Microstructure, Hardness, and Linear Reciprocating Sliding Wear Response of Directionally Solidified Al–(2.5, 3.5, 4.5)Cu–(0.25, 0.50)Cr Alloys
Abstract
:1. Introduction
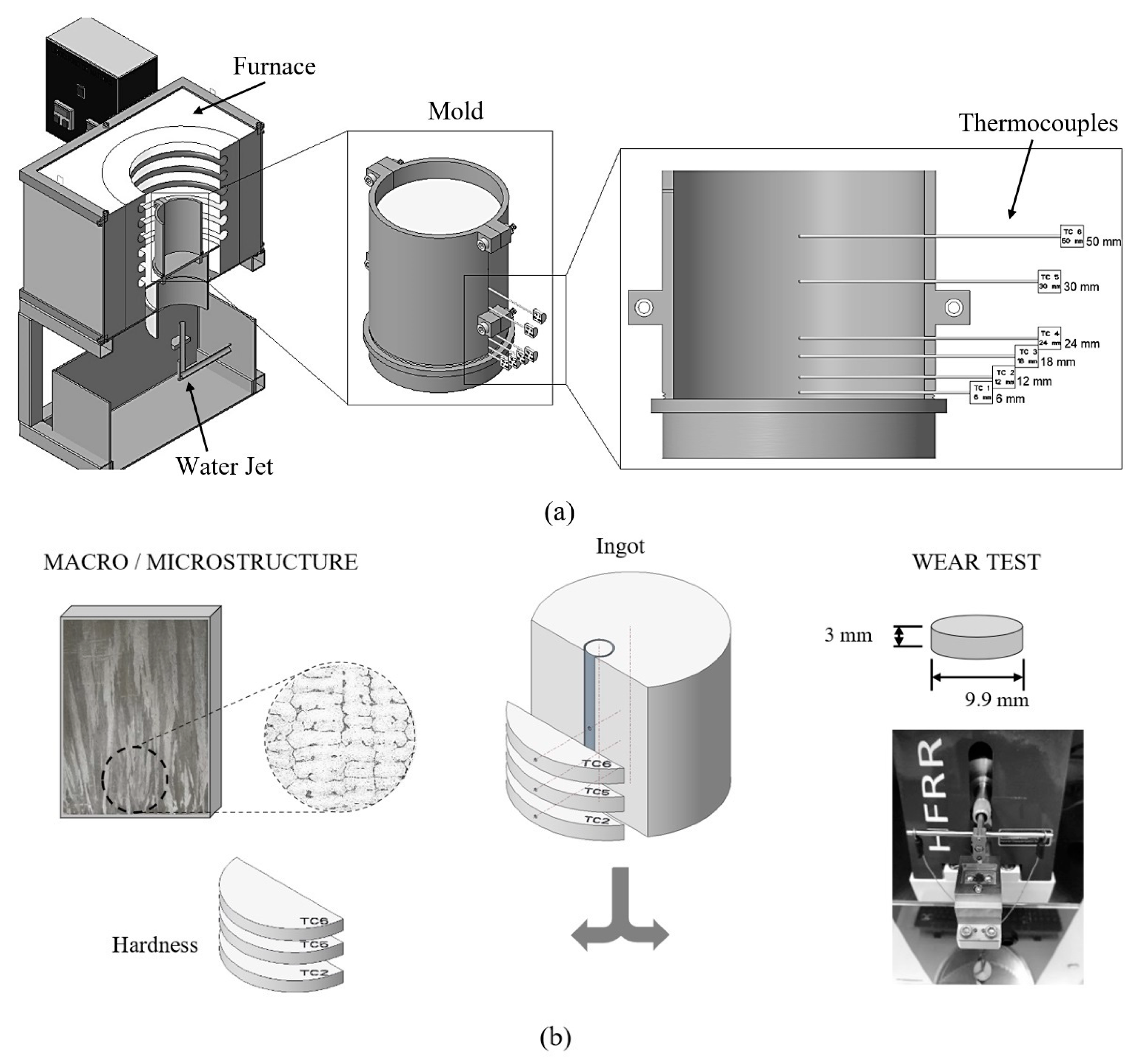
2. Materials and Methods
3. Results and Discussion
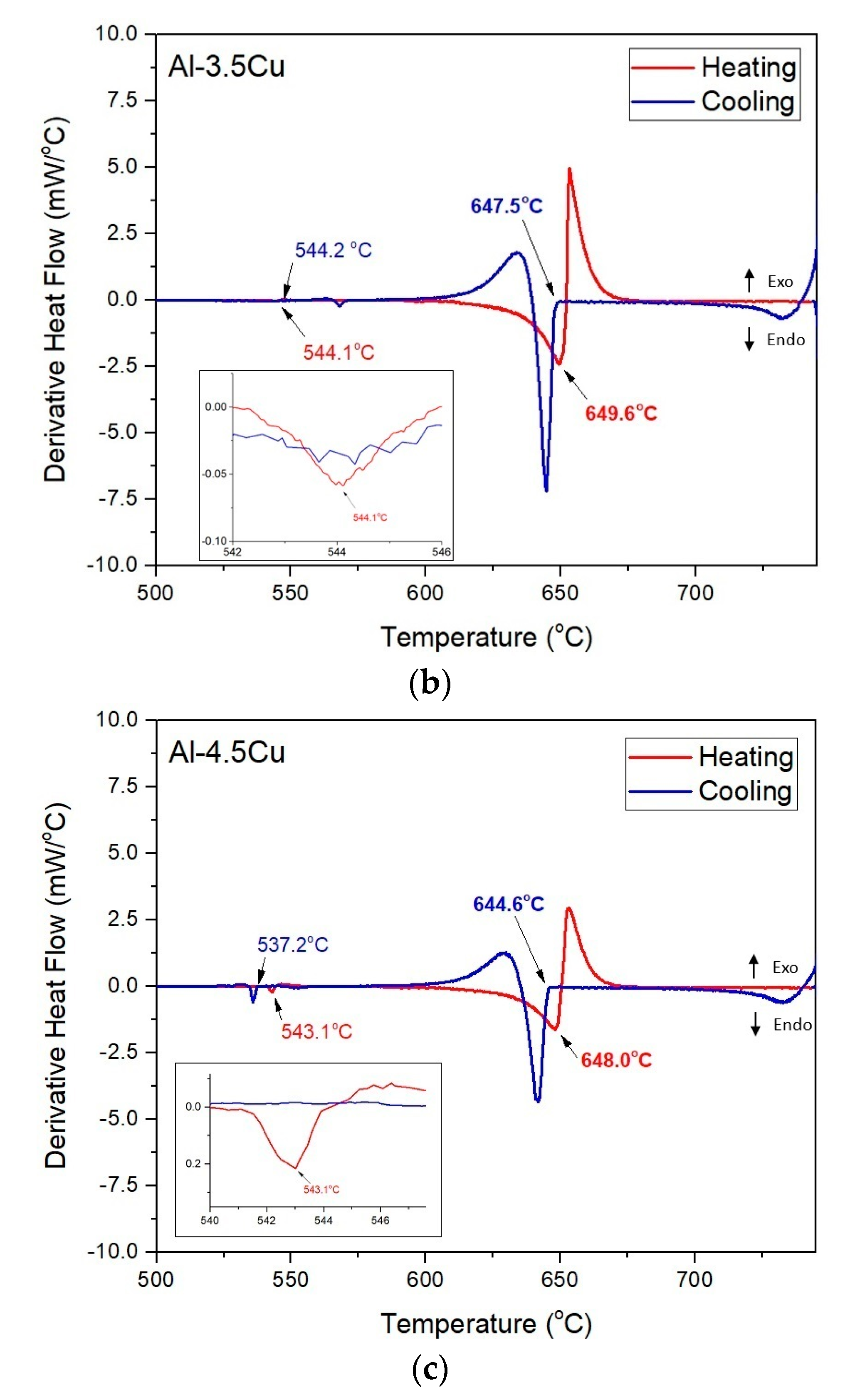
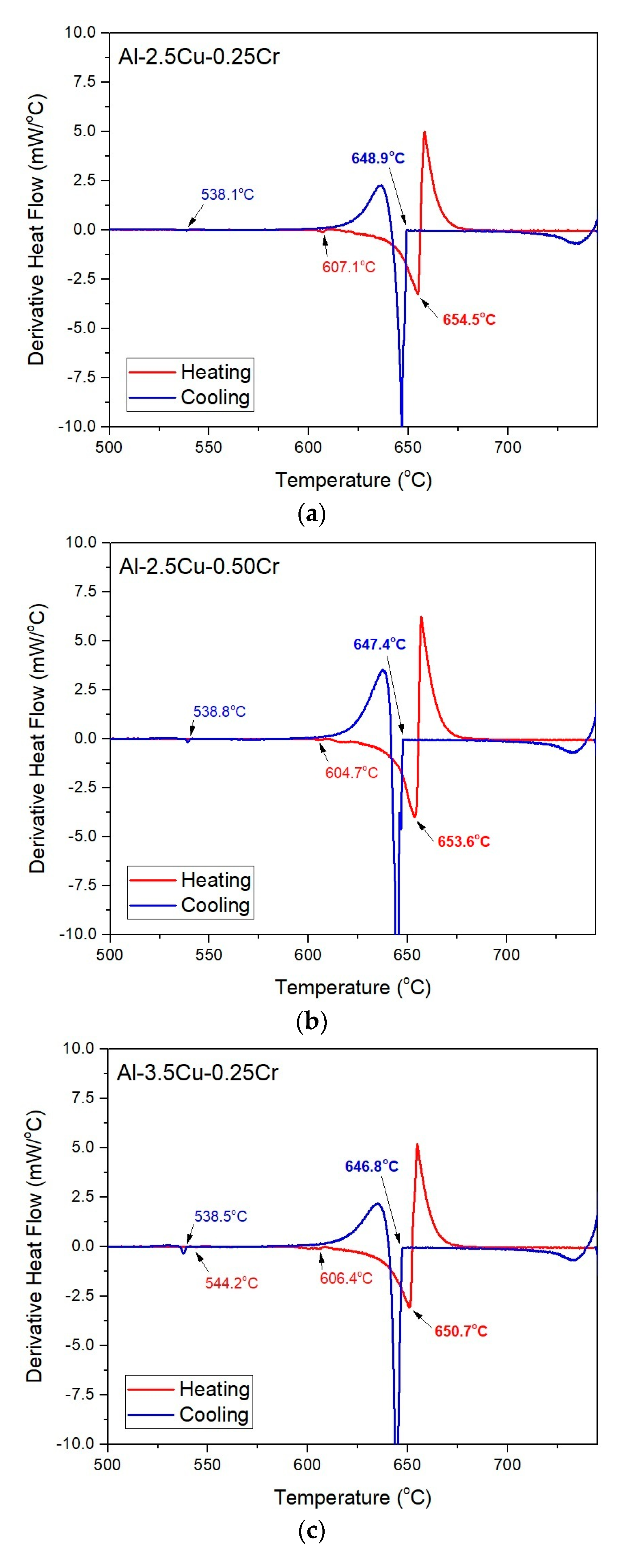
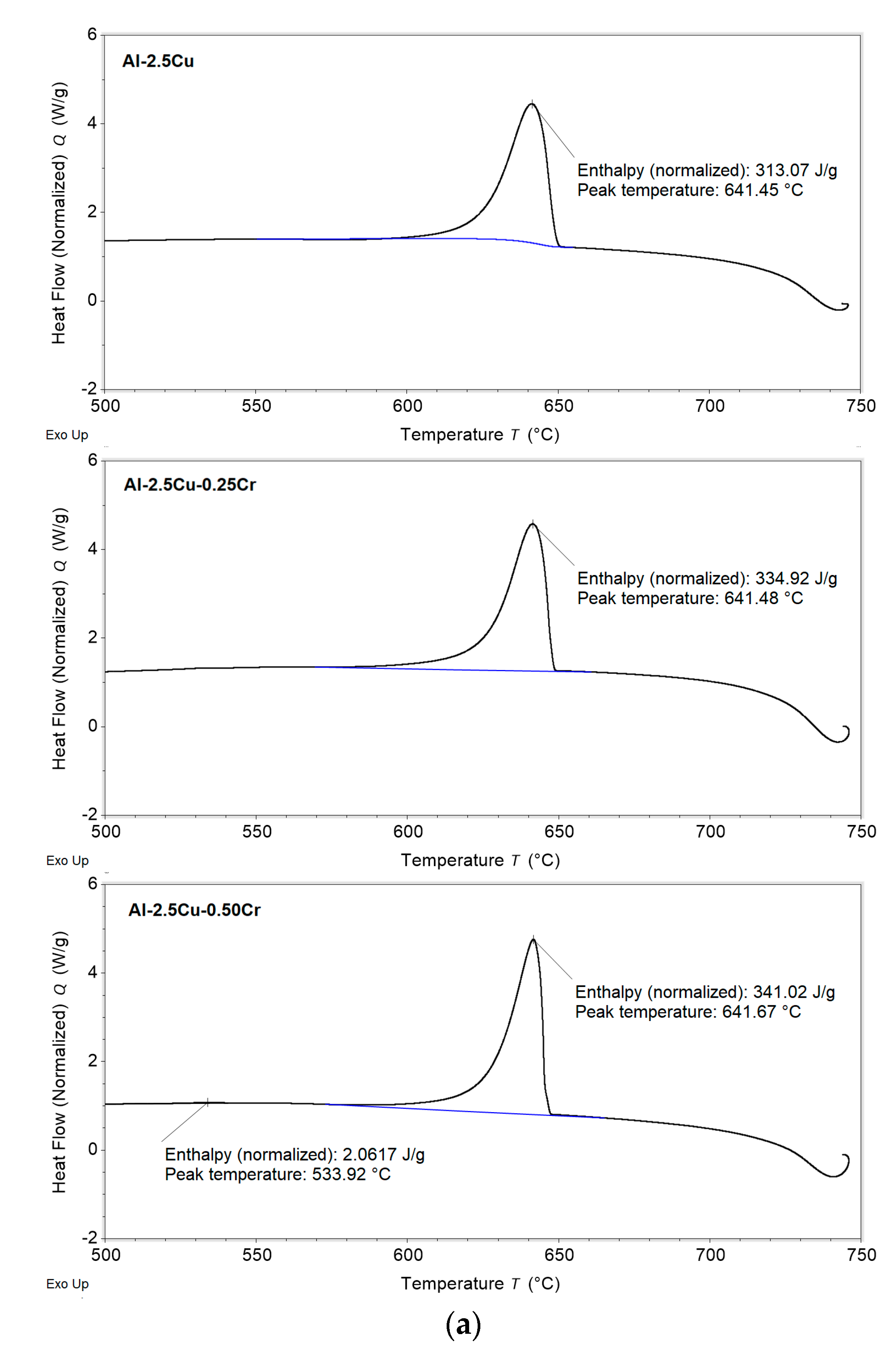
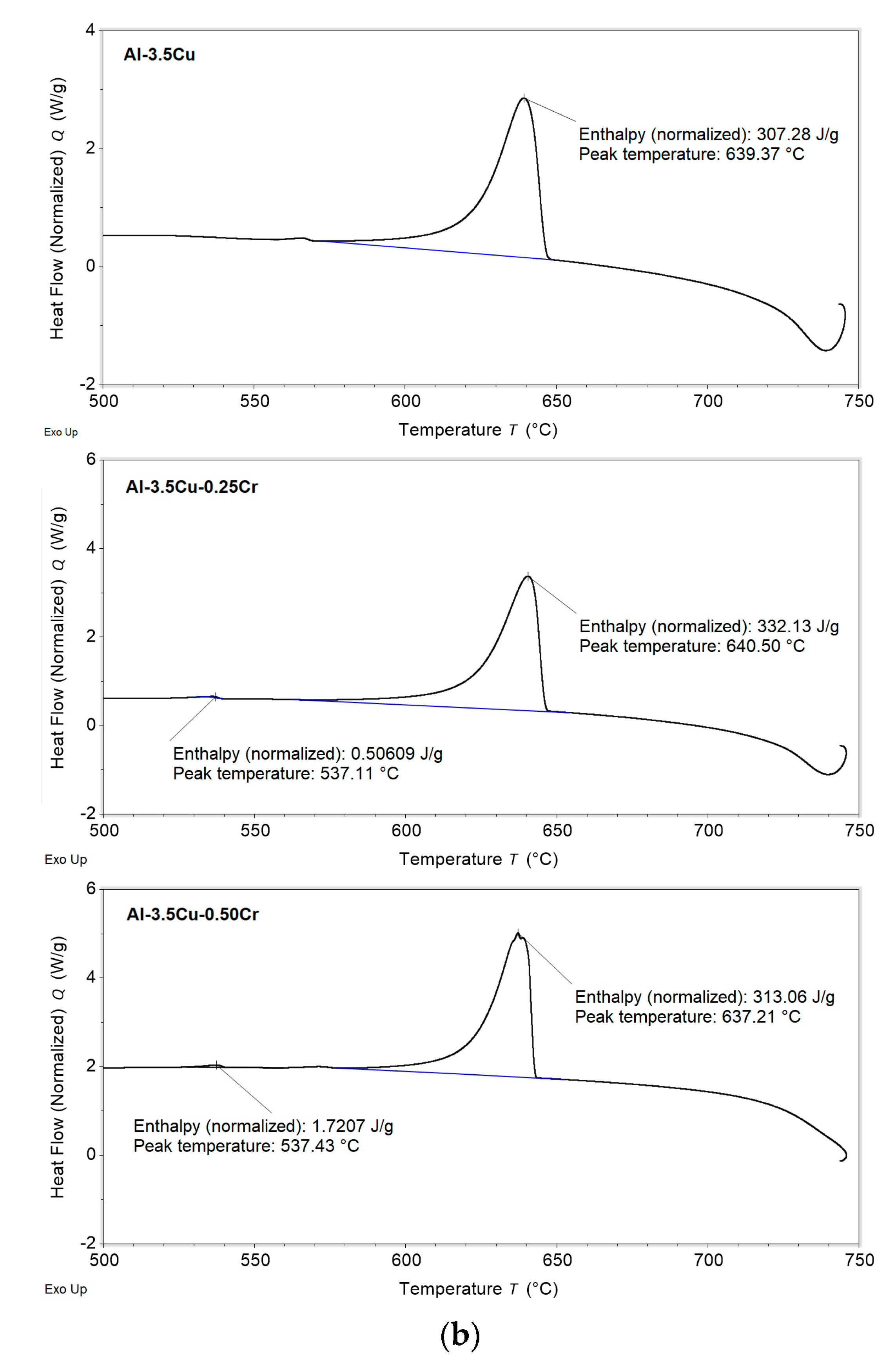
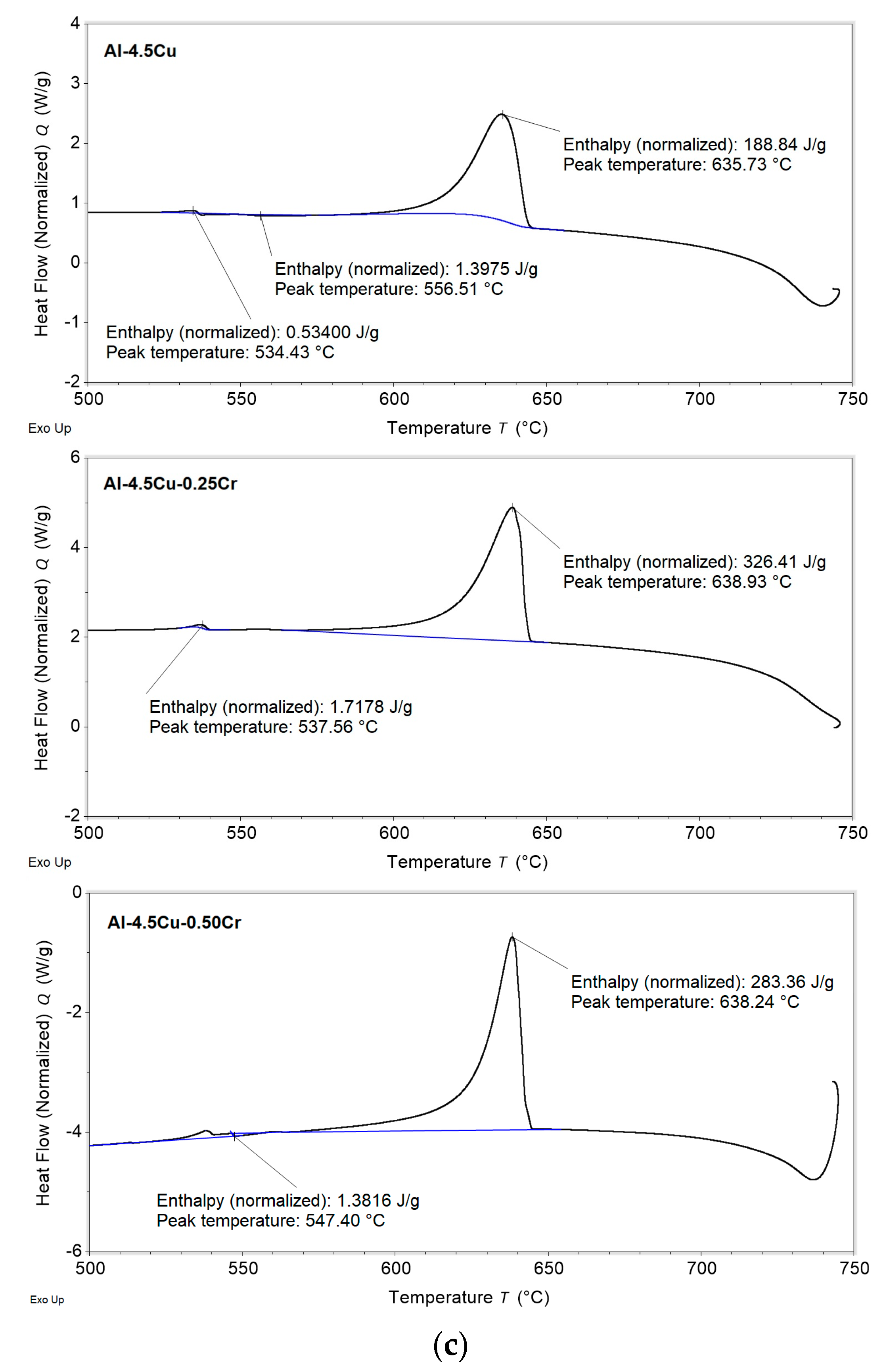
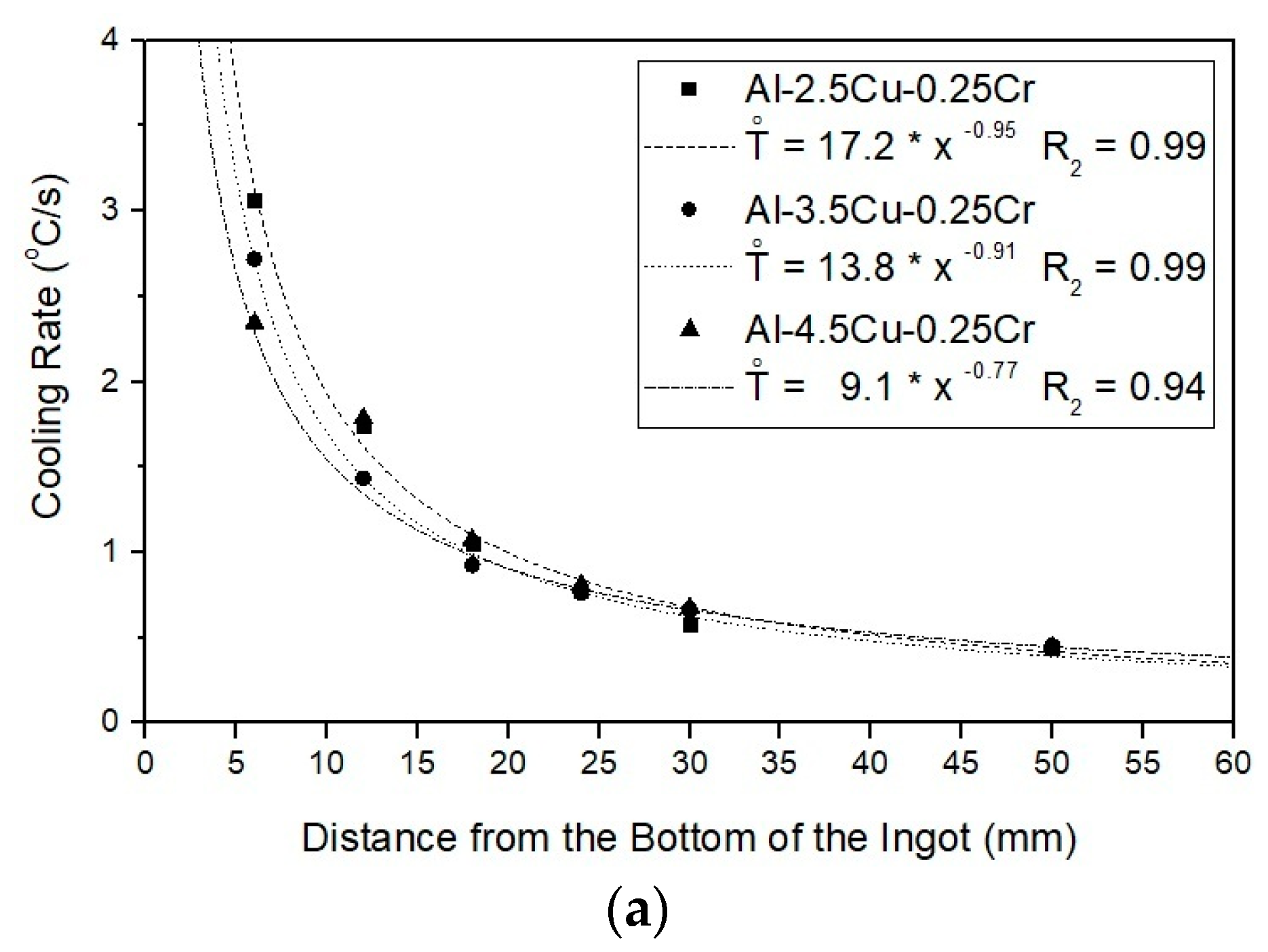
3.1. Solidification Thermal Parameters
3.2. Directional Solidification
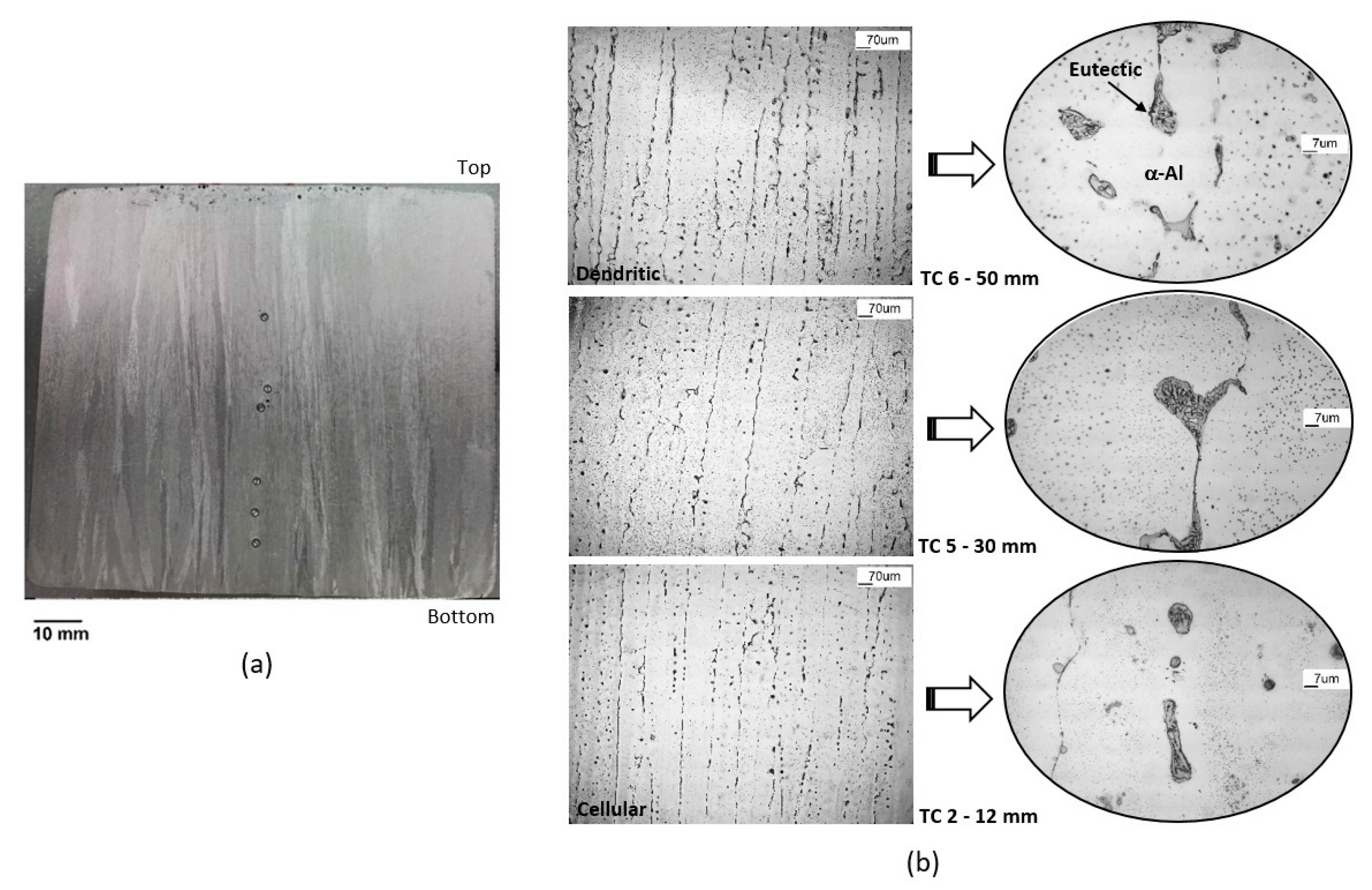
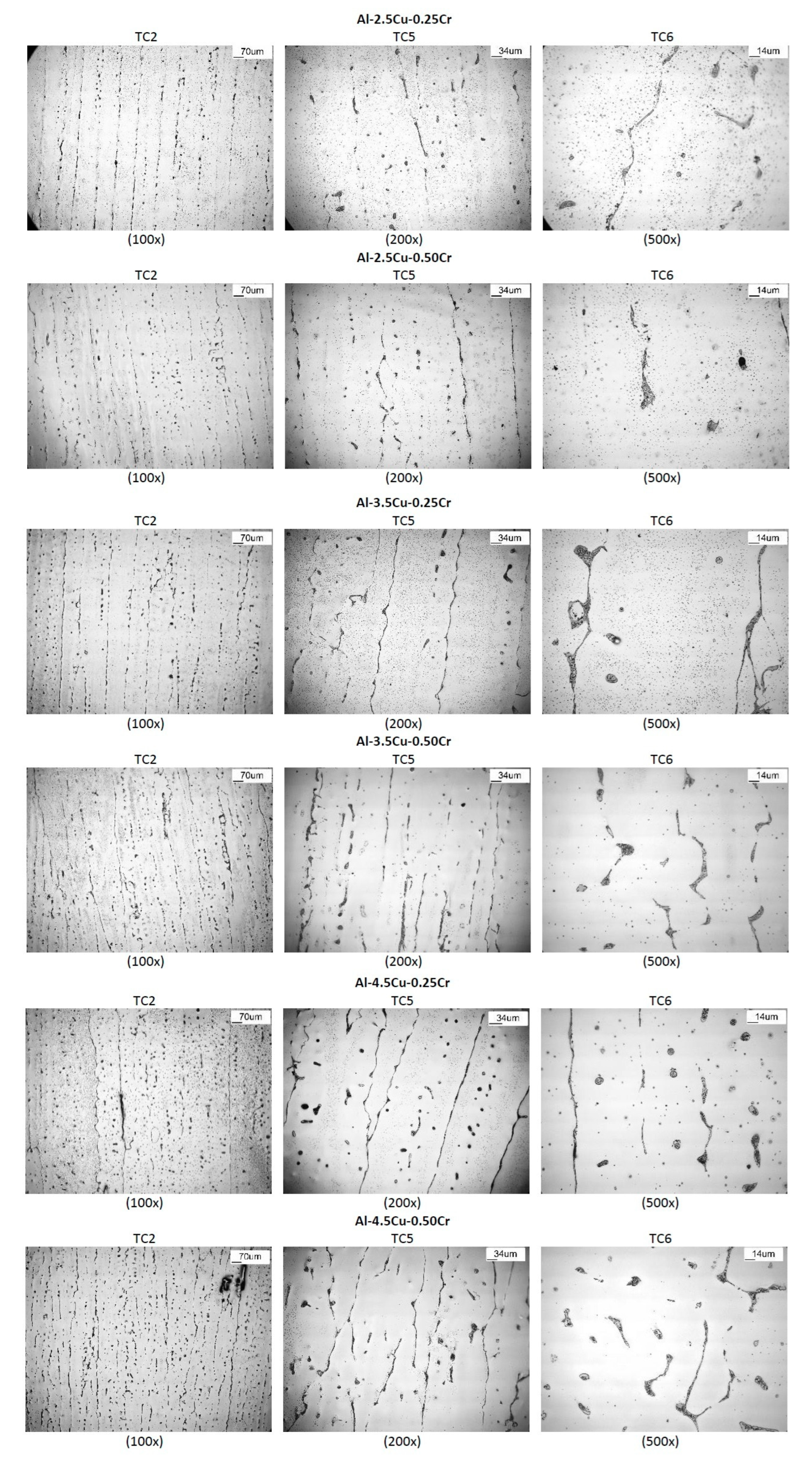
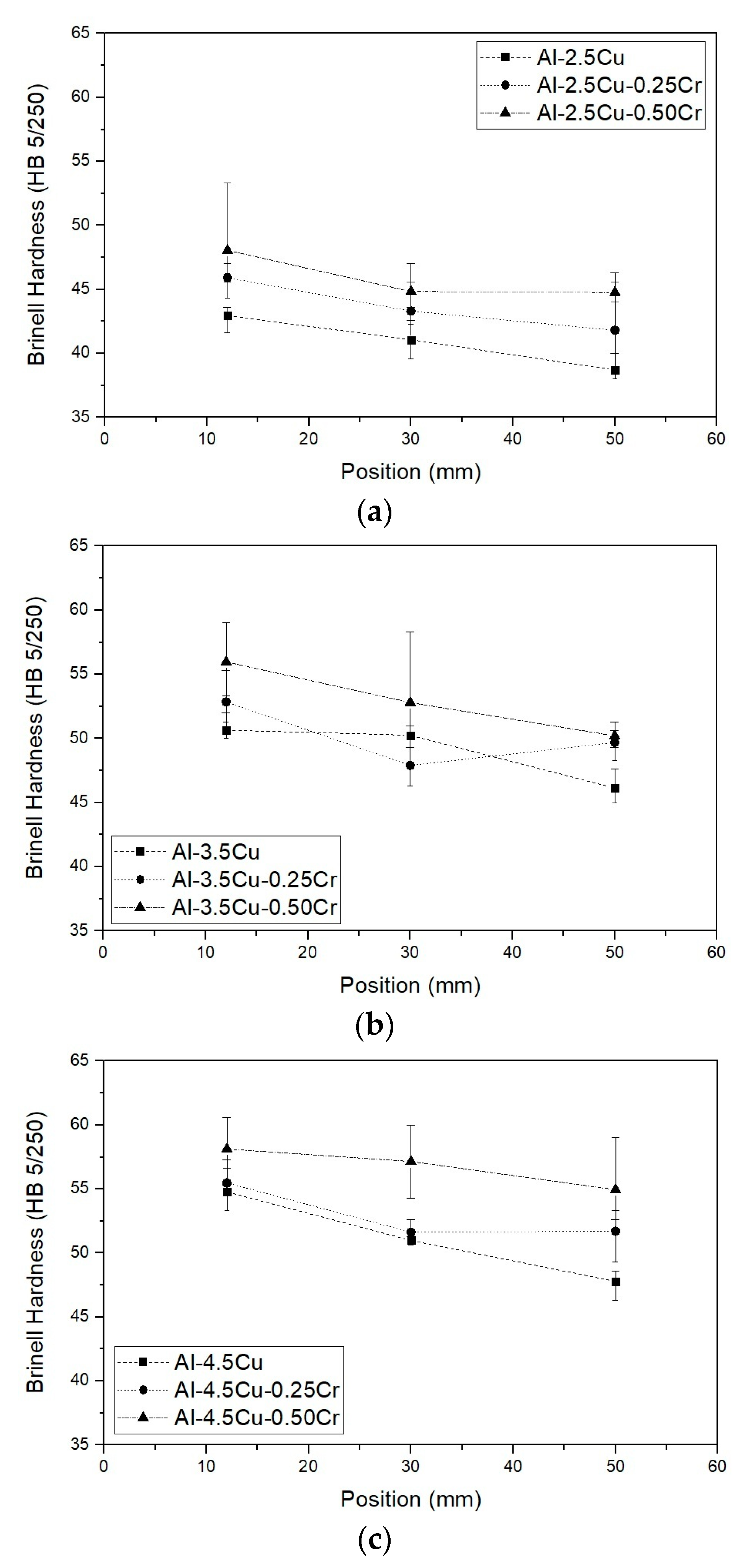
3.3. Microstructure and Hardness
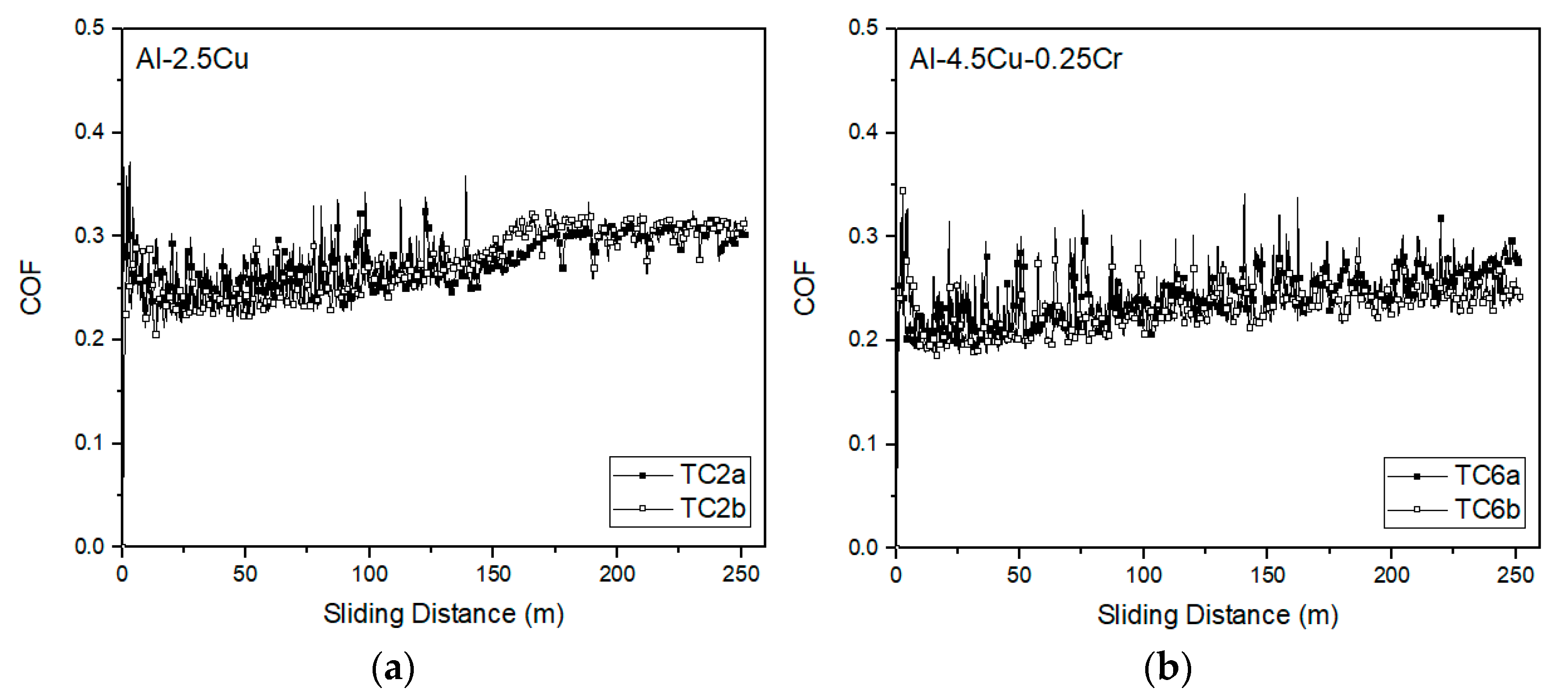
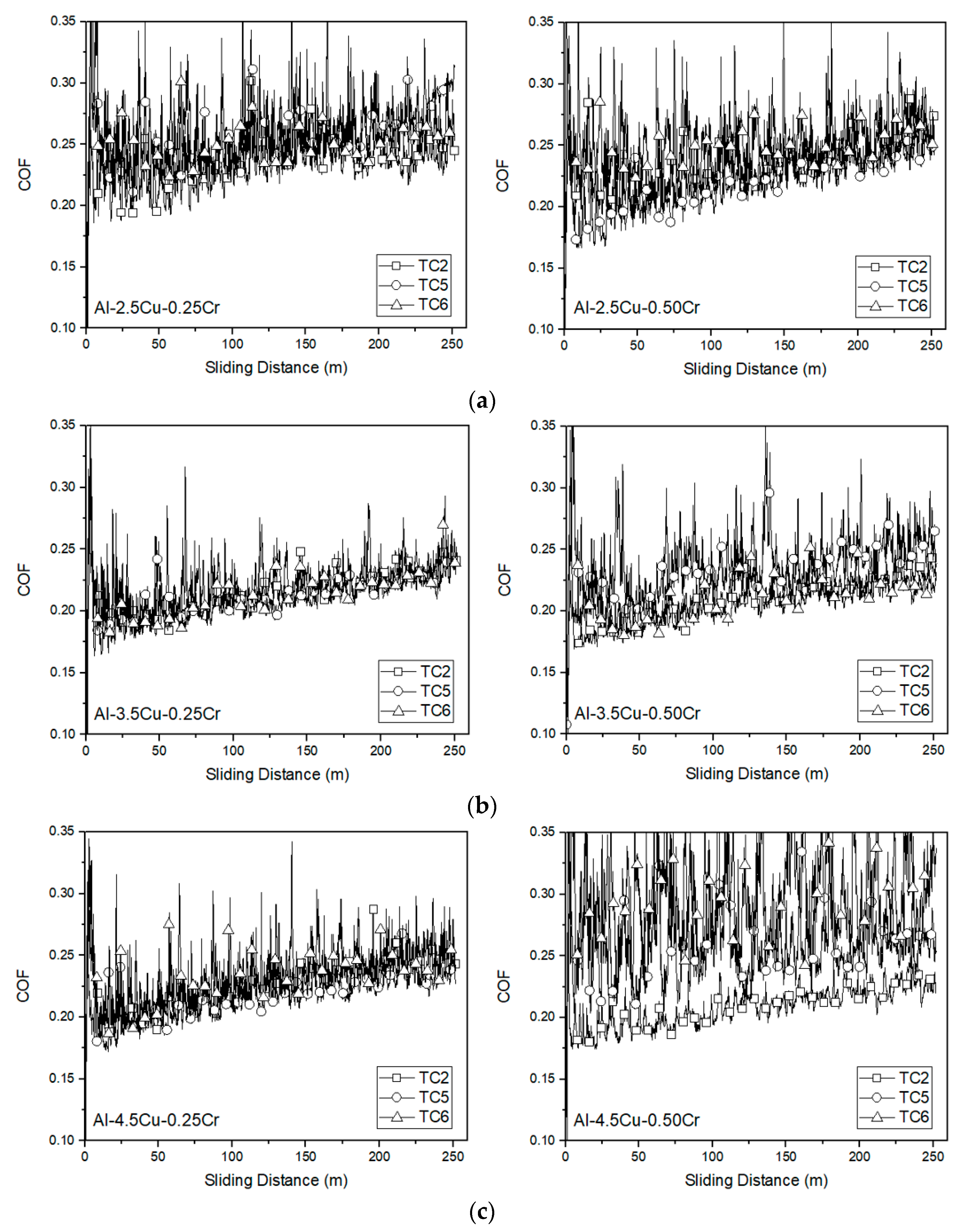
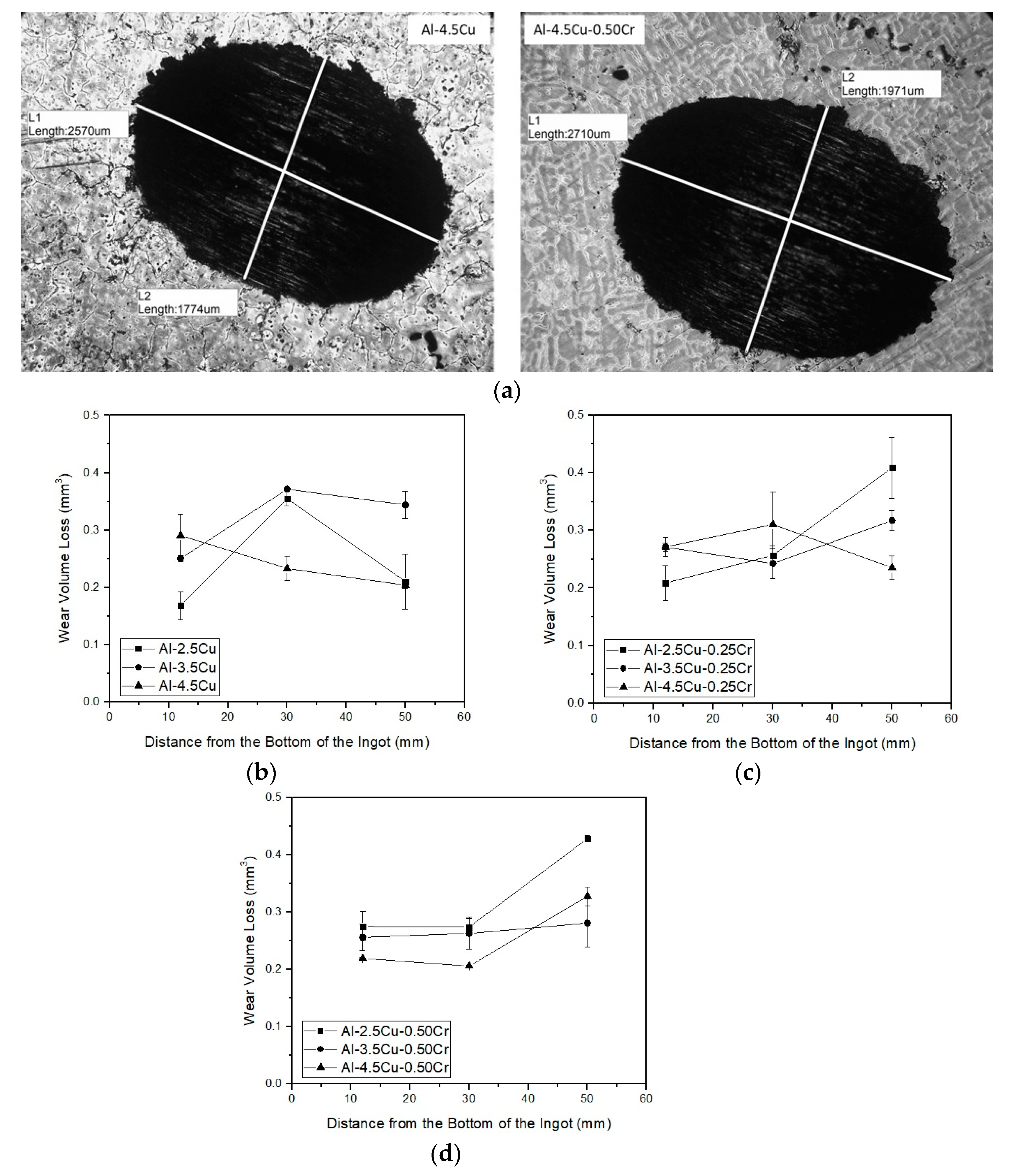
3.4. Sliding Wear Responses
4. Conclusions
- -
- Additions of Cu and Cr decreased the Liquidus temperatures (from 648.9 and 647.4 °C for the Al–2.5Cu alloys with 0.25 and 0.50%Cr, respectively, to 644.8 and 643.9 °C for the Al–4.5Cu alloys with 0.25 and 0.50%Cr, respectively) and the enthalpies of transformation of the alloys;
- -
- The addition of Cu significantly affected cooling rates, decreasing as the Cu content increased, from 3.1 °C/s (Al–2.5Cu–0.25Cr alloy) to 2.3 °C/s (Al–4.5Cu–0.25Cr alloy), and from 3.3 °C/s (Al–2.5Cu–0.50Cr alloy) to 2.5 °C/s (Al–4.5Cu–0.50Cr alloy) at those positions close to the bottom of the ingots). On the other hand, higher Cr contents increased cooling rates;
- -
- When comparing alloys with the same Cu content, Cr addition improved hardness, especially for lower Cu-containing alloys (≅10% at those positions near the bottom of the ingots). In all alloys, hardness decreased with increasing λ1;
- -
- Additions of Cu and Cr to the alloys resulted in a slight decrease in COF, with average values ranging from 0.30 to 0.20. Analyzing individually the effect of Cu and Cr additions on the wear volume loss, insignificant changes were observed, regardless of microstructure refinement. However, it is important to note that, when analyzed together, there is a tendency of decreasing wear volume loss with the coarsening of the microstructure.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties, 1st ed.; Butterworth-Heinemann: Oxfordshire, UK, 1976; 971p. [Google Scholar]
- Kim, H.T.; Nam, S.W.; Hwang, S.H. Study on the solidification cracking behaviour of high strength aluminum alloy welds: Effects of alloying elements and solidification behaviours. J. Mater. Sci. 1996, 31, 2859–2864. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the Influences of Alloying elements on the Microstructure and Mechanical Properties of Aluminum Alloys and Aluminum Alloy Composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Razumovskiy, V.I.; Ruban, A.V.; Razumovskii, I.M.; Lozovoi, A.Y.; Butrim, V.N.; Vekilov, Y.K. The effect of alloying elements on grain boundary and bulk cohesion in aluminum alloys: An ab initio study. Scr. Mater. 2011, 65, 926–929. [Google Scholar] [CrossRef]
- Zakharov, V.V. About alloying of aluminum alloys with transition metals. Met. Sci. Heat Treat. 2017, 59, 67–71. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Lamb, J.; Langan, T. Effect of Sc and Zr additions on the microstructure/strength of Al-Cu binary alloys. Mater. Sci. Eng. A 2017, 707, 58–64. [Google Scholar] [CrossRef]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications, 1st ed.; ASM International: Novelty, OH, USA, 2004. [Google Scholar]
- Kimura, T.; Nakamoto, T.; Ozaki, T.; Miki, T. Microstructures and mechanical properties of aluminum-transition metal binary alloys (Al-Fe, Al-Mn, and Al-Cr) processed by laser powder bed fusion. J. Alloys Compd. 2021, 872, 159680. [Google Scholar] [CrossRef]
- Predel, B. Al-Cr (Aluminum-Chromium) Datasheet from Landolt-Börnstein-Group IV Physical Chemistry. In “Ac-Ag... Au-Zr”-Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys; Springer Materials: Cham, Switzerland, 2006; Volume 12A. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q.; Wang, Y.; Qiang, J.; Dong, C. Composition rule for Al–transition metal binary quasicrystals. Philos. Mag. 2010, 90, 3935–3946. [Google Scholar] [CrossRef]
- Grushko, B.; Przepiórzyński, B.; Pavlyuchkov, D.; Mi, S.; Kowalska-Strzęciwilk, E.; Surowiec, M. Complex intermetallics in Al–Cu–Cr system. J. Alloys Compd. 2007, 442, 114–116. [Google Scholar] [CrossRef]
- Sugiyama, K.; Saito, H.; Hiraga, K. On the crystal structures of the Al–Cu–Cr alloy system. J. Alloys Compd. 2002, 342, 148–152. [Google Scholar] [CrossRef]
- Grushko, B. A contribution to the Al–Cu–Cr phase diagram. J. Alloys Compd. 2017, 729, 426–437. [Google Scholar] [CrossRef]
- Sviridova, T.A.; Schevchukov, A.P.; Shelekhov, E.V.; Diakonov, D.L.; Tcherdyntsev, V.V.; Kaloshkin, S.D. The quasicrystalline phaseformation in Al–Cu–Cr alloys produced by mechanicalalloying. J. Alloys Compd. 2011, 509S, S299–S303. [Google Scholar] [CrossRef]
- Fu, Y.; Kang, N.; Liao, H.; Gao, Y.; Coddet, C. An investigation onselective laser melting of Al–Cu–Fe–Cr quasicrystal: Fromsingle layer to multilayers. Intermetallics 2017, 86, 51–88. [Google Scholar] [CrossRef]
- Salimon, A.L.; Shevchukov, A.P.; Stepashkin, A.A.; Tcherdyntsev, V.V.; Olifirov, L.K. Mechanical alloying as a solid state route forfabrication of Al–Cu–M(=Fe, Cr) quasicrystalline phases. J. Alloys Compd. 2017, 707, 315–320. [Google Scholar] [CrossRef]
- El-Nasser, G.A.; Samy, S.M.; Nassef, A. Effect of chromium additions on formability and mechanical behavior of Al-Cu alloy. In Proceedings of the 10th International Mining, Petroleum, and Metallurgical Engineering Conference, Suez, Egypt, 6–8 March 2007; pp. 84–94. [Google Scholar]
- Ravikumar, A.; Sellamuthu, R.; Saravanan, R. Effect of Cr addition on mechanical properties and wear rate of cast Al–Cu alloy. Indian J. Sci. Technol. 2016, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.M.; Catellan, E.; Garcia, A.; Santos, C.A. The effects of Cr addition on microstructure, hardness and tensile properties of as-cast Al–3.8wt.%Cu–(Cr) alloys. J. Mater. Res. Technol. 2020, 9, 6620–6631. [Google Scholar] [CrossRef]
- Amer, S.M.; Glavatskikh, M.V.; Barkov, R.Y.; Loginova, I.S.; Pozdniakov, A.V. Effect of Cr on the Microstructure and Mechanical Properties of the Al-Cu-Y-Zr Alloy. Metals 2023, 13, 349. [Google Scholar] [CrossRef]
- Monti, C.; Turani, M.; Papis, K.; Bambach, M. A new Al-Cu alloy for LPBF developed via ultrasonic atomization. Mater. Des. 2023, 229, 111907. [Google Scholar] [CrossRef]
- Ache, C.T.; Lopes, M.M.; Reis, B.P.; Garcia, A.; Santos, C.A. Dendritic Spacing/Columnar Grain Diameter of Al–2Mg–Zn Alloys Affecting Hardness, Tensile Properties, and Dry Sliding Wear in the As-Cast/Heat-Treated Conditions. Adv. Eng. Technol. 2020, 22, 1901145. [Google Scholar] [CrossRef]
- Rosso, E.; Santos, C.A.; Garcia, A. Microstructure, Hardness, Tensile Strength, and Sliding Wear of Hypoeutectic Al–Si Cast Alloys with Small Cr Additions and Fe-Impurity Content. Adv. Eng. Mater. 2021, 24, 2001552. [Google Scholar] [CrossRef]
- Quaresma, J.M.V.; Santos, C.A.; Garcia, A. Correlation between unsteady-state solidification conditions, dendrite spacings, and mechanical properties of Al-Cu alloys. Metall. Mater. Trans. A 2000, 31, 3167–3178. [Google Scholar] [CrossRef] [Green Version]
- Reis, B.P.; Lopes, M.M.; Garcia, A.; Santos, C.A. The correlation of microstructure features, dry sliding wear behavior, hardness and tensile properties of Al-2wt%Mg-Zn alloys. J. Alloys Compd. 2018, 764, 267–278. [Google Scholar] [CrossRef]
- ASTM E3; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM E407; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM E10; Standard Test Method for Brinell Hardness of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM G133; Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Jaradeh, M.; Carlberg, T. Differential thermal analysis and differential scanning calorimetry studies of aluminum 3003 alloys with Zn and Cu additions. Metall. Mater. Trans. A 2007, 38, 2138–2147. [Google Scholar] [CrossRef]
- Liu, B.; Yang, X.G.; Huang, H.J.; Guo, Z. The effect of alloying elements on the microstructure of Al–5Fe alloys. J. Miner. Metals Mater. Soc. 2012, 64, 316–322. [Google Scholar] [CrossRef]
- Santos, C.A.; Quaresma, J.M.V.; Garcia, A. Determination of transient interfacial heat transfer coefficients in chill mold castings. J. Alloys Compd. 2001, 319, 174–186. [Google Scholar] [CrossRef]
- Santos, C.A.; Siqueira, C.A.; Garcia, A.; Quaresma, J.M.V.; Spim, J.A. Metal–mold heat transfer coefficients during horizontal and vertical Unsteady-State solidification of Al–Cu and Sn–Pb Alloys. Inv. Probl. Sci. Eng. 2004, 12, 279–296. [Google Scholar] [CrossRef]
- Souza, E.N.; Cheung, N.; Santos, C.A.; Garcia, A. Factors affecting solidification thermal variables along the cross-section of horizontal cylindrical ingots. Mater. Sci. Eng. A 2005, 397, 239–248. [Google Scholar] [CrossRef]
- Alfaia, M.Â.O.; Oliveira, R.; Lima, T.S.; Mariani, F.E.; Casteletti, L.C.; Cheung, N.; Garcia, A. Effects of cooling rate and microstructure scale on wear resistance of unidirectionally solidified Al-3.2wt.%Bi-(1; 3) wt.%Pb alloys. Mater. Today Comm. 2020, 25, 101659. [Google Scholar] [CrossRef]
- He, C.; Yu, W.; Li, Y.; Wang, Z.; Wu, D.; Xu, G. Relationship between cooling rate, microstructure evolution, and performance improvement of an Al–Cu alloy prepared using different methods. Mater. Res. Express 2020, 7, 116501. [Google Scholar] [CrossRef]
- Júnior, P.F.; de Olivé Ferreira, L.; Garção, W.J.L.; de Paula Almeida, R.; Melo, C.M.; Ferreira, A.F. Heat-flow parameters affecting microstructure and mechanical properties of Al–Cu and Al–Ni alloys in directional solidification: An experimental comparative study. Int. J. Mater. Res. 2022, 113, 181–193. [Google Scholar] [CrossRef]
- Stapóri, S.; Górny, M.; Kawalec, M.; Gracz, B. Effect of manganese content on microstructure of Al-Cu alloys. Arch. Metall. Mater. 2020, 65, 1377–1383. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Ma, Z.; Bai, Y.; Duan, H.; Tao, D.; Shi, G.; Zhang, J.; Wang, Z.; Li, J. Influence of cerium content on the microstructure and thermal expansion properties of suction cast Al-Cu-Fe alloys. R. Soc. Open Sci. 2021, 8, 210584. [Google Scholar] [CrossRef] [PubMed]
- Czerwinski, F. Thermal stability of aluminum alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef]






| Alloys | Cu | Cr | Fe | Others | Al |
|---|---|---|---|---|---|
| 2.5Cu | 2.57 | - | 0.057 | 0.072 | 97.3 |
| 2.5Cu–0.25Cr | 2.48 | 0.264 | 0.060 | 0.095 | 97.1 |
| 2.5Cu–0.50Cr | 2.55 | 0.464 | 0.070 | 0.115 | 96.8 |
| 3.5Cu | 3.56 | - | 0.062 | 0.177 | 96.2 |
| 3.5Cu–0.25Cr | 3.49 | 0.255 | 0.063 | 0.191 | 96.0 |
| 3.5Cu–0.50Cr | 3.52 | 0.490 | 0.084 | 0.105 | 95.8 |
| 4.5Cu | 4.54 | - | 0.065 | 0.094 | 95.3 |
| 4.5Cu–0.25Cr | 4.48 | 0.247 | 0.062 | 0.110 | 95.1 |
| 4.5Cu–0.50Cr | 4.58 | 0.485 | 0.074 | 0.160 | 94.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lantmann, R.V.; Mariante, A.M.S.; Pinheiro, T.V.; da Costa, E.M.; dos Santos, C.A. Microstructure, Hardness, and Linear Reciprocating Sliding Wear Response of Directionally Solidified Al–(2.5, 3.5, 4.5)Cu–(0.25, 0.50)Cr Alloys. Metals 2023, 13, 1178. https://doi.org/10.3390/met13071178
Lantmann RV, Mariante AMS, Pinheiro TV, da Costa EM, dos Santos CA. Microstructure, Hardness, and Linear Reciprocating Sliding Wear Response of Directionally Solidified Al–(2.5, 3.5, 4.5)Cu–(0.25, 0.50)Cr Alloys. Metals. 2023; 13(7):1178. https://doi.org/10.3390/met13071178
Chicago/Turabian StyleLantmann, Rafael V., André M. S. Mariante, Tiago V. Pinheiro, Eleani M. da Costa, and Carlos A. dos Santos. 2023. "Microstructure, Hardness, and Linear Reciprocating Sliding Wear Response of Directionally Solidified Al–(2.5, 3.5, 4.5)Cu–(0.25, 0.50)Cr Alloys" Metals 13, no. 7: 1178. https://doi.org/10.3390/met13071178
APA StyleLantmann, R. V., Mariante, A. M. S., Pinheiro, T. V., da Costa, E. M., & dos Santos, C. A. (2023). Microstructure, Hardness, and Linear Reciprocating Sliding Wear Response of Directionally Solidified Al–(2.5, 3.5, 4.5)Cu–(0.25, 0.50)Cr Alloys. Metals, 13(7), 1178. https://doi.org/10.3390/met13071178







