Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies
Abstract
:1. Introduction
2. Polyphenols as Rust Converter Layers on Steel Substrates

3. Coatings for Corrosion Protection with Polyphenols
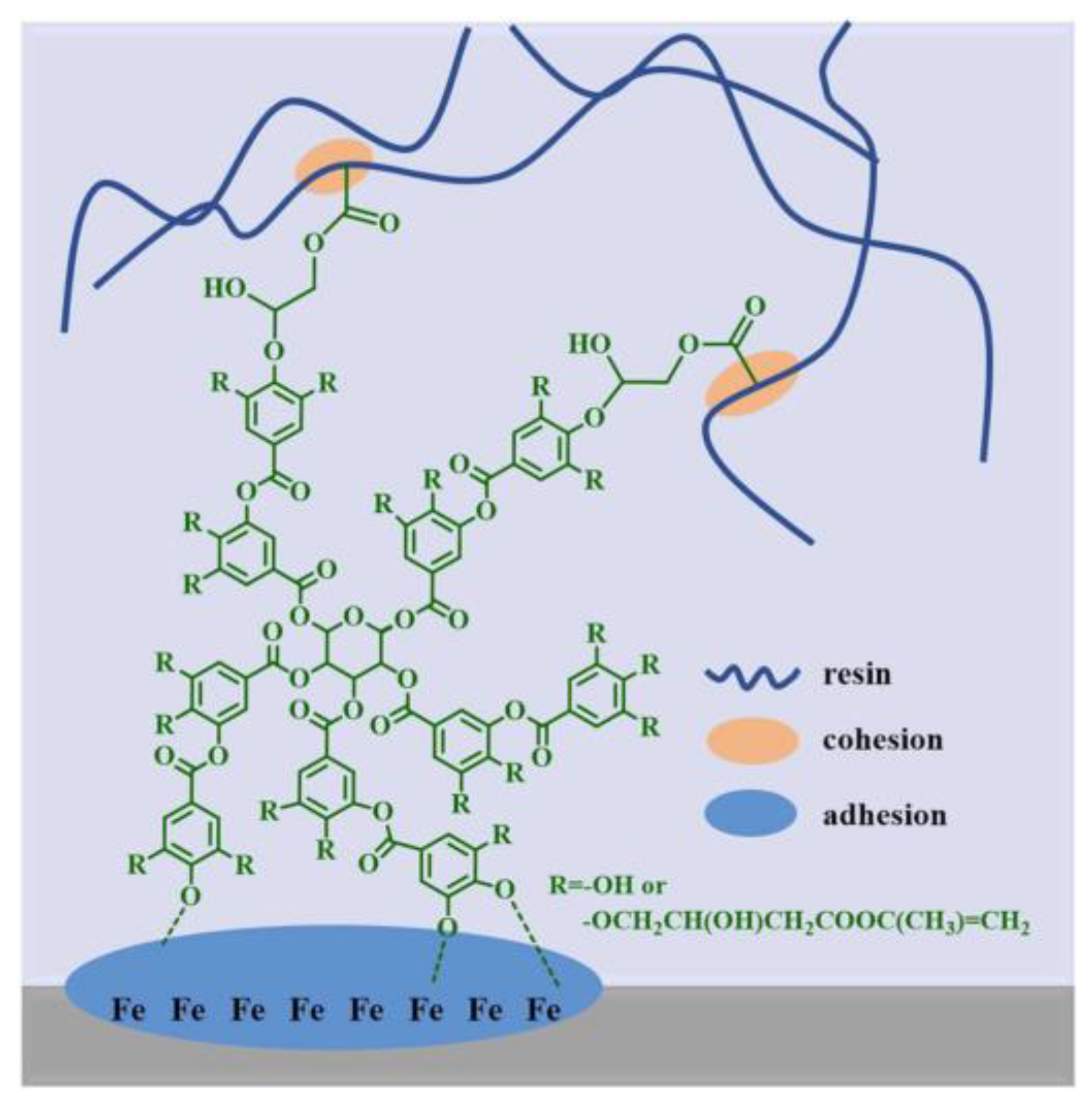
4. Polyphenols as Adhesion Promoters/Primers for Anticorrosion Coatings
5. Polyphenols as Additives to Corrosion Protection Polymeric Coatings
| Substrate | Polymeric Coating | Additive | Application Strategy | Main Results | Reference |
|---|---|---|---|---|---|
| JIS G3141 mild steel (composition—0.15% C, 0.60% Mn, 0.05% S, 0.10% P) polished using a grinder and then manually with an emery paper no. 100, and then degreased with ethanol | Alkyd paint | Zinc tannate pigment prepared with tara powder and zinc oxide | Spray gun application of 2 coats of alkyd paint pigmented with zinc tannate (ATZ) | Results from salt spray tests proved that samples covered by ATZ paints had outstanding anticorrosive behavior compared to those with paints containing conventional pigments. The system with the ATZ paint and the topcoat of a synthetic, commercial, and white alkyd paint showed similar degrees of rusting and blistering to a system with zinc chromate. The adhesion was significantly increased by using ATZ paints. | [80] |
| 1020 Steel with a surface preparation according to the SSPC SP5 and SP1 standards | Bisphenol A solvent-based epoxy resin, amide-based hardener, acrylic diluent as a solvent, R1 titanium dioxide, mica, calcined aluminum silicate, pigment dispersant, fumed silica, and a film leveling agent | The water-soluble fraction (WSF) and water-insoluble fraction (WIF) from industrial by-products of Pinus radiata | Pure tannins applications followed by drying in an environmental chamber at 25 °C, with a relative humidity of 60% and pH of 7, for periods of 12 h, 24 h, and 48 h. Tannins addition to an epoxy resin and spray coating (100 µm) | Polyphenols formed a protective film on steel, and the WSF fraction was more effective at this purpose and also at increasing the corrosion protection of epoxy-resin coating. | [88] |
| Steel grinded (600, 1200 grit) and degreased with acetone | Epoxy-ester resin (EER) (type EE-430CS, solid content: 60 wt%) and driers/accelerators, including cobalt (57%, as primary drier and autoxidation catalyst), lead (72%, as a secondary drier which activates the crosslinking steps of the coating), and calcium (41%, as an auxiliary drier) | Ce-TAA pigment obtained by the formation of a complex between the tannic acid and cerium nitrate | Introduction of the additive into the resin before curing, and resin application on the steel substrate. | The corrosion protection was increased. The release of the active inhibitor (Ce-TAA pigment) after the coating damage guaranteed corrosion protection. | [113] |
| Carbon steel samples (composition—0.108% C, 0.42% Mn, 0.12% Cr, 0.053% S, 0.016% P) degreased with acetone and polished with #150 sandpaper | Araldite® GZ 7071 × 75 epoxy resin and Aradur® 450 curing agent | Zinc tannate prepared by mixing black wattle tannins and 1 M Zn(NO3)·6H2O solution. Magnesium tannate prepared by mixing black wattle tannins and 1 M MnSO4 solution. | Brush-application | From pull-off test, no influence on the coating adherence by the introduction of zinc tannate and magnesium tannate pigments was proved. Results of EIS analysis and salt spray tests indicated the resin with zinc tannate as an excellent anticorrosion agent. | [114] |
| SAE 1010 steel panels degreased with toluene and sandblasted | Polyvinyl chloride (PVC) water-based anticorrosive system | Quebracho tannin-based pigment containing zinc oxide | Application of two coats of anticorrosive paints and then two coats of a topcoat white paint (PVC as a paint matrix and titanium dioxide as a unique pigment) by brushing | The best adhesion values were obtained for samples with the tannin-based coating. No brushing and rusting phenomena and no adhesion losses were noticed for samples coated with a paint pigmented with tannin after the humidity cabinet test as well as the salt fog test. The zinc tannate pigment was the most efficient in corrosion protection together with zinc phosphomolybdate. | [115] |
| SAE 1020 carbon steel blasted and degreased | DGEBA epoxy resin | Acetylated Kraft lignin | Addition of acetylated lignin to an epoxy resin before curing and coating application by paintbrush | The presence of lignin (7.5 and 15%) did not alter the curing process. Lignin added UV adsorption ability to the coating conferring UV protection of the resin. The corrosion protection of the coating was comparable with conventional coal tar epoxy. | [116] |
| Q235 steel pre-treated with a sandblasting machine | Epoxy matrix | Graphene nanosheets functionalized by tea polyphenols (TP/GE) | Addition of TP/GE (0.1, 0.3, and 0.6 wt.%) in an epoxy matrix (EP/TPG) and coating process on steel samples by immersion | TPG additive in an epoxy resin improved the coating barrier properties. The addition of 0.3 wt.% TP/GE widened the impedance arc, indicating the enhanced impenetrability and corrosion resistance of the coating. EP/TPG (0.3 wt.%) showed the lowest value of corrosion current density due to its excellent anticorrosive ability and it had the fewest corrosion products and bubbles after the spray test. | [117] |
| Mild steel cleaned by mechanical buffing and dilution with HCl | Phenol-formaldehyde matrix (PF) | Recovered tannins from wastewater from the vegetable tanning process | Application of a PF-recovered tannin resin (PFT) above mild steel | The introduction of tannins into a PF matrix enhanced the coating adhesion property. Thus, the PFT resin showed great resistance to alkali and acid conditions. | [118] |
| Rusted carbon steel | Epoxy resin E51 | Tannic acid, limonene, and nano-ZrO2 | Tannic acid used, at first, as rust converted and also added to the resin together with limonene and nano-ZrO2 | The rust conversion layer protected the substrate and improved coating adhesion, tannic acid increased the stability of the coating, acting as a crosslinker and forming ferric tannates, limonene was able to adsorb to both steel substrate and tannic acid, improving coating performances, and finally, nano-ZrO2 was effectively able to bind to tannic acid and limonene and fill the pinholes of the coating, improving the corrosion resistance. | [119] |
| ASTM A36 steel, surface preparation in accordance with SSPC SP5 and SP1 standards | Araldite GY 9513 (resin derived from Bisphenol-A) crosslinked with a polyamine Versamid® | Tannins from Pinus radiata and zinc oxide nanoparticles bare or silanized with 3-aminopropyltriethoxysilane (APTES) | Addition of Pinus radiata tannins and zinc oxide nanoparticles bare or silanized with 3-aminopropyltriethoxysilane (APTES) to the resin. Coating application by spray painting (150–200 µm) | Tannins increased the abrasion resistance of the coating while the ZnO nanoparticles were detrimental to this property. Functionalized ZnO nanoparticles increased the contact angle. Composite coatings improved steel corrosion resistance and exhibited self-healing properties. | [120] |
| Mild steel panels (Q panels from TQC sheen) | Epoxy resin-based coating | Calcium tannate obtained by the filtration of a mixture of commercial hydrolysable tannin and 1 M calcium nitrate (Ca(NO3)2) | Application of a calcium tannate 10 wt.% epoxy coating (CTE) by using a draw-down film applicator | In potentiodynamic polarization tests, a corrosion potential shift to more positive values was observed for the CTE-coated steel, suggesting the efficient anodically inhibition of the corrosion by calcium tannates. No adhesion losses for the CTE coating after 21 days of exposure to the salt spray chamber. | [132] |
| Q235 steel | Alkyd varnish coatings | Tannic acid loaded to silica mesoporous nanoparticles attached to Ti3AlC2 powder as an additive of the polymeric coating | Inclusion in the coating mixture and deposition on the substrate | The corrosion resistance was improved, and self-healing ability occurred due to the tannic acid released in the case of coating damage. | [133] |
| Q235 steel sheets | Waterborne polyurethane coating | Tannic acid functionalized cerium montmorillonite nanocomposites (TA@Ce-MMT) | Uniformly coating on steel surface at room temperature for 24 h and drying step at 50 °C for 2 days | The release of tannic acid and Ce3+ ions increased as the pH decreased and allowed a positive corrosion resistance to steel, producing TA-Fe3+ complexes. The TA@Ce-MMT markedly enhanced the long-term protection against corrosion due to the synergistic effect of tannic acid and Ce3+ ions. | [134] |
| Mild steel (composition -wt.%—0.22 C, 1.30 Mn, 0.035 S, 0.35 silicone, 0.035 phosphate) | Paralux P268HS, an epoxy high solid zinc phosphate primer (Paralux P268HS) | Tannin extracted from Rhizophora apiculate mangrove bark | Zinc tannates (TZn) were produced by mixing 1 M zinc nitrate and tannin solution at pH 4 for 24 h. Five paints with different amounts of TZn were obtained by stirring for 1 h and cured in an oven at 35 °C for 3 h after their application on the steel samples | Weight loss measurements in 3.1 wt.% NaCl in 20 L of artificial seawater for 45 days showed that paint with tannin exhibited an excellent corrosion inhibition effect. The efficiency of the corrosion protection increased along with the tannin content in the paint (93.9% for paint with 6 g of TZn). EIS results demonstrated the improvement of coating barrier properties and layer strengthening due to the TZn addition to the paint. A decrease in the damage of TZn-epoxy coated steel was observed through SEM analysis. | [135] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, D. Automotive Corrosion and Accelerated Corrosion Tests for Zinc Coated Steels. ISIJ Int. 2018, 58, 1562–1568. [Google Scholar] [CrossRef] [Green Version]
- Gerengi, H.; Sen, N.; Uygur, I.; Solomon, M.M. Corrosion response of ultra-high strength steels used for automotive applications. Mater. Res. Express 2019, 6, 0865a6. [Google Scholar] [CrossRef]
- Koch, G. 1-Cost of Corrosion. In Trends in Oil and Gas Corrosion Research and Technologies: Production and Trasmission, 1st ed.; El-Sherik, A.M., Ed.; Woodhead Publishing Series in Energy: Sawston, UK, 2017; pp. 3–30. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. The carbon footprint of steel corrosion. NPJ Mater. Degrad. 2022, 6, 101. [Google Scholar] [CrossRef]
- Shang, Z.; Zhu, J. Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol. 2021, 15, 5078–5094. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Commun. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Bagale, U.D.; Sonawane, S.H.; Bhanvase, B.A.; Kulkarni, R.D.; Gogate, P.R. Green synthesis of nanocapsules for self-healing anticorrosion coating using ultrasound-assisted approach. Green Process. Synth. 2018, 7, 147–159. [Google Scholar] [CrossRef]
- Bagale, U.D.; Sonawane, S.H. Synthesis of nanocapsules using safflower oil for self-healing material. Nanomater. Energy 2019, 8, 42–50. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.; Dagdag, O.; El Gouri, M.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-Alhosseini, A. New Promising Ceramic Coatings for Corrosion and Wear Protection of Steels: A Review. Surf. Interfaces 2021, 23, 100997. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Mu, J.; Gao, F.; Cui, G.; Wang, S.; Tang, S.; Li, Z. A comprehensive review of anticorrosive graphene-composite coatings. Prog. Org. Coat. 2021, 157, 106321. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Wang, S.; Liu, J.; Xing, X.; Li, Z.; Wang, B. A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coat. 2020, 148, 105821. [Google Scholar] [CrossRef]
- Montemor, M. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- George, J.S.; Hoang, A.T.; Kalarikkal, N.; Nguyen-Tri, P.; Thomas, S. Recent advances in bio-inspired multifunctional coatings for corrosion protection. Prog. Org. Coat. 2022, 168, 106858. [Google Scholar] [CrossRef]
- Spriano, S.; Dmitruk, A.; Naplocha, K.; Ferraris, S. Tannic Acid Coatings to Control the Degradation of AZ91 Mg Alloy Porous Structures. Metals 2023, 13, 200. [Google Scholar] [CrossRef]
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Okumura, H. Application of phenolic compounds in plants for green chemical materials. Curr. Opin. Green Sustain. Chem. 2021, 27, 100418. [Google Scholar] [CrossRef]
- Xua, L.Q.; Neohd, K.G.; Kang, E.T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Riccucci, G.; Ferraris, S.; Reggio, C.; Bosso, A.; Örlygsson, G.; Ng, C.H.; Spriano, S. Polyphenols from Grape Pomace: Functionalization of Chitosan-Coated Hydroxyapatite for Modulated Swelling and Release of Polyphenols. Langmuir 2021, 37, 14793–14804. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications–a review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Rambaran, T.F. Nanopolyphenols: A review of their encapsulation and anti-diabetic effects. SN Appl. Sci. 2020, 2, 1335. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Beconcini, D.; Felice, F.; Fabiano, A.; Sarmento, B.; Zambito, Y.; Di Stefano, R. Antioxidant and Anti-Inflammatory Properties of Cherry Extract: Nanosystems-Based Strategies to Improve Endothelial Function and Intestinal Absorption. Foods 2020, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 3–44. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef] [Green Version]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Sesia, R.; Ferraris, S.; Sangermano, M.; Spriano, S. UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water. Polymers 2022, 14, 4645. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.; Pell, A. Analysis of condensed tannins: A review. Anim. Feed. Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. South Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Ocampo, L.; Margarit, I.; Mattos, O.; de Torresi, S.C.; Fragata, F. Performance of rust converter based in phosphoric and tannic acids. Corros. Sci. 2004, 46, 1515–1525. [Google Scholar] [CrossRef]
- Guo, S.; Si, R.; Dai, Q.; You, Z.; Ma, Y.; Wang, J. A critical review of corrosion development and rust removal techniques on the structural/environmental performance of corroded steel bridges. J. Clean. Prod. 2019, 233, 126–146. [Google Scholar] [CrossRef]
- Saji, V.S. Progress in rust converters. Prog. Org. Coat. 2019, 127, 88–99. [Google Scholar] [CrossRef]
- Feliu, S.; Galvàn, J.C.; Feliu, S., Jr.; Bastidas, J.M.; Simancas, J.; Morcillo, M.; Almeida, E.M. An electrochemical impedance study of the behaviour of some pretreatments applied to rusted steel surfaces. Corros. Sci. 1993, 35, 1351–1358. [Google Scholar] [CrossRef]
- Marco, J.F.; Dávalos, J.; Gracia, M.; Gancedo, J.R. Corrosion studies of iron and its alloys by means of57Fe Mössbauer spectroscopy. Hyperfine Interact. 1994, 83, 111–123. [Google Scholar] [CrossRef]
- Díaz, B.; Figueroa, R.; Nóvoa, X.; Pérez, C.; Pintos, A. The corrosion protection afforded by a commercial rust converter doped with graphene oxide. Electrochim. Acta 2020, 342, 136096. [Google Scholar] [CrossRef]
- Díaz, B.; Nóvoa, X.R.; Pérez, C.; Rodríguez-Morgado, M. Influence of Graphene Oxide Additions on the Corrosion Resistance of a Rust Converter Primer. Coatings 2022, 12, 345. [Google Scholar] [CrossRef]
- Barrero, C.; Ocampo, L.; Arroyave, C. Possible improvements in the action of some rust converters. Corros. Sci. 2001, 43, 1003–1018. [Google Scholar] [CrossRef]
- Nasrazadani, S. The application of infrared spectroscopy to a study of phosphoric and tannic acids interactions with magnetite (Fe3O4), goethite (α-FEOOH) and lepidocrocite (γ-FeOOH). Corros. Sci. 1997, 39, 1845–1859. [Google Scholar] [CrossRef]
- Morcillo, M.; Feliu, S.; Simancas, J.; Bastidas, J.M.; Galvan, J.C.; Almeida, E.M. Corrosion of Rusted Steel in Aqueous Solutions of Tannic Acid. Corrosion 1992, 48, 1032–1039. [Google Scholar] [CrossRef]
- Jia, Y.; Ren, N.; Yue, H.; Deng, J.; Liu, Y. Preparation and properties of natural gallic acid based rust conversion emulsion. Pigment. Resin Technol. 2016, 45, 191–198. [Google Scholar] [CrossRef]
- Li, J.; Ge, S.; Wang, J.; Du, H.; Song, K.; Fei, Z.; Shao, Q.; Guo, Z. Water-based rust converter and its polymer composites for surface anticorrosion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 334–342. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Liu, X.; Hou, B. Rust Conversion Performance of Phosphoric Acid-Gallic Acid in Vinyl Chloride Acrylic Emulsion. Coatings 2021, 11, 152. [Google Scholar] [CrossRef]
- Ross, T.; Francis, R. The treatment of rusted steel with mimosa tannin. Corros. Sci. 1978, 18, 351–361. [Google Scholar] [CrossRef]
- Rahim, A.A.; Kassim, J. Recent Development of Vegetal Tannins in Corrosion Protection of Iron and Steel. Recent Pat. Mater. Sci. 2008, 100, 223–231. [Google Scholar] [CrossRef]
- Beltrán, J.J.; Novegil, F.J.; García, K.E.; Barrero, C.A. On the reaction of iron oxides and oxyhydroxides with tannic and phosphoric acid and their mixtures. Hyperfine Interact. 2010, 195, 133–140. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Adnan, R.; Sani Ibrahim, M. Mangrove tannins and their flavonoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros. Sci. 2007, 49, 402–427. [Google Scholar] [CrossRef]
- Gust, J.; Suwalski, J. Use of Mössbauer Spectroscopy to Study Reaction Products of Polyphenols and Iron Compounds. Corrosion 1994, 50, 355–365. [Google Scholar] [CrossRef]
- Zhao, X.D.; Cheng, Y.F.; Fan, W.; Vladimir, C.; Volha, V.; Alla, T. Inhibitive Performance of a Rust Converter on Corrosion of Mild Steel. J. Mater. Eng. Perform. 2014, 23, 4102–4108. [Google Scholar] [CrossRef]
- Yahya, S.; Shah, A.M.; Abdul Rahim, A.; Abd Aziz, N.H.; Roslan, R. Phase transformation of rust in the presence of various tannins. J. Phys. Sci. 2008, 19, 31–41. [Google Scholar]
- Collazo, A.; Nóvoa, X.; Pérez, C.; Puga, B. EIS study of the rust converter effectiveness under different conditions. Electrochim. Acta 2008, 53, 7565–7574. [Google Scholar] [CrossRef]
- Gust, J. Application of Infrared Spectroscopy for Investigation of Rust Phase Component Conversion by Agents Containing Oak Tannin and Phosphoric Acid. Corrosion 1991, 47, 453–457. [Google Scholar] [CrossRef]
- A Rahim, A.; Kassim, M.J.; Rocca, E.; Steinmetz, J. Mangrove (Rhizophora apiculata) tannins: An eco-friendly rust converter. Corros. Eng. Sci. Technol. 2011, 46, 425–431. [Google Scholar] [CrossRef]
- Merino, S.F.; Caprari, J.J.; Torres, L.V.; Ramos, L.F.; Girola, A.H. Inhibitive action of tara tannin in rust converter formulation. Anti Corros. Methods Mater. 2017, 64, 136–147. [Google Scholar] [CrossRef]
- Jaén, J.A.; De Obaldía, J.; Rodriguez, M.V. Application of Mössbauer spectroscopy to the study of tannins inhibition of iron and steel corrosion. Hyperfine Interact. 2011, 202, 25–38. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J. Inhibitive action of mangrove tannins and phosphoric acid on pre-rusted steel via electrochemical methods. Corros. Sci. 2008, 50, 1546–1550. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S. Role of tannic acid based rust converter on formation of passive film on zinc rich coating exposed in simulated concrete pore solution. Surf. Coat. Technol. 2008, 202, 1526–1542. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Zhang, B.; Lei, B.; Li, Y. Enhancement the Adhesion between Epoxy Coating and Rusted Structural Steel by Tannic Acid Treatment. Acta Met. Sin. Engl. Lett. 2014, 27, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Tüken, T.; Yazıcı, B.; Erbil, M. A new multilayer coating for mild steel protection. Prog. Org. Coat. 2004, 50, 115–122. [Google Scholar] [CrossRef]
- Tüken, T.; Arslan, G.; Yazıcı, B.; Erbil, M. The corrosion protection of mild steel by polypyrrole/polyphenol multilayer coating. Corros. Sci. 2004, 46, 2743–2754. [Google Scholar] [CrossRef]
- Samet, Y.; Kraiem, D.; Abdelhédi, R. Electropolymerization of phenol, o-nitrophenol and o-methoxyphenol on gold and carbon steel materials and their corrosion protection effects. Prog. Org. Coat. 2010, 69, 335–343. [Google Scholar] [CrossRef]
- Koerner, C.M.; Hopkinson, D.P.; Ziomek-Moroz, M.E.; Rodriguez, A.; Xiang, F. Environmentally Friendly Tannic Acid Multilayer Coating for Reducing Corrosion of Carbon Steel. Ind. Eng. Chem. Res. 2021, 60, 243–250. [Google Scholar] [CrossRef]
- Cheng, X.; Lu, R.; Zhang, X.; Zhu, Y.; Wei, S.; Zhang, Y.; Zan, X.; Geng, W.; Zhang, L. Silanization of a Metal–Polyphenol Coating onto Diverse Substrates as a Strategy for Controllable Wettability with Enhanced Performance to Resist Acid Corrosion. Langmuir 2021, 37, 3637–3647. [Google Scholar] [CrossRef]
- Jain, R.; Bhagawati, B.; Khandagiri, P.; Shamshoddin, S.; Bhadu, M.K.; Rout, T.K.; Das, S. Anticorrosive and lubricating polyphenol coatings on galvanneal steel. Surf. Eng. 2017, 33, 410–427. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, O.; Selmi, G.J.; Deyá, C.; Di Sarli, A.; Romagnoli, R. Lanthanum Derivative from “Tara” Tannin for Steel Temporary Protection. Ind. Eng. Chem. Res. 2018, 57, 3215–3226. [Google Scholar] [CrossRef]
- Byrne, C.; D’alessandro, O.; Selmi, G.; Romagnoli, R.; Deyá, C. Primers based on tara and quebracho tannins for poorly prepared steel surfaces. Prog. Org. Coat. 2019, 130, 244–250. [Google Scholar] [CrossRef]
- Sack, S.H.; Romagnoli, R.; Vetere, V.F.; Elsner, C.I.; Pardini, O.; Amalvy, J.I.; Di Sarli, A.R. Evaluation of steel/primer based on chestnut tannin/paint film systems by EIS. J. Coat. Technol. 2002, 74, 63–69. [Google Scholar] [CrossRef]
- Pardini, O.R.; Amalvy, J.I.; Di Sarli, A.R.; Romagnoli, R.; Vetere, V.F. Formulation and testing of a waterborne primer containing chestnut tannin. J. Coat. Technol. 2001, 73, 99–106. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, J.; Liu, R.; Luo, J. Synthesis of acrylated tannic acid as bio-based adhesion promoter in UV-curable coating with improved corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128834. [Google Scholar] [CrossRef]
- Hadzich, A.; Flores, S.; Caprari, J.; Romagnoli, R. Study of zinc tannates prepared with Tara powder (Caesalpinia spinosa) as anticorrosive pigments in alkyd paints and wash primer formulations. Prog. Org. Coat. 2018, 117, 35–46. [Google Scholar] [CrossRef]
- Peres, R.S.; Cassel, E.; Ferreira, C.A.; Azambuja, D.S. Grain Refiner Effect of Black Wattle Tannin in Iron and Zinc Phosphate Coatings. Ind. Eng. Chem. Res. 2014, 53, 2706–2712. [Google Scholar] [CrossRef]
- Matamala, G.; Smeltzer, W.; Droguett, G. Use of Tannin Anticorrosive Reaction Primer to Improve Traditional Coating Systems. Corrosion 1994, 50, 270–275. [Google Scholar] [CrossRef]
- Matamala, G.; Smeltzer, W.; Droguett, G. Comparison of steel anticorrosive protection formulated with natural tannins extracted from acacia and from pine bark. Corros. Sci. 2000, 42, 1351–1362. [Google Scholar] [CrossRef]
- D’alessandro, O.; Selmi, G.J.; Deyá, C.; Di Sarli, A.; Romagnoli, R. Formulation and Assessment of a Wash-Primer Containing Lanthanum “Tannate” for Steel Temporary Protection. J. Mater. Eng. Perform. 2017, 27, 687–704. [Google Scholar] [CrossRef]
- Glück, S. Parts Cleaning in Pre-Treatment Processes. JOT Int. Surf. Technol. 2016, 9, 50–53. [Google Scholar] [CrossRef]
- Rahimi, S.K.; Potrekar, R.; Dutta, N.K.; Choudhury, N.R. Anticorrosive interfacial coatings for metallic substrates. Surf. Innov. 2013, 1, 112–137. [Google Scholar] [CrossRef]
- Šolić, T.; Marić, D.; Novoselović, D.; Samardžić, I. Optimization of Parameters for Protection of Materials by Primer Application. Coatings 2022, 12, 413. [Google Scholar] [CrossRef]
- Montoya, L.; Contreras, D.; Jaramillo, A.; Carrasco, C.; Fernández, K.; Schwederski, B.; Rojas, D.; Melendrez, M. Study of anticorrosive coatings based on high and low molecular weight polyphenols extracted from the Pine radiata bark. Prog. Org. Coat. 2019, 127, 100–109. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–principles, mechanisms and applications. Dev. Corros. Prot. 2014, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Zandi-Zand, R.; Ershad-Langroudi, A.; Rahimi, A. Silica based organic–inorganic hybrid nanocomposite coatings for corrosion protection. Prog. Org. Coat. 2005, 53, 286–291. [Google Scholar] [CrossRef]
- Dar, M.A. A review: Plant extracts and oils as corrosion inhibitors in aggressive media. Ind. Lubr. Tribol. 2011, 63, 227–233. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crop. Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Ramírez, J.; Díaz-Gómez, A.; Montoya, L.F.; Samhitha, S.S.; Rojas, D.; Oñate, Á.; Jaramillo, A.F.; Melendrez, M.F. Evaluation in Real Conditions of New Anticorrosive Formulations Based on Polyphenols from Natural Sources and Encapsulated Nanoparticles. Coatings 2023, 13, 8. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M. Studying the corrosion resistance and hydrolytic degradation of an epoxy coating containing ZnO nanoparticles. Mater. Chem. Phys. 2011, 130, 1208–1219. [Google Scholar] [CrossRef]
- Banczek, E.; Rodrigues, P.; Costa, I. Investigation on the effect of benzotriazole on the phosphating of carbon steel. Surf. Coat. Technol. 2006, 201, 3701–3708. [Google Scholar] [CrossRef]
- Pérez, M.; Garcia, M.; Blustein, G.; Stupak, M. Tannin and tannate from the quebracho tree: An eco-friendly alternative for controlling marine biofouling. Biofouling 2007, 23, 151–159. [Google Scholar] [CrossRef]
- Peres, R.S.; Armelin, E.; Alemán, C.; Ferreira, C.A. Modified tannin extracted from black wattle tree as an environmentally friendly antifouling pigment. Ind. Crop. Prod. 2015, 65, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhuang, J.; Yu, Y.; Zeng, X. Research on anti-corrosion property of rare earth inhibitor for X70 steel. J. Rare Earths 2013, 31, 734–740. [Google Scholar] [CrossRef]
- Forsyth, M.; Wilson, K.; Behrsing, T.; Deacon, G.B.; Phanasgoankar, A.; Forsyth, C. Effectiveness of Rare-Earth Metal Compounds as Corrosion Inhibitors for Steel. Corrosion 2002, 58, 953–960. [Google Scholar] [CrossRef]
- Montemor, M.; Trabelsi, W.; Zheludevich, M.; Ferreira, M. Modification of bis-silane solutions with rare-earth cations for improved corrosion protection of galvanized steel substrates. Prog. Org. Coat. 2006, 57, 67–77. [Google Scholar] [CrossRef]
- Motte, C.; Poelman, M.; Roobroeck, A.; Fedel, M.; Deflorian, F.; Olivier, M.-G. Improvement of corrosion protection offered to galvanized steel by incorporation of lanthanide modified nanoclays in silane layer. Prog. Org. Coat. 2012, 74, 326–333. [Google Scholar] [CrossRef]
- Noè, C.; Iannucci, L.; Malburet, S.; Graillot, A.; Sangermano, M.; Grassini, S. New UV-Curable Anticorrosion Coatings from Vegetable Oils. Macromol. Mater. Eng. 2021, 306, 2100029. [Google Scholar] [CrossRef]
- Pezzana, L.; Mousa, M.; Malmström, E.; Johansson, M.; Sangermano, M. Bio-based monomers for UV-curable coatings: Allylation of ferulic acid and investigation of photocured thiol-ene network. Prog. Org. Coat. 2021, 150, 105986. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Zhu, J.; Liu, X.; Wang, Z.; Yan, J. UV-Curable Coatings from Multiarmed Cardanol-Based Acrylate Oligomers. ACS Sustain. Chem. Eng. 2015, 3, 1313–1320. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Sanai, Y.; Ninomiya, T.; Arimitsu, K. Improvements in the physical properties of UV-curable coating by utilizing type II photoinitiator. Prog. Org. Coat. 2021, 151, 106038. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A. Tannin-Based Adhesives. J. Macromol. Sci. Part C 1980, 18, 247–315. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Banche, G.; Di Confiengo, G.G.; Geobaldo, F.; Novara, C.; Spriano, S. Innovative Coatings Based on Peppermint Essential Oil on Titanium and Steel Substrates: Chemical and Mechanical Protection Ability. Materials 2020, 13, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armelin, E.; Pla, R.; Liesa, F.; Ramis, X.; Iribarren, J.I.; Alemán, C. Corrosion protection with polyaniline and polypyrrole as anticorrosive additives for epoxy paint. Corros. Sci. 2008, 50, 721–728. [Google Scholar] [CrossRef]
- Moghaddam, P.N.; Amini, R.; Kardar, P.; Ramezanzadeh, B. Epoxy-ester coating reinforced with cerium (III)-tannic acid-based hybrid pigment for effective mild-steel substrate corrosion protection. Prog. Org. Coat. 2021, 161, 106485. [Google Scholar] [CrossRef]
- Zmozinski, A.V.; Peres, R.S.; Freiberger, K.; Ferreira, C.A.; Tamborim, S.M.M.; Azambuja, D.S. Zinc tannate and magnesium tannate as anticorrosion pigments in epoxy paint formulations. Prog. Org. Coat. 2018, 121, 23–29. [Google Scholar] [CrossRef]
- Amalvy, J.I.; Aznar, A.C.; Pardini, O.R.; Guzman, G.A. Waterborne Anticorrosive Systems for Steel Protection—Part 1: Formulation and Testing. Corrosion 2002, 58, 871–880. [Google Scholar] [CrossRef]
- Diógenes, O.B.; de Oliveira, D.R.; da Silva, L.R.; Pereira, G.; Mazzetto, S.E.; Araujo, W.S.; Lomonaco, D. Development of coal tar-free coatings: Acetylated lignin as a bio-additive for anticorrosive and UV-blocking epoxy resins. Prog. Org. Coat. 2021, 161, 106533. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, G.; Zhang, W.; Zhang, N.; Chen, C.; Fan, Y.; Li, H.; Liu, X.; He, Y. One-Step Method for Preparing Dispersive Tea Polyphenol/Graphene Nanosheets Enhanced with Anticorrosion Performance. Coatings 2019, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Sekaran, G.; Thamizharasi, S.; Ramasami, T. Physicochemical and Thermal Properties of Phenol-Formaldehyde-Modified Polyphenol Impregnate. J. Appl. Polym. Sci. 2001, 81, 1567–1571. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Han, E.; Liang, X.; Wang, G.; Yi, Z.; Li, N. Corrosion resistance of tannic acid, d-limonene and nano-ZrO2 modified epoxy coatings in acid corrosion environments. J. Mater. Sci. Technol. 2021, 65, 137–150. [Google Scholar] [CrossRef]
- Jaramillo, A.; Montoya, L.; Prabhakar, M.; Sanhueza, J.P.; Fernández, K.; Rohwerder, M.; Rojas, D.; Montalba, C.; Melendrez, M. Formulation of a multifunctional coating based on polyphenols extracted from the Pine radiata bark and functionalized zinc oxide nanoparticles: Evaluation of hydrophobic and anticorrosive properties. Prog. Org. Coat. 2019, 135, 191–204. [Google Scholar] [CrossRef]
- The Association for Materials Protection and Performance (AMPP). Available online: https://blogs.ampp.org/surface-prep-standards-a-quick-summary (accessed on 20 September 2021).
- Auepattana-Aumrung, K.; Phakkeeree, T.; Crespy, D. Polymer-corrosion inhibitor conjugates as additives for anticorrosion application. Prog. Org. Coat. 2022, 163, 106639. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Bethencourt, M.; Botana, F.J.; Marcos, M.; Osuna, R.M.; Sánchez-Amaya, J.M. Inhibitor properties of ‘green’ pigments for paints. Prog. Org. Coat. 2003, 46, 280–287. [Google Scholar] [CrossRef]
- Askari, F.; Ghasemi, E.; Ramezanzadeh, B.; Mahdavian, M. The corrosion inhibitive properties of various kinds of potassium zinc phosphate pigments: Solution phase and coating phase studies. Prog. Org. Coat. 2015, 85, 109–122. [Google Scholar] [CrossRef]
- Sinko, J. Challenges of chromate inhibitor pigments replacement in organic coatings. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Lahodny-Šarc, O.; Kapor, F. Corrosion inhibition of carbon steel in the near neutral media by blends of tannin and calcium gluconate. Mater. Corros. 2002, 53, 264–268. [Google Scholar] [CrossRef]
- EU Regulations, Directive 2006/11/EC of the European Parliament and of the Council of 15 February 2006 on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community. 2006. Available online: http://europa.eu/legislation_summaries/environment/water_protection_management/l2807a_es.htm (accessed on 15 February 2006).
- Lamprakou, Z.; Bi, H.; Weinell, C.E.; Dam-Johansen, K. Tannin-based inhibitive pigment for sustainable epoxy coatings formulation. Prog. Org. Coat. 2022, 167, 106841. [Google Scholar] [CrossRef]
- Sun, X.; Ma, C.; Ma, F.; Wang, T.; Feng, C.; Wang, W. A novel coating with SiO2 anchored on MXene loading tannic acid for self-healing anticorrosive performance. J. Alloys Compd. 2022, 928, 167202. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Waterborne polyurethane coating based on tannic acid functionalized Ce-MMT nanocomposites for the corrosion protection of carbon steel. Prog. Org. Coat. 2022, 163, 106613. [Google Scholar] [CrossRef]
- Idora, M.S.N.; Quen, L.K.; Kang, H.S. Effect of tannin from Rhizophora apiculate as corrosion inhibitor for epoxy paint on mild steel. J. Phys. Conf. Ser. 2017, 890, 12062. [Google Scholar] [CrossRef] [Green Version]



| Substrate | Coating | Application Strategy | Main Results | Reference |
|---|---|---|---|---|
| Mild steel (composition—wt.%—0.098 C, 0.35 Mn, 0.031 S, 0.017 P, and 99.334% Fe). Pre-treatment of the surface: polishing abrasive papers up to 1200, degreasing with 1:1 ethanol/acetone mixture, washing with bi-distilled water, and drying with warm air | Polypyrrole (PPy) and polyphenol (PPhe) films | Electrochemical synthesis by cyclic voltammetry technique | The synthesis of a thin PPhe coating on PPy one increased the corrosion protection, acting as a barrier able to block the solution penetration in the PPy pores. | [68] |
| Mild steel (composition—wt%—0.098 C, 0.35 Mn, 0.031 S, 0.017 P, and 99.334% Fe) polished with abrasive papers up to 1200 grit, degreased with 1/1 ethanol/acetone mixture, washed with distilled water, and dried | Polypyrrole (PPy) and polyphenol (PPhe) | Cyclic voltammetry technique. Deposition of PPhe in acidic conditions | The thin PPhe coating on top of the PPy one increased the corrosion protection in an acid environment. The PPhe coating covered the PPy pores, increasing the protection ability. | [69] |
| Carbon steel | Phenol, o-methoxyphenol, and o-nitrophenol | Electropolymerization by cyclic voltammetry | O-methoxyphenol resulted to be the most effective in the synthesis of a corrosion protection coating. The coating adhered well to the carbon steel, but was non-completely homogeneous and continuous; consequently, the corrosion protection was not complete. | [70] |
| Carbon steel and corroded carbon steel by exposure to air for 6 months | Branched polyethylenimine (PEI) and tannic acid (TA) | Alternating steps of dipping in a TA and PEI solution to obtain a layer-by-layer deposition (TA/PEI multilayer coating) | TA/PEI multilayer assembly presented long-term corrosion resistance. Negatively charged TA and positively charged PEI joined to form a very thin multilayer coating. The high water stability was due to ionic bonding and strong adhesion to the steel surface and, as a result, outstanding corrosion resistance was proved. | [71] |
| Several substrates, including iron sheets and stainless steel | Metal-polyphenolic network-based (MNP) coatings and silane-MNP coatings prepared by proanthocyanidin and iron(III) nitrate nonahydrate | Dipping of the substrates in a reactor for the coatings’ preparation | Substrates with MNP coatings showed lower values in water contact angle compared to the original substrates, while silane-MNPs coatings increased the surface hydrophobicity. The silane-MNP coating was more stable than MPN coatings under strong acid conditions, showing excellent resistance to acid corrosion. | [72] |
| Galvanneal steel degreased with degreasing solution (20 wt.% cetylctrimethylammonium bromide mixed with demineralised water) | Coating based on phosphorylated polyphenol and organic–inorganic silane compounds with different additives | Application by sponge roller | The silane-phosphorylated polyphenol coating enhanced the corrosion resistance, and 0.5 wt.% of manganese phosphate as an additive in a silane-phosphorylated polyphenol coating improved the corrosion resistance and lubricant properties. | [73] |
| Substrate | Adhesion Promoter/Primer | Corrosion Protective Coating | Application Strategy | Main Results | Reference |
|---|---|---|---|---|---|
| Sandblasted SAE 1010 steel panels degreased with toluene | Two commercial tannins from tara: T40 and T80 | An anticorrosive alkyd paint pigmented with zinc molybdenum phosphate and titanium dioxide, and an alkyd topcoat containing titanium dioxide | Preparation in a two-pack system of tara tannins primer (TT40 and TT80) and lanthanum tannates (LT40 and LT80) in a polyvinyl butyral resin-based formulation and a subsequent application by brushing | LT40 highly reduced the steel corrosion rate, and its efficiency was 95%, while an efficiency of 86% was assessed for LT80. TT40 and LT40 had similar steel corrosion rates, whereas the anticorrosive performance of LT80 was better than TT80. The ionic resistance was increased by the use of tannin-based primers and in particular, TT40 and LT40 showed ten times higher resistance than TT80 and LT80. The anticorrosive performance of a complete painting system was enhanced by the primer application. LT40 performed as the one with zinc tetroxychromate (ZTC) in accelerated tests and had better behavior than the other tannin-based primers formulations. | [75] |
| SAE 1010 steel degreased with isopropyl alcohol | Tannins from tara (T40, INDUNOR, abbreviated as T) and quebracho (UNITAN CROWN ATO, abbreviated as Q) | - | Preparation in a two-pack system of tannins primers (T and Q) and lanthanum tannates (TL and QL) in a polyvinyl butyral resin-based formulation and a subsequent application by brushing | Results from humidity chamber experiments proved that tara-based primers and quebracho-based ones had good adhesion to a steel substrate at the beginning of the test. In detail, after 5 days, the adhesion of T and TL primers was better than Q and QL, while the rusting degrees were similar (T and Q were more protective than TL and QL). T-coated panels had ionic resistance with an order of magnitude greater than steel without primer, and steels with a TL primer layer showed 2–4 times higher values. Q and QL primers on steels presented 3–4 times higher ionic resistance values than the metal without primer. Tannin-based primers had similar adhesion to the zinc tetroxychromate (ZTC) one and better barrier properties than ZTC. | [76] |
| SAE 1010 steel panels degreased with toluene | Tannins from chestnut | An alkyd paint pigmented with zinc molybdenum and/or an alkyd topcoat pigmented with titanium dioxide | Mixing of filtered tannins with a 40% aqueous solution of an acrylic resin, Texanol, and 10% ammonium molybdate solution, and brush application of the primer | The water permeation was not considerably influenced by the primer treatment. Impedance data showed a significant corrosion delay due to the iron tannates’ formation. The corrosion protection provided by the tannin primer was limited to a short immersion period in non-aggressive electrolytes (the tannin primer layer was more effective in NaCl than in NaClO4). | [77] |
| SAE 1010 steel panels degreased with toluene | Tannins from chestnut | An anticorrosive alkyd paint pigmented with zinc molybdenum phosphate and with a 0.8 PVC/CPVC ratio, and an alkyd topcoat pigmented with titanium dioxide | Mixing of filtered tannins with a 40% aqueous solution of an acrylic binder, Texanol, and 10% ammonium molybdate solution, and brush application of the primer | The anticorrosive performance was improved by a complete paint system (tannin primer + anticorrosive coating + topcoat). The tannin treatment prevented the formation of oxides on the scratch marks, where the corrosive process was more intense. The tannin-based primer on the steel showed high adhesion (20 ± 5 kg/cm2) and good paint adhesion promotion even after the salt fog chamber test. | [78] |
| Low-carbon steel and aluminum plates degreased with acetone | Acrylated tannic acid | A UV curable resin containing Irgacure 1173 (photoinitiator), polyester acrylate (DR-E524), tetrahydrofurfuryl acrylate (THFA), standard bisphenol-A epoxy acrylate (G500), and a leveling agent (BYK333) | Addition of the adhesion promoter to the resin formulation and application of the mixture on the substrate by bar coating | Tannic acid-based adhesion promoters significantly increased the adhesion of the polymeric coating to the metallic substrates and their corrosion protection ability. An optimal acrylation degree was identified. | [79] |
| JIS G3141 mild steel (composition—0.15% C, 0.60% Mn, 0.05% S, 0.10% P) polished using a grinder, manually with an emery paper no. 100, and then degreased with ethanol | Zinc tannate pigment prepared with tara powder and zinc oxide | Isopropanol-based wash primer | Spray gun application of two coats of a wash primer containing zinc tannate (WTZ) | WTZ, alone and with a topcoat of a synthetic, commercial, and white alkyd paint, exhibited an anticorrosive property that was similar to primers containing zinc phosphate. However, the zinc chromate-based primer had the best corrosion protection capacity and higher adhesion to steel. | [80] |
| Carbon steel (composition—wt.%—0.103 C, 0.46 Mn, 0.013 P, 0.096 S, 0.01 Cu, 0.18 Cr) degreased with an acetone/chloroform mixture and polished with sandpaper up to grade #1200 | Condensed tannin from black wattle barks | Epoxy coating (Intergard 269) as a finish paint for adherence tests | Addition of different black wattle tannin concentrations (0, 2, 4, and 8 g L−1) to a zinc phosphating bath and 0.5 g L−1 of tannin to an iron phosphating bath. Dipping of a steel sample in the bath for 10 min at 80 °C after an acid pickling treatment | The tannin addition affected the zinc phosphate crystals’ orientation and size over the steel surface, modifying the substrate’s active sites. The grain size reduction was confirmed in iron phosphating. From EIS analysis, better corrosion resistance was found when the tannin concentration in the zinc phosphating bath was 2 g L−1. | [81] |
| Plain or shot-blasted plates of AISI 1010 steel | Tannins from radiata pine bark | Three anticorrosive top coatings: alkyd, vinylic, and epoxy paint systems | Application of a 5 µm thick layer of a tannin-based primer containing polyvinyl butyral as a ligand element by brush and the use of spraying topcoats | The difference in rusting kinetics indicated the tannin primer’s corrosion inhibitor capacity over the anticorrosive alone. Particularly, no rusting or color change occurred when a 25 µm-thick anticorrosive epoxy coating was applied over a tannin primer layer. The corrosion current of the steel treated with the tannin primer stabilized at 2.7 µA/cm2 against 8.5 µA/cm2 of the steel protected by a chromated conversion coating. The adherence test on the epoxy paint applied in combination with a tannin primer achieved values of up to 35 kg/cm2. | [82] |
| 10 × 15 cm plain or shot-blasted plates of AISI 1010 steel | Tannins from radiata pine bark and from black acacia | Three anticorrosive top coatings: alkyd, vinylic, and epoxy paint systems | Application of a 5 µm thick layer of tannin-based primer containing polyvinyl butyral as a ligand element and the use of spraying topcoats | During the salt spray chamber exposure, the percentage of the rusted steel area was notably decreased by the use of pine tannin under the topcoat compared to samples without a primer layer and samples with an acacia tannin primer layer. Both types of tannins acted as adhesion promoters, although the acacia tannin-based primer slightly decreased paint adherence. | [83] |
| Sandblasted SAE 1010 steel panels, previously degreased with toluene | Tannins from quebracho | Anticorrosive alkyd paint and finish alkyd topcoat | Brush application of a wash-primer composed of quebracho tannin (QT) or lanthanum “tannate” (LT), which was obtained as a precipitated mass from a mixture of tannin, potassium nitrate (KNO3), and lanthanum(III) nitrate (La(NO3)3) | Results from steel corrosion potential measurements in pigment suspensions in 0.025 M NaCl showed that LT was the best inhibitor for steel corrosion. LT significantly decreased the steel corrosion rate with an efficiency of about 94%. LT in a primer formulation inhibited the development of globular oxides as zinc tetroxychromate (ZTC) did. The electrochemical evaluation revealed that the presence of a primer in the paint system delayed the corrosion, and the LT primer showed a similar anticorrosive property to a traditional ZTC primer. | [84] |
| ASTM A36 Steel | Tannins from radiata pine | Topcoat containing Poliol 112, BYK 333, BYK 425, additives (BYK 333, Purmol 333, Antiterra), zinc oxide nanoparticles (ZnO-NPs), thinner, and crosslinking agents | Application of a mixture of tannins with an epoxy resin formulation containing zinc oxide nanoparticles (PT-ZnO NPs) and a topcoat by an air gun. | After accelerated corrosion chamber experiments, no type of failure occurred for the PT-ZnO NPs primer according to ISO 4628: better adhesion and excellent interlay adhesion than commercial coatings (polyurethane and transurethane paints). The PT-ZnO NPs coating showed similar or superior mechanical properties than commercial coatings, except for the impact resistance. The impedance modulus of PT-ZnO NPs was measured at 6.7 × 108 Ω cm2, which indicated high resistance against aggressive agents. Therefore, the functional properties (film, mechanical, and corrosion) were improved by the addition of tannins in the primer formulation. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesia, R.; Spriano, S.; Sangermano, M.; Ferraris, S. Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies. Metals 2023, 13, 1070. https://doi.org/10.3390/met13061070
Sesia R, Spriano S, Sangermano M, Ferraris S. Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies. Metals. 2023; 13(6):1070. https://doi.org/10.3390/met13061070
Chicago/Turabian StyleSesia, Rossella, Silvia Spriano, Marco Sangermano, and Sara Ferraris. 2023. "Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies" Metals 13, no. 6: 1070. https://doi.org/10.3390/met13061070
APA StyleSesia, R., Spriano, S., Sangermano, M., & Ferraris, S. (2023). Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies. Metals, 13(6), 1070. https://doi.org/10.3390/met13061070









