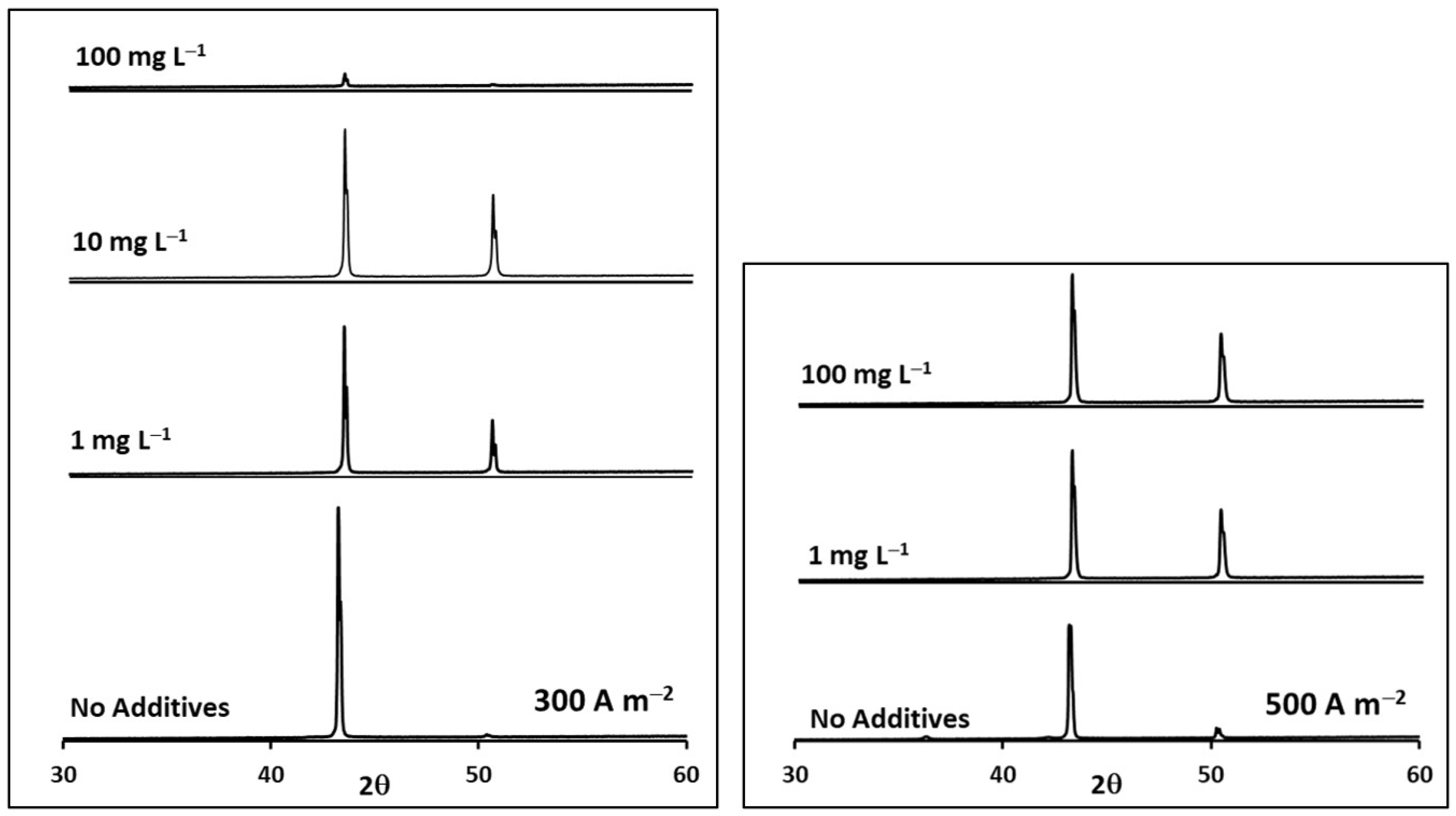
| i (A m−2) | Thiourea Concentration(mg L−1) | Relative Intensity P(111)/P(200) | TC(111) | TC(200) |
|---|---|---|---|---|
| 300 | 0 | 0.97 | 0.94 | 0.06 |
| 1 | 0.72 | 0.55 | 0.45 | |
| 10 | 0.64 | 0.45 | 0.55 | |
| 100 | 0.73 | 0.55 | 0.45 | |
| 500 | 0 | 0.90 | 0.80 | 0.20 |
| 1 | 0.81 | 0.66 | 0.34 | |
| 100 | 0.64 | 0.45 | 0.55 |
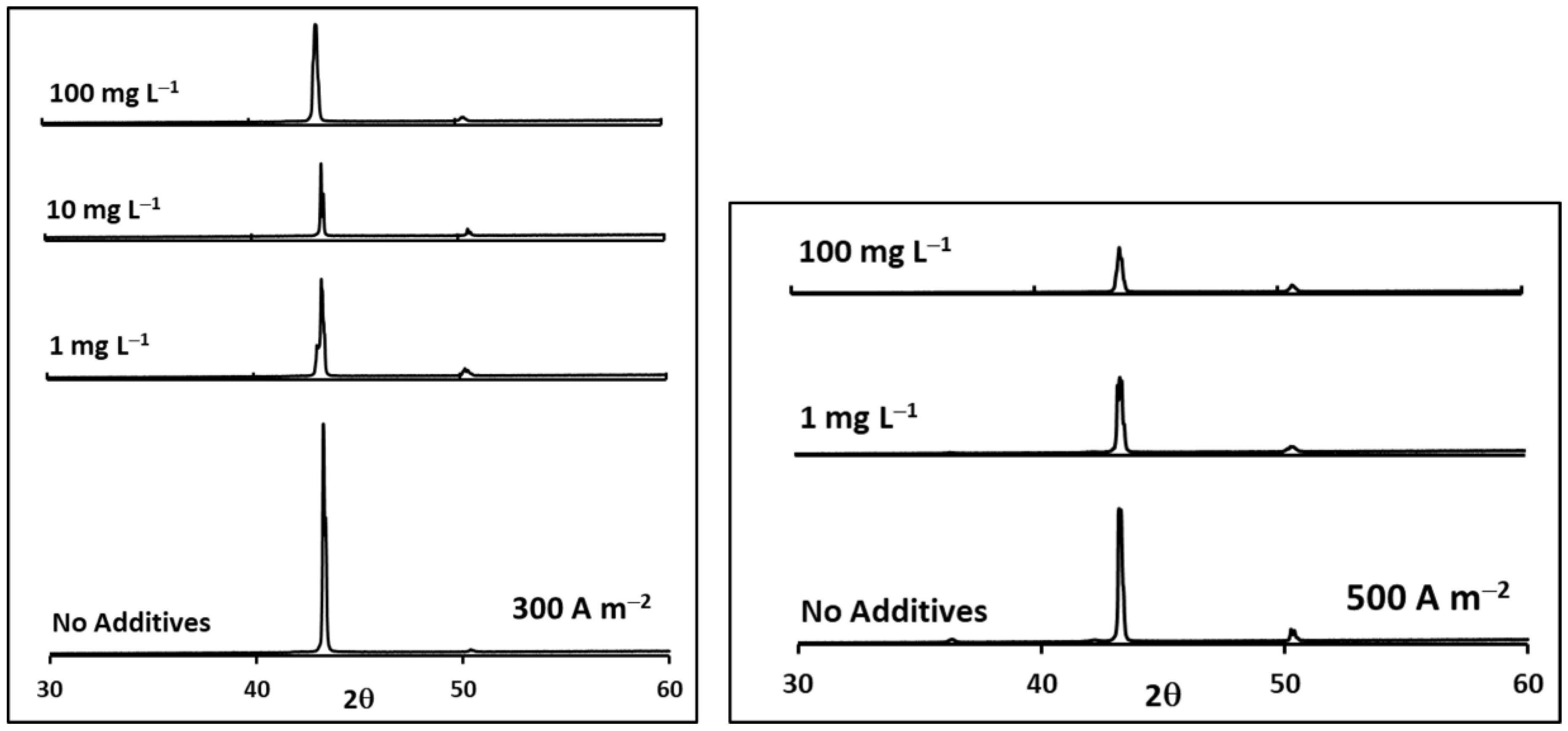
| i (A m−2) | Chloride Concentration (mg L−1) | Relative Intensity P(111)/P(200) | TC(111) | TC(200) |
|---|---|---|---|---|
| 300 | 0 | 0.97 | 0.94 | 0.06 |
| 1 | 0.90 | 0.80 | 0.20 | |
| 10 | 0.87 | 0.75 | 0.25 | |
| 100 | 0.93 | 0.85 | 0.15 | |
| 500 | 0 | 0.90 | 0.80 | 0.20 |
| 1 | 0.89 | 0.80 | 0.20 | |
| 100 | 0.84 | 0.71 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevárez-Llamas, É.D.; Araneda-Hernández, E.A.; Parra-Sánchez, V.R.; Villagrán-Guerra, E.A. Effect of Glue, Thiourea, and Chloride on the Electrochemical Reduction in CuSO4–H2SO4 Solutions. Metals 2023, 13, 891. https://doi.org/10.3390/met13050891
Nevárez-Llamas ÉD, Araneda-Hernández EA, Parra-Sánchez VR, Villagrán-Guerra EA. Effect of Glue, Thiourea, and Chloride on the Electrochemical Reduction in CuSO4–H2SO4 Solutions. Metals. 2023; 13(5):891. https://doi.org/10.3390/met13050891
Chicago/Turabian StyleNevárez-Llamas, Érika D., Eugenia A. Araneda-Hernández, Víctor R. Parra-Sánchez, and Eduardo A. Villagrán-Guerra. 2023. "Effect of Glue, Thiourea, and Chloride on the Electrochemical Reduction in CuSO4–H2SO4 Solutions" Metals 13, no. 5: 891. https://doi.org/10.3390/met13050891
APA StyleNevárez-Llamas, É. D., Araneda-Hernández, E. A., Parra-Sánchez, V. R., & Villagrán-Guerra, E. A. (2023). Effect of Glue, Thiourea, and Chloride on the Electrochemical Reduction in CuSO4–H2SO4 Solutions. Metals, 13(5), 891. https://doi.org/10.3390/met13050891





