A Facile and Surfactant-Free Electrochemical Synthesis of PtIr Nanocubes towards Ammonia Electro-Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of Pt Nanocubes and PtIr Nanoparticles
2.3. Materials’ Characterization
2.4. Electrochemical Tests
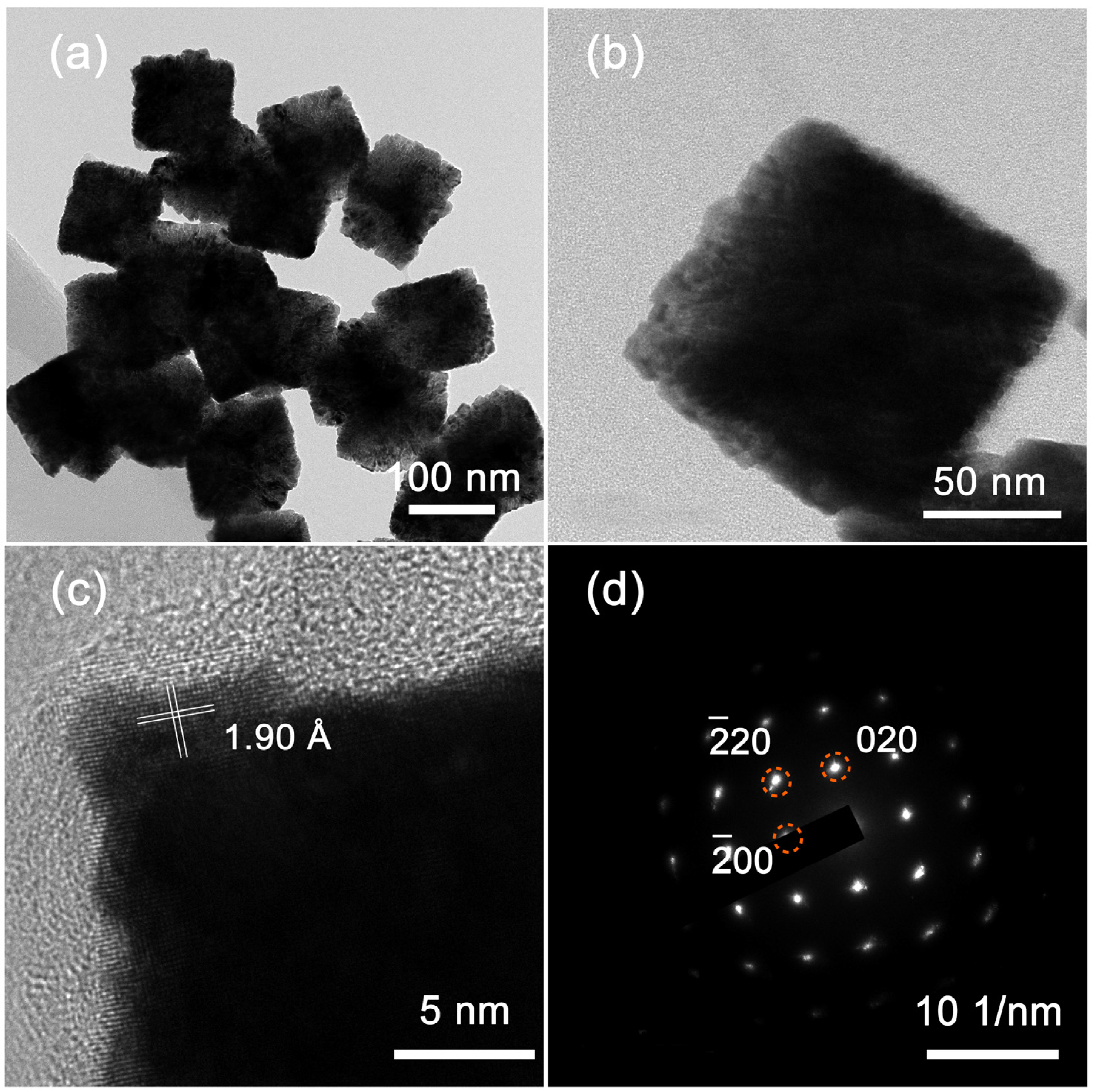
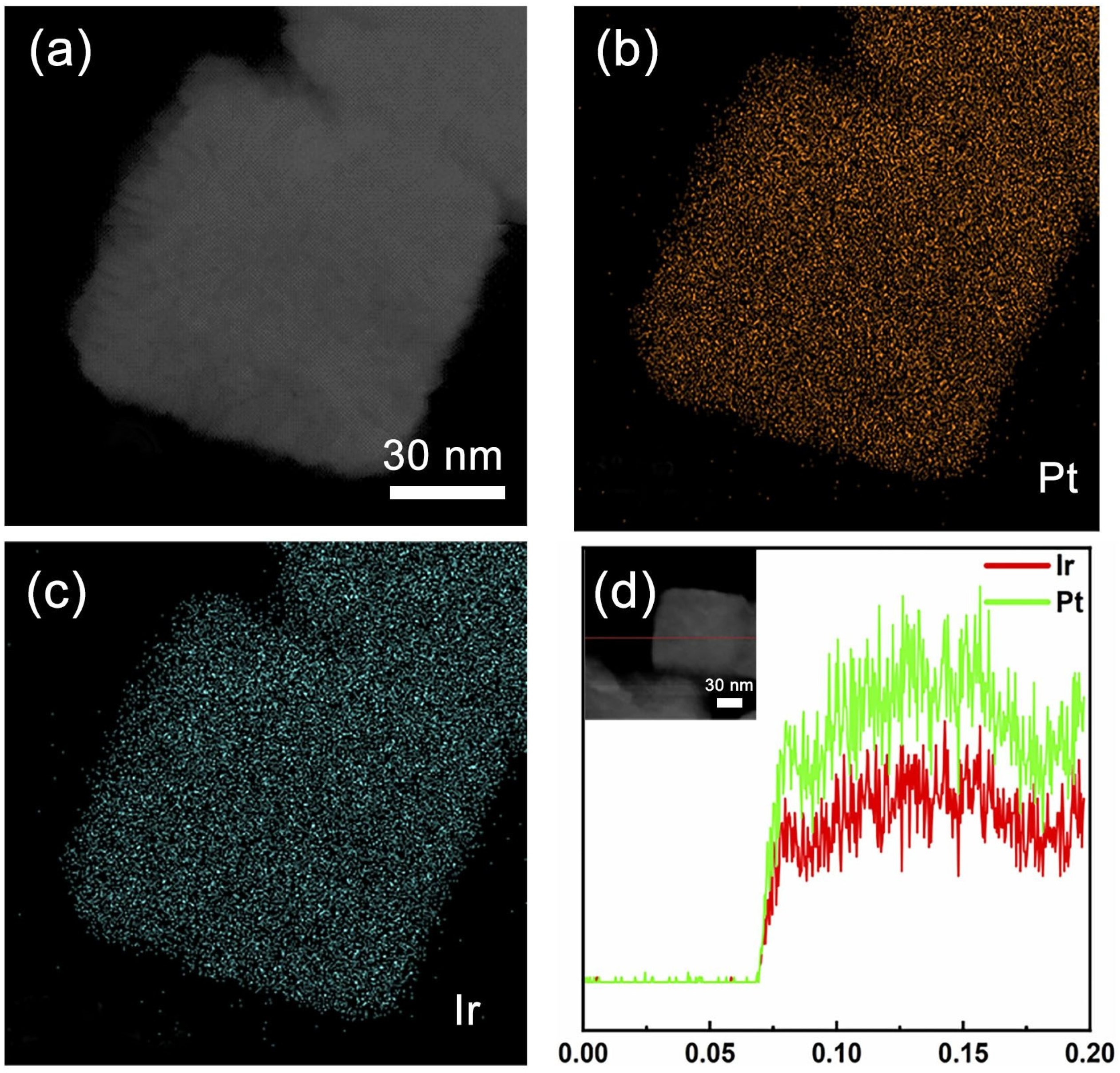
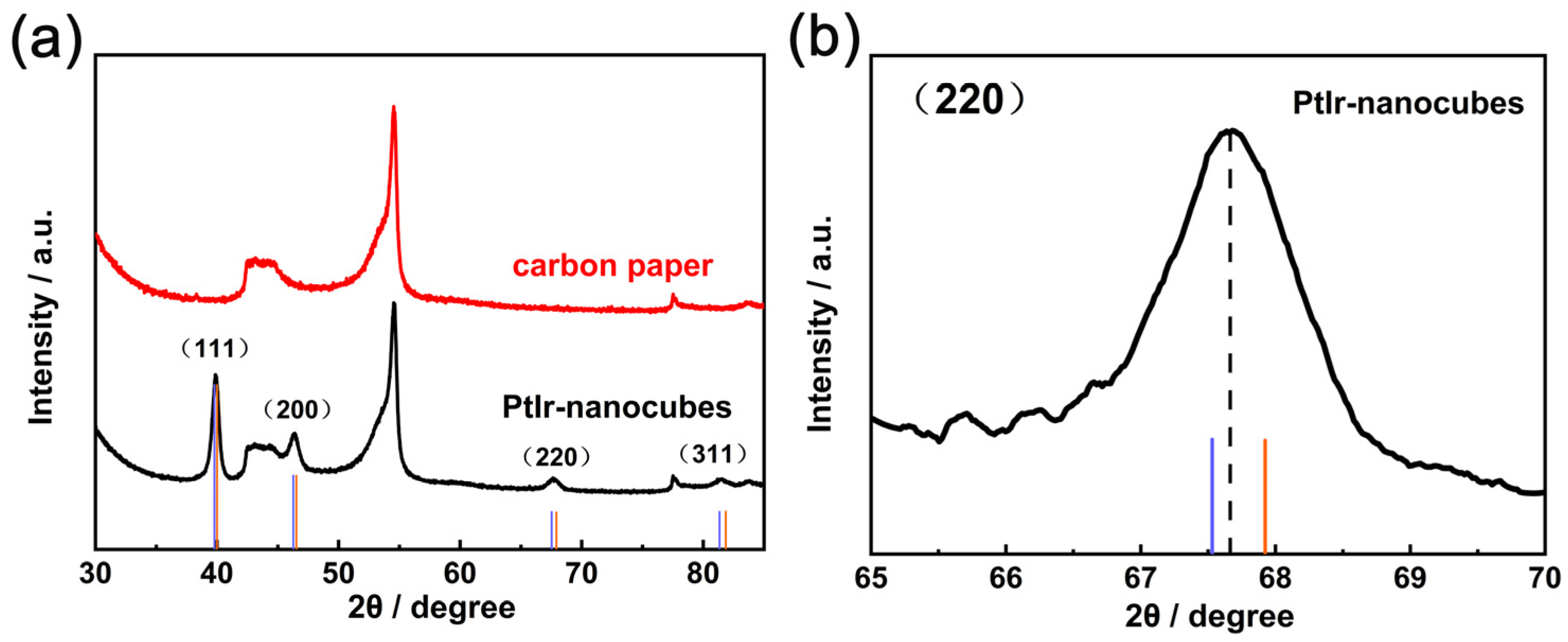
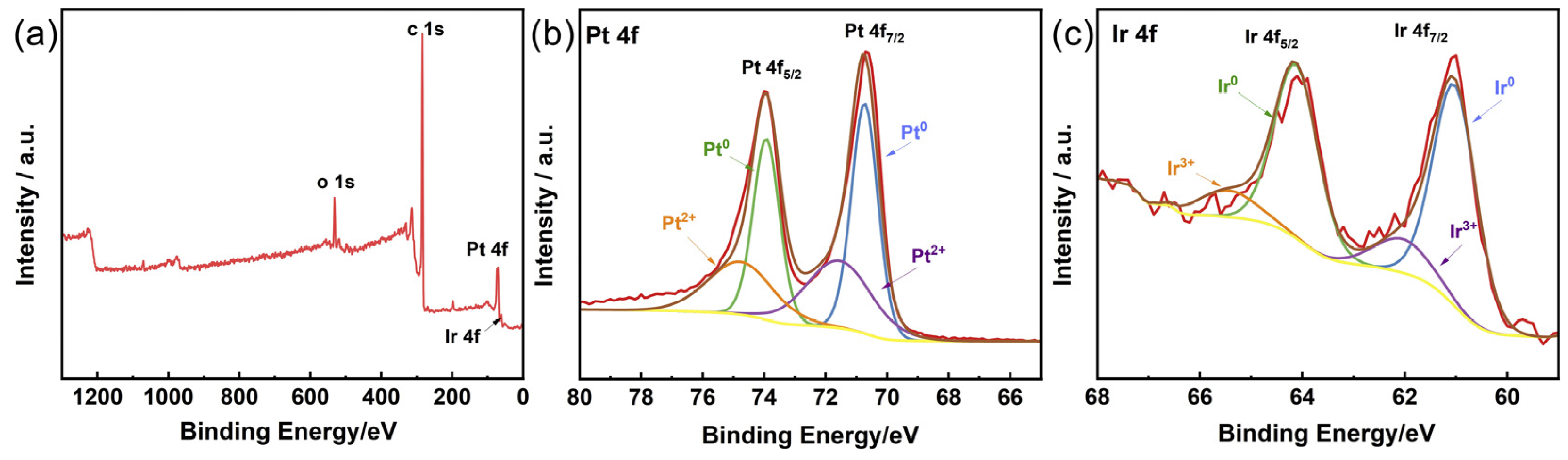
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Pillai, H.S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells. Energy Environ. Sci. 2021, 14, 1449–1460. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Z.; Esan, O.C.; Xu, X.; An, L. Performance Characteristics of a Direct Ammonia Fuel Cell with an Anion Exchange Membrane. Energy Fuels 2022, 36, 13203–13211. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Mandal, P.; Yadav, M.K.; Gupta, A.K.; Dubey, B.K. Chlorine mediated indirect electro-oxidation of ammonia using non-active PbO2 anode: Influencing parameters and mechanism identification. Sep. Purif. Technol. 2020, 247, 116910. [Google Scholar] [CrossRef]
- de Mishima, B.A.L.; Lescano, D.; Holgado, T.M.; Mishima, H.T. Electrochemical oxidation of ammonia in alkaline solutions: Its application to an amperometric sensor. Electrochim. Acta 1998, 43, 395–404. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.; Kim, H.; Jun, Y.; Kim, S.Y.; Ahn, S.H. Recent progress in Pt-based electrocatalysts for ammonia oxidation reaction. Appl. Mater. Today 2022, 29, 101640. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Zhang, X.; Ye, J.; Xue, F.; Zhen, C.; Liao, X.; Li, H.; Li, P.; Liu, M.; et al. Quatermetallic Pt-based ultrathin nanowires intensified by Rh enable highly active and robust electrocatalysts for methanol oxidation. Nano Energy 2020, 71, 104623. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhang, X.; Rao, S.; Li, J.; Wang, W.; Sun, Z.; Yang, J. Surface Structure Engineering of PtPd Nanoparticles for Boosting Ammonia Oxidation Electrocatalysis. Acs Appl. Mater. Interfaces 2022, 14, 28816–28825. [Google Scholar] [CrossRef]
- Wallace, S.W.; McCrum, I.T.; Janik, M.J. Ammonia electro-oxidation mechanism on the platinum (100) surface. Catal. Today 2021, 371, 50–57. [Google Scholar] [CrossRef]
- Chan, Y.T.; Siddharth, K.; Shao, M. Investigation of cubic Pt alloys for ammonia oxidation reaction. Nano Res. 2020, 13, 1920–1927. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Liu, X.; Song, Z.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Synthesis of Cubic-Shaped Pt Particles with (100) Preferential Orientation by a Quick, One-Step and Clean Electrochemical Method. Acs Appl. Mater. Interfaces 2017, 9, 18856–18864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hwang, S.Y.; Peng, Z. Shape-enhanced ammonia electro-oxidation property of a cubic platinum nanocrystal catalyst prepared by surfactant-free synthesis. J. Mater. Chem. A 2013, 1, 14402–14408. [Google Scholar] [CrossRef]
- Fu, G.-T.; Liu, C.; Wu, R.; Chen, Y.; Zhu, X.-S.; Sun, D.-M.; Tang, Y.-W.; Lu, T.-H. l-Lysine mediated synthesis of platinum nanocuboids and their electrocatalytic activity towards ammonia oxidation. J. Mater. Chem. A 2014, 2, 17883–17888. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, R.A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Cabrera, C.R.; Feliu, J.M. Synthesis and Electrocatalytic Properties of H2SO4-Induced (100) Pt Nanoparticles Prepared in Water-in-Oil Microemulsion. ChemPhysChem 2014, 15, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. Available online: https://www.science.org/doi/10.1126/science.aah6133 (accessed on 18 April 2023). [CrossRef] [PubMed]
- Liu, C.-W.; Wei, Y.-C.; Wang, K.-W. Preparation and characterization of carbon-supported Pt-Au cathode catalysts for oxygen reduction reaction. J. Colloid Interface Sci. 2009, 336, 654–657. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Methanol electrooxidation on a colloidal PtRu-alloy fuel-cell catalyst. Electrochem. Commun. 1999, 1, 1–4. [Google Scholar] [CrossRef]
- Kugai, J.; Moriya, T.; Seino, S.; Nakagawa, T.; Ohkubo, Y.; Nitani, H.; Daimon, H.; Yamamoto, T.A. CeO2-supported Pt-Cu alloy nanoparticles synthesized by radiolytic process for highly selective CO oxidation. Int. J. Hydrogen Energy 2012, 37, 4787–4797. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Li, H.-H.; Yu, S.-H.; Heggen, M.; Strasser, P. Octahedral PtNi Nanoparticle Catalysts: Exceptional Oxygen Reduction Activity by Tuning the Alloy Particle Surface Composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Li, N.; Yan, Y.; Lou, X.W.; Wang, X. One-Pot Synthesis of Pt–Co Alloy Nanowire Assemblies with Tunable Composition and Enhanced Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 3797–3801. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.-S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled Synthesis of Pd–Pt Alloy Hollow Nanostructures with Enhanced Catalytic Activities for Oxygen Reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Zhang, H.; Li, M. Potentiostatic Electrodeposition of Pt-Fe Alloy Catalyst and Application in PEMFC Cathode. J. Inorg. Mater. 2013, 28, 1217–1222. Available online: http://www.jim.org.cn/en/10.3724/sp.j.1077.2013.13118 (accessed on 18 April 2023). [CrossRef]
- Zhang, T.; Li, S.-C.; Zhu, W.; Zhang, Z.-P.; Gu, J.; Zhang, Y.-W. Shape-tunable Pt–Ir alloy nanocatalysts with high performance in oxygen electrode reactions. Nanoscale 2017, 9, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Won, D.; Nahm, K.S.; Kim, P. Facile Preparation of Hollow Pt- and PtIr-Nanostructures with Spiky Surface for the Electro-Oxidation of Ammonia. Catal. Lett. 2014, 144, 469–477. [Google Scholar] [CrossRef]
- Lomocso, T.L.; Baranova, E.A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M = Ir, Pd, SnOx) nanoparticles. Electrochim. Acta 2011, 56, 8551–8558. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, J.; Ni, Z.; Deng, Y.; Chen, B.; Hu, W. Shape-controlled synthesis of Pt-Ir nanocubes with preferential (100) orientation and their unusual enhanced electrocatalytic activities. Sci. China-Mater. 2014, 57, 13–25. [Google Scholar] [CrossRef]
- Allagui, A.; Oudah, M.; Tuaev, X.; Ntais, S.; Almomani, F.; Baranova, E.A. Ammonia electro-oxidation on alloyed PtIr nanoparticles of well-defined size. Int. J. Hydrogen Energy 2013, 38, 2455–2463. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhang, P.; Chen, S.; Xu, J. Effects of the composition on the active carbon supported Pd-Pt bimetallic catalysts for HI decomposition in the iodine-sulfur cycle. Int. J. Hydrogen Energy 2013, 38, 6586–6592. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Aziz, F.; Ajid, A.Z.A. Advanced ternary RGO/bimetallic Pt-Pd alloy/CeO2 nanocomposite electrocatalyst by one-step hydrothermal-assisted formic acid reduction reaction for methanol electrooxidation. J. Environ. Chem. Eng. 2021, 9, 104991. [Google Scholar] [CrossRef]
- He, Q.; Mukerjee, S. Electrocatalysis of oxygen reduction on carbon-supported PtCo catalysts prepared by water-in-oil micro-emulsion. Electrochim. Acta 2010, 55, 1709–1719. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Leon, M.N.; Fernandes, C.M.; Antoniassi, R.M.; Alves, O.C.; Ponzio, E.A.; Silva, J.C.M. PtSnO2/C and Pt/C with preferential (100) orientation: High active electrocatalysts for ammonia electro-oxidation reaction. Appl. Catal. B-Environ. 2020, 264, 118458. [Google Scholar] [CrossRef]
- Chin, T.-K.; Liao, M.-W.; Perng, T.-P. Enabling higher electrochemical activity of Pt nanoparticles uniformly coated on cubic titanium oxynitride by vertical forced-flow atomic layer deposition. J. Power Sources 2019, 434, 226716. [Google Scholar] [CrossRef]
- Le Vot, S.; Roue, L.; Belanger, D. Electrodeposition of iridium onto glassy carbon and platinum electrodes. Electrochim. Acta 2012, 59, 49–56. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, G.; Yu, M.; Xu, J.; Qiao, S.; Cheng, X.; Yang, F. High Mass and Specific Activity for Ammonia Electro-oxidation through Optimization of Dispersion Degree and Particle Size of Pt-Ir Nanoparticles over N-Doped Reductive Graphene Oxide. Chemistryselect 2018, 3, 3433–3443. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer, Physical Electronics Division: Eden Prairie, MN, USA, 1992; pp. 178–181. [Google Scholar]
- Chen, W.; Chen, S. Iridium-platinum alloy nanoparticles: Composition-dependent electrocatalytic activity for formic acid oxidation. J. Mater. Chem. 2011, 21, 9169–9178. [Google Scholar] [CrossRef]
- Dai, H.; Dong, K.; Zhang, T.; Pu, H.; Wang, Y.; Deng, Y. Electrodeposition of shaped PtIr alloy nanocrystals with high–index facets for the electro–catalytic oxidation of alcohols. Appl. Surf. Sci. 2023, 609, 155225. [Google Scholar] [CrossRef]
- Ponrouch, A.; Garbarino, S.; Bertin, E.; Andrei, C.; Botton, G.A.; Guay, D. Highly Porous and Preferentially Oriented {100} Platinum Nanowires and Thin Films. Adv. Funct. Mater. 2012, 22, 4172–4181. [Google Scholar] [CrossRef]
- Shibata, S.; Sumino, M.P. The electrochemical Peltier heat for the adsorption and desorption of hydrogen on a platinized platinum electrode in sulfuric acid solution. J. Electroanalytical. Chem. Interfacial Electrochem. 1985, 193, 135–143. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Sources 1999, 77, 136–142. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Zhong, C.; Cheng, Y.F. Surfactant-free electrochemical synthesis of hierarchical platinum particle electrocatalysts for oxidation of ammonia. J. Power Sources 2013, 223, 165–174. [Google Scholar] [CrossRef]
- Gerischer, H.; Mauerer, A. Untersuchungen Zur anodischen Oxidation von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. Interfacial Electrochem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Le Vot, S.; Roué, L.; Bélanger, D. Study of the electrochemical oxidation of ammonia on platinum in alkaline solution: Effect of electrodeposition potential on the activity of platinum. J. Electroanal. Chem. 2013, 691, 18–27. [Google Scholar] [CrossRef]
- Novell-Leruth, G.; Valcárcel, A.; Clotet, A.; Ricart, J.M.; Pérez-Ramírez, J. DFT Characterization of Adsorbed NHx Species on Pt(100) and Pt(111) Surfaces. J. Phys. Chem. B 2005, 109, 18061–18069. [Google Scholar] [CrossRef] [PubMed]
- Rosca, V.; Koper, M.T.M. Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces. Phys. Chem. Chem. Phys. 2006, 8, 2513–2524. [Google Scholar] [CrossRef]
- Rosca, V.; Koper, M.T.M. Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim. Acta 2008, 53, 5199–5205. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Solla-Gullón, J.; Montiel, V.; Feliu, J.M.; Aldaz, A. Screening of electrocatalysts for direct ammonia fuel cell: Ammonia oxidation on PtMe (Me: Ir, Rh, Pd, Ru) and preferentially oriented Pt(100) nanoparticles. J. Power Sources 2007, 171, 448–456. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Kou, Y.; Liu, Z.; Chen, X.; Li, Y.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Pt-Decorated highly porous flower-like Ni particles with high mass activity for ammonia electro-oxidation. J. Mater. Chem. A 2016, 4, 11060–11068. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Koper, M.T.M.; van Santen, R.A.; van Veen, J.A.R. The role of adsorbates in the electrochemical oxidation of ammonia on noble and transition metal electrodes. J. Electroanal. Chem. 2001, 506, 127–137. [Google Scholar] [CrossRef]
- Le Vot, S.; Roué, L.; Bélanger, D. Synthesis of Pt–Ir catalysts by coelectrodeposition: Application to ammonia electrooxidation in alkaline media. J. Power Sources 2013, 223, 221–231. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Geng, D.; Cheng, Z.; Han, X.; Han, L.; Zhang, J.; Xia, D.; Liu, J. A Facile and Surfactant-Free Electrochemical Synthesis of PtIr Nanocubes towards Ammonia Electro-Oxidation. Metals 2023, 13, 901. https://doi.org/10.3390/met13050901
Tang Y, Geng D, Cheng Z, Han X, Han L, Zhang J, Xia D, Liu J. A Facile and Surfactant-Free Electrochemical Synthesis of PtIr Nanocubes towards Ammonia Electro-Oxidation. Metals. 2023; 13(5):901. https://doi.org/10.3390/met13050901
Chicago/Turabian StyleTang, Yusu, Dinglei Geng, Zhihao Cheng, Xin Han, Liying Han, Jinfeng Zhang, Dahai Xia, and Jie Liu. 2023. "A Facile and Surfactant-Free Electrochemical Synthesis of PtIr Nanocubes towards Ammonia Electro-Oxidation" Metals 13, no. 5: 901. https://doi.org/10.3390/met13050901
APA StyleTang, Y., Geng, D., Cheng, Z., Han, X., Han, L., Zhang, J., Xia, D., & Liu, J. (2023). A Facile and Surfactant-Free Electrochemical Synthesis of PtIr Nanocubes towards Ammonia Electro-Oxidation. Metals, 13(5), 901. https://doi.org/10.3390/met13050901







