First-Principles Study of Oxygen in ω-Zr
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
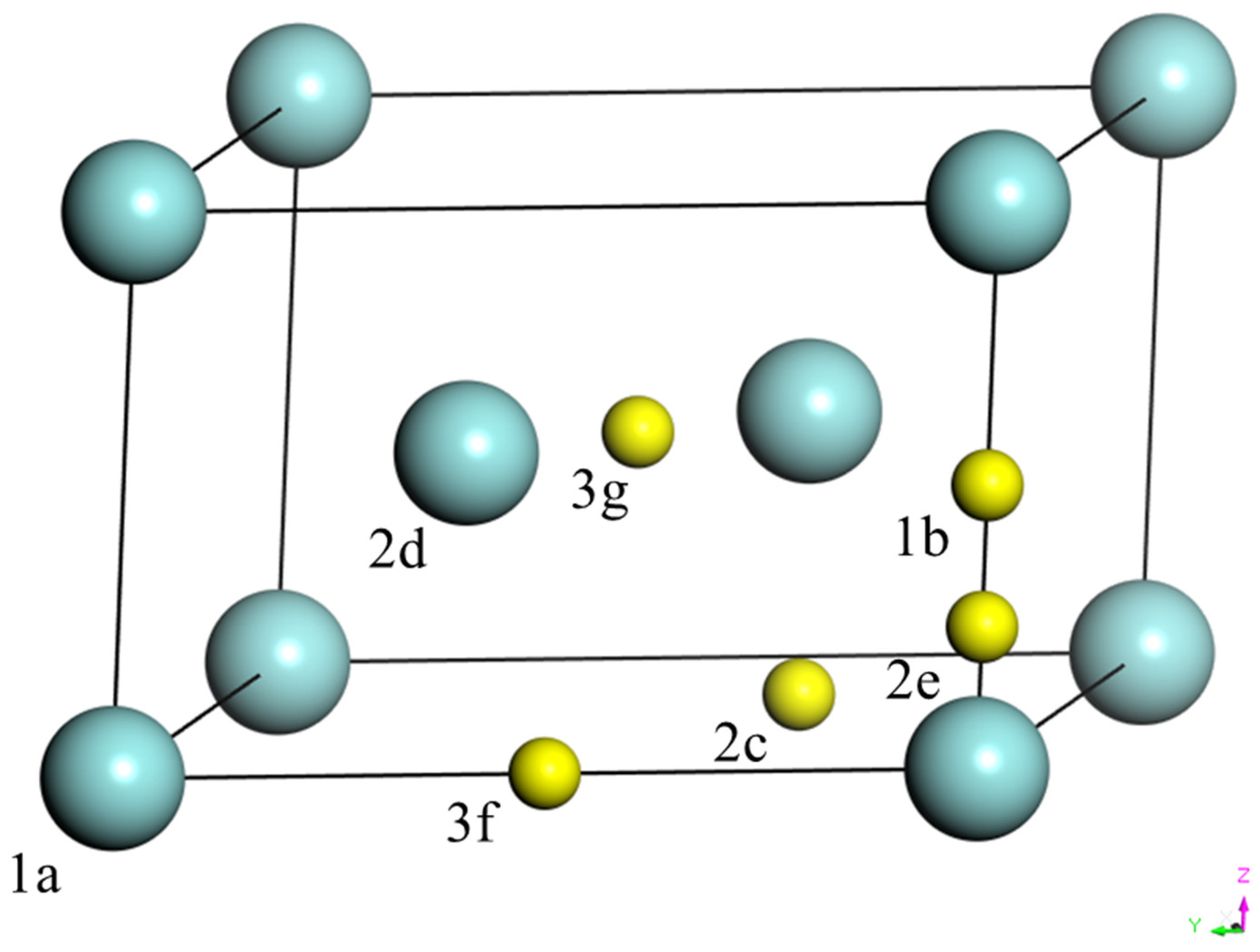
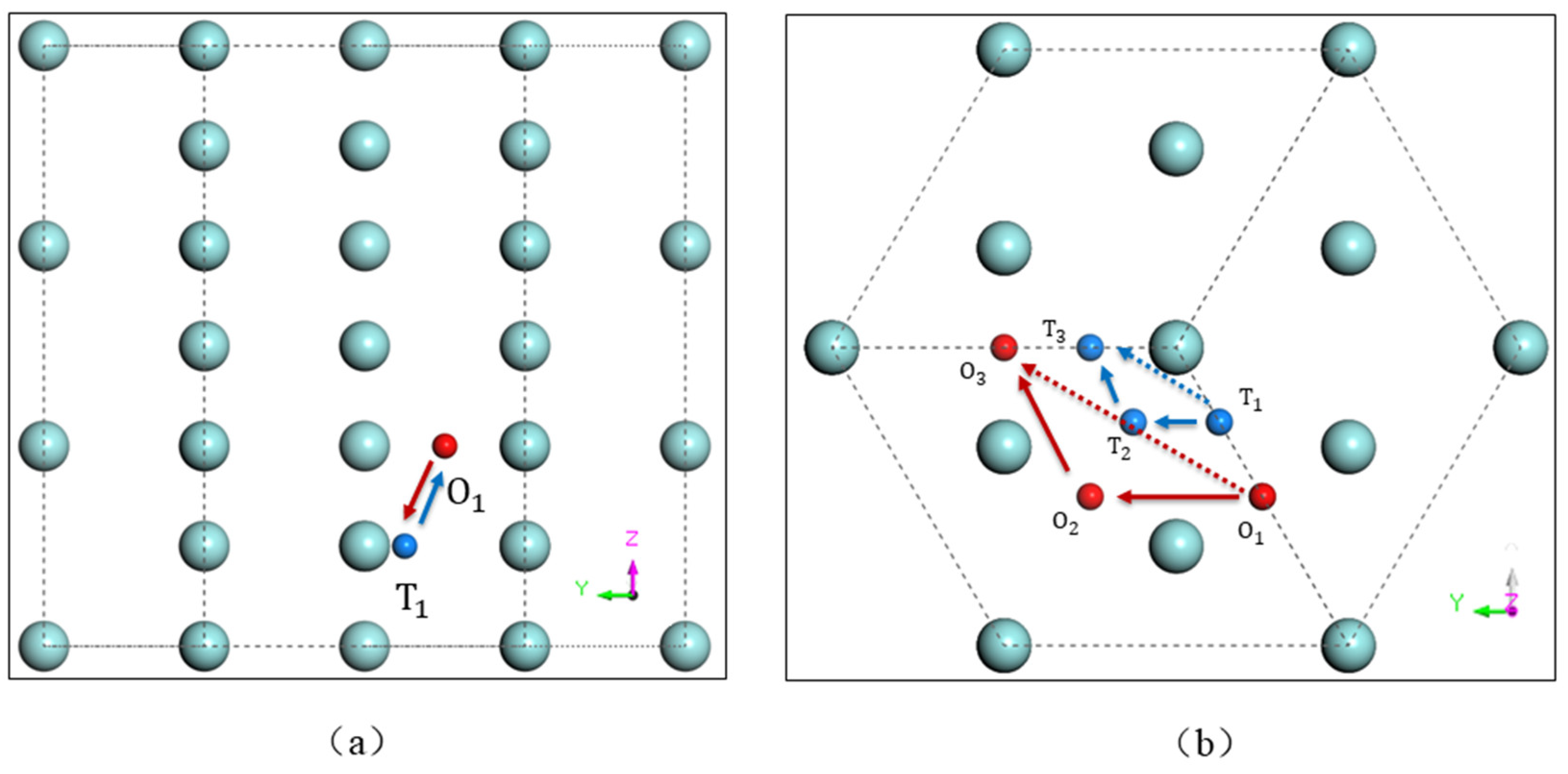
3.1. Oxygen in ω-Zr
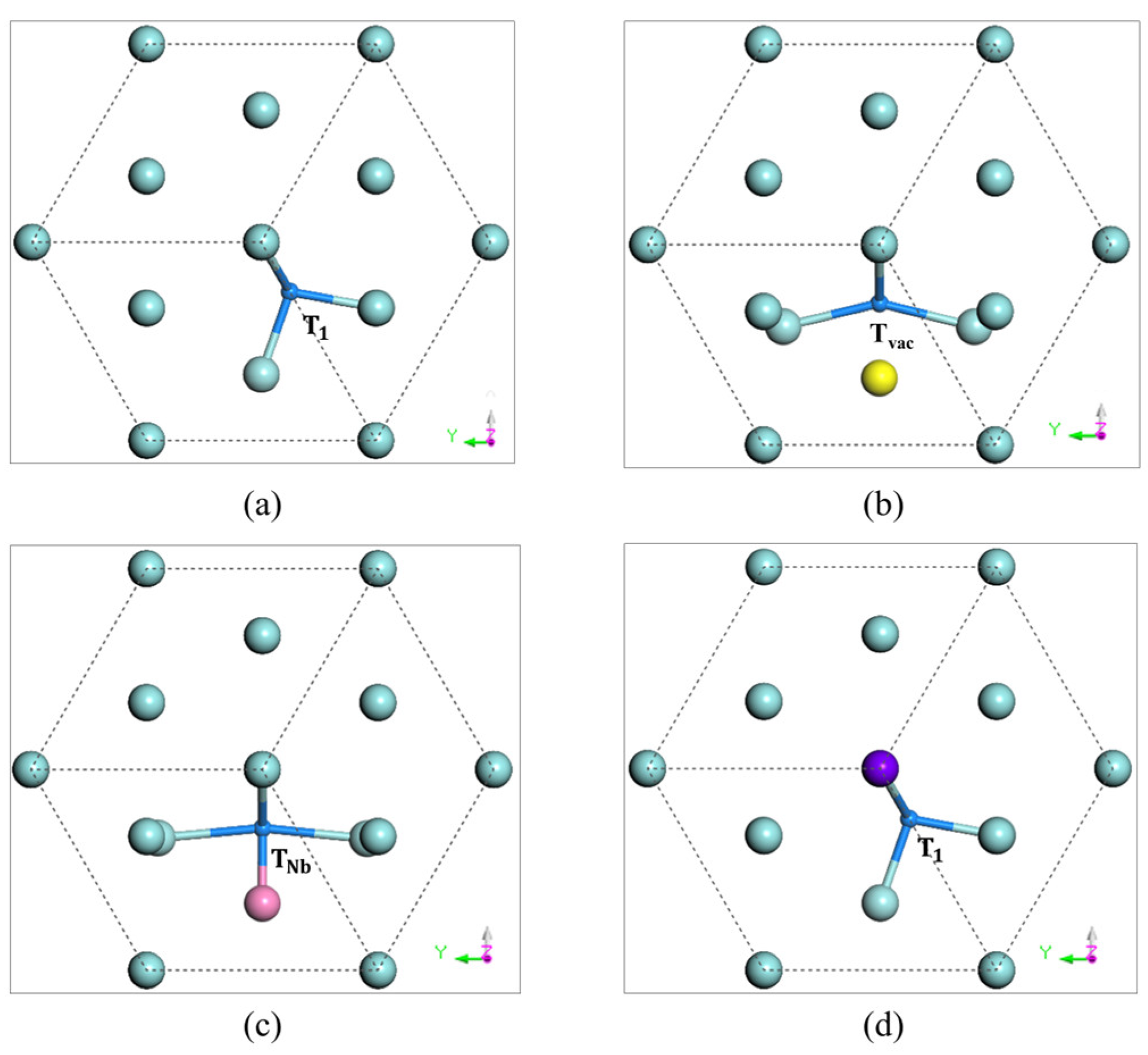
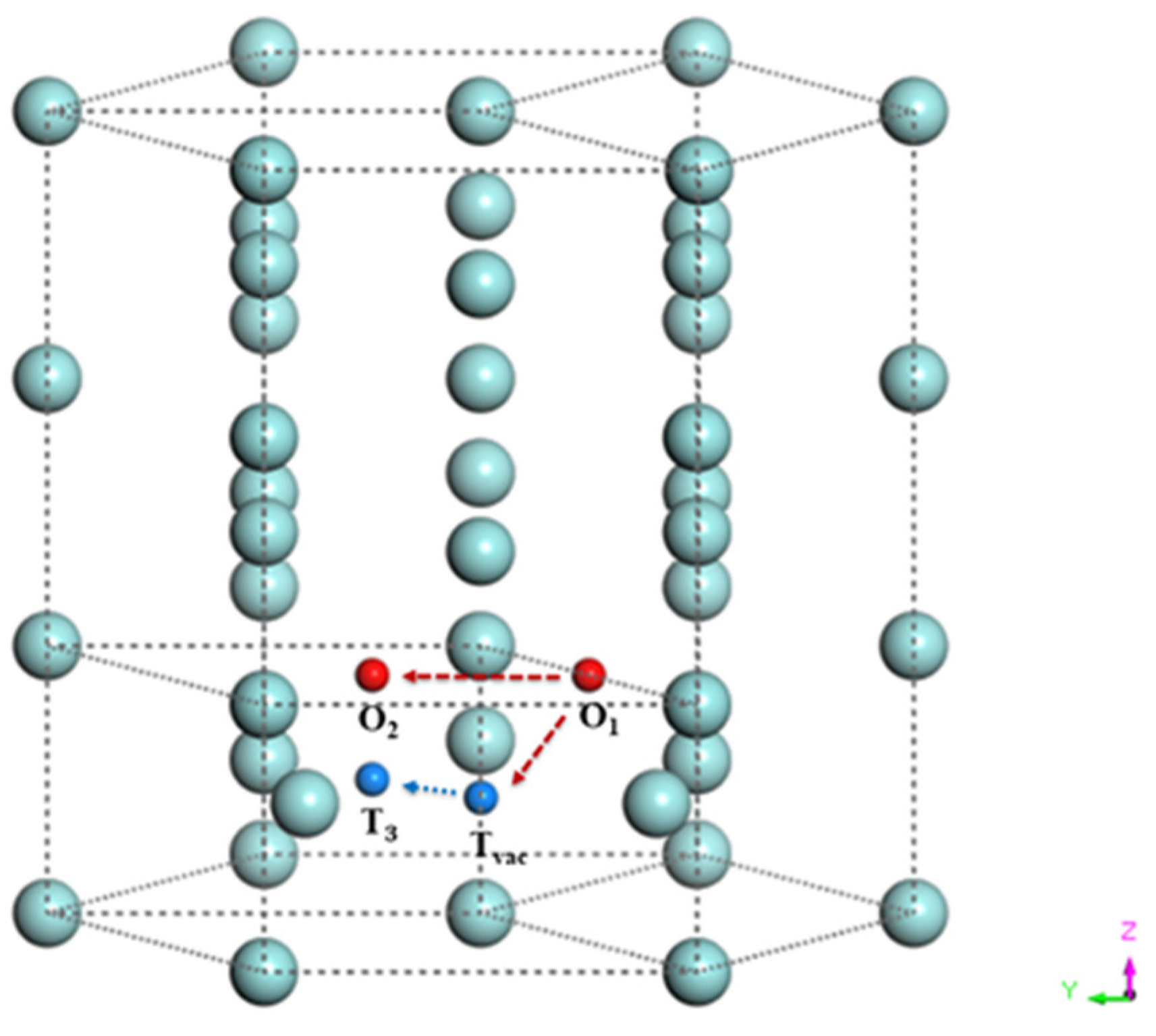
3.2. Oxygen-Vacancy Interaction in -Zr
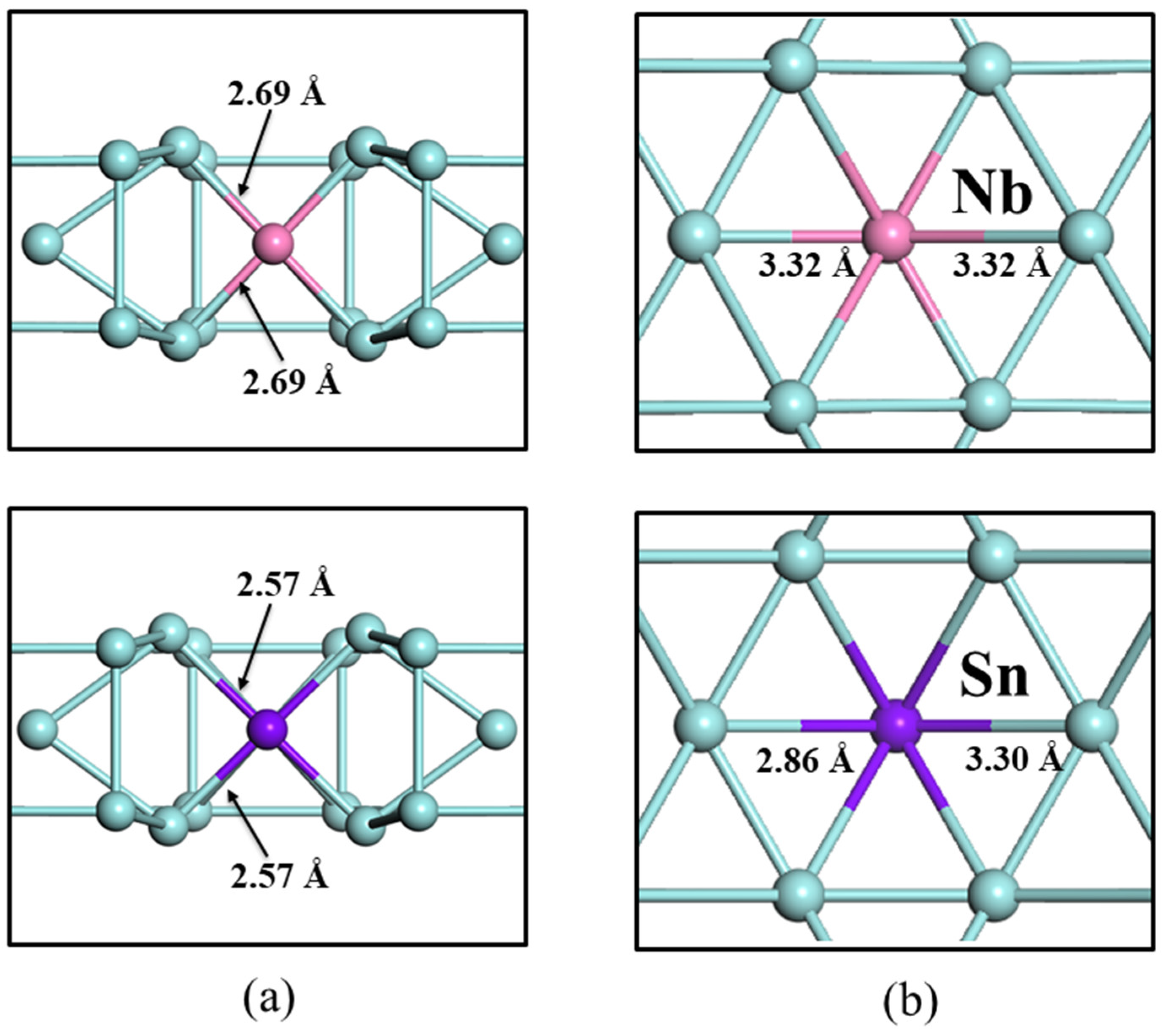
3.3. Effect of Alloying Elements (Nb, Sn) on Oxygen in ω-Zr
3.4. O Atoms Clustering in ω-Zr
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshi, P.P.; Kumar, N.; Murty, K.L. Materials for Nuclear Reactors. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Oxford, UK, 2022; pp. 364–376. [Google Scholar] [CrossRef]
- Yang, J.; Steinbrück, M.; Tang, C.; Grosse, M.; Liu, J.; Zhang, J.; Yun, D.; Wang, S. Review on chromium coated zirconium alloy accident tolerant fuel cladding. J. Alloys Compd. 2022, 895, 162450. [Google Scholar] [CrossRef]
- Anzellini, S.; Bottin, F.; Bouchet, J.; Dewaele, A. Phase transitions and equation of state of zirconium under high pressure. Phys. Rev. B 2020, 102, 184105. [Google Scholar] [CrossRef]
- Arul Kumar, M.; Hilairet, N.; McCabe, R.J.; Yu, T.; Wang, Y.; Beyerlein, I.J.; Tomé, C.N. Role of twinning on the omega-phase transformation and stability in zirconium. Acta Mater. 2020, 185, 211–217. [Google Scholar] [CrossRef]
- Zwolinski, A.; Letocha, A.; Cyboron, J.; Noga, P.; Skrzekut, T.; Podsiadlo, M.; Lis, L.; Jaworska, L.; Boczkal, G. Influence of the high-pressure ω—Zr phase on selected properties of sintered zirconium powder materials. Int. J. Refract. Met. Hard Mater. 2023, 110, 106036. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J. Enhancement of yield strength in zirconium metal through high-pressure induced structural phase transition. Appl. Phys. Lett. 2007, 91, 201907. [Google Scholar] [CrossRef]
- Catledge, S.A.; Spencer, P.T.; Vohra, Y.K. Nanoindentation hardness and atomic force microscope imaging studies of pressure-quenched zirconium metal. Appl. Phys. Lett. 2000, 77, 3568–3570. [Google Scholar] [CrossRef]
- Silcock, J.M. An X-ray examination of the to phase in TiV, TiMo and TiCr alloys. Acta Metall. 1958, 6, 481–493. [Google Scholar] [CrossRef]
- Jaworska, L.; Cyboron, J.; Cygan, S.; Zwolinski, A.; Onderka, B.; Skrzekut, T. Zirconium phase transformation under static high pressure and ω-Zr phase stability at high temperatures. Materials 2019, 12, 2244. [Google Scholar] [CrossRef]
- Zong, H.; He, P.; Ding, X.; Ackland, G.J. Nucleation mechanism for hcp → bcc phase transformation in shock-compressed Zr. Phys. Rev. B 2020, 101, 144105. [Google Scholar] [CrossRef]
- Zoologische Garten Zool; GartenAnpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, Z. Characterisation and reactivity of oxygen species at the surface of metal oxides. J. Catal. 2021, 393, 259–280. [Google Scholar] [CrossRef]
- Trush, V.S.; Stoev, P.I.; Luk’yanenko, A.G.; Voyevodin, V.M.; Pohrelyuk, I.M.; Fedirko, V.M.; Kovtun, K.V.; Kravchyshyn, T.M. Influence of Oxidation on the Properties of Near-Surface Layers of Metals from Group IV (Ti, Zr, and Hf). Mater. Sci. 2022, 57, 649–655. [Google Scholar] [CrossRef]
- Abriata, J.; Versaci, R.; Garces, J. Alloy Phase Diagrams. Bull. Alloy Phase Diagr. 1989, 10, 606–616. [Google Scholar] [CrossRef]
- Yamanaka, S.; Tanaka, T.; Miyake, M. Effect of oxygen on hydrogen solubility in zirconium. J. Nucl. Mater. 1989, 167, 231–237. [Google Scholar] [CrossRef]
- Liu, Z.; Welsch, G. Effects of oxygen and heat treatment on the mechanical properties of alpha and beta titanium alloys. Metall. Trans. A 1988, 19, 527–542. [Google Scholar] [CrossRef]
- Paton, N.E.; Williams, J.C. The influence of oxygen content on the athermal β-ω transformation. Scr. Metall. 1973, 7, 647–649. [Google Scholar] [CrossRef]
- Williams, J.C.; Hickman, B.S.; Leslie, D.H. The effect of ternary additions on the decompositon of metastable beta-phase titanium alloys. Metall. Trans. 1971, 2, 477–484. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hendrickson, M.; Nandwana, P.; Alam, T.; Choudhuri, D.; Banerjee, R. Influence of oxygen on omega phase stability in the Ti-29Nb-13Ta-4.6Zr alloy. Scr. Mater. 2016, 123, 144–148. [Google Scholar] [CrossRef]
- Joshi, P.; Tillman, M.; Kumar, N.; Murty, K.; Cinbiz, N. Biaxial Creep Behavior of Nb-Modified Zircaloys. Nucl. Technol. 2020, 206, 706–716. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Liu, L.; Ding, X.; Kuang, W.; Wang, M. Oxidation of typical Zr-1 Nb alloy in high-temperature air under 700–900 °C. Corros. Sci. 2022, 209, 110701. [Google Scholar] [CrossRef]
- Moorehead, M.; Yu, Z.; Borrel, L.; Hu, J.; Cai, Z.; Couet, A. Comprehensive investigation of the role of Nb on the oxidation kinetics of Zr-Nb alloys. Corros. Sci. 2019, 155, 173–181. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, J.; Hu, P.; Qiu, J.; Xie, S.; Meng, R.; Liu, W.; Lu, J.; Cui, Y.; Li, C.; et al. Role of microchannels in breakaway oxidation of Zr alloy under high-temperature steam oxidation at 1000 °C. Corros. Sci. 2022, 199, 110204. [Google Scholar] [CrossRef]
- Xu, S.; Huang, J.; Pei, W.; Yao, M.; Hu, L.; Xie, Y.; Lin, X.; Liang, X.; Peng, J.; Xu, J.; et al. Effect of oxygen content in 400 °C super-heated steam on the corrosion resistance of Zr-xSn-0.35Fe-0.15Cr-0.15 Nb alloys. Corros. Sci. 2022, 198, 110135. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, T.; Liao, J.; Song, P.; Peng, X.; Liao, Z.; Peng, Q.; He, X. High-temperature oxidation behavior of Zr-4 and Zr-Sn-Nb alloy in different oxidation ambient. J. Alloys Compd. 2021, 887, 161396. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B Condens Matter 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Sheppard, D.; Xiao, P.; Chemelewski, W.; Johnson, D.D.; Henkelman, G. A generalized solid-state nudged elastic band method. J. Chem. Phys. 2012, 136, 074103. [Google Scholar] [CrossRef]
- Smidstrup, S.; Pedersen, A.; Stokbro, K.; Jonsson, H. Improved initial guess for minimum energy path calculations. J. Chem. Phys. 2014, 140, 214106. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Pantea, C.; Qian, J.; Daemen, L.L.; Rigg, P.A.; Hixson, R.S.; Gray, G.T.; Yang, Y.; Wang, L.; et al. Thermal equations of state of the alpha, beta, and omega phases of zirconium. Phys. Rev. B 2005, 71, 184119. [Google Scholar] [CrossRef]
- Greeff, C.W. Phase changes and the equation of state of Zr. Model. Simul. Mater. Sci. Eng. 2005, 13, 1015. [Google Scholar] [CrossRef]
- You, D.; Ganorkar, S.; Joo, M.; Park, D.; Kim, S.; Kang, K.; Lee, D. Ab initio study of H, B, C, N, O, and self-interstitial atoms in hcp-Zr. J. Alloys Compd. 2019, 787, 631–637. [Google Scholar] [CrossRef]
- Yang, J.; Youssef, M.; Yildiz, B. Oxygen self-diffusion mechanisms in monoclinic ZrO2 revealed and quantified by density functional theory, random walk analysis, and kinetic Monte Carlo calculations. Phys. Rev. B 2018, 97, 024114. [Google Scholar] [CrossRef]
- Wu, H.H.; Wisesa, P.; Trinkle, D.R. Oxygen diffusion in hcp metals from first principles. Phys. Rev. B 2016, 94, 014307. [Google Scholar] [CrossRef]
- Wollenberger, H. Point defects. In Physical Metallurgy, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Fang, H.Z.; Shang, S.L.; Wang, Y.; Liu, Z.K.; Alfonso, D.; Alman, D.E.; Shin, Y.K.; Zou, C.Y.; van Duin, A.C.T.; Lei, Y.K.; et al. First-principles studies on vacancy-modified interstitial diffusion mechanism of oxygen in nickel, associated with large-scale atomic simulation techniques. J. Appl. Phys. 2014, 115, 043501. [Google Scholar] [CrossRef]
- Christensen, M.; Wolf, W.; Freeman, C.M.; Wimmer, E.; Adamson, R.B.; Hallstadius, L.; Cantonwine, P.E.; Mader, E.V. Effect of alloying elements on the properties of Zr and the Zr–H system. J. Nucl. Mater. 2014, 445, 241–250. [Google Scholar] [CrossRef]
- Lu, H.-J.; Wu, H.; Zou, N.; Lu, X.-G.; He, Y.-L.; Morgan, D. First-principles investigation on diffusion mechanism of alloying elements in dilute Zr alloys. Acta Mater. 2018, 154, 161–171. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Liu, F.; Shu, J.; Liu, Y.; Dong, Z.; Liu, Y. Alloying Effects on the Oxygen Diffusion in Nb Alloys: A First-Principles Study. Metall. Mater. Trans. A 2021, 52, 270–283. [Google Scholar] [CrossRef]
| This Work | Zhao [35] | Greeff [36] | |
|---|---|---|---|
| a (Å) | 5.056 | 5.039 | 5.050 |
| c/a | 0.623 | 0.658 | 0.623 |
| Initial Position | Coordinates | Final Position | Formation Energies | Ref. [37] |
|---|---|---|---|---|
| 1b | 1b | −2.76 | - | |
| 2c | , 0 | Octa | −5.97 | - |
| 2e | 0, 0, z | Tetra | −4.62 | −4.51 |
| 3f | , 0 | Octa | −5.96 | −5.44 |
| 3g | 3g | −2.11 | - |
| Migration Path | ω-Zr | α-Zr [39] |
|---|---|---|
| O1 → O2 | 2.39 | 2.94 |
| O1 → O3 | 2.40 | - |
| O1 → T1 | 2.00 | 1.82 |
| T1 → T2 | 0.01 | - |
| T1 → T3 | 0.01 | - |
| T1 → O1 | 0.66 | - |
| Paths | With Vacancy | Pure |
|---|---|---|
| Tvac → T3 | 1.03 | 0.01 |
| O1 → Tvac | 0.50 | 2.00 |
| O1 → O2 | 1.50 | 2.40 |
| Elements | Substitution | Interstitial | ||
|---|---|---|---|---|
| 1b | 2d | Octa | Tetra | |
| Nb | 0.78 | 0.42 | 3.90 | 3.45 |
| Sn | −0.97 | −0.74 | 3.24 | 3.38 |
| Systems | Octa | Tetra |
|---|---|---|
| Pure | −5.96 | −4.62 |
| With vacancy | −5.49 | −5.53 |
| With Nb | −11.04 | −9.77 |
| With Sn | −12.12 | −9.84 |
| Path | with Nb | with Sn | Pure | Ref. [44] |
|---|---|---|---|---|
| O1 → TNb | 1.32 | - | - | 1.65 |
| TNb → T3 | 0.38 | - | - | - |
| O1 → O2 | 1.05 | 3.36 | 2.40 | 0.96 |
| O1 → T1 | - | 2.86 | 2.00 | - |
| T1 → T2 | - | 0.88 | 0.01 | - |
| Number of O Atoms | System | 1 nn | 2 nn | 3 nn |
|---|---|---|---|---|
| O2 | Pure | −0.32 | −0.08 | 0.01 |
| With Vacancy | −1.32 | −1.38 | −1.19 | |
| With Nb | −0.50 | −0.06 | 0.04 | |
| With Sn | −0.52 | −0.45 | −0.05 | |
| O3 | Pure | −0.33 | −0.08 | −0.33 |
| With Nb | −0.51 | −0.06 | −0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Z.; Wang, D.; Zhao, Y. First-Principles Study of Oxygen in ω-Zr. Metals 2023, 13, 1042. https://doi.org/10.3390/met13061042
Chen Y, Liu Z, Wang D, Zhao Y. First-Principles Study of Oxygen in ω-Zr. Metals. 2023; 13(6):1042. https://doi.org/10.3390/met13061042
Chicago/Turabian StyleChen, Yonghao, Zhixiao Liu, Dong Wang, and Yi Zhao. 2023. "First-Principles Study of Oxygen in ω-Zr" Metals 13, no. 6: 1042. https://doi.org/10.3390/met13061042
APA StyleChen, Y., Liu, Z., Wang, D., & Zhao, Y. (2023). First-Principles Study of Oxygen in ω-Zr. Metals, 13(6), 1042. https://doi.org/10.3390/met13061042






