Abstract
The effects of double-step homogenization processes on the precipitation of Al3Zr dispersoids and the dissolution of the primary phases of 2196 aluminum alloy were studied by optical microscopy, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). It was revealed that first-step homogenization facilitates the nucleation of Al3Zr, while second-step homogenization results in the dissolution of the primary phases and the growth of Al3Zr dispersoids. The nanosized θ’ precipitates formed in the first-step homogenization are dissolved after the second-step homogenization. The optimum homogenization process was selected as 400 °C/10 h + 520 °C/24 h, which effectively dissolves the primary phases and promotes the formation of refined distribution of Al3Zr dispersoids. This phenomenon is mainly caused by the highest nucleation rate of the Al3Zr phase at 400 °C. While reducing the heating rate of the homogenization process can increase the number density of the Al3Zr dispersoids and reduce the precipitate free zone (PFZ), it does not alleviate the inhomogeneity of the Al3Zr precipitation. These results are expected to be meaningful for tailoring the industrial homogenization processing of as-cast Al-Cu-Li alloy.
1. Introduction
Al-Li alloys have emerged as the most promising lightweight materials for aerospace and space applications due to their excellent comprehensive properties, such as low density, high strength, high stiffness, and corrosion resistance [1,2,3,4]. As a typical third-generation Al-Li alloy, 2196 Al-Cu-Li alloy has been widely used in manufacturing aircraft floor beams, fuselage beams, trusses, and pillars, exhibiting obvious advantages compared to conventional 2xxx and 7xxx aluminum alloys [4,5,6].
Homogenization is a crucial step in the processing of 2196 alloy and achieving the desired properties. This process is effective in dissolving non-equilibrium coarse constituents, thereby reducing microsegregation and dendrites and improving the processability of the alloy. Moreover, homogenization plays an important role in controlling the recrystallization behavior of the alloys by forming nanosized coherent Al3Zr dispersoids [7]. The presence of these dispersoids produces dispersion strengthening through the Orowan mechanism and inhibits recrystallization through grain boundary pinning, which ultimately, improves the mechanical properties of the alloy [8,9,10]. However, the homogenization process faces two major challenges: (1) Conventional single-step homogenization cannot fully dissolve all the coarse constituents with different melting points, resulting in an inadequate strengthening potential. (2) Due to the low diffusion rate and solubility of Zr in the Al matrix, Zr is prone to segregation at the dendrite center during solidification, resulting in low Zr concentration between dendrite arms and grain boundaries [11,12,13,14]. The uneven distribution of the Al3Zr dispersoids is unfavorable for the recrystallization resistance of the alloy [13,14,15,16]. Additionally, the presence of coarse primary Al3Zr constituent can even promote recrystallization [8,17,18].
Intensive efforts have been dedicated to optimizing the homogenization process of Al-Cu-Li alloys [10,11,12,13,19,20,21]. Based on homogenization kinetic analysis, Li et al. [19] proposed that the optimal homogenization process of Al-2.8Cu-1.4Li-0.3Mn-0.12Zr alloy was 510 °C/18 h and that the dendritic network as well as the microsegregation of the alloying elements were effectively eliminated. Guo et al. [16] proposed that the double-step homogenization exhibited great advantages compared to the conventional single-step homogenization for the 2195 alloy. Double-step homogenization not only prevents the occurrence of overburn but also provides more favorable conditions for the uniform precipitation of Al3Zr dispersoids. The optimal homogenization parameter was selected as 460 °C/16 h + 515 °C/20 h. Deng et al. [20] further demonstrated that the double-step homogenization promoted the uniform distribution of Al3Zr dispersoids and the precipitation of the T1 phase during the following aging treatment. Conversely, Liu et al. [10] proposed a double-step homogenization process of 350 °C/12 h + 470 °C/24 h for 2195 alloy, which resulted in fine and dense Al3Zr distribution and, thus, a significant improvement in its properties. Therefore, inconsistent homogenization processes were reported for the Al-Cu-Li alloy, especially the first stage of the double-step homogenization, which limited the properties improvement in this type of alloy. In order to optimize the homogenization process for the Al-Cu-Li alloy system, a systematical investigation into the effect of double-step homogenization temperature on the precipitation of Al3Zr dispersoids and dissolution of constituent phases is essential, especially for the first step of this homogenization.
In the present work, a commercial 2196 alloy with a low Cu/Li ratio of 1.67 (much lower than the 2195 alloy with a Cu/Li ratio of 4) was selected due to its extensive application of extrusion profiles. A series of double-step homogenization processes with different first-stage temperatures were designed to explore its effect on the microstructural evolution of the 2196 alloy. The dissolution of the constituent and precipitation of Al3Zr dispersoids were systematically studied. Finally, the diffusion behavior of the solute atoms was studied through microstructural characterization and kinetic analysis, and the classical nucleation theory related to thermodynamics and kinetics was used to verify the model.
2. Materials and Methods
The experimental material was the as-cast 2196 Al-Cu-Li alloy with Φ125 mm in diameter and 250 mm in length, received from CHINALCO Research Institute of Material Application and Technology in Beijing, China. The alloy ingot was prepared by a casting metallurgy method under an argon atmosphere, and the composition of the studied alloy (wt.%) was examined by an inductively coupled plasma optical emission spectrometer (ICP-OES). The chemical composition of the alloy is shown in Table 1. Various homogenization treatments were carried out on the alloy. Firstly, first-step homogenization with 4 different temperatures (350 °C/10 h, 375 °C/10 h, 400 °C/10 h, and 425 °C/10 h) with a heating rate of 30 °C/h was performed. A heating rate of 30 °C/hour relates to heating from room temperature to the specified temperature at a rate of 30 °C/hour, after which the sample was held at this temperature for the specified time. After then, all the ingots were second-step homogenized at 520 °C/24 h and air-cooled to room temperature. Another sample with a heating rate of 300 °C/h at the first stage of homogenization was selected for comparison. The schematic diagram of the entire experimental route is shown in Figure 1.

Table 1.
The chemical compositions (Wt.%) of 2196 aluminum alloy.
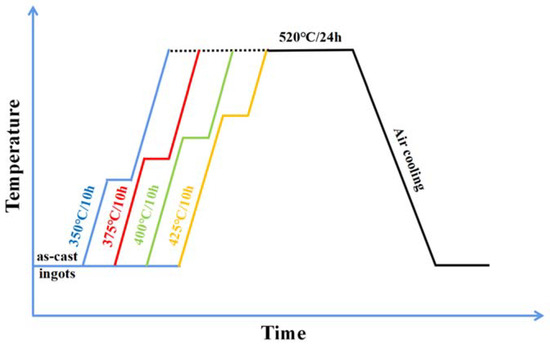
Figure 1.
Schematic diagram for the whole experimental route of the experimental alloy.
The grain size of the samples was observed by polarized light optical microscopy (PLOM) performed using a ZEISS Axio Observer 3 material microscope (Carl Zeiss Microscopy Gmb, Jena, Germany). The second phases were characterized by scanning electron microscopy performed using a TESCAN S8000 GMH SEM (Carl Zeiss Microscopy Gmb, Jena, Germany) with an accelerating voltage of 20 kV. The morphology and structure of the Al3Zr dispersoid were characterized using a FEI Tecnai G2 F20 transmission electron microscope (TEM, FEI, Portland, OR, USA) operated at 200 KV. The TEM samples were prepared by twin-jet polishing with an electrolyte solution of 30% HNO3 and 70% CH3OH. The operating temperature and voltage were set to −25 °C and 12 V, respectively. For the quantification of the dispersoids, more than 500 particles were counted based on 10 TEM images. The average thicknesses of samples were measured by convergent beam electron diffraction (CBED).
3. Results
3.1. As-Cast Microstructure
Figure 2 shows the representative SEM image and EDS quantification results of the as-deposited alloy. As shown in Figure 2a, the as-deposited alloy consisted of fine equiaxed grains with grain sizes of about 30–50 µm. Some pores were visible in the alloy, which is the main drawback of a spray-formed alloy. Some discontinuous or semi-continuously network primary phases were distributed along the grain boundaries. Additionally, abundant plate-like and irregular-morphology phases were observed in the grain interior. Figure 2b shows the SEM image of primary phases under higher magnification. The compositions of these second phases were analyzed by energy dispersive spectroscopy (EDS), and the results are shown in Figure 2c. Various types of primary phases, including Al-Cu, Al-Cu-Fe, Al-Cu-Mg, and Al-Cu-Zr phases can be identified. By comparing the results here with the results reported in the literature, it can be judged that the Al-Cu phase is θ(Al2Cu), the Mg-containing phase is S(Al2CuMg), and the Fe-containing phase is Al7Cu2Fe [18,19,21]. The plate-like particles in the interior of the grains can be identified as the TB phase, as shown in Figure 2h.

Figure 2.
(a) PLOM image of as-cast alloy; (b,c) BSE images: the orange arrow is the selected area of point scanning, and the red rectangle is the area of mapping in (c); (d–g) EDS mapping analyses of as-cast alloy; (h) bright-filed TEM image and corresponding selected area diffraction (SAD) pattern of the TB phase.
The PLOM image of the as-cast alloy is shown in Figure 2a. The alloy exhibited a typical as-cast eutectic structure with a clear dendritic network within the grains. The average grain size of the as-cast alloy was measured as 142.5 ± 16.6 µm. From the backscatter electron (BSE) image shown in Figure 2b, large numbers of coarse continuous non-equilibrium eutectic phases were distributed along the grain boundaries, and some spherical or elongated particles were formed in the grain interior. The enlarged SEM image and corresponding EDS mappings are shown in Figure 2c–g. The EDS analysis results of points A, B, and C are shown in Table 2. It is clear that the dendrite network was mainly enriched with Cu, and some Mg signals can be detected in the regions with lower contrast. Based on the point analysis shown in Table 2, two primary phases can be identified: θ (Al2Cu) and S (Al2CuMg) phases. Moreover, some particles with high contrast can be identified as the Al7Cu2Fe-phase based on the EDS point results and the mapping shown in Figure 2d,f. The Mn element was partially detected in the Al7Cu2Fe phase, which was caused by the mutual replacement between Fe and Mn. The primary Al3Zr phase was not detected in this alloy due to its low Zr concentration.

Table 2.
Chemical compositions (at.%) of intermetallic phases in Figure 2.
3.2. Microstructural Evolution during First-Step Homogenization
Figure 3 shows the PLOM and BSE images of the alloy after first-stage homogenization at different temperatures. The dendrite structure was generally reduced as the homogenization temperatures increased. The BSE images of Figure 3b,d,f,h show that the continuous primary phases distributed along the grain boundaries were gradually broken up and some spherical or elongated particles in the grain interior dissolved in the matrix. However, most of the dendritic network still existed, and the area fractions of the primary phases remained almost unchanged (the area fractions decreased from 3.45% to 3.10% as the first-stage homogenization temperatures increased from 350 °C to 425 °C), indicating that the low temperature in the first-step homogenization could not eliminate the macrosegregation and dendrites of the as-cast alloy. Except for the dissolution of the primary phases, some precipitations can be observed from the enlarged SEM images shown in Figure 4. Most of the precipitates exhibit a plate-like morphology. Based on previous literature [22,23,24], these precipitates can be identified as the θ’ phase. The distributions of the θ’ phase were inhomogeneous for all the homogenization temperatures, and a precipitate-free zone was observed in the core of the grains. Additionally, the number of densities in the θ’ phase generally decreased as the homogenization temperatures increased, which is mainly caused by the low drive force for precipitation during high-temperature homogenization.
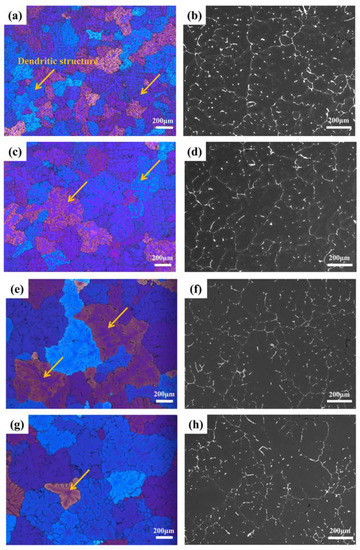
Figure 3.
Optical and BSE-SEM microstructures of specimens after first-step homogenization at different temperatures for 10 h: (a,b) 350 °C, (c,d) 375 °C, (e,f) 400 °C, and (g,h) 425 °C. The orange arrows are dendritic structures.
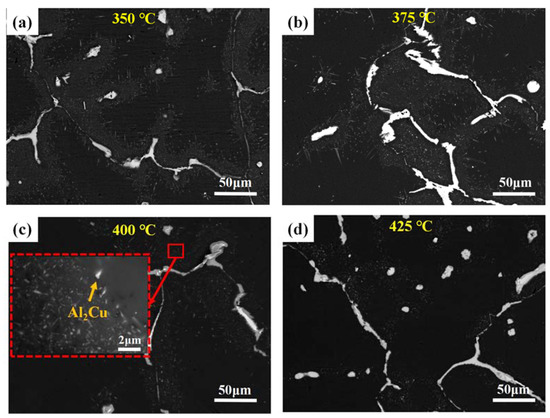
Figure 4.
BSE images after first-step homogenization at different temperatures for 10 h: (a) 350 °C, (b) 375 °C, (c) 400 °C, and (d) 425 °C and the red dotted line in (c) is the enlarged area.
3.3. Microstructural Evolution during Second-Step Homogenization
After second-step homogenization at 520 °C/24 h, regardless of the different first-step homogenization temperatures, most of the microsegregation was eliminated (shown in the PLOM images in Figure 5). BSE-SEM images of the four samples are shown in Figure 6, combined with the EDS results shown in Table 3, the low melting point (Cu, Mg, and Ag)-enriching phases were dissolved in the matrix, which left merely the insoluble Al7Cu2Fe phases. The Al7Cu2Fe phase was almost unchanged after the homogenization, which was mainly caused by the low solubility of Fe in the Al matrix. The S-Al2CuMg phases were fully dissolved into the matrix, with only a limited number of Al2Cu phases remaining in the Al matrix. This occurrence was likely due to insufficient homogenization time during production. The relatively low content of the residual Al2Cu phase suggests that its impact on the precipitation process of the alloy may be negligible. Additionally, the nanosized θ’ precipitates formed in the first-step homogenization dissolved after the second-step homogenization at higher temperatures. At this stage, no clear difference was observed in the four samples with different first-step homogenization temperatures. The area fractions of the residual second phase in the four samples were basically the same (0.50%, 0.18%, 0.21%, and 0.26% for first-step homogenization at 350 °C, 375 °C, 400 °C, and 425 °C, respectively).
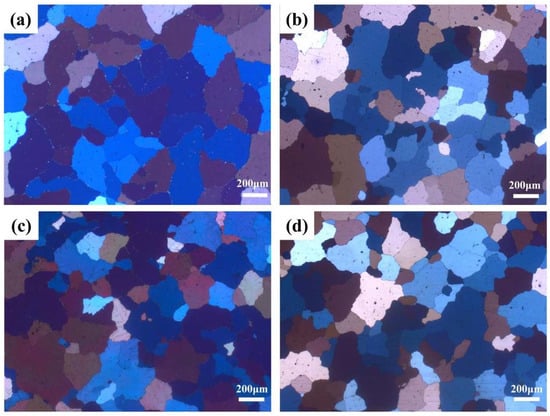
Figure 5.
PLOM images after different double-step homogenizations: (a) 350 °C/10 h + 520 °C/24 h, (b) 375 °C/10 h + 520 °C/24 h, (c) 400 °C/10 h + 520 °C/24 h, and (d) 425 °C/10 h + 520 °C/24 h.
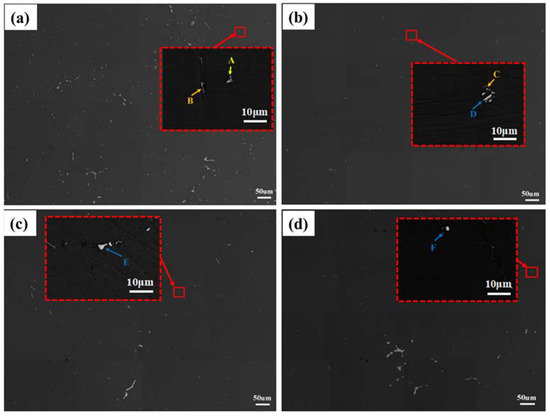
Figure 6.
BSE images after different double-step homogenizations, the red dotted square is the enlarged area: (a) 350 °C/10 h + 520 °C/24 h, (b) 375 °C/10 h + 520 °C/24 h, (c) 400 °C/10 h + 520 °C/24 h, and (d) 425 °C/10 h + 520 °C/24 h.

Table 3.
Chemical compositions (at.%) of intermetallic phases in Figure 6.
3.4. Precipitation Behavior of Al3Zr Dispersoids during Different Homogenization Processes
Figure 7 shows the high-angle angular dark-field scanning transmission electron microscopy image and corresponding selected area diffraction (SAD) pattern of the Al3Zr dispersoids formed after homogenization at 400 °C/10 h + 520 °C/24 h. The additional diffraction spots at the center of the Al spots confirmed the L12 structure of the Al3Zr dispersoids.
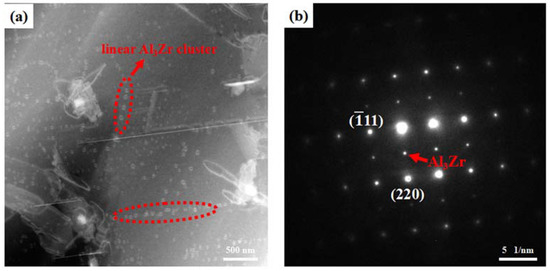
Figure 7.
TEM analysis after the 400 °C/10 h + 520 °C/24 h homogenization: (a) dark-fields TEM micrograph of the Al3Zr dispersoids; (b) corresponding SAD pattern along [112] Al zone axis.
To reveal the precipitation of the Al3Zr dispersoids after the different homogenization processes, SEM characterization was performed on the four double-step homogenized samples. Figure 8 shows the distribution of the Al3Zr dispersoids at the different regions of the grains. For the four samples, three different regions were observed. In the region closest to grain boundaries, designated as area 1, a few of the Al3Zr dispersoids can be observed. This phenomenon can be attributed primarily to the diminished concentration of Zr within the region adjacent to grain boundaries. In area 2, a low density of Al3Zr dispersoids with relatively larger sizes can be observed. Conversely, in the vicinity of the grain center, referred to as region 3, highly dense Al3Zr dispersoids were generated. Therefore, the precipitation of the Al3Zr dispersoids was inhomogeneous for all the homogenization processes. Figure 9 shows the HAADF-STEM images of the Al3Zr dispersoids formed in the grain center (region three) of the four samples. The average particle size (r), number density (Nv), and volume fraction (fv) of the Al3Zr dispersoids formed in the four different double-step heat-treated samples are shown in Table 4. The sample homogenized at 400 °C/10 h + 520 °C/24 h exhibited the highest number density, volume fraction, and smallest average size. The number density of Al3Zr in this process was more than 25% higher than in other processes, meaning it was selected as the optimum homogenization process in this work. For all four samples, the Al3Zr dispersoids exhibited two different configurations: linear clusters and blocky clusters, which was consistent with previous reports [16,25].
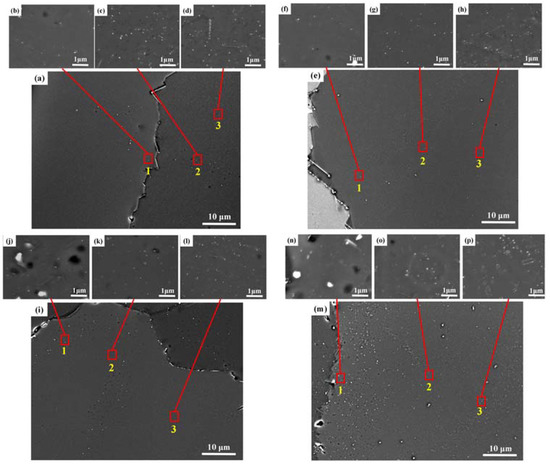
Figure 8.
BSE images of Al3Zr distributions at nearby grain boundaries after different double-step homogenizations, the red square is the enlarged area: (a) 350 °C/10 h + 520 °C/24 h, (b–d) The magnified regions of area 1, 2, and 3 in (a) respectively; (e) 375 °C/10 h + 520 °C/24 h, (f–h) The magnified regions of area 1, 2, and 3 in (e) respectively; (i) 400 °C/10 h + 520 °C/24 h, (j–l) The magnified regions of area 1, 2, and 3 in (i) respectively and (m) 425 °C/10 h + 520 °C/24 h, (n–p) The magnified regions of area 1, 2, and 3 in (m) respectively.
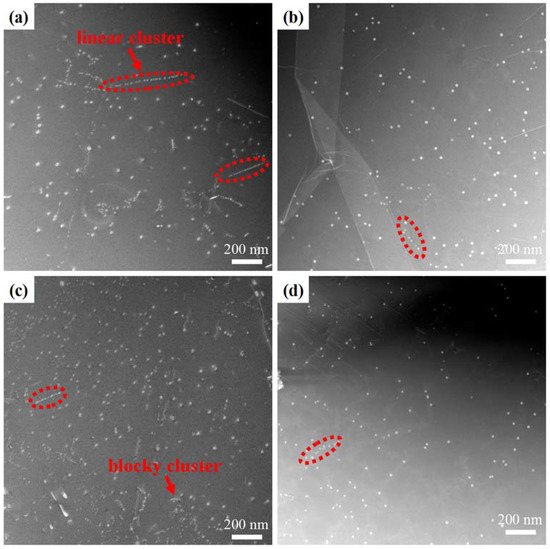
Figure 9.
The typical HAADF-STEM images of Al3Zr distributions and morphology after different double-step homogenizations: (a) 350 °C/10 h + 520 °C/24 h, (b) 375 °C/10 h + 520 °C/24 h, (c) 400 °C/10 h + 520 °C/24 h, and (d) 425 °C/10 h + 520 °C/24 h.

Table 4.
Average radius, number density, and volume fraction of Al3Zr dispersoids in the four different homogenized samples.
3.5. Precipitation of Al3Zr Dispersoids during Different Heating Rates
To study the effect of heating rate on the precipitation of Al3Zr dispersoids, different heating rates (30 °C/h and 300 °C/h) were adopted for the optimized homogenization process of 400 °C/10 h + 520 °C/24 h. SEM images of the two samples with different heating rates are shown in Figure 10. The sample with a slow heating rate of 30 °C/h demonstrates a much higher number density of Al3Zr dispersoids compared to the sample with a rapid heating rate of 300 °C/h, while the width of PFZ was smaller, indicating that the slow heating rate was beneficial in the precipitation of the Al3Zr dispersoids. However, both kinds of samples exhibited the inhomogeneous distribution of the Al3Zr phase in grains, implying that regulation of the heating rate could not efficiently improve the uniformity of the Al3Zr phase.
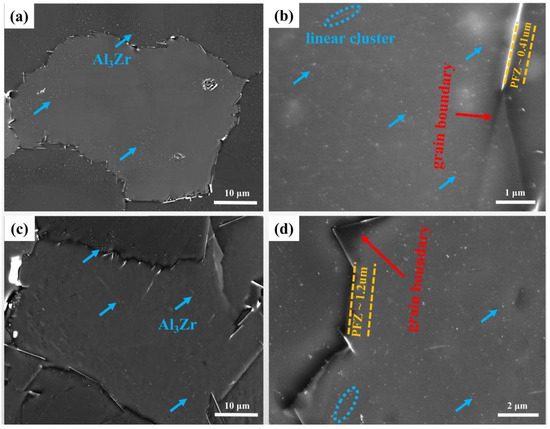
Figure 10.
Al3Zr distributions and micrographs after different heating modes: (a,b) slowly-heated specimen; (c,d) rapidly-heated specimen.
4. Discussions
Based on the above results, it was demonstrated that the regulation of the first-step homogenization temperature can significantly promote the precipitation of Al3Zr dispersoids. Specifically, a low-temperature treatment during the first stage of the double-step homogenization promotes the nucleation of Al3Zr due to the large driving force, while a subsequent high-temperature treatment facilitates the diffusion of Zr atoms and promotes the formation of numerous Al3Zr dispersoids. The homogenization treatment mainly consisted of the nucleation of Al3Zr and the dissolution of primary phases, as discussed below.
4.1. Nucleation Model of Al3Zr Dispersoids
The precipitation kinetics model of Al3Zr dispersoids is based on the Kampmann–Wagner numerical (KWN) model [26], which has been widely used by previous researchers [15,26,27,28,29]. In the KWN model, the evaluation of the precipitate nucleation rate is based on the classical nucleation theory [30]. This theory applies to binary alloys and is generally considered as not strictly applicable to multicomponent alloys [31].
In the present work, an approximate method to calculate the nucleation rate was used, which ignored the incubation time before nucleation. The nucleation rate can be expressed by [18]:
where J is the nucleation rate per unit volume. N0 is the number of nucleation sites per unit volume, which is calculated by the number of Zr atoms per unit volume in this paper. The values of average radius and numerical density are shown in Table 4. Q is the activation energy for the transfer of Zr across the nucleus interface (assumed to be approximately 2.07 eV atom−1 [32]). The k and h are the Boltzmann constant and Planck constant, respectively. T is the thermodynamic temperature and σ is the interface energy, which is 0.1 J/m2 [10,16]. The r* is the critical radius.
The critical radius is calculated using the Gibbs–Thomson equation [18,28]. The critical radius r* can be written as:
where Va is the atomic volume, c is the instantaneous concentration of Zr in the matrix, and is the concentration of Zr in the matrix when it is in equilibrium with Al3Zr. The value is obtained from the solvent line of the metastable Al3Zr phase in the binary Al-Zr system calculation [18]. The radius of each newly formed particle is set to be slightly larger than the critical radius so that these particles can grow. According to the KWN procedure, newly nucleated particles are arbitrarily taken as 10% greater than r*.
After normalization, the nucleation rate of Al3Zr dispersoids alongside temperature is shown in Figure 11. The peak nucleation rate occurs at 400 °C, and then, the nucleation rate decreased rapidly alongside the increase in temperature. Therefore, the nucleation rate of Al3Zr dispersoids at 400 °C was the largest, which was more consistent with the experimental results, in which the highest number density of Al3Zr dispersoids was formed using this condition.
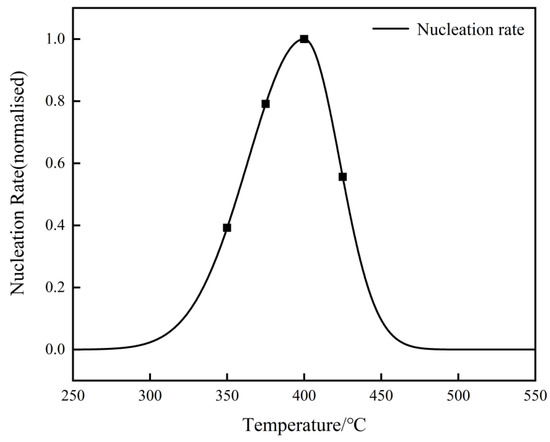
Figure 11.
The normalized nucleation rates with temperature.
4.2. Homogenization Kinetic Analysis
The experimental results show that the nonequilibrium eutectic phase and dendrite segregation gradually disappear in the homogenization process. The distributions of the major elements (Cu, Mg, and Ag) in the grain and at the grain boundaries are uneven. Therefore, it is of great significance to study the diffusion law in the dendritic region for studying the distribution of elements in the homogenization process. According to References [33,34], the initial concentration of the elements in the interdendritic region can be expressed by the Fourier series components of the cosine function:
where L is the interdendritic spacing, is the initial amplitude of the composition segregation, and is the average concentration of the element.
According to Fick’s second law [35] and boundary conditions, w (x,t) is:
where D is the diffusion coefficient of the elements in the matrix, and t is the diffusion time.
The attenuation law of the cosine distribution in Equation (4) can be described by the attenuation function [36]:
whereby it is generally believed that when the component segregation amplitude decreases to 1%, the element distribution is uniform, then:
the diffusion coefficient D is:
where D0 is the independent coefficient, Q is the diffusion activation energy, R is the gas constant, and T is the absolute temperature.
Replace Equation (7) and substitute Equation (6), the equation can be written as:
assuming A = R/Q, B = 4.6/4π2D0, the homogeneous dynamic equation can be obtained:
previous studies have shown that at the same temperature, the diffusion coefficient of Cu was much lower than for Mg, Zn, and Mn. Therefore, it can be considered that the homogenization process is mainly affected by Cu diffusion [21,34].
Set D0 (Cu) = 0.084 cm2·s−1, Q (Cu) = 136.8 kJ·mol−1, and R = 8.314 J/(mol·K), as introduced in Equation (9), and homogenization kinetics curves of 2196 Al-Cu-Li alloy at different dendrite spacings can be obtained, as shown in Figure 12. Here, as the temperature increased, the diffusion time of elements shortened, and the microstructure greatly improved.
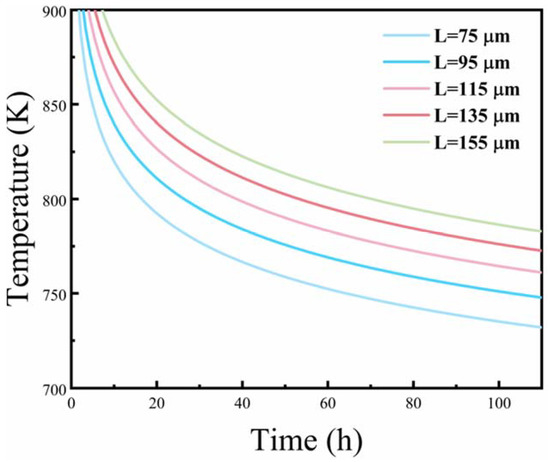
Figure 12.
Corresponding kinetic curves of homogenized alloy.
Quantitative metallographic analysis shows that the average dendrite spacing in the as-cast alloy was about 100 ± 15 μm, calculated from Figure 3. According to the homogenization kinetic curves, at a homogenization temperature of 520 °C, the corresponding diffusion time was about 24.8 h. This is in good agreement with the experimental results. Therefore, second-step homogenization at 520 °C/24 h can effectively dissolve the primary phases formed during casting, even though a low content of Al2Cu phase can still be observed. Compared to the previous literature, and specifically reference [10], the optimal homogenization process for 2195 alloy was determined as 350 °C/12 h + 470 °C/24 h, with quantitative statistics indicating an average particle size of Al3Zr at 22.6 ± 4.1 nm. Meanwhile, in reference [16], the optimum two-step homogenization parameter for as-cast 2195 Al-Li alloy was found to be 460 °C/16 h + 515 °C/20 h, which facilitates uniform precipitation of Al3Zr particles with a diameter of 25.23 nm. After undergoing two-step homogenization at 460 °C/12 h + 510 °C/24 h, the Al3Zr dispersoids in Al-Cu-Li-Zr alloy exhibited a finer and more uniform microstructure with an average size of 24.9 ± 1.1 nm, while the width of PFZ narrowed, as reported in reference [20]. In this paper, the optimized homogenization process of 400 °C/10 h + 520 °C/24 h can effectively dissolve the primary phases and refine the distribution of Al3Zr dispersoids. As a result, the average particle size of the Al3Zr dispersoids was reduced to 17.7 nm, which is expected to suppress the recrystallization and improve the mechanical properties of the alloys during the subsequent processing.
5. Conclusions
In this work, the influences of two-step homogenization processes on the dissolution of primary phases and precipitation of Al3Zr dispersoids of an as-cast 2196 alloy were investigated. The main conclusions can be drawn as follows:
- During the second-step homogenization, regardless of the different first-step homogenization temperatures, most of the microsegregation is eliminated, and the θ’ precipitates formed during first-step homogenization are dissolved. The different first-step homogenization temperatures can result in a different distribution of Al3Zr dispersoids after a second-step homogenization at 520 °C. The optimized homogenization process of 400 °C/10 h + 520 °C/24 h can effectively dissolve the primary phases and refine the distribution of Al3Zr dispersoids.
- Decreasing the heating rate of the homogenization process can greatly increase the number density of the Al3Zr dispersoids. However, regulation of the heating rate could not improve the uniformity of the Al3Zr dispersoids.
- The corresponding precipitation of the Al3Zr dispersoids model and dissolution kinetics of the primary phases have been developed for the homogenization process of the 2196 alloy, which corresponds well with these experimental results.
Author Contributions
Conceptualization, H.Z. and L.D.; Methodology, H.Z. and L.D.; Investigation, H.Z., X.L. and M.L.; writing—original draft preparation, H.Z. and L.D.; writing—review and editing, L.D., Y.Z., Y.W., K.W. and Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (U2141215; U22A20187; 52004227; 51871035), and the Natural Science Foundation of Jiangsu Province grant number (BK20202010, BE2022159).
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abd El-Aty, A.; Xu, Y.; Guo, X.; Zhang, S.; Ma, Y.; Chen, D. Strengthening mechanisms, deformation behavior, and anisotropic mechanical properties of Al-Li alloys: A review. J. Adv. Res. 2018, 10, 49–67. [Google Scholar] [CrossRef]
- Yu, X.; Dai, H.; Li, Z.; Sun, J.; Zhao, J.; Li, C.; Liu, W. Improved Recrystallization Resistance of Al-Cu-Li-Zr Alloy through Ce Addition. Metals 2018, 8, 1035. [Google Scholar] [CrossRef]
- Ta, N.; Bilal, M.U.; Hausler, I.; Saxena, A.; Lin, Y.Y.; Schleifer, F.; Fleck, M.; Glatzel, U.; Skrotzki, B.; Darvishi Kamachali, R. Simulation of the θ′ precipitation process with interfacial anisotropy effects in Al-Cu alloys. Materials 2021, 14, 1280. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, X.; Xi, H.; Zhao, G.; Wang, Y.; Xu, X. Effects of heat treatment on the microstructure and mechanical properties of extruded 2196 Al-Cu-Li alloy. Mater. Des. 2020, 192, 108746. [Google Scholar] [CrossRef]
- Xu, X.; Ma, X.; Yu, S.; Zhao, G.; Wang, Y.; Chen, X. Bonding mechanism and mechanical properties of 2196 Al-Cu-Li alloy joined by hot compression deformation. Mater. Charact. 2020, 167, 110486. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, G.; Liu, G.; Sun, L.; Chen, L.; Zhang, C. Microstructure evolution and mechanical properties of 2196 Al-Li alloy in hot extrusion process. J. Mater. Process. Technol. 2020, 275, 116348. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, X.; Huang, Y.; Ding, L.; Lei, X.; Weng, Y.; Cao, L.; Zhang, K.; Jia, Z. The Effect of Thermomechanical Processing Sequence on the Dispersoid Distribution and Final Mechanical Properties of Spray-Formed Al-Cu-Li Alloy. Metals 2022, 12, 1893. [Google Scholar] [CrossRef]
- Lue, X.; Guo, E.; Rometsch, P.; Wang, L. Effect of one-step and two-step homogenization treatments on distribution of Al3Zr dispersoids in commercial AA7150 aluminium alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 2645–2651. [Google Scholar] [CrossRef]
- Li, H.; Yu, W.; Wang, X.; Du, R.; You, W. Investigation on Microstructural Evolution and Properties of an Al-Cu-Li Alloy with Mg and Zn Microalloying during Homogenization. Metals 2018, 8, 1010. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, G.; Tan, Z.; Li, Z.; Zhang, D.; Wang, J.; Zhang, H. Precipitation of Al3Zr by two-step homogenization and its effect on the recrystallization and mechanical property in 2195 Al-Cu-Li alloys. Mater. Sci. Eng. A 2021, 821, 141637. [Google Scholar] [CrossRef]
- Jia, Z.; Hu, G.; Forbord, B.; Solberg, J.K. Effect of homogenization and alloying elements on recrystallization resistance of Al-Zr-Mn alloys. Mater. Sci. Eng. A 2007, 444, 284–290. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Nucleation and precipitation strengthening in dilute Al-Ti and Al-Zr alloys. Metall. Mater. Trans. A 2007, 38, 2552–2563. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Ronson, J.D.; Sigli, C.; Prangnell, P.B. Interactions between zirconium and manganese dispersoid-forming elements on their combined addition in Al-Cu-Li alloys. Acta Mater. 2012, 60, 5245–5259. [Google Scholar] [CrossRef]
- Honaramooz, M.T.; Morak, R.; Pogatscher, S.; Fritz-Popovski, G.; Kremmer, T.M.; Meisel, T.C.; Österreicher, J.A.; Arnoldt, A.; Paris, O. Characterization of Zr-Containing Dispersoids in Al–Zn–Mg–Cu Alloys by Small-Angle Scattering. Materials 2023, 16, 1213. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.D. Optimizing the homogenization of zirconium containing commercial aluminium alloys using a novel process model. Mater. Sci. Eng. A 2002, 338, 219–229. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Lu, D.; Deng, S.; Zeng, G.; Ma, Y.; You, W.; Chen, Y.; Zhang, X.; Zhang, R. Characterization of Al3Zr precipitation via double-step homogenization and recrystallization behavior after subsequent deformation in 2195 Al-Li alloy. Mater. Charact. 2021, 182, 111549. [Google Scholar] [CrossRef]
- Pourkia, N.; Emamy, M.; Farhangii, H.; Ebrahimi, S.S. The effect of Ti and Zr elements and cooling rate on the microstructure and tensile properties of a new developed super high-strength aluminum alloy. Mater. Sci. Eng. A 2010, 527, 5318–5325. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Dispersoid precipitation and process modelling in zirconium containing commercial aluminium alloys. Acta Mater. 2001, 49, 599–613. [Google Scholar] [CrossRef]
- Li, S.; Wei, B.; Yu, C.; Li, Y.; Xu, G.; Li, Y. Evolution of microstructure and properties during homogenization of the novel Al-Li alloy fabricated by electromagnetic oscillation twin-roll casting. J. Mater. Res. Technol. 2020, 9, 3304–3317. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, J.; Chen, J.; Guo, X. Effect of double-step homogenization treatments on the microstructure and mechanical properties of Al-Cu-Li-Zr alloy. Mater. Sci. Eng. A 2020, 795, 139975. [Google Scholar] [CrossRef]
- Yang, S.; Shen, J.; Yan, X.; Li, X.; Zhang, F.; Sun, B. Homogenization treatment parameter optimization and microstructural evolution of Al-Cu-Li alloy. Rare Met. Mater. Eng. 2017, 46, 28–34. [Google Scholar]
- Wang, Y.; Ma, X.; Zhao, G.; Xu, X.; Chen, X.; Zhang, C. Microstructure evolution of spray deposited and as-cast 2195 Al-Li alloys during homogenization. J. Mater. Sci. Technol. 2021, 82, 161–178. [Google Scholar] [CrossRef]
- Wang, S.; Starink, M.J. Precipitates and intermetallic phases in precipitation hardening Al-Cu-Mg-(Li) based alloys. Int. Mater. Rev. 2005, 50, 193–215. [Google Scholar] [CrossRef]
- Li, J.; Ning, H.; Liu, D.; Zheng, Z. Alloying and micro-alloying in Al-Cu-Li series alloys. Chin. J. Nonferrous Met. 2021, 31, 258–279. [Google Scholar]
- Tsivoulas, D.; Robson, J.D. Heterogeneous Zr solute segregation and Al3Zr dispersoid distributions in Al-Cu-Li alloys. Acta Mater. 2015, 93, 73–86. [Google Scholar] [CrossRef]
- Khan, I.N.; Starink, M.J.; Yan, J. A model for precipitation kinetics and strengthening in Al-Cu-Mg alloys. Mater. Sci. Eng. A 2008, 472, 66–74. [Google Scholar] [CrossRef]
- Werenskiold, J.C.; Deschamps, A.; Brechet, Y. Characterization and modeling of precipitation kinetics in an Al-Zn-Mg alloy. Mater. Sci. Eng. A 2000, 293, 267–274. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Modelling Al3Zr dispersoid precipitation in multicomponent aluminium alloys. Mater. Sci. Eng. A 2003, 352, 240–250. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Modeling the precipitation kinetics and tensile properties in Al-7Si-Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 403–416. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys; Newnes; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Kamp, N.; Sullivan, A.; Tomaasi, R.; Robsonn, J.D. Modelling of heterogeneous precipitate distribution evolution during friction stir welding process. Acta Mater. 2006, 54, 2003–2014. [Google Scholar] [CrossRef]
- Ronson, J.D. A new model for prediction of dispersoid precipitation in aluminium alloys containing zirconium and scandium. Acta Mater. 2004, 52, 1409–1421. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Q.; Fan, X.; He, Y.; Li, B.; Liang, W. Microstructural evolution of Al-Cu-Mg-Ag alloy during homogenization. J. Alloys Compd. 2009, 484, 790–794. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Yan, X.; Sun, J.; Sun, X.; Wang, Y. Homogenization heat treatment of 2099 Al-Li alloy. Rare Met. 2014, 33, 28–36. [Google Scholar] [CrossRef]
- Ujihara, T.; Fujiwara, K.; Sazaki, G.; Usami, N.; Nakajima, K. New method for measurement of interdiffusion coefficient in high temperature solutions based on Fick’s first law. J. Cryst. Growth 2002, 241, 387–394. [Google Scholar] [CrossRef]
- Liu, G. Fundamentals of Metallurgy; Metallurgical Industry Press: Beijing, China, 1980; p. 283. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).