Effect of Main Composition on the Viscosity and Thermal Stability of BaO-Containing Slag
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials Preparation
2.2. Viscosity Measurements
2.3. Thermodynamic Calculations
3. Results and Discussion
3.1. The Characteristic of Slag Viscosity and Equilibrium Phase
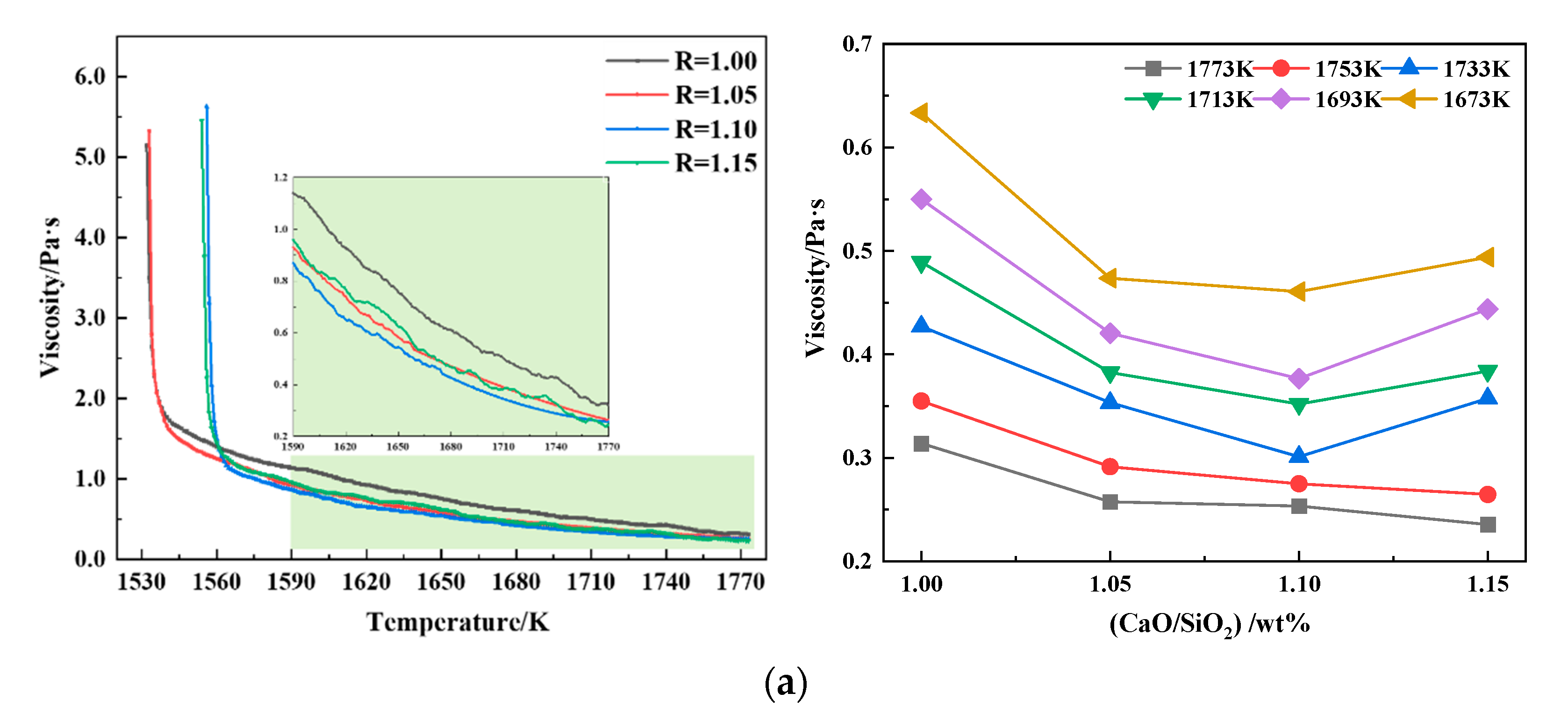
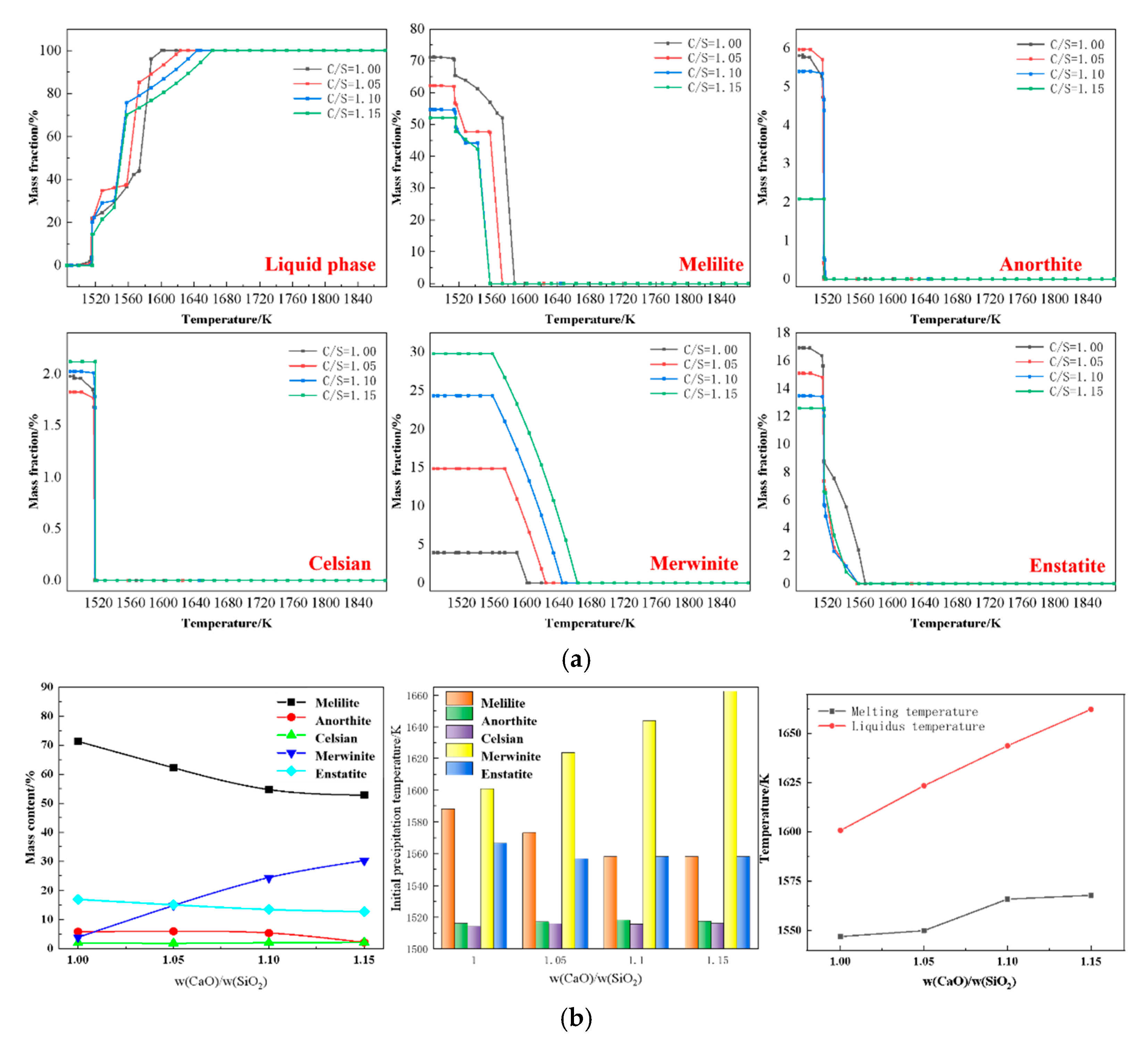
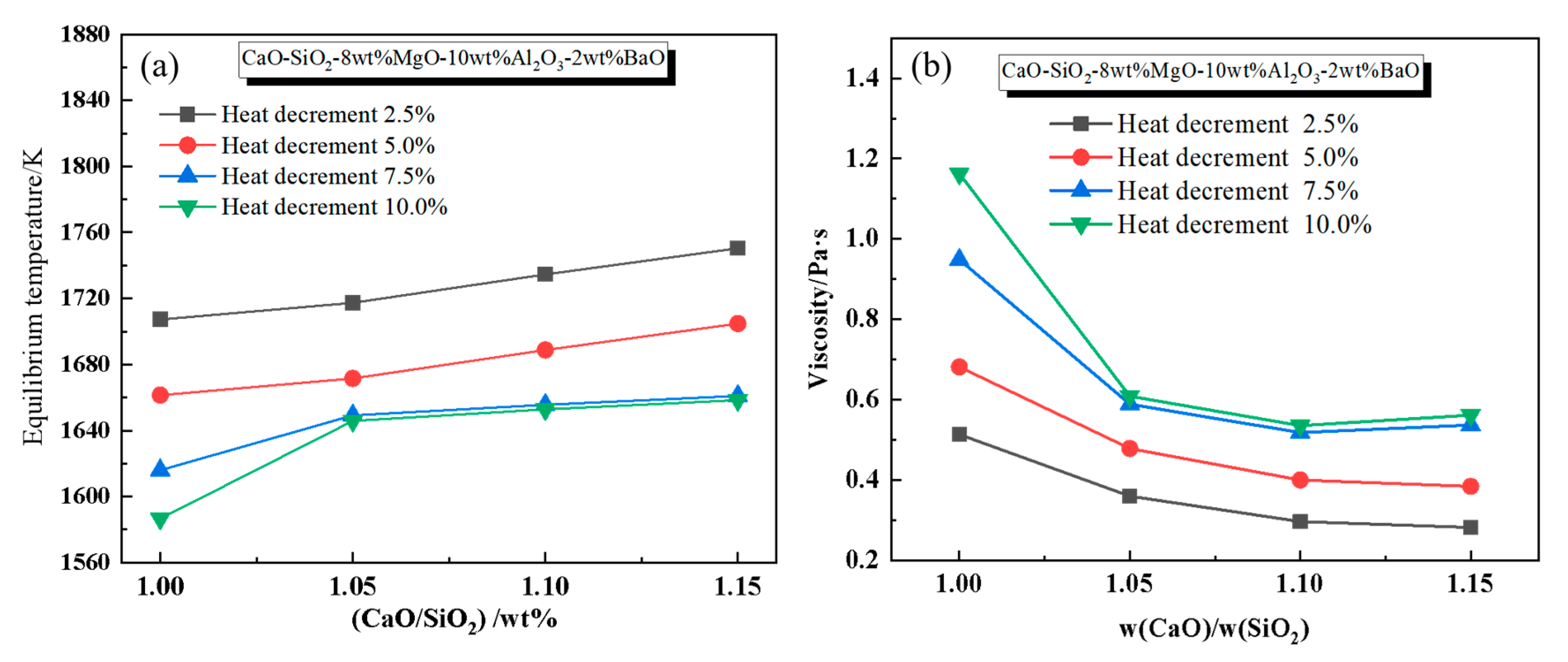
3.1.1. Effect of Basicity on Slag Viscosity and Phase Evolution
3.1.2. Effect of Al2O3 on Slag Viscosity and Phase Evolution
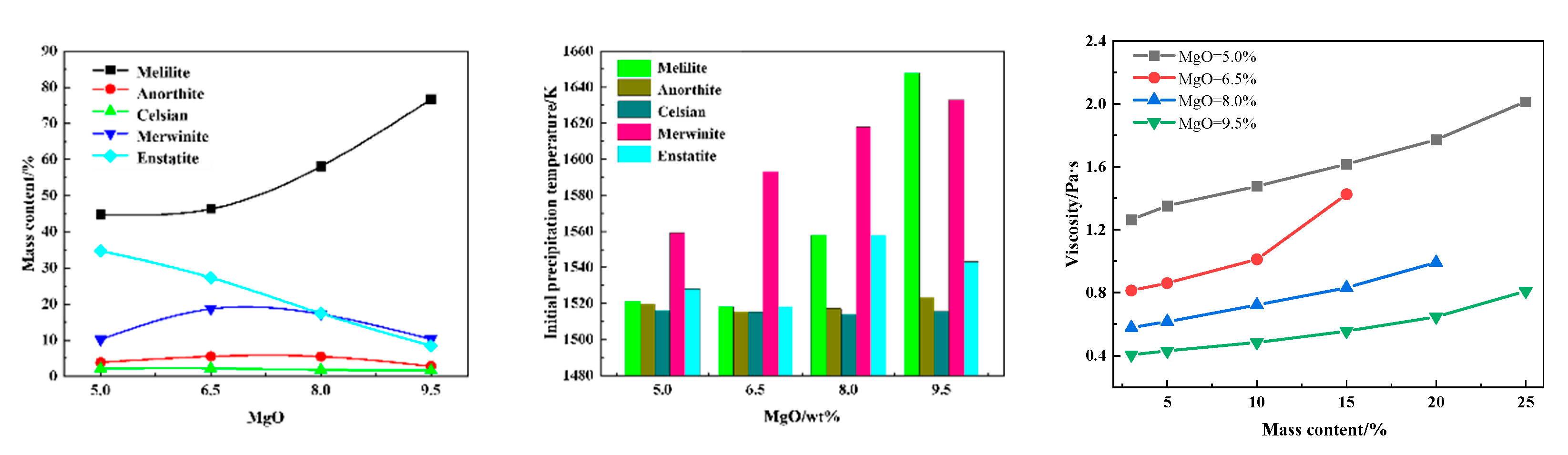
3.1.3. Effect of MgO on Slag Viscosity and Phase Evolution
3.2. The Characteristic of Heat Capacity and Thermal Stability of Slag
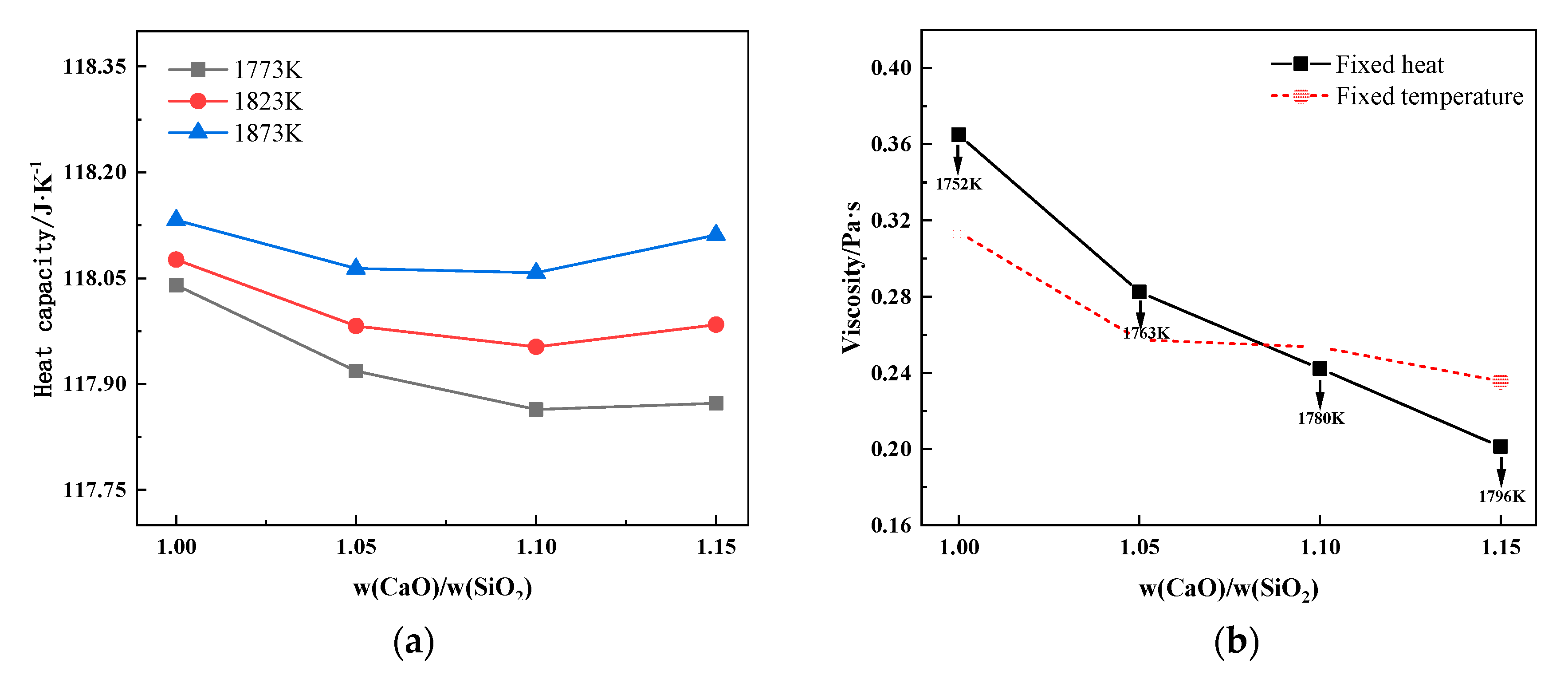
3.2.1. Effect of Basicity on Heat Capacity and Thermal Stability of Slag
3.2.2. Effect of Al2O3 on Heat Capacity and Thermal Stability of Slag
3.2.3. Effect of MgO on Heat Capacity and Thermal Stability of Slag
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Zuo, H.; Wang, Y.; Wang, J.; Xue, Q. Review of green and low-carbon ironmaking technology. Ironmak. Steelmak. 2020, 47, 296–306. [Google Scholar] [CrossRef]
- Hu, Y.; Rufford, T.E.; Chen, J.; HaO, L.; Li, M.; Qiu, Y.; Garg, S.; Rudolph, V.; Wang, G. Opportunities to reduce energy consumption and CO₂ emissions from ironmaking blast furnace using CO2 electrolysis to CO for carbon recycling. J. Clean. Prod. 2023, 389, 135997. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Lingiardi, O. Modern Blast Furnace Ironmaking: An Introduction; Ios Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Hazra, S.; Pal, S.; Biswajeet, D.D.; Sahoo, M.; Tarachand, G.S.; Bhattacharyya, D.; Nag, S.; Seetharaman, S. Optimization of high alumina slag practice in blast furnace ironmaking: An industrial approach (PART 1: Fundamental aspects). In Ironmaking & Steelmaking; Taylor and Francis: Abingdon, UK, 2023; pp. 1–14. [Google Scholar]
- Wang, C.; Zhang, J.; Gao, K.; Jiao, K.X.; Liu, Z.J.; Zhang, X.W. Interface reaction between carbon composite brick and blast furnace slag. In Ironmaking & Steelmaking; Taylor and Francis: Abingdon, UK, 2023; pp. 1–10. [Google Scholar]
- Chen, B.; Jiang, T.; Wen, J.; Li, L.; Hu, P. Review of pellets and blast furnace slag research progress: The effects of MgO on metallurgical properties. In Ironmaking & Steelmaking; Taylor and Francis: Abingdon, UK, 2023; pp. 1–15. [Google Scholar]
- Liao, J.; Qing, G.; Zhao, B. Phase equilibria studies in the CaO-MgO-Al2O3-SiO2 system with Al2O3/SiO2 weight ratio of 0.4. Metals 2023, 13, 224. [Google Scholar] [CrossRef]
- Liao, J.; Qing, G.; Zhao, B. Phase Equilibrium Studies of the CaO-MgO-Al2O3-SiO2 System for Iron Blast Furnace Slag: A Review. Metals 2023, 13, 801. [Google Scholar] [CrossRef]
- Webster, R.I.; Opila, E.J. Viscosity of CaO-MgO-Al2O3-SiO2 (CMAS) melts: Experimental measurements and comparison to model calculations. J. Non-Cryst. Solids 2022, 584, 121508. [Google Scholar] [CrossRef]
- Lu, Y.; Shan, R.; Wang, X.; Liu, Q.; Liu, J. Effect of Al2O3, MgO, and CaO/SiO2 on viscosity of high alumina blast furnace slag. Steel Res. Int. 2016, 87, 241–249. [Google Scholar]
- Zhang, J.; Wang, C.; Jiao, K.; Zhang, J.; Liu, Z.; Ma, H.; Fan, X.; Guo, Z. Effect of BaO and MnO on high-temperature properties and structure of blast furnace slag. J. Non-Cryst. Solids 2021, 571, 121066. [Google Scholar] [CrossRef]
- Xing, X.; Pang, Z.; Mo, C.; Wang, S.; Ju, J. Effect of MgO and BaO on viscosity and structure of blast furnace slag. J. Non-Cryst. Solids 2020, 530, 119801. [Google Scholar] [CrossRef]
- Xu, R.Z.; Zhang, J.L.; Han, W.X.; Chang, Z.Y.; Jiao, K.X. Effect of BaO and Na2O on the viscosity and structure of blast furnace slag. Ironmak. Steelmak. 2018, 47, 168–172. [Google Scholar] [CrossRef]
- Wang, Z.J.; Sohn, I. Effect of substituting CaO with BaO on the viscosity and structure of CaO-BaO-SiO2-MgO-Al2O3 slags. J. Am. Ceram. Soc. 2018, 101, 4285–4296. [Google Scholar] [CrossRef]
- Wang, P.; Meng, Q.-M.; Long, H.-M.; Li, J.-X. Influence of basicity and MgO on fluidity and desulfurization ability of high aluminum slag. High Temp. Mater. Process. 2016, 35, 669–675. [Google Scholar] [CrossRef]
- Jiao, K.-X.; Chang, Z.-Y.; Chen, C.; Zhang, J.-L. Thermodynamic properties and viscosities of CaO-SiO2-MgO-Al2O3 slags. Metall. Mater. Trans. B 2019, 50, 1012–1022. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Kondratiev, A.; Jak, E. A quasi-chemical viscosity model for fully liquid slags in the Al2O3-CaO-‘FeO’-SiO2 system. Metall. Mater. Trans. B 2005, 36, 623–638. [Google Scholar] [CrossRef]
- Kou, M.; Wu, S.; Ma, X.; Wang, L.; Chen, M.; Cai, Q.; Zhao, B. Phase equilibrium studies of CaO-SiO2-MgO-Al2O3 system with binary basicity of 1.5 related to blast furnace slag. Metall. Mater. Trans. B 2016, 47, 1093–1102. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Park, J.H.; Min, D.J. A study on the effect of Na2O on the viscosity for ironmaking slags. Steel Res. Int. 2010, 81, 17–24. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. FT-IR spectroscopic study on structure of CaO-SiO2 and CaO-SiO2-CaF2 slags. ISIJ Int. 2002, 42, 344–351. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.; Sohn, I. Influence of CaF2 and Li2O on the viscous behavior of calcium silicate melts containing 12wtpctNa2O. Metall. Mater. Trans. B 2011, 42, 324–330. [Google Scholar] [CrossRef]
- Sohn, I.; Min, D.J. A Review of the relationship between viscosity and the structure of calcium-silicate-based slags in ironmaking. Steel Res. Int. 2012, 83, 611–630. [Google Scholar] [CrossRef]
- Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wang, S.; Hong, C.; Ma, X.J.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt% Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Liu, W.; Pang, Z.; Wang, J.; Zuo, H.; Xue, Q. Investigation of viscosity and structure of CaO-SiO2-MgO-Al2O3-BaO-B2O3 slag melt. Ceram. Int. 2022, 48, 17123–17130. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Wu, H.; Xue, Q.; Zuo, H. Investigation on the crystallization properties of BaO-bearing blast furnace slag through a confocal laser scanning microscope. Constr. Build. Mater. 2022, 352, 129010. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, T.; Xue, X.; Hu, B. Influence of MgO/Al2O3 ratio on viscosity of blast furnace slag with high Al2O3 content. Steel Res. Int. 2016, 87, 87–94. [Google Scholar] [CrossRef]
- Wu, J.J.; Wang, H.; Zhu, X.; Liao, Q.; Ding, B. Centrifugal granulation performance of liquid with various viscosities for heat recovery of blast furnace slag. Appl. Therm. Eng. 2015, 89, 494–504. [Google Scholar] [CrossRef]
- Sadek, D.M. Effect of cooling technique of blast furnace slag on the thermal behavior of solid cement bricks. J. Clean. Prod. 2014, 79, 134–141. [Google Scholar] [CrossRef]
- Zhang, L.; Jahanshahi, S. Review and modeling of viscosity of silicate melts: Part I. Viscosity of binary and ternary silicates containing CaO, MgO, and MnO. Metall. Mater. Trans. B 1998, 29, 177–186. [Google Scholar] [CrossRef]
- Kim, J.B.; Sohn, I. Influence of TiO2/SiO2 and MnO on the viscosity and structure in the TiO2-MnO-SiO2 welding flux system. J. Non-Cryst. Solids 2013, 379, 235–243. [Google Scholar] [CrossRef]
- Kim, J.B.; Sohn, I. Effect of alumina and extended basicity on the viscosity and structure in the TiO2-MnO-Al2O3-8.64ZrO2-2.77Na2O welding flux system. Trans. Iron Steel Inst. Jpn. 2014, 54, 2014. [Google Scholar] [CrossRef] [Green Version]
- Ning, X.J.; Li, P.C. Effects of MgO/Al2O3 ratio and basicity on the viscosities of CaO-MgO-SiO2-Al2O3 Slags: Experiments and modeling. Metall. Mater. Trans. B 2016, 47, 446–457. [Google Scholar]
- Mudersbach, D.; Drissen, P.M.; Kühn, M.; Geiseler, J. Viscosity of slags. Steel Res. 2001, 72, 86–90. [Google Scholar] [CrossRef]
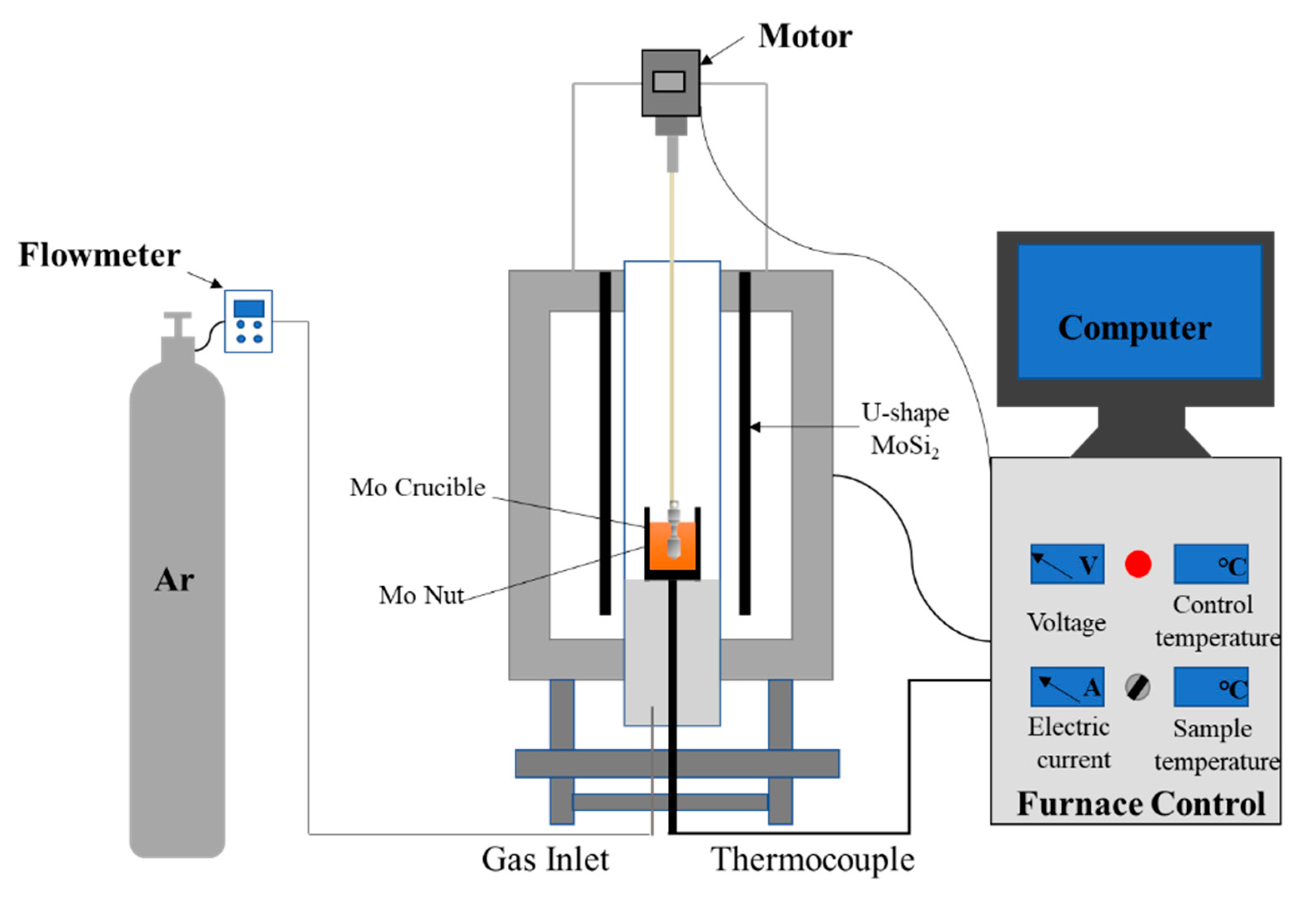






| No. | CaO/SiO2 (R2) | CaO/% | SiO2/% | MgO/% | Al2O3/% | BaO/% |
|---|---|---|---|---|---|---|
| A-1 | 1.05 | 41.49 | 39.51 | 8.00 | 9.00 | 2.00 |
| A-2 | 1.05 | 40.98 | 39.02 | 8.00 | 10.00 | 2.00 |
| A-3 | 1.05 | 40.46 | 38.54 | 8.00 | 11.00 | 2.00 |
| A-4 | 1.05 | 39.44 | 37.56 | 8.00 | 13.00 | 2.00 |
| B-1 | 1.00 | 40.00 | 40.00 | 8.00 | 10.00 | 2.00 |
| B-2 | 1.05 | 40.98 | 39.02 | 8.00 | 10.00 | 2.00 |
| B-3 | 1.10 | 41.90 | 38.10 | 8.00 | 10.00 | 2.00 |
| B-4 | 1.15 | 42.79 | 37.21 | 8.00 | 10.00 | 2.00 |
| M-1 | 1.05 | 43.02 | 40.98 | 5.00 | 9.00 | 2.00 |
| M-2 | 1.05 | 42.26 | 40.24 | 6.50 | 9.00 | 2.00 |
| M-3 | 1.05 | 41.49 | 39.51 | 8.00 | 9.00 | 2.00 |
| M-4 | 1.05 | 40.72 | 38.78 | 9.50 | 9.00 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, J. Effect of Main Composition on the Viscosity and Thermal Stability of BaO-Containing Slag. Metals 2023, 13, 1170. https://doi.org/10.3390/met13071170
Fan X, Zhang J. Effect of Main Composition on the Viscosity and Thermal Stability of BaO-Containing Slag. Metals. 2023; 13(7):1170. https://doi.org/10.3390/met13071170
Chicago/Turabian StyleFan, Xiaoyue, and Jianliang Zhang. 2023. "Effect of Main Composition on the Viscosity and Thermal Stability of BaO-Containing Slag" Metals 13, no. 7: 1170. https://doi.org/10.3390/met13071170




