Manufacturing of Novel Nanostructured TiCrC Carbides Using Mechanical Alloying and Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of (Ti,Cr)C Carbides Using Mechanical Alloying and Spark Plasma Sintering
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Bulk Samples Prepared via SPS
3.2. Electrochemical Properties of Bulk Samples Prepared via SPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mhadhbi, M.; Driss, M. Titanium Carbide: Synthesis, Properties and Applications. J. Brill. Eng. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Komratov, G.N. Oxidation Kinetics of Double Titanium-chromium Carbide and Chromium Carbide. Poroshk. Metall. 1999, 9–10, 52–57. [Google Scholar]
- Chen, K.; Zhao, L. Elastic Properties, Thermal Expansion Coefficients and Electronic Structures of Ti0.75X0.25C Carbides. J. Phys. Chem. Solids 2007, 68, 1805–1811. [Google Scholar] [CrossRef]
- Umanskii, A.P.; Lavrenko, V.A.; Chuprov, S.S.; Konoval, V.P. High-temperature Oxidation of Composites Based on Titanium Carbonitride and Double Titanium–chromium Carbide. Nov. Ogneup. 2006, 8, 42–46. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Polkowski, W. Synthesis and Characterization of Mechanically Alloyed Nanostructured (Ti,Cr)C Carbide for Cutting Tools Application. Crystals 2022, 12, 1280. [Google Scholar] [CrossRef]
- Chuev, I.I.; Kovalev, D.Y. Effects of Titanium High-energy Ball Milling on the Solid-phase Reaction Ti+C. Mater. Chem. Phys. 2022, 283, 126025. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, Y.; Gu, J.; Yan, X.; Li, X.; Yu, P.; Wang, L. Synthesis of Nano (Ti,W)C Powder with Preferred Orientation and Twin Boundary Structure. Adv. Powder Technol. 2022, 33, 103550. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Dutta, H.; Pradhan, S.K. XRD and HRTEM Characterization of Mechanosynthesized Ti0.9W0.1C Cermet. J. Alloys Compd. 2013, 581, 710–716. [Google Scholar] [CrossRef]
- Li, F.; Wang, W.; Dang, W.; Xu, Z.; Zhao, K.; Tang, Y. Microstructural Features and Oxidation Resistance of (Ti, Zr)C Solid Solution Nanofibers Fabricated Using Polymeric Precursors. Ceram. Int. 2019, 45, 24941–24945. [Google Scholar] [CrossRef]
- Ke, D.; Pan, Y.; Wu, R.; Xu, Y.; Wang, P.; Wu, T. Effect of Initial Co Content on the Microstructure, Mechanical Properties and High-Temperature Oxidation Resistance of WCoB–TiC Ceramic Composites. Ceram. Int. 2018, 44, 1213–1219. [Google Scholar] [CrossRef]
- Yung, D.L.; Maaten, B.; Antonov, M.; Hussainova, I. Oxidation of Spark Plasma Sintered ZrC–Mo and ZrC–TiC Composites. Int. J. Refract. Metals Hard Mater. 2017, 66, 244–251. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xu, Y.; Yi, M.; Xiao, G.; Chen, Z.; Zhang, J.; Chen, H.; Xu, C. Microstructure and Mechanical Properties of (Ti,W)C Cermets Prepared by Ultrafast Spark Plasma Sintering. Ceram. Int. 2022, 48, 15613–15621. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, S.; Aversano, F.; Bortolotti, M.; Zhang, H.; Hu, C.; Grasso, S. Arc Melting: A Novel Method to Prepare Homogeneous Solid Solutions of Transition Metal Carbides (Zr, Ta, Hf). Ceram. Int. 2019, 45, 9316–9319. [Google Scholar] [CrossRef]
- Pellizzari, M.; Cipolloni, G. Tribological Behavior of Cu Based Materials Produced by Mechanical milling/Alloying and Spark Plasma Sintering. Wear 2017, 376–377, 958–967. [Google Scholar] [CrossRef]
- Dudina, D.V.; Vidyuk, T.M.; Gavrilov, A.I.; Ukhina, A.V.; Bokhonov, B.B.; Legan, M.A.; Matvienko, A.A.; Korchagin, M.A. Separating the Reaction and Spark Plasma Sintering Effects During the Formation of TiC–Cu Composites from Mechanically Milled Ti–C–3Cu Mixtures. Ceram. Int. 2021, 47, 12494–12504. [Google Scholar] [CrossRef]
- Esteki, S.; Saeidi, R.; Dini, G.; Milani, M. Fabrication of Silicon Carbide Ceramics by Combination of Slip Casting and Spark Plasma Sintering. Mater. Chem. Phys. 2023, 297, 127418. [Google Scholar] [CrossRef]
- Dudina, D.V.; Grigoreva, T.F.; Devyatkina, E.T.; Vosmerikov, S.V.; Ukhina, A.V.; Markushin, V.V.; Lyakhov, N.Z. Structural Features of Tantalum Carbide-copper Composites Obtained by Liquid Phase-assisted Spark Plasma Sintering. Ceram. Int. 2022, 48, 32556–32560. [Google Scholar] [CrossRef]
- Lamim, D.D.; de Oliveira, H.C.P.; Batista, A.C.; Guimarães, R.S.; Filgueira, M. Use of Ti in Hard Metal Alloys—Part I: Structural and Microstructural Analysis. Mater. Werkst. 2010, 41, 198–201. [Google Scholar] [CrossRef]
- Ouar, N.; Bousnina, M.A.; Schoenstein, F.; Mercone, S.; Brinza, O.; Farhat, S.; Jouini, N. Spark Plasma Sintering of Co80Ni20 Nanopowders Synthesized by Polyol Process and their Magnetic and Mechanical Properties. J. Alloys Compd. 2014, 615, S269–S275. [Google Scholar] [CrossRef]
- Fang, G.; Tang, H.; Ren, Z.; Cheng, Y.; Yu, Y.; Wang, L.; Li, T.; Zhang, Y.; Qiao, Z. Effect of Grain Size on Oxidation Resistance of WC–6wt% Co Cemented Carbide Sintered by Spark Plasma Sintering. Int. J. Refract. Met. Hard Mater. 2023, 111, 106108. [Google Scholar] [CrossRef]
- Podbolotov, K.; Moskovskikh, D.; Abedi, M.; Suvorova, V.; Nepapushev, A.; Ostrikov, K.; Khort, A. Low-temperature Reactive Spark Plasma Sintering of Dense SiC–Ti3SiC2 Ceramics. J. Eur. Ceram. Soc. 2023, 43, 1343–1351. [Google Scholar] [CrossRef]
- Tripathy, H.; Sudha, C.; Thomas Paul, V.; Thirumurugesan, R.; Prasanthi, T.N.; Sundar, R.; Vijayashanthi, N.; Parameswaran, P.; Raju, S. High Temperature Thermophysical Properties of Spark Plasma Sintered Tungsten Carbide. Int. J. Refract. Met. Hard Mater. 2022, 104, 105804. [Google Scholar] [CrossRef]
- Ke, B.; Ji, W.; Zou, J.; Wang, W.; Fu, Z. Densification Mechanism, Microstructure and Mechanical Properties of ZrC Ceramics Prepared by High–pressure Spark Plasma Sintering. J. Eur. Ceram. Soc. 2023, 43, 3053–3061. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Yang, L.; Cheng, Y.; Cai, Z.; Mehrizi, M.Z. Microstructure and Properties of NiAl/TiC Composite Synthesized by Spark Plasma Sintering of Mechanically Activated Elemental Powders. Ceram. Int. 2023, 49, 15710–15716. [Google Scholar] [CrossRef]
- Lee, J.; Jang, K.; Lee, S.; Mo, C.B.; Kim, H.; Park, K.R.; Kim, J.; Bang, J.; Jung, I.C.; Kim, J.C.; et al. Mechanical Properties of TiC Reinforced MgO–ZrO2 Composites via Spark Plasma Sintering. Ceram. Int. 2023; in press. [Google Scholar] [CrossRef]
- Lou, Z.; Li, Y.; Zou, Q.; Luo, W.; Gu, H.; Li, Z.; Luo, Y. In-situ Fabrication and Characterization of TiC Matrix Composite Reinforced by SiC and Ti3SiC2. Ceram. Int. 2023; in press. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line Profiles of Neutron Powder-Diffraction Peaks for Structure Refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Wiles, D.B.; Young, R.A. A New Computer Program for Rietveld Analysis of X-ray Powder Diffraction Patterns. J. Appl. Cryst. 1981, 14, 149–151. [Google Scholar] [CrossRef]
- Young, R.A.; Wiles, D.B. Profile Shape Functions in Rietveld Refinements. J. Appl. Cryst. 1982, 15, 430–438. [Google Scholar] [CrossRef]
- Young, R.A. (Ed.) The Rietveld Method; Oxford University Press/IUCr: Oxford, UK, 1996; pp. 1–38. [Google Scholar]
- Lutterotti, L.; Scardi, P.; Maistrelli, P. LSI-a Computer Program for Simultaneous Refinement of Material Structure and Microstructure. J. Appl. Cryst. 1992, 25, 459–462. [Google Scholar] [CrossRef]
- Ghosh, B.; Dutta, H.; Pradhan, S.K. Microstructure Characterization of Nanocrystalline Ni3C Synthesized by High-energy Ball Milling. J. Alloys Comp. 2009, 479, 193–200. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction; Chapter 13; Addison-Wesley, Reading: Boston, MA, USA, 1969. [Google Scholar]
- Oldfield, J.W. Electrochemical Theory of Galvanic Corrosion, STP978 Galvanic Corrosion; Hack, H.P., Ed.; ASTM Int. West Conshohocken: Conshohocken, PA, USA, 1988; pp. 5–22. [Google Scholar]
- Nelson, E.E. Discussion of the Mechanism of Passivating-Type Inhibitors [M. Stern (pp. 638–647, Vol. 105)]. J. Electrochem. Soc. 1959, 106, 540. [Google Scholar] [CrossRef]
- Luiz, L.A.; de Andrade, J.; Pesqueira, C.M.; Siqueira, I.B.d.A.F.; Sucharski, G.B.; de Sousa, M.J. Corrosion Behavior and Galvanic Corrosion Resistance of WC and Cr3C2 Cermet Coatings in Madeira River Water. J. Therm. Spray Technol. 2021, 30, 205–221. [Google Scholar] [CrossRef]
- Kunrath, A.O.; Reimanis, I.E.; Moore, J.J. Microstructural Evolution of Titanium Carbide–Chromium Carbide (TiC–Cr3C2) Composites Produced via Combustion Synthesis. J. Am. Ceram. Soc. 2002, 85, 1285–1290. [Google Scholar] [CrossRef]
- Booker, P.H.; Kunrath, A.O.; Hepworth, M.T. Experimental Determination of the Ternary Diagram of the Ti-Cr-C System. Acta Mater. 1997, 45, 1625–1632. [Google Scholar] [CrossRef]
- Feng, P.; Xiong, W.; Yu, L.; Zheng, Y.; Xia, Y. Phase Evolution and Microstructure Characteristics of Ultrafine Ti(C,N) –based Cermet by Spark Plasma Sintering. Int. J. Refract. Met. Hard Mater. 2004, 22, 133–138. [Google Scholar] [CrossRef]
- Soundaraj, P.V.; Sembulingam, S.S.; Shanmugavel, B.P. On the Role of B4C on Hardness and Toughness of TiCN–SiC–TiN–Cr3C2–Co Cermet. Int. J. Refract. Met. Hard Mater. 2020, 90, 105252. [Google Scholar] [CrossRef]
- Soria, T.; Lopez, B.; Lozada, L.; Moseley, S.; Alveen, P.; Elsen, M.; Müller-Grunz, A.; Magin, M.; Useldinger, R.; Sánchez, J.M. An Investigation into the Effects of HIP After Sintering of WC–ZrC–Co–Cr3C2 Cemented Carbides. Int. J. Refract. Met. Hard Mater. 2020, 87, 105164. [Google Scholar] [CrossRef]
- Bousnina, M.A.; Schoenstein, F.; Mercone, S.; Jouini, N. From Ni–P Metastable Alloy Nanoparticles to Bulk Submicrometer Grain-Sized MMCs with Tunable Mechanical and Magnetic Properties. Metals. 2020, 10, 112. [Google Scholar] [CrossRef]
- Shankar, E.; Prabu, S.B.; Padmanabhan, K.A. Mechanical Properties and Microstructures of TiCN/nano-TiB2/TiN Cermets Prepared by Spark Plasma Sintering. Ceram. Int. 2018, 44, 9384–9394. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, Y.; Li, Z.; Zhou, K. New Production of (Ti, W)C–based Cermets Toughened by In-situ Formed WC and Twinned (Ti, W)C Platelets: Carbonization of The Nix(Ti0.6, W0.4)4C–type η Phases. J. Alloys Compd. 2018, 731, 253–263. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, Q.; Gu, J.; Zhong, Z.; Long, J.; Wang, C. Strengthening and Toughening of Ti(C,N)–based Cermets: (Ti,W)C Additive Design and The Mechanism. Int. J. Refract. Met. Hard Mater. 2022, 103, 105758. [Google Scholar] [CrossRef]
- Available online: https://www.gordonengland.co.uk/hardness/hvconv.htm (accessed on 9 January 2023).
- Abderrazak, H.; Schoenstein, F.; Abdellaoui, M.; Jouini, N. Spark Plasma Sintering Consolidation of Nanostructured TiC Prepared by Mechanical Alloying. Int. J. Refract. Met. Hard Mater. 2011, 29, 170–176. [Google Scholar] [CrossRef]
- Wan, W.; Xiong, J.; Yang, M.; Guo, Z.; Dong, G.; Yi, C. Effects of Cr3C2 Addition on the Corrosion Behavior of Ti(C, N)-based Cermets. Int. J. Refract. Met. Hard Mater. 2012, 31, 179–186. [Google Scholar] [CrossRef]
- Chen, S.; Xiong, W.; Yao, Z.; Zhang, G.; Chen, X.; Huang, B.; Yang, Q. Corrosion Behavior of Ti(C,N)-Ni/Cr Cermets in H2SO4 Solution. Int. J. Refract. Met. Hard Mater. 2014, 47, 139–144. [Google Scholar] [CrossRef]
- Vorotilo, S.; Kiryukhantsev-Korneev, P.V.; Seplyarskii, B.S.; Kochetkov, R.A.; Abzalov, N.I.; Kovalev, I.D.; Lisina, T.G.; Zaitsev, A.A. (Ti,Cr)C–Based Cermets with Varied NiCr Binder Content via Elemental SHS for Perspective Cutting Tools. Crystals 2020, 10, 412. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Li, X. Corrosion of Metallic Materials Fabricated by Selective Laser Melting. NPJ Mater. Degrad. 2019, 3, 24. [Google Scholar] [CrossRef]
- He, L.; Gao, Y.; Li, Y.; Liu, Z.; Huo, X.; Zhai, W. Effect of Milling Time on Powder’s Structure Evolution of Ti(C,N)–304 Stainless Steel Cermet. Mater. Res. Express 2018, 5, 36516. [Google Scholar] [CrossRef]
- Memarrashidi, Z.; Plucknett, K.P. Factors Influencing the Aqueous Electrochemical Response of TiC–Ni3Al Cermets. J. Mater. Res. 2017, 32, 3333–3343. [Google Scholar] [CrossRef]
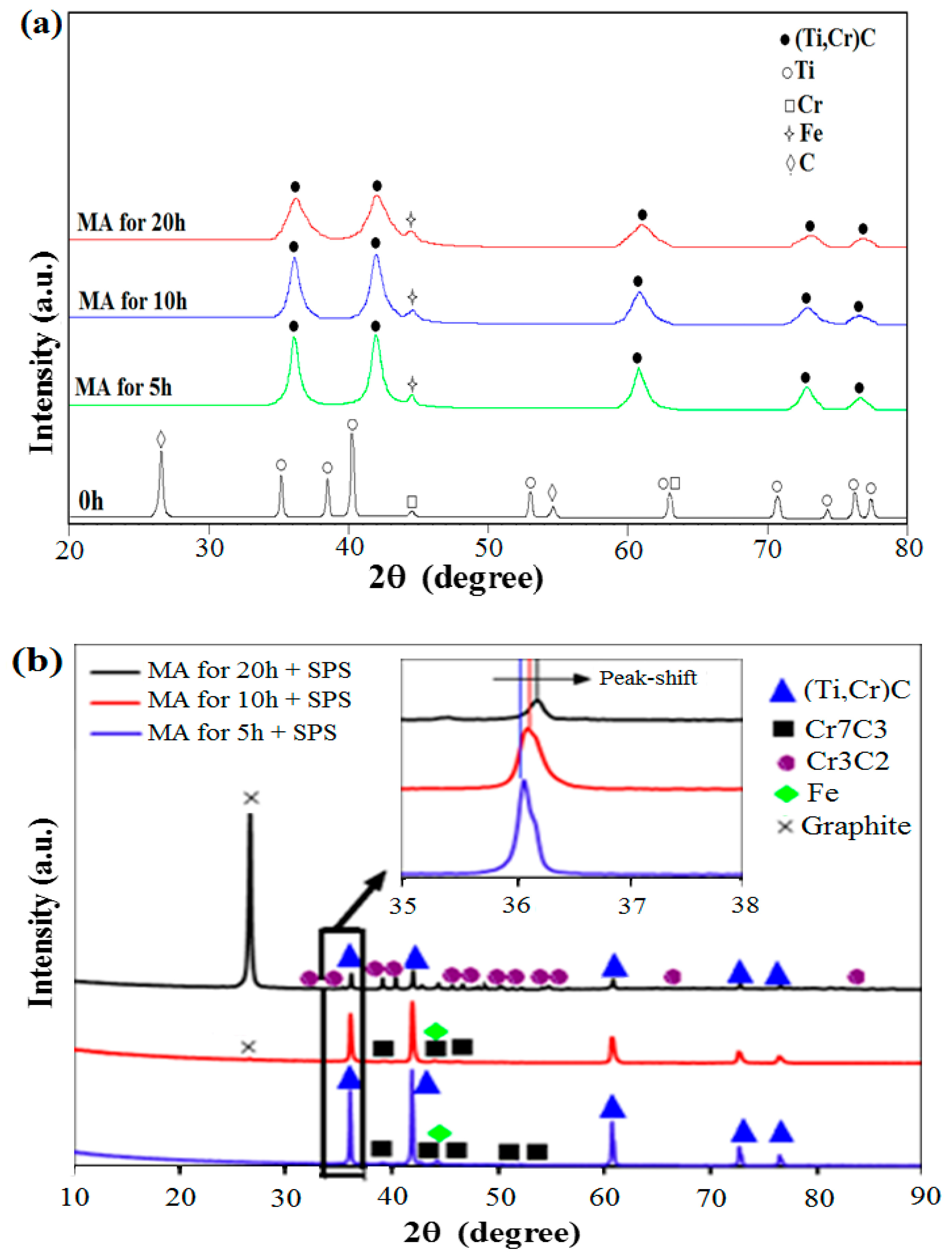


| Milling Time (h) | Crystallite Size in Powder (nm) | Crystallite Size in SPS Sample (nm) |
|---|---|---|
| 5 | 22 | 219 |
| 10 | 15 | 58 |
| 20 | 11 | 100 |
| Sample | Density (g/cm3) | Relative Density (%) | Rockwell Hardness (HRA) |
|---|---|---|---|
| 5 h + SPS | 5.029 | 98.43 | 93.3 ± 0.3 |
| 10 h + SPS | 4.452 | 87.14 | 83.2 ± 1.7 |
| 20 h + SPS | 5.033 | 98.51 | 91.5 ± 0.4 |
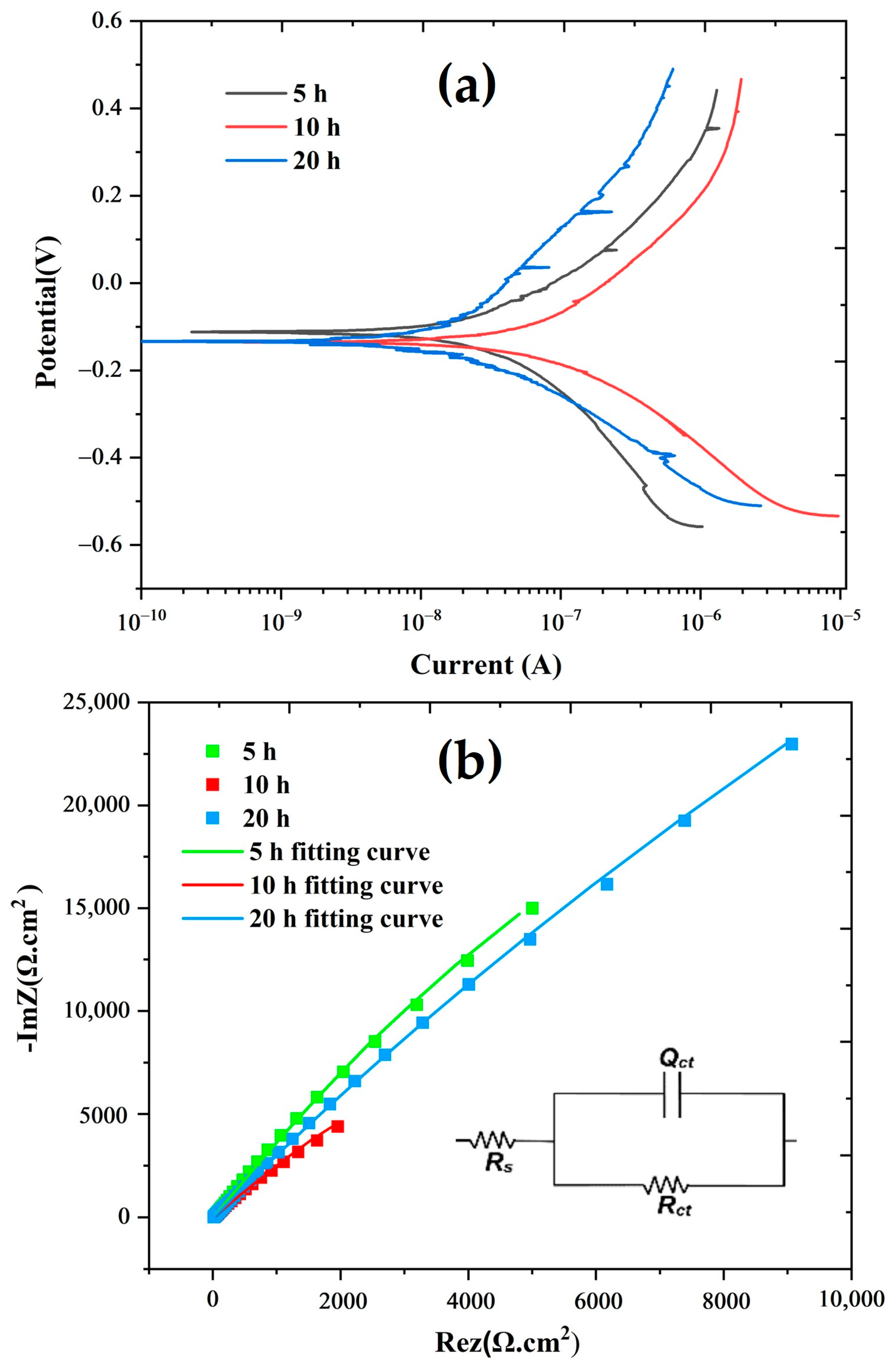
| Sample | Ecorr (V) | icorr (µA/cm2) | BetaA (V/decade) | BetaC (V/decade) | CR (mm/year) | Rp (kΩ.cm2) | Rct (kΩ.cm2) |
|---|---|---|---|---|---|---|---|
| 5 h + SPS | −0.112 | 0.2742 | 0.2819 | 0.3295 | 0.00236 | 240.57 | 302.24 |
| 10 h + SPS | −0.135 | 0.5654 | 0.3189 | 0.2132 | 0.00487 | 98.12 | 128.99 |
| 20 h + SPS | −0.133 | 0.1270 | 0.3698 | 0.1685 | 0.00110 | 395.63 | 371.68 |
| TiC-Ni3Al [55] | −0.049 | 0.0137 | - | - | 0.00045 | - | - |
| SPS-TiC pure [55] | 0.005 | 0.0422 | - | - | 0.00029 | - | - |
| SPS-TiWC [55] | −0.176 | 1.28 | - | - | 0.00781 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhadhbi, M.; Dağ, İ.E.; Avar, B.; Khitouni, M.; Bousnina, M.A.; Schoenstein, F.; Jouini, N. Manufacturing of Novel Nanostructured TiCrC Carbides Using Mechanical Alloying and Spark Plasma Sintering. Metals 2023, 13, 1040. https://doi.org/10.3390/met13061040
Mhadhbi M, Dağ İE, Avar B, Khitouni M, Bousnina MA, Schoenstein F, Jouini N. Manufacturing of Novel Nanostructured TiCrC Carbides Using Mechanical Alloying and Spark Plasma Sintering. Metals. 2023; 13(6):1040. https://doi.org/10.3390/met13061040
Chicago/Turabian StyleMhadhbi, Mohsen, İlker Emin Dağ, Barış Avar, Mohamed Khitouni, Mohamed Ali Bousnina, Frédéric Schoenstein, and Noureddine Jouini. 2023. "Manufacturing of Novel Nanostructured TiCrC Carbides Using Mechanical Alloying and Spark Plasma Sintering" Metals 13, no. 6: 1040. https://doi.org/10.3390/met13061040






