Study of Structure and Phase Transformations in Rejuvenated Rapidly Quenched TiNiCu Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystallization Temperature Range and Pattern
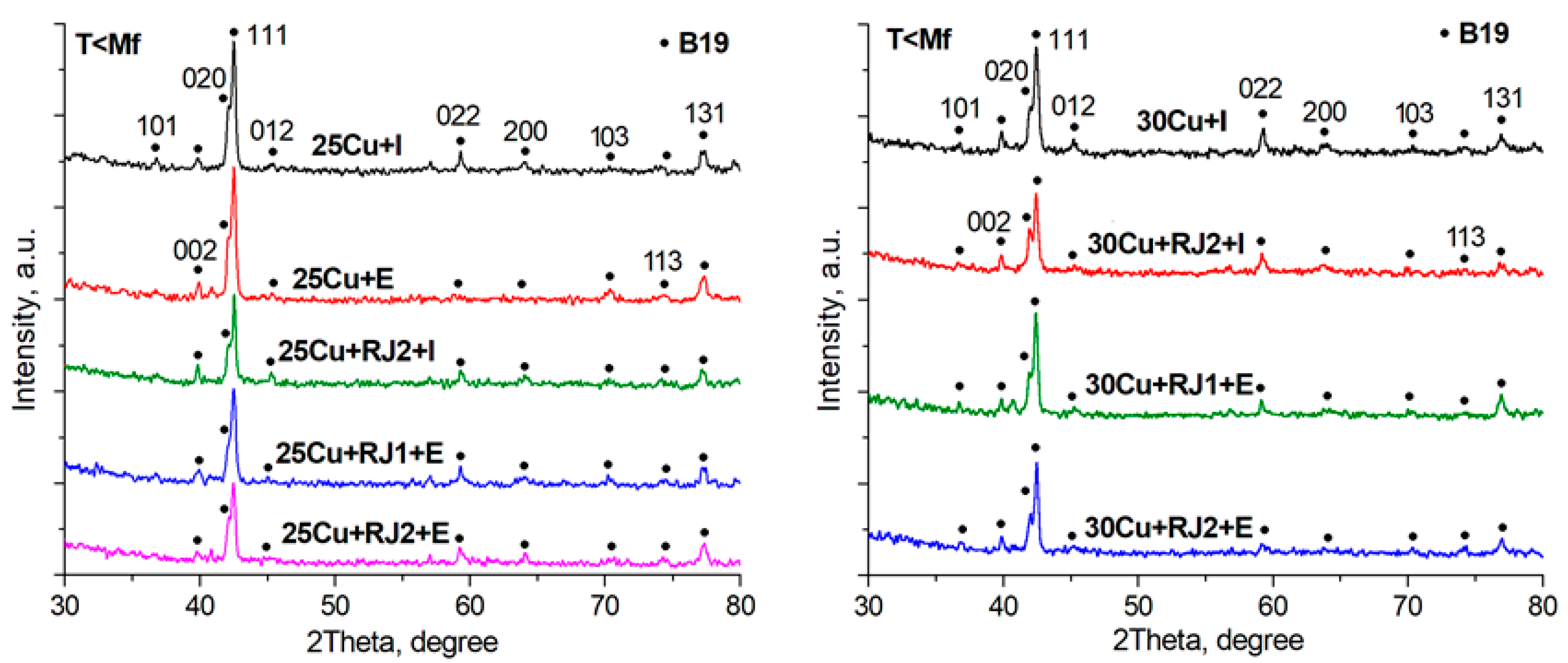
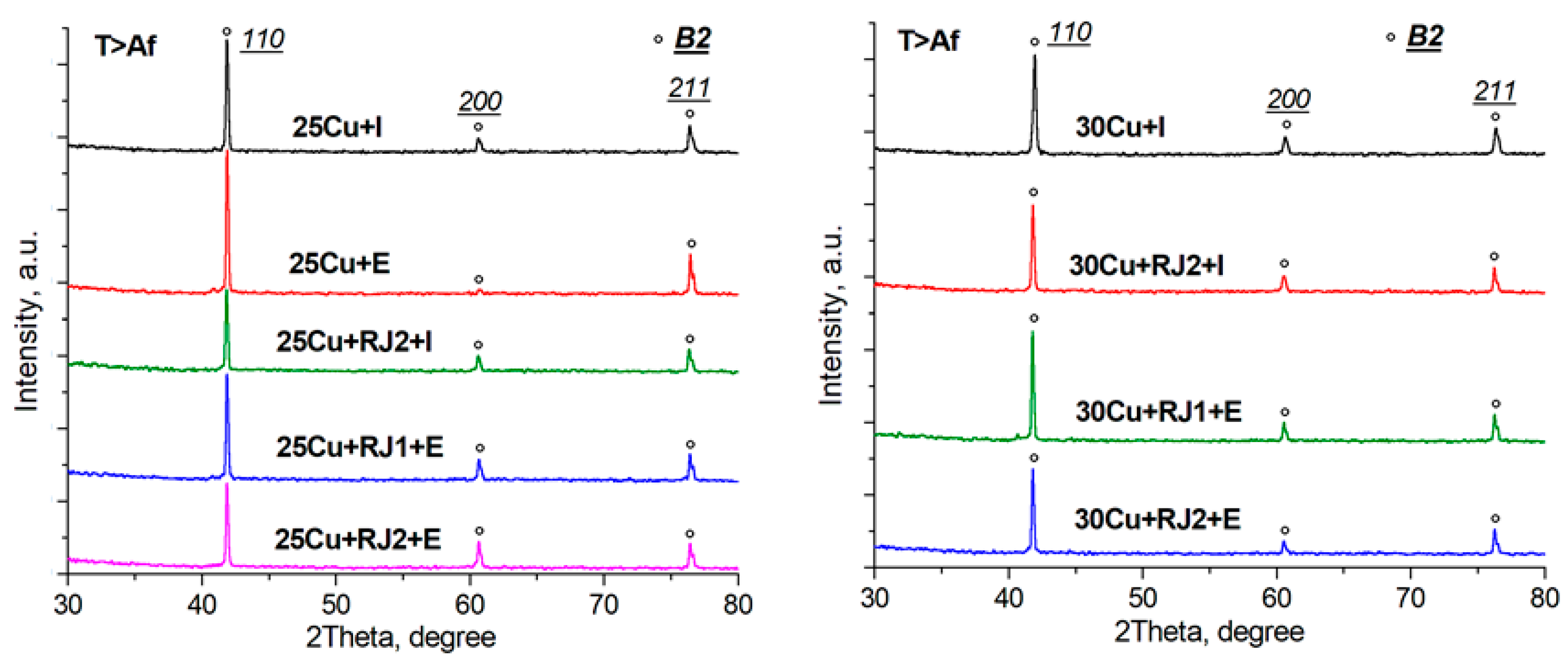
3.2. X-ray Diffraction Analysis
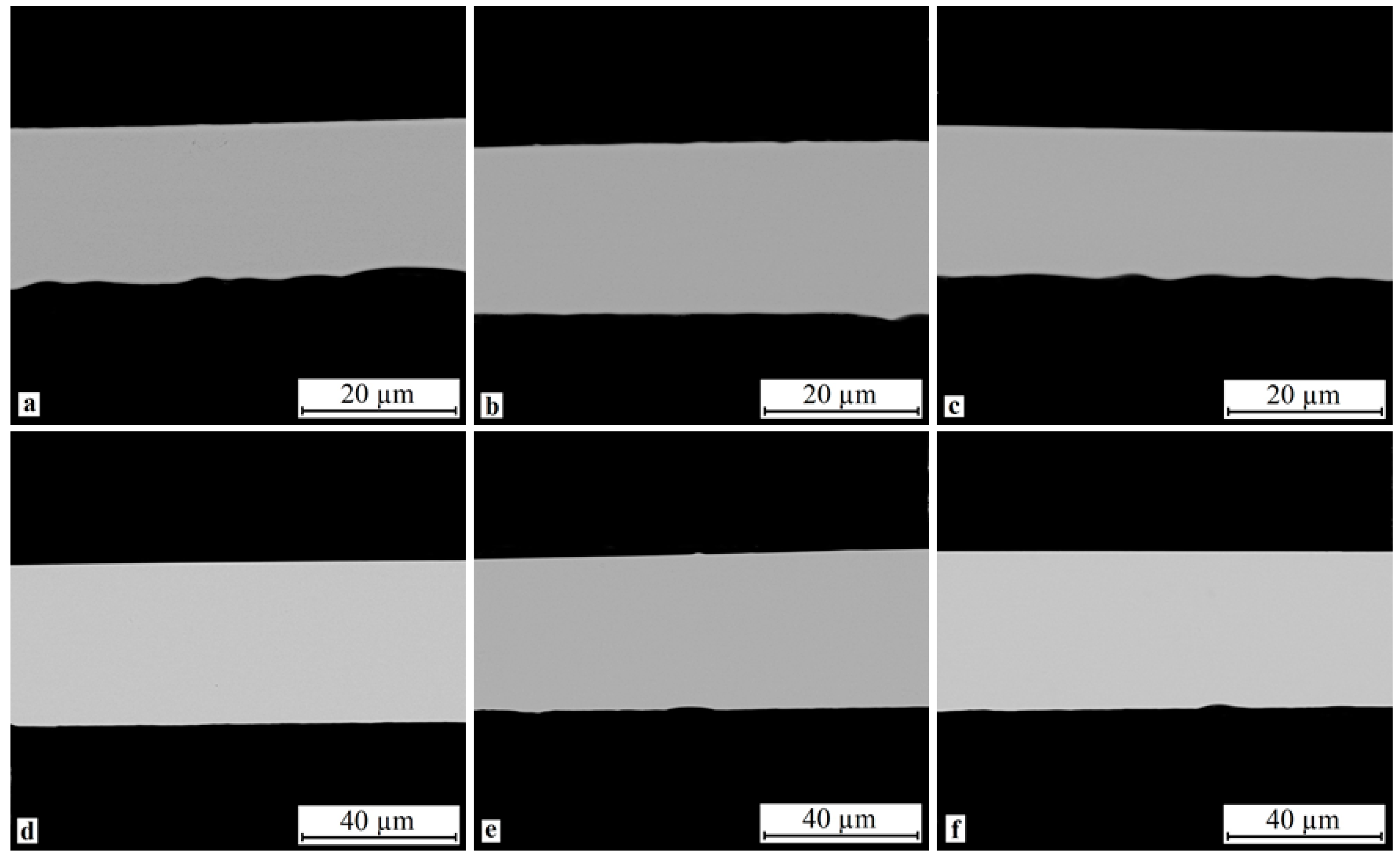
3.3. Cross-Sectional Microstructure of Rapidly Quenched Ribbons
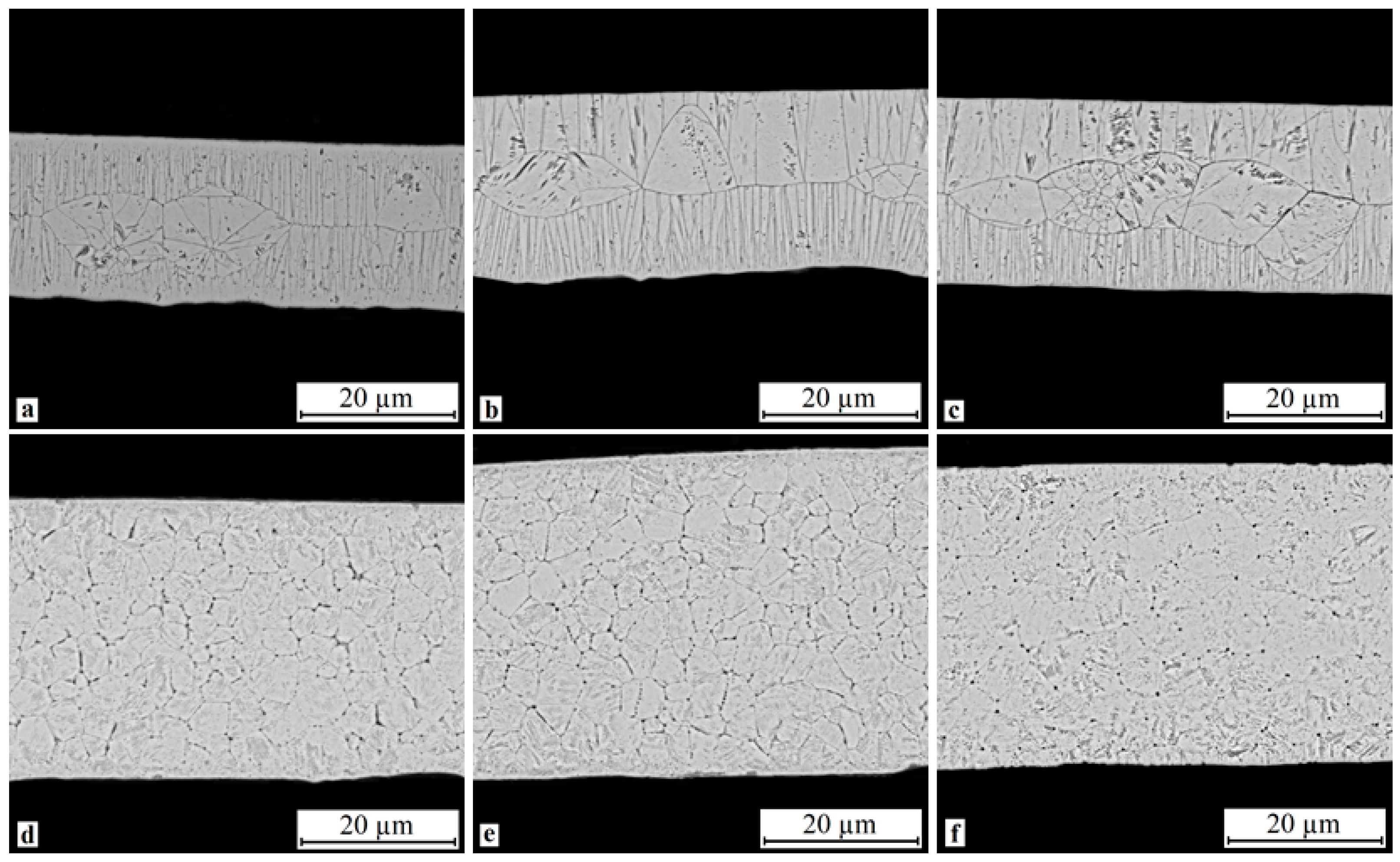
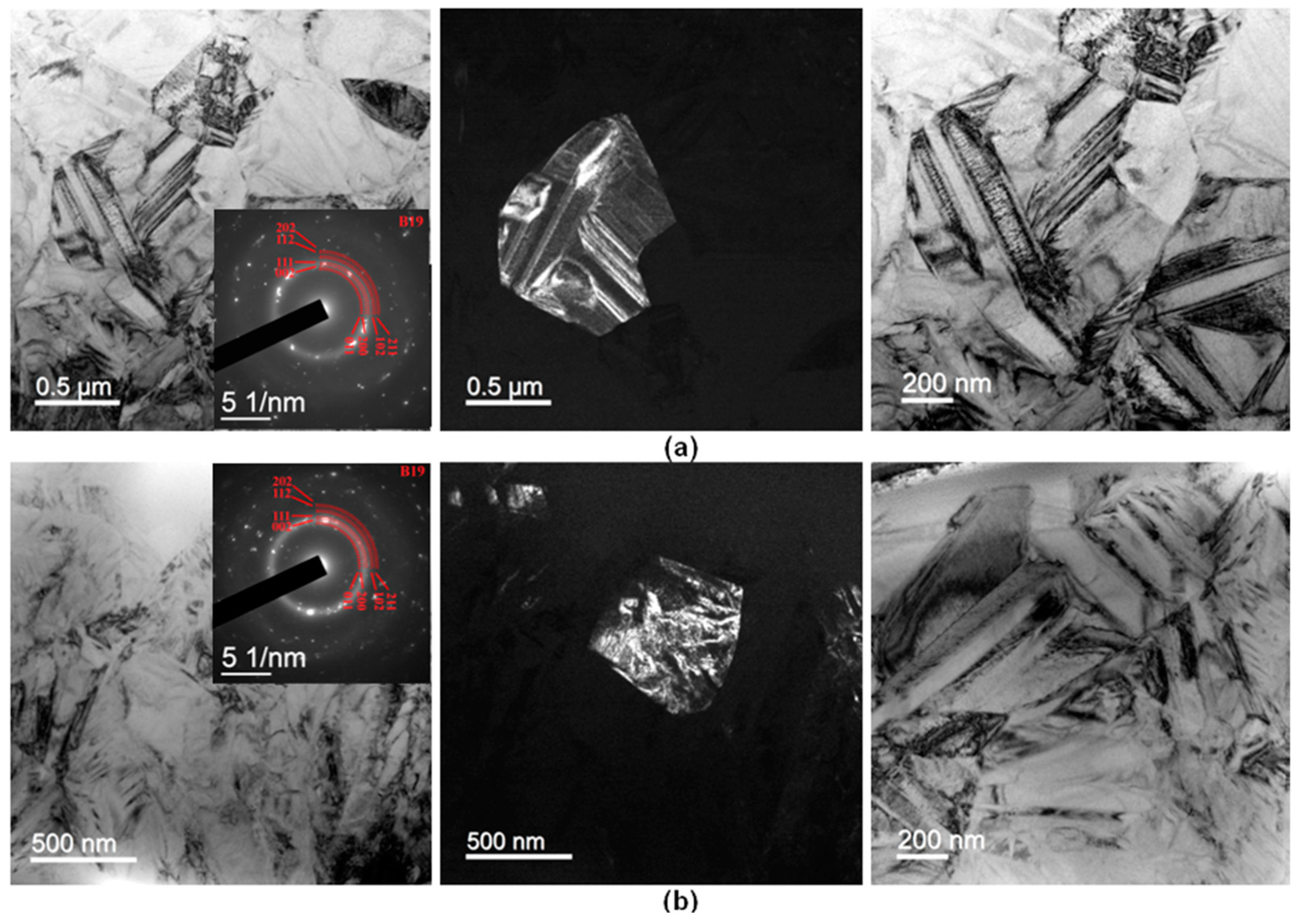
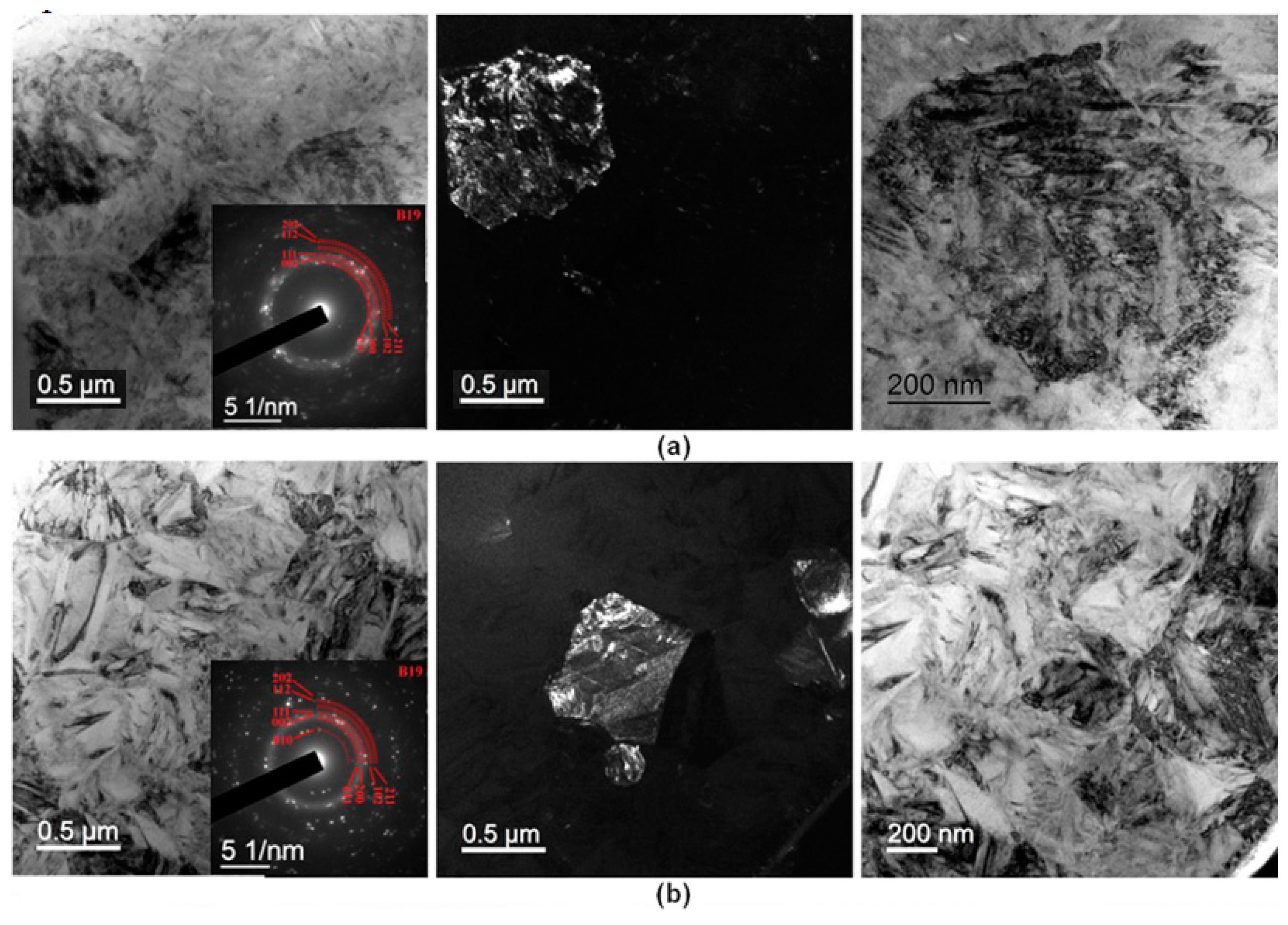
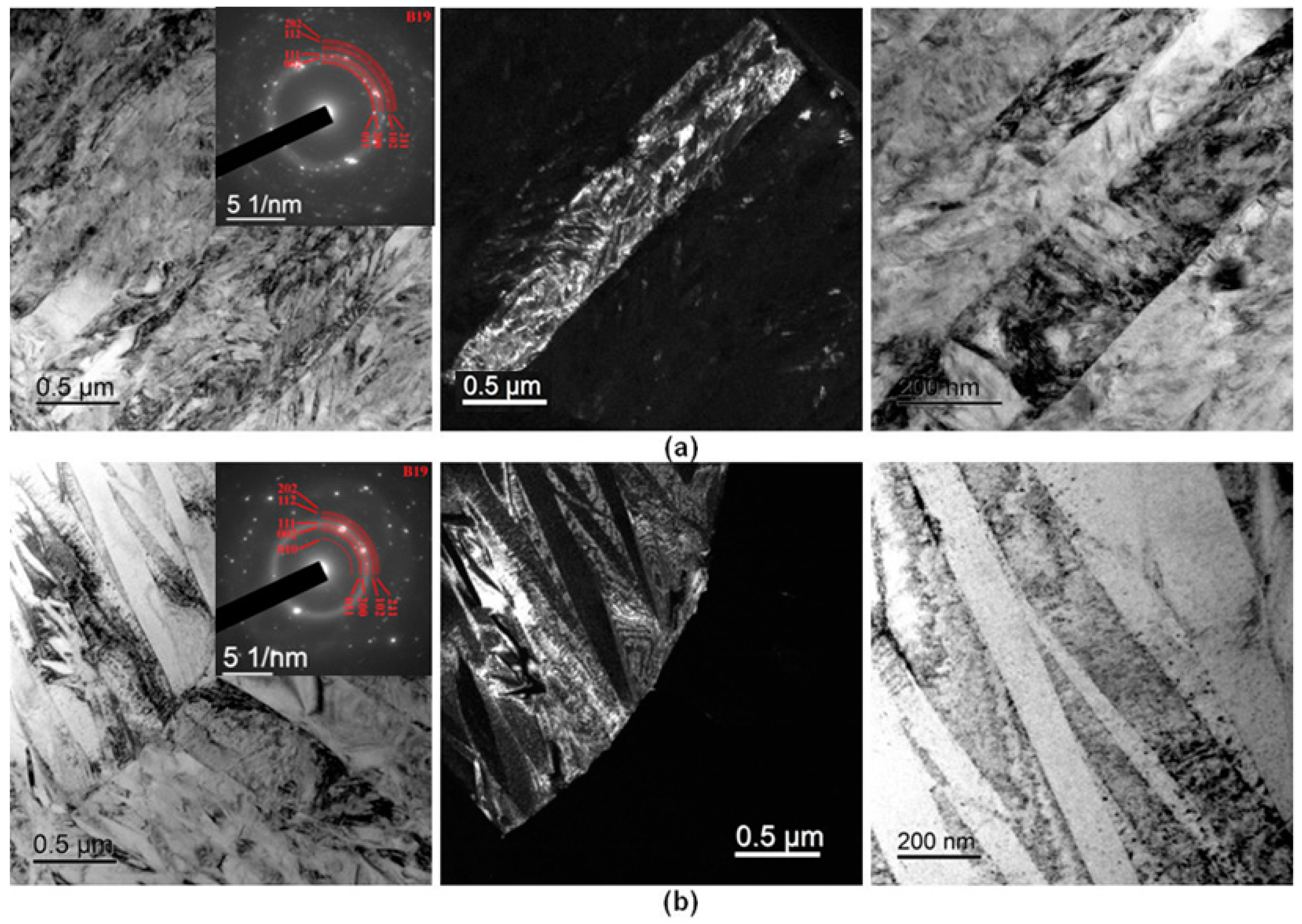
3.4. Fine Structure of Rapidly Quenched Ribbons
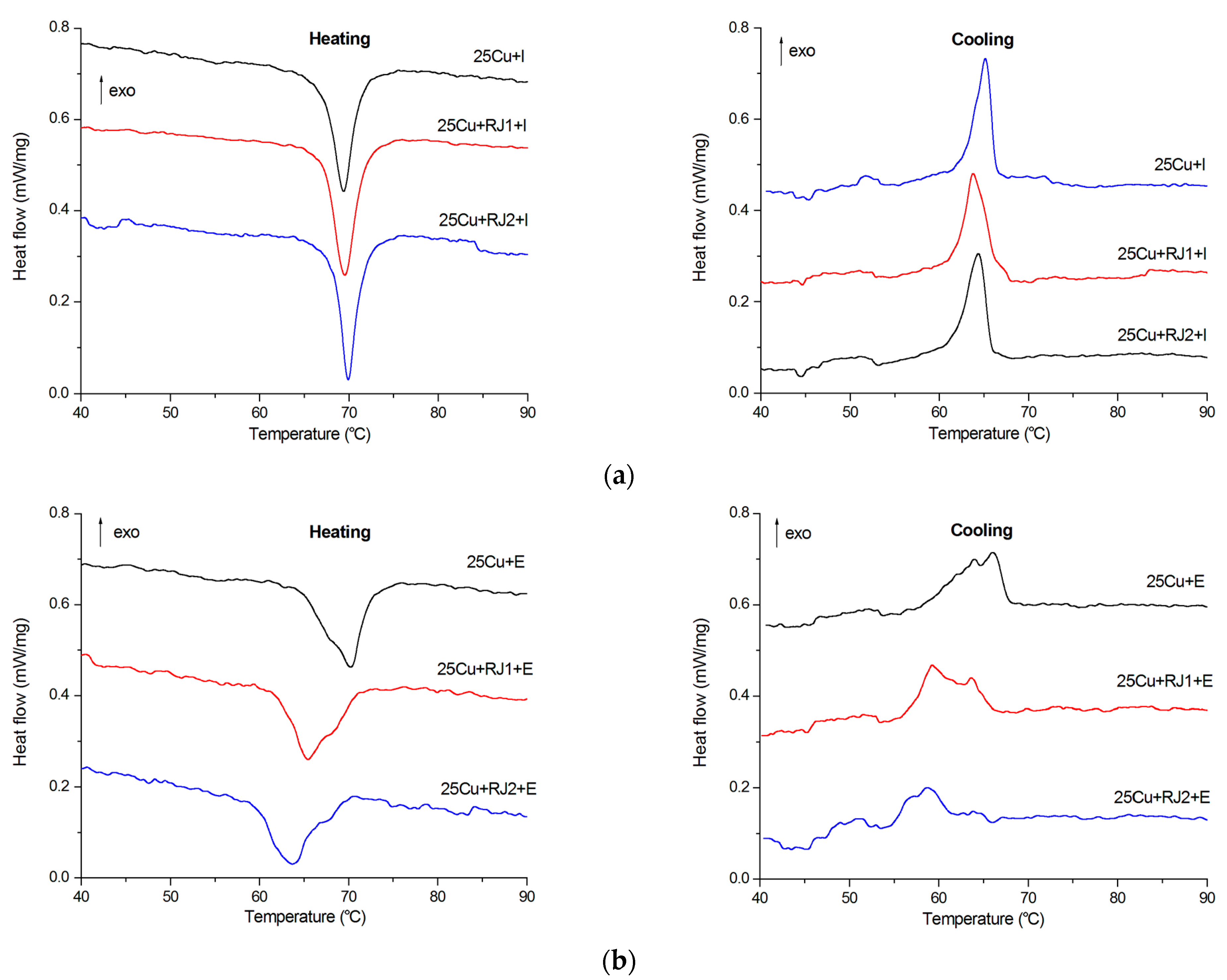
3.5. Martensitic Transformation Temperature Ranges and Pattern
4. Conclusions
- -
- a slight decrease in glass transition of 30 at.% Cu alloy and crystallization onset temperatures of 25 at.% Cu alloy;
- -
- a moderate decrease in the quantity of the B19 martensite phase, the decrease being somewhat greater after RJ2 treatment than after RJ1 treatment;
- -
- an increase in grain sizes, decrease in thickness of martensite plates, and higher degree of structural imperfection;
- -
- an increase in characteristic martensitic transformation temperatures of isothermally annealed 25 at.% Cu alloy and of electropulse-crystallized 30 at.% Cu alloy by 3–7 °C, and vice versa, i.e., their decrease for electropulse-treated 25 at.% Cu alloy by 3–4 °C;
- -
- an increase in martensitic transformation enthalpy for RJ1-treated alloys and its decrease for RJ2-treated alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stachiv, I.; Alarcon, E.; Lamac, M. Shape Memory Alloys and Polymers for MEMS/NEMS Applications: Review on Recent Findings and Challenges in Design, Preparation, and Characterization. Metals 2021, 11, 415. [Google Scholar] [CrossRef]
- Rao, A.; Srinivasa, A.R.; Reddy, J.N. Design of Shape Memory Alloy (SMA) Actuators; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Kohl, M.; Ossmer, H.; Gueltig, M.; Megnin, C. SMA Foils for MEMS: From Material Properties to the Engineering of Microdevices. Shape Mem. Superelasticity 2018, 4, 127–142. [Google Scholar] [CrossRef]
- Nespoli, A.; Besseghini, S.; Pittaccio, S.; Villa, E.; Viscuso, S. The high potential of shape memory alloys in developing miniature mechanical devices: A review on shape memory alloy mini-actuators. Sens. Actuators A Phys. 2010, 158, 149–160. [Google Scholar] [CrossRef]
- Mohd Jani, J.; Leary, M.; Subic, A.; Gibson, M.A. A review of Shape Memory Alloy research, applications and opportunities. Mater. Des. 2013, 56, 1078–1113. [Google Scholar] [CrossRef]
- Ishida, A.; Sato, M. Microstructure and shape memory behaviour of annealed Ti51.5Ni(48.5-x)Cux (x = 6.5–20.9) thin films. Philos. Mag. 2007, 87, 5523–5538. [Google Scholar] [CrossRef]
- Shelyakov, A.V.; Sitnikov, N.N.; Borodako, K.A.; Sevryukov, O.N. Two-Way Shape Memory Effect in Rapidly-Quenched High-Copper TiNiCu Alloys Deformed in the Martensitic State. Russ. Metall. 2020, 2020, 345–350. [Google Scholar] [CrossRef]
- Kang, S.W.; Lim, Y.M.; Lee, Y.H.; Moon, H.J.; Kim, Y.W.; Nam, T.H. Microstructures and shape memory characteristics of a Ti–20Ni–30Cu (at.%) alloy strip fabricated by the melt overflow process. Scr. Mater. 2010, 62, 71–74. [Google Scholar] [CrossRef]
- Shelyakov, A.V.; Sitnikov, N.N.; Borodako, K.A.; Khabibullina, I.A.; Dyadechko, A.A. Structure and functional properties of rapidly quenched TiNiCu alloys with high copper contents. J. Phys. Conf. Ser. 2021, 1758, 012036. [Google Scholar] [CrossRef]
- Sitnikov, N.; Shelyakov, A.; Rizakhanov, R.; Mitina, N.; Khabibullina, I. The effect of copper on structure of TiNiCu melt-spun ribbons. Mater. Today Proc. 2017, 4, 4680–4684. [Google Scholar] [CrossRef]
- Liu, Y. Mechanical and thermomechanical properties of a Ti50Ni25Cu25 melt spun ribbon. Mater. Sci. Eng. A. 2003, 354, 286–291. [Google Scholar] [CrossRef]
- Borodako, K.A.; Shelyakov, A.V.; Sitnikov, N.N.; Zaletova, I.A.; Sevryukov, O.N. Influence of copper content and thermal treatment on shape memory effect in rapidly quenched TiNiCu alloys. J. Phys. Conf. Ser. 2021, 2036, 012013. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, S.K.; Kimura, H. Annealing effects on the crystallization and shape memory effect of Ti50Ni25Cu25 melt-spun ribbons. Intermetallics 2007, 15, 233–240. [Google Scholar] [CrossRef]
- Shelyakov, A.; Sitnikov, N.; Khabibullina, I.; Borodako, K.; Sevryukov, O. Shape Memory Behavior of Rapidly Quenched High-copper TiNiCu Alloys. U. Porto J. Eng. 2021, 7, 2–10. [Google Scholar] [CrossRef]
- Shelyakov, A.; Sitnikov, N.; Zaletova, I.; Tabachkova, N.; Andreev, N. Effect of External Impacts on the Structure and Martensitic Transformation of Rapidly Quenched TiNiCu Alloys. Metals 2021, 11, 1528. [Google Scholar] [CrossRef]
- Gong, P.; Yin, G.; Jamili-Shirvan, Z.; Ding, H.; Wang, X. Influence of deep cryogenic cycling on the rejuvenation and plasticization of TiZrHfBeCu high-entropy bulk metallic glass. Mater. Sci. Eng. A 2020, 797, 140078. [Google Scholar] [CrossRef]
- Abrosimova, G.; Volkov, N.; Pershina, E.; Tuan, T.V.; Aronin, A. Amorphous structure rejuvenation under cryogenic treatment of Al-based amorphous-nanocrystalline alloys. J. Non-Cryst. Solids 2020, 528, 119751. [Google Scholar] [CrossRef]
- Jiang, J.; Ko, W.-S.; Joo, S.-H.; Wei, D.X.; Wada, T.; Kato, H.; Louzguine-Luzgin, D.V. Experimental and molecular dynamics studies of phase transfor ations during cryogenic thermal cycling in complex TiNi based crystalline/amorphous alloys. J. Alloys Compd. 2021, 854, 155379. [Google Scholar] [CrossRef]
- Priezjev, N.V. The effect of thermal history on the atomic structure and mechanical properties of amorphous alloys. Comput. Mater. Sci. 2020, 174, 109477. [Google Scholar] [CrossRef] [Green Version]
- Ketov, S.V.; Sun, Y.H.; Nachum, S.; Lu, Z.; Checchi, A.; Beraldin, A.R.; Bai, H.Y.; Wang, W.H.; Louzguine-Luzgin, D.V.; Carpenter, M.A.; et al. Rejuvenation of metallic glasses by non-affine thermal strain. Nature 2015, 524, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.J.; Cao, Q.P.; Liu, J.; Tang, Y.; Wang, X.D.; Zhang, D.X.; Ahn, I.S.; Caron, A.; Jiang, J.Z. Intermediate structural state for maximizing the rejuvenation effect in metallic glass via thermo-cycling treatment. J. Alloys Comp. 2019, 795, 493–500. [Google Scholar] [CrossRef]
- Saida, J.; Yamada, R.; Wakeda, M.; Ogata, S. Thermal rejuvenation in metallic glasses. Sci. Technol. Adv. Mater. 2017, 18, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Hua, Y.; Wang, X.; Zhao, X.; Chen, L.; Zhou, H.; Wang, J.; Berndt, C.C.; Li, W. Application of High-Density Electropulsing to Improve the Performance of Metallic Materials: Mechanisms, Microstructure and Properties. Materials 2018, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Ishida, A.; Sato, M. Microstructures of crystallized Ti51.5Ni48.5−xCux (x = 23.4–37.3) thin films. Intermetallics 2011, 19, 900–907. [Google Scholar] [CrossRef]
- Shelyakov, A.V.; Sitnikov, N.N.; Khabibullina, I.A.; Sundeev, R.V.; Sevryukov, O.N. Specifics of Crystallization of Amorphous TiNiCu Alloys with High Concentrations of Copper. Phys. Solid State 2020, 62, 937–941. [Google Scholar] [CrossRef]
- Zarinejad, M.; Liu, Y.; Liu, T.; White, T.; Yang, P.; Chen, Q. Evolution of local atomic structure in a melt-spun Ti50Ni25Cu25 shape memory alloy during crystallization. Philos. Mag. 2011, 91, 404–420. [Google Scholar] [CrossRef]
- Chernysheva, O.; Shelyakov, A.; Sitnikov, N.; Veligzhanin, A.; Borodako, K.; Sundeev, R. Local atomic and crystal structure of rapidly quenched TiNiCu shape memory alloys with high copper content. Mater. Lett. 2021, 285, 129104. [Google Scholar] [CrossRef]
- Shelyakov, A.; Sitnikov, N.; Khabibullina, I.; Tabachkova, N.; Fominski, V.; Andreev, N. Effect of high-rate annealing on microstructure, martensitic transformation and shape memory behavior of TiNiCu melt-spun ribbons. Mater. Lett. 2019, 248, 48–51. [Google Scholar] [CrossRef]








| Specimen | Tg, °C | Toneset, °C | Tp, °C | Tend, °C | ΔH, J/g |
|---|---|---|---|---|---|
| 25Cu | 435.9 | 449.5 | 453.4 | 457.7 | 28.4 |
| 25Cu + RJ1 | 435.0 | 449.4 | 453.4 | 457.7 | 28.3 |
| 25Cu + RJ2 | 435.8 | 448.0 | 453.4 | 457.3 | 28.7 |
| 30Cu | 425.1 | 442.1 | 445.2 | 449.5 | 30.1 |
| 30Cu + RJ1 | 423.0 | 441.3 | 445.2 | 449.2 | 29.7 |
| 30Cu + RJ2 | 422.2 | 442.0 | 445.2 | 450.0 | 29.4 |
| Specimen | Ms, °C | Mf, °C | As, °C | Af, °C | HM, J/g | HA, J/g |
|---|---|---|---|---|---|---|
| 25Cu + I | 65.9 | 61.4 | 66.8 | 71.8 | 10.1 | −10.7 |
| 25Cu + RJ1 + I | 67.4 | 61.4 | 67.1 | 72.3 | 11.5 | −11.4 |
| 25Cu + RJ2 + I | 66.4 | 62.4 | 68.0 | 72.3 | 10.0 | −10.7 |
| 25Cu + E | 67.8 | 58.2 | 64.2 | 73.5 | 10.8 | −10.9 |
| 25Cu + RJ1 + E | 66.1 | 55.2 | 61.6 | 71.5 | 11.4 | −10.6 |
| 25Cu + RJ2 + E | 65.2 | 54.5 | 60.0 | 70.5 | 8.7 | −10.6 |
| 30Cu + I | 53.6 | 49.1 | 54.3 | 63.7 | 9.5 | −8.1 |
| 30Cu + RJ1 + I | 53.5 | 49.2 | 54.2 | 63.8 | 9.4 | −8.0 |
| 30Cu + RJ2 + I | 53.5 | 48.4 | 55.6 | 63.1 | 10.8 | −8.7 |
| 30Cu + E | 62.3 | 54.3 | 56.9 | 65.4 | 9.8 | −9.2 |
| 30Cu + RJ1 + E | 63.6 | 56.5 | 61.8 | 67.5 | 10.5 | −9.5 |
| 30Cu + RJ2 + E | 66.9 | 57.7 | 60.9 | 72.3 | 9.3 | −8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelyakov, A.; Sitnikov, N.; Zaletova, I.; Borodako, K.; Tabachkova, N. Study of Structure and Phase Transformations in Rejuvenated Rapidly Quenched TiNiCu Alloys. Metals 2023, 13, 1175. https://doi.org/10.3390/met13071175
Shelyakov A, Sitnikov N, Zaletova I, Borodako K, Tabachkova N. Study of Structure and Phase Transformations in Rejuvenated Rapidly Quenched TiNiCu Alloys. Metals. 2023; 13(7):1175. https://doi.org/10.3390/met13071175
Chicago/Turabian StyleShelyakov, Alexander, Nikolay Sitnikov, Irina Zaletova, Kirill Borodako, and Natalia Tabachkova. 2023. "Study of Structure and Phase Transformations in Rejuvenated Rapidly Quenched TiNiCu Alloys" Metals 13, no. 7: 1175. https://doi.org/10.3390/met13071175





