Combustion Behavior and Microstructure of TC17 Titanium Alloy under Oxygen-Enriched Atmosphere
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Material
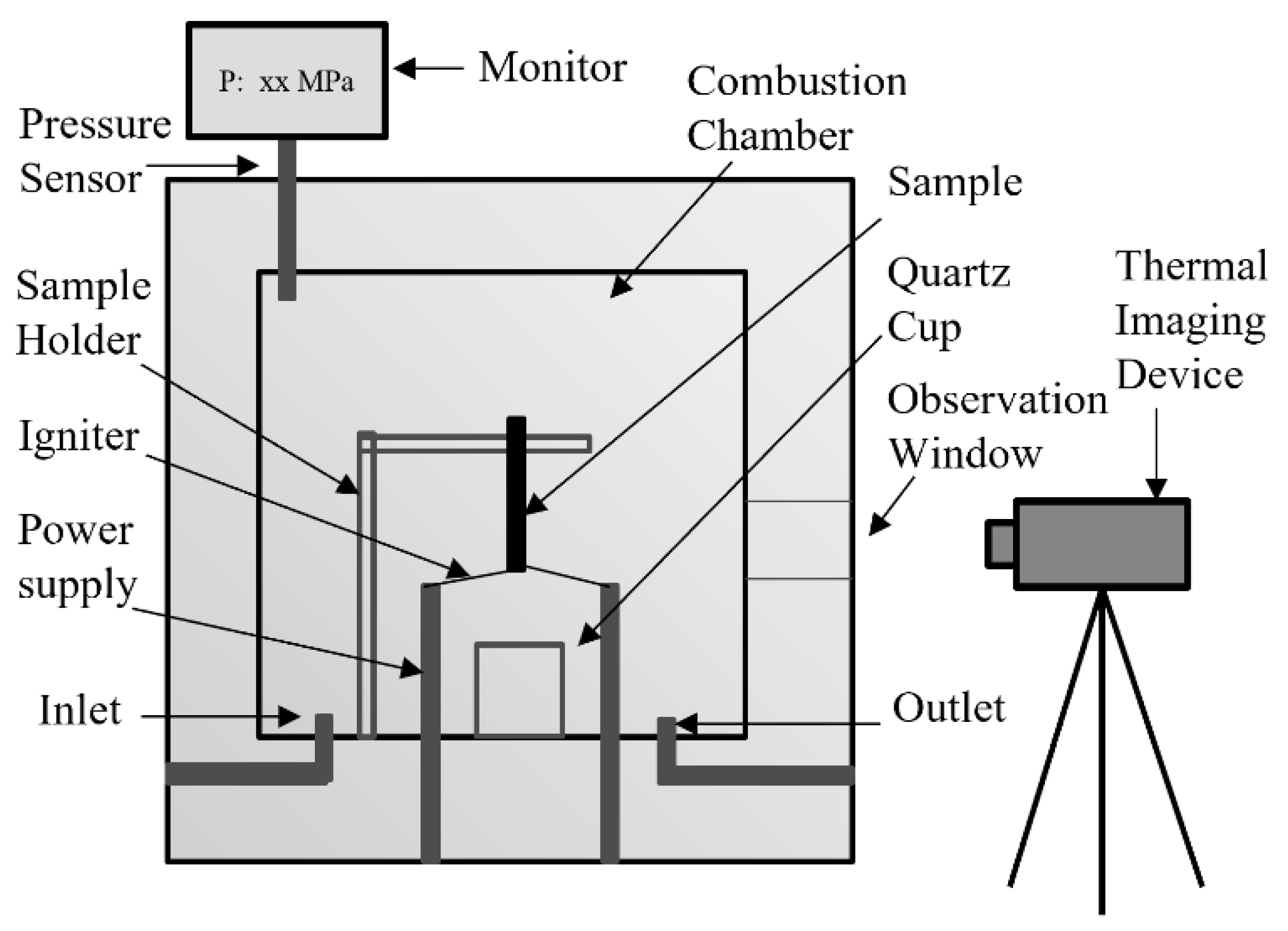
2.2. Promoted Ignition–Combustion (PIC) Test
2.3. Microstructural Characterization
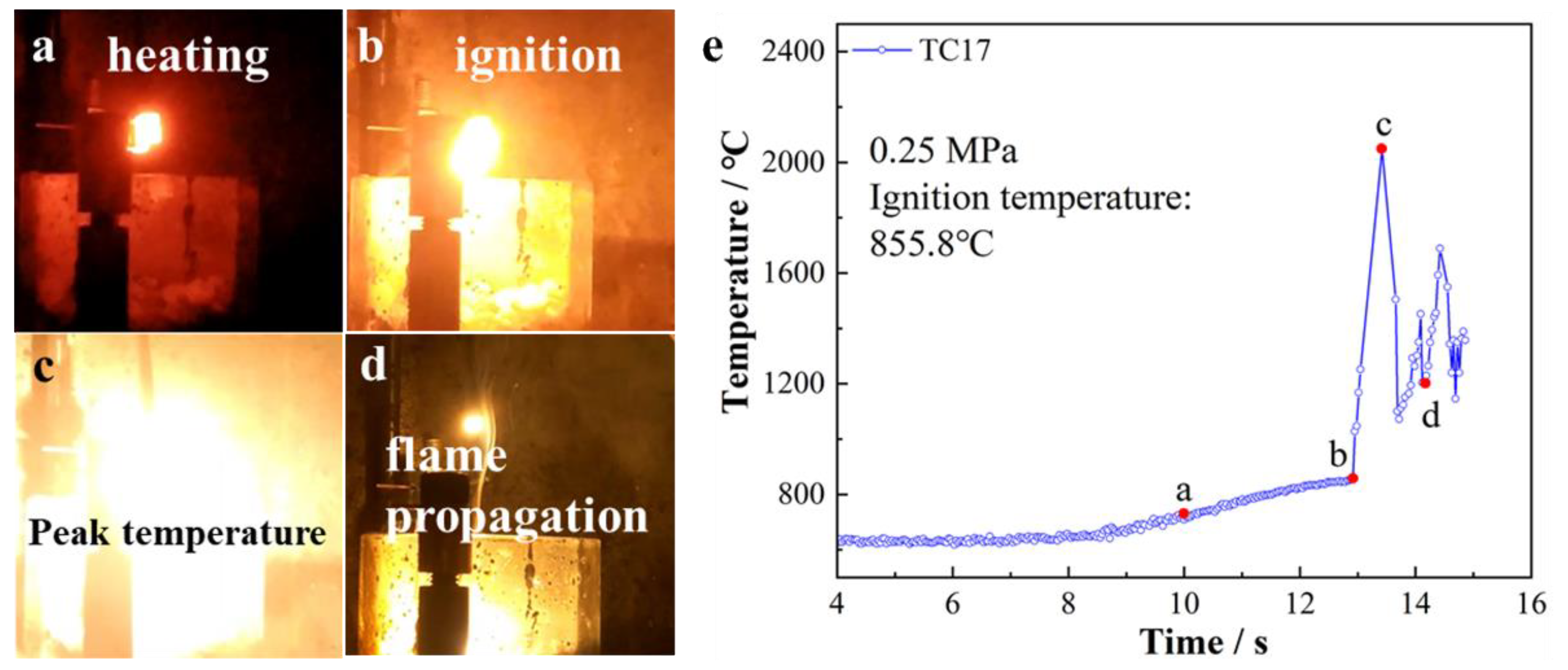
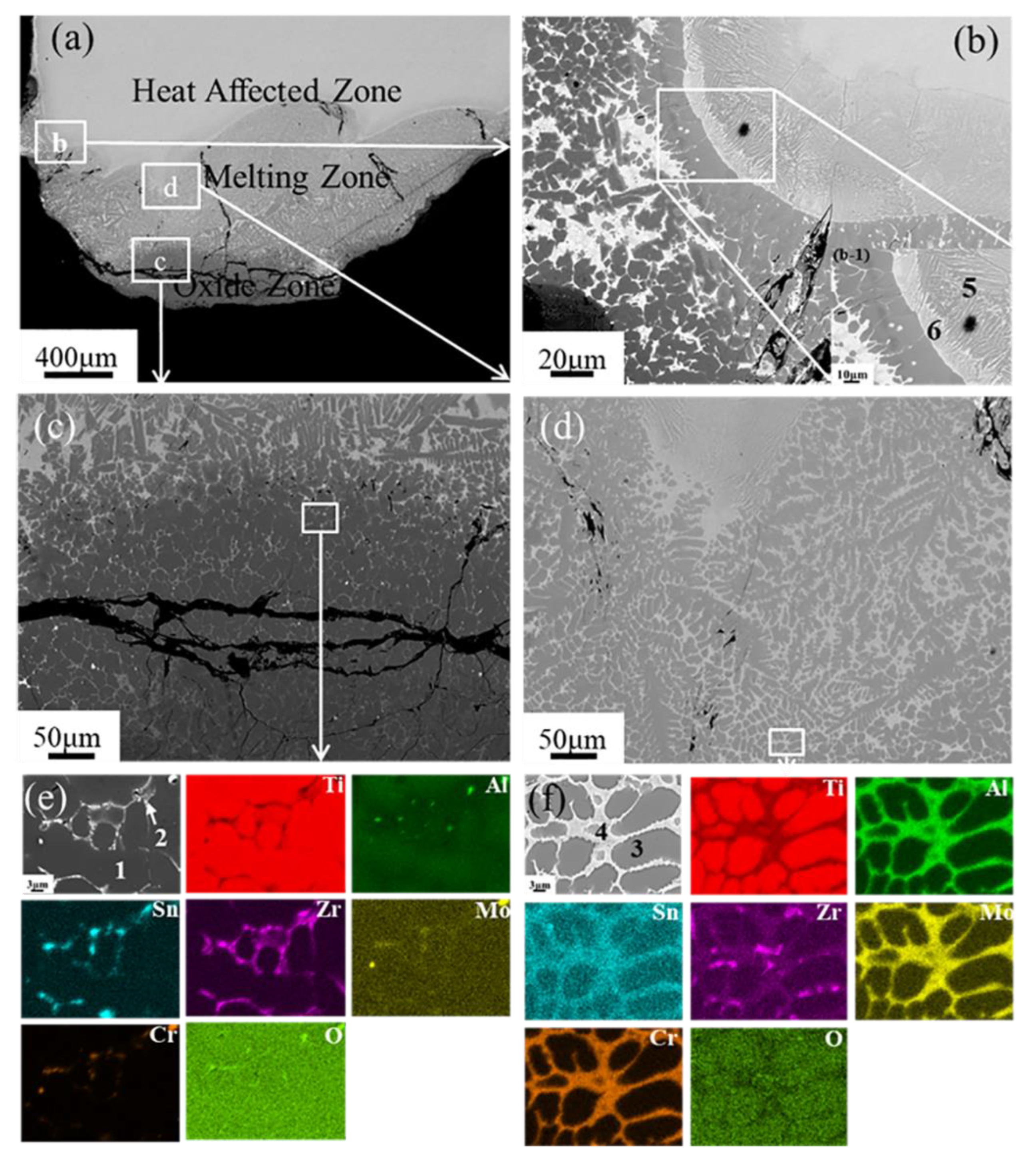
3. Results
4. Discussion
5. Conclusions
- The combustion of the TC17 alloy consists of the ignition, splash, and flame propagation stages. The threshold oxygen pressure for the combustion of the TC17 alloy is comparable to that of TC11; however, the combustion velocity of the TC17 alloy is obviously faster. The combustion velocity and oxygen pressure follow a power law relationship;
- The oxide zone, melting zone, and heat-affected zone are observed in the TC17 alloy after extinguishing the combustion process. The segregation of Cr, Mo, and Al is observed in the interdendritic phase in the melting zone and the interface between the melting zone and the heat-affected zone;
- The enhanced combustion velocity of the TC17 alloy may be caused by the segregation of Cr at the liquid/solid interface, which lowers the interfacial temperature and speeds up the migration of the liquid/solid interface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Luo, Q.S.; Li, S.F.; Pei, H.P. Progress in Titanium Fire Resistant Technology for Aero-Engine. J. Aerosp. Power 2012, 27, 2763–2768. [Google Scholar]
- Shao, L.; Xie, G.; Zhang, C.; Liu, X.; Lu, W.; He, G.; Huang, J. Combustion of Metals in Oxygen-Enriched Atmospheres. Metals 2020, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.F.; Moulder, J.C.; Runyan, C.C. Combustion of Bulk Titanium in Oxygen. Symp. Combust. 1975, 15, 489–499. [Google Scholar] [CrossRef]
- Littman, F.E.; Church, F.M.; Kinderman, E.M. A Study of Metal Ignitions I. The Spontaneous Ignition of Titanium. J. Less Common Met. 1961, 3, 367–378. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Zhang, Q.; Wang, L.; Ji, K.; Peng, L.; Gao, W. Combustion Behaviors and Flame Microstructures of Micro- and Nano-Titanium Dust Explosions. Fuel 2016, 181, 785–792. [Google Scholar] [CrossRef]
- Dreizin, E.L. Phase Changes in Metal Combustion. Prog. Energy Combust. Sci. 2000, 26, 57–78. [Google Scholar] [CrossRef]
- Gaohui, Z.; Zhang, P.Z.; Guoqing, H.; Xu, P.; Shunqi, Z. Study on the Burn-Resistant Properties of Titanium Alloy Ti6Al4V Surface by Diffusing Copper. Rare Met. Mater. Eng. 2011, 40, 286–289. [Google Scholar]
- Molodetsky, I.E.; Vicenzi, E.P.; Dreizin, E.L.; Law, C.K. Phases of Titanium Combustion in Air. Combust. Flame 1998, 112, 522–532. [Google Scholar] [CrossRef]
- Shafirovich, E.; Teoh, S.K.; Varma, A. Combustion of Levitated Titanium Particles in Air. Combust. Flame 2008, 152, 262–271. [Google Scholar] [CrossRef]
- Schutz, R.W. Environmental Behavior of Beta Titanium Alloys. J. Miner. Met. Mater. Soc. 1994, 46, 24–29. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Makarov, K.M.; Shteinberg, A.S.; Drozhzhin, P.F. Compact-Specimen Burning with Fresh Metal Surface Production. Combust. Explos. Shock Waves 1992, 28, 457–459. [Google Scholar] [CrossRef]
- Bolobov, V.I. Mechanism of Self-Ignition of Titanium Alloys in Oxygen. Combust. Explos. Shock Waves 2002, 38, 639–645. [Google Scholar] [CrossRef]
- Shao, L.; Xie, G.; Liu, X.; Wu, Y.; Yu, J.; Hao, Z.; Lu, W.; Liu, X. Combustion Behaviour and Mechanism of TC4 and TC11 Alloys. Corros. Sci. 2020, 168, 108564. [Google Scholar] [CrossRef]
- Shao, L.; Li, Z.; Yu, J.; Yang, G.; Zhang, C.; Zou, Y.; Huang, J. Combustion Behavior and Mechanisms of Ti2AlNb Compared to an α + β Ti Alloy. Corros. Sci. 2021, 192, 109868. [Google Scholar] [CrossRef]
- Shao, L.; Wang, Y.; Xie, G.; Li, H.; Xiong, J.; Yu, J.; He, G.; Huang, J. Combustion Mechanism of Alloying Elements Cr in Ti-Cr-V Alloys. Materials 2019, 12, 3206. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.J.; Wang, B.; Gao, Y. Investigation of TC4 and TC11 Titanium Alloys’ Combustion Resistance Properties. J. Mater. Eng. 2004, 33–35. [Google Scholar]
- Shao, L.; Xie, G.; Liu, X.; Wu, Y.; Tan, Q.; Xie, L.; Xin, S.; Hao, F.; Yu, J.; Xue, W.; et al. Combustion Behavior and Mechanism of Ti-25V-15Cr Compared to Ti-6Al-4V Alloy. Corros. Sci. 2022, 194, 109957. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Bo, A.; Zhan, H.; Zhang, F.; Zhao, Y.; Zhao, Q.; Wan, M.; Gu, Y. Underlying Burning Resistant Mechanisms for Titanium Alloy. Mater. Des. 2018, 156, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huo, Y.; Song, X.; Bi, Z.; Gao, Y.; Zhao, Y. Burn-Resistant Behavior and Mechanism of Ti14 Alloy. Int. J. Miner. Metall. Mater. 2016, 23, 215–221. [Google Scholar] [CrossRef]
- Ding, M.C.; Zhang, Y.L.; Lu, H.T. Fatigue Life Prediction of TC17 Titanium Alloy Based on Micro Scratch. Int. J. Fatigue 2020, 139, 105793. [Google Scholar] [CrossRef]
- Liang, S.; Li, J.; Zhang, Q.; Chen, J. Microstructural Evolution in the TC17 Titanium Alloy Processed During Laser Stereo Forming. J. Mater. Eng. Perform. 2021, 30, 2967–2976. [Google Scholar] [CrossRef]
- Sato, J.; Hirano, T. Fire Spread Limits along Metal Pieces in Oxygen. In Flammability and Sensitivity of Materials in Oxygen-Enriched Atmospheres: Third Volume, ASTM STP, 986; ASTM: Philadelphia, PA, USA, 1988; pp. 158–171. [Google Scholar] [CrossRef]
- Zhao, P.; Fu, L.; Zhong, D. Numerical Simulation of Transient Temperature and Axial Deformation during Linear Friction Welding between TC11 and TC17 Titanium Alloys. Comput. Mater. Sci. 2014, 92, 325–333. [Google Scholar] [CrossRef]
- Xie, L.; Liu, C.; Song, Y.; Guo, H.; Wang, Z.; Hua, L.; Wang, L.; Zhang, L.C. Evaluation of Microstructure Variation of TC11 Alloy after Electroshocking Treatment. J. Mater. Res. Technol. 2020, 9, 2455–2466. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Qiao, H. Microstructure Characteristics and Formation Mechanism of TC17 Titanium Alloy Induced by Laser Shock Processing. J. Alloys Compd. 2017, 722, 509–516. [Google Scholar] [CrossRef]
- Chepulskii, R.V.; Curtarolo, S. Calculation of Solubility in Titanium Alloys from First Principles. Acta Mater. 2009, 57, 5314–5323. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.L. The Cr−Ti (Chromium-Titanium) System. Bull. Alloy Phase Diagr. 1981, 2, 174–181. [Google Scholar] [CrossRef]



| wt.% | Al | Mo | Zr | Sn | Cr | Fe | Ti |
| 4.6 | 4.1 | 1.9 | 2.0 | 4.0 | 0.15 | Bal. |
| Region | Composition | ||||||
|---|---|---|---|---|---|---|---|
| Ti | Al | Sn | Zr | Mo | Cr | O | |
| Phase 1 (at %) | 39.93 | 2.87 | 0.02 | 0.09 | 0 | 0.07 | 57.02 |
| Phase 2 (at %) | 33.4 | 1.46 | 3.27 | 14.92 | 0.41 | 1.32 | 45.23 |
| Phase 3 (at %) | 63.07 | 3.96 | 0.45 | 0 | 0 | 0.56 | 31.96 |
| Phase 4 (at %) | 50.54 | 18.92 | 1.14 | 0.7 | 3.29 | 9.88 | 15.53 |
| Phase 5 (at %) | 64.43 | 8.09 | 0.8 | 1.1 | 1.58 | 3.6 | 20.4 |
| Phase 6 (at %) | 51.02 | 15.2 | 0.98 | 1.08 | 4.47 | 7.77 | 19.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Xing, P.; Li, Z.; Wang, C.; Dou, C.; Jiao, Y.; Li, J.; Wang, B.; He, G.; Huang, J. Combustion Behavior and Microstructure of TC17 Titanium Alloy under Oxygen-Enriched Atmosphere. Metals 2023, 13, 1020. https://doi.org/10.3390/met13061020
Zhang C, Xing P, Li Z, Wang C, Dou C, Jiao Y, Li J, Wang B, He G, Huang J. Combustion Behavior and Microstructure of TC17 Titanium Alloy under Oxygen-Enriched Atmosphere. Metals. 2023; 13(6):1020. https://doi.org/10.3390/met13061020
Chicago/Turabian StyleZhang, Cheng, Peng Xing, Zhibin Li, Congzhen Wang, Caihong Dou, Yuxuan Jiao, Jianjun Li, Biao Wang, Guangyu He, and Jinfeng Huang. 2023. "Combustion Behavior and Microstructure of TC17 Titanium Alloy under Oxygen-Enriched Atmosphere" Metals 13, no. 6: 1020. https://doi.org/10.3390/met13061020
APA StyleZhang, C., Xing, P., Li, Z., Wang, C., Dou, C., Jiao, Y., Li, J., Wang, B., He, G., & Huang, J. (2023). Combustion Behavior and Microstructure of TC17 Titanium Alloy under Oxygen-Enriched Atmosphere. Metals, 13(6), 1020. https://doi.org/10.3390/met13061020






