Production of a Reinforced Refractory Multielement Alloy via High-Energy Ball Milling and Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
3. Results
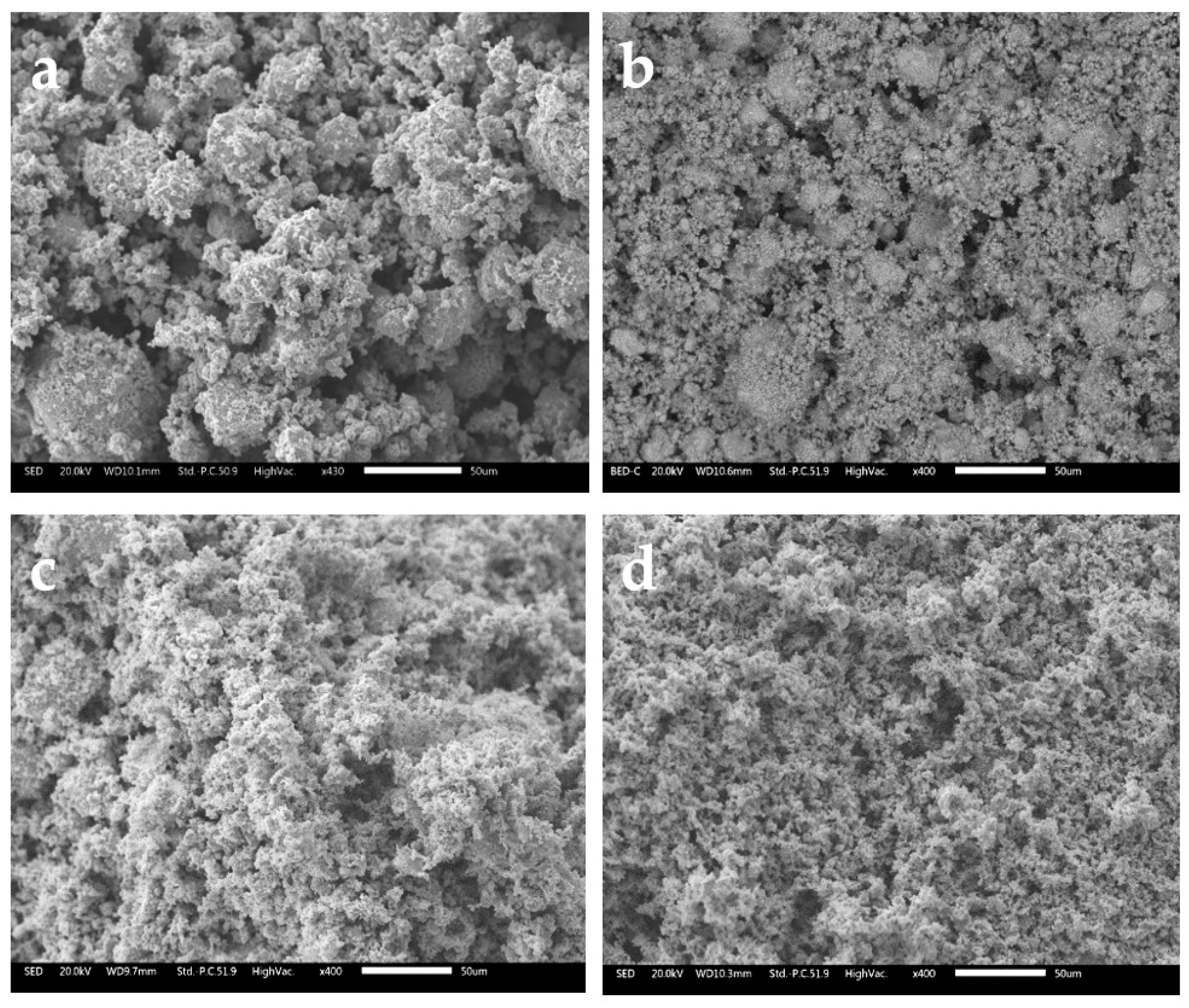
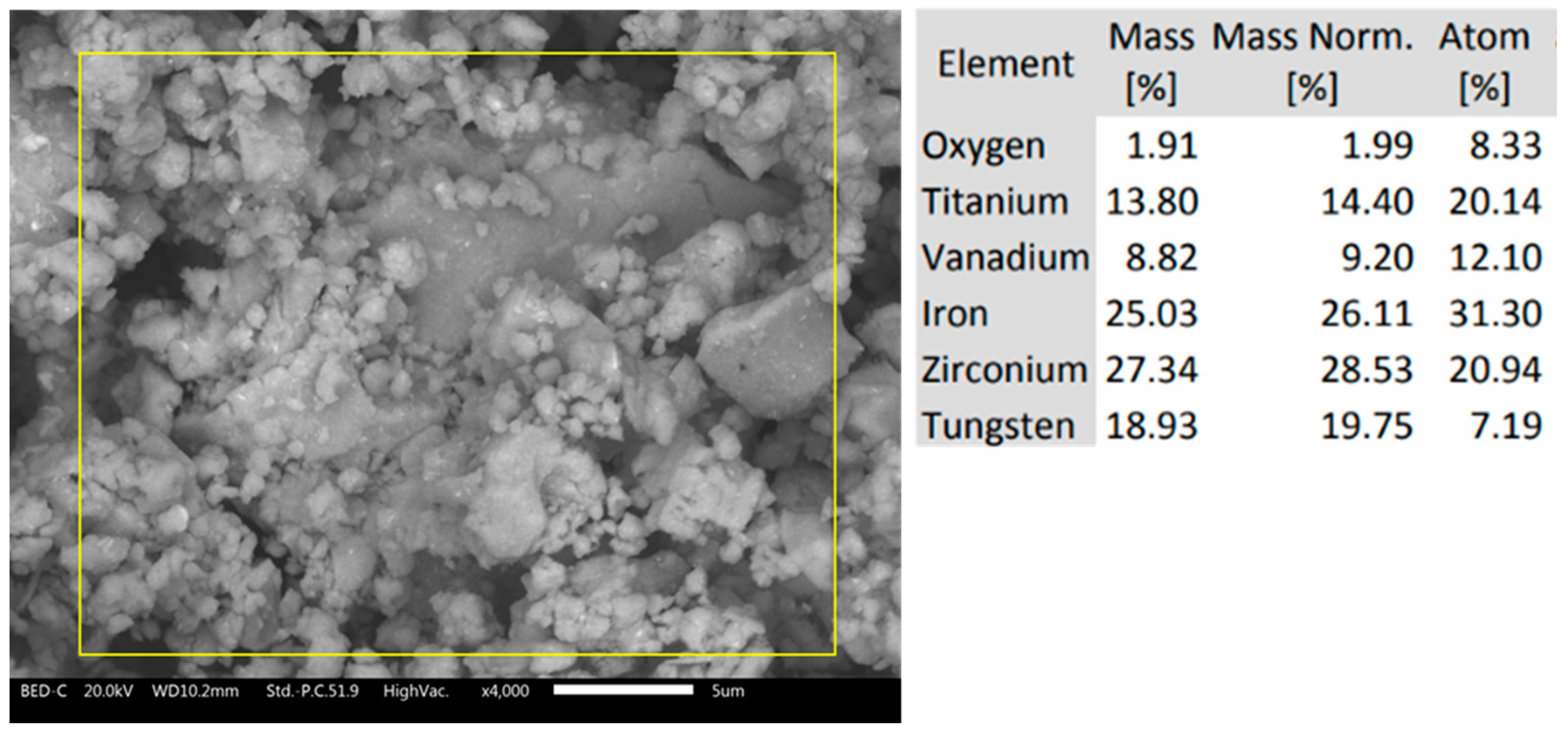
3.1. Milled Powders
3.2. Sintered Samples
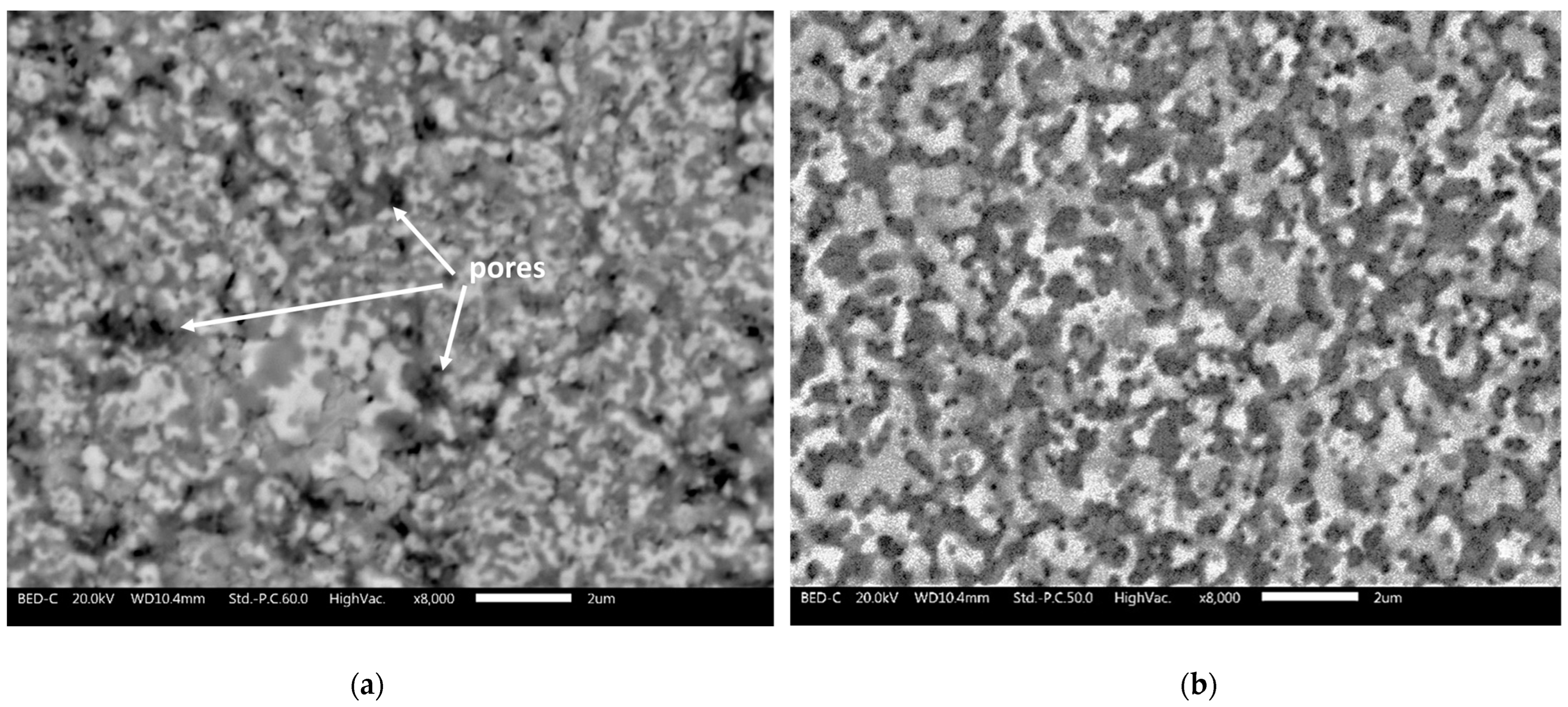
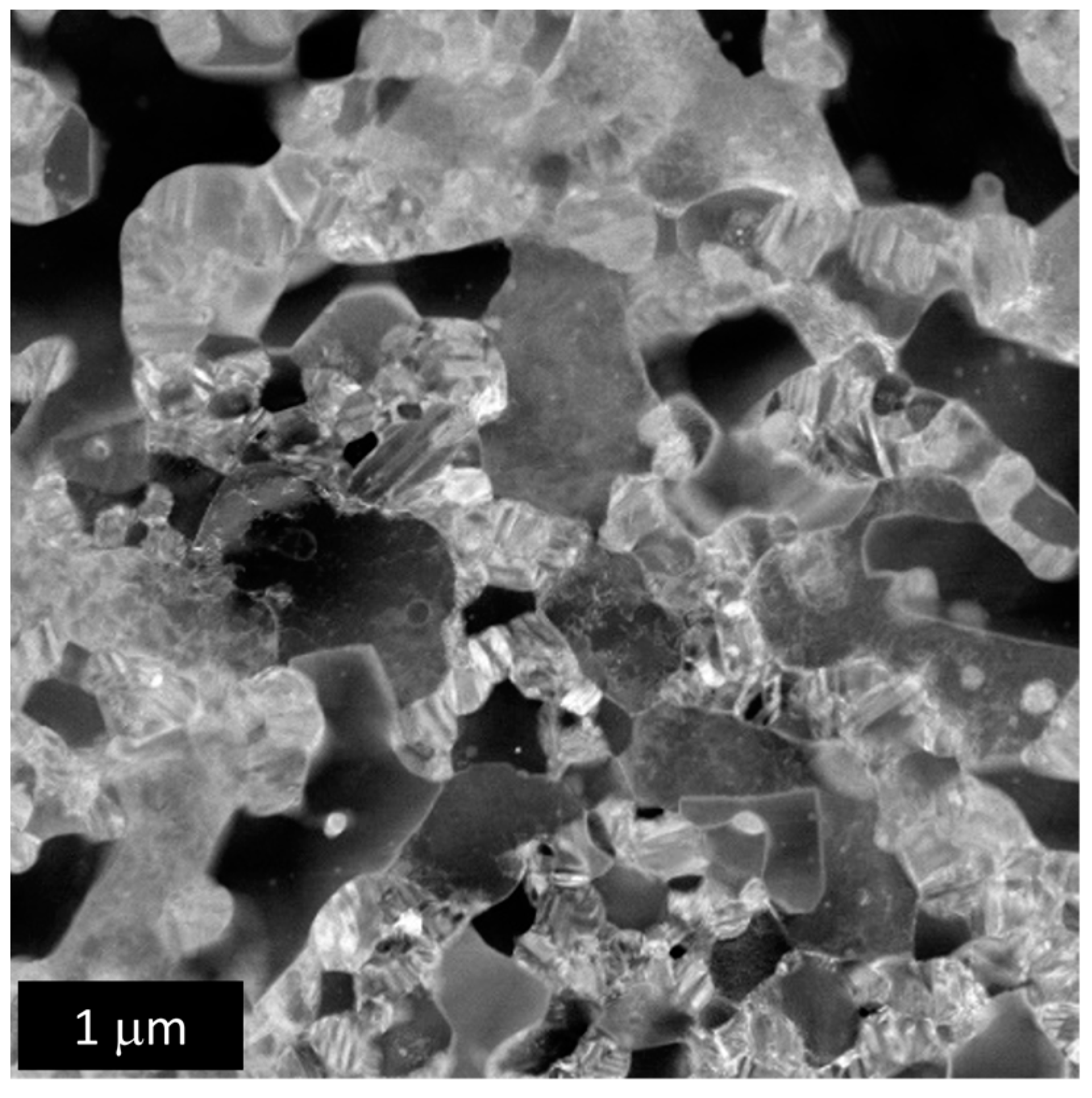
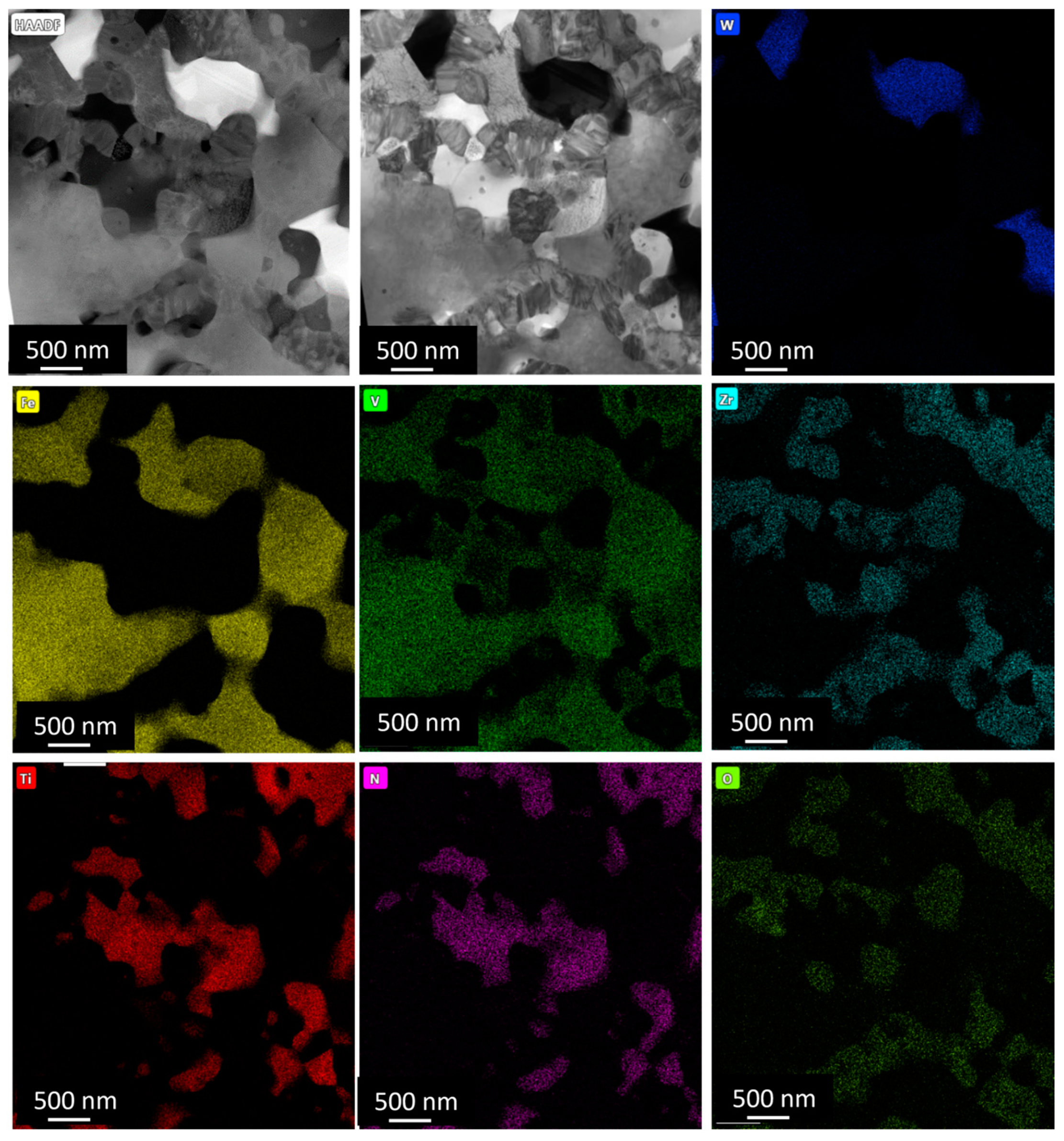
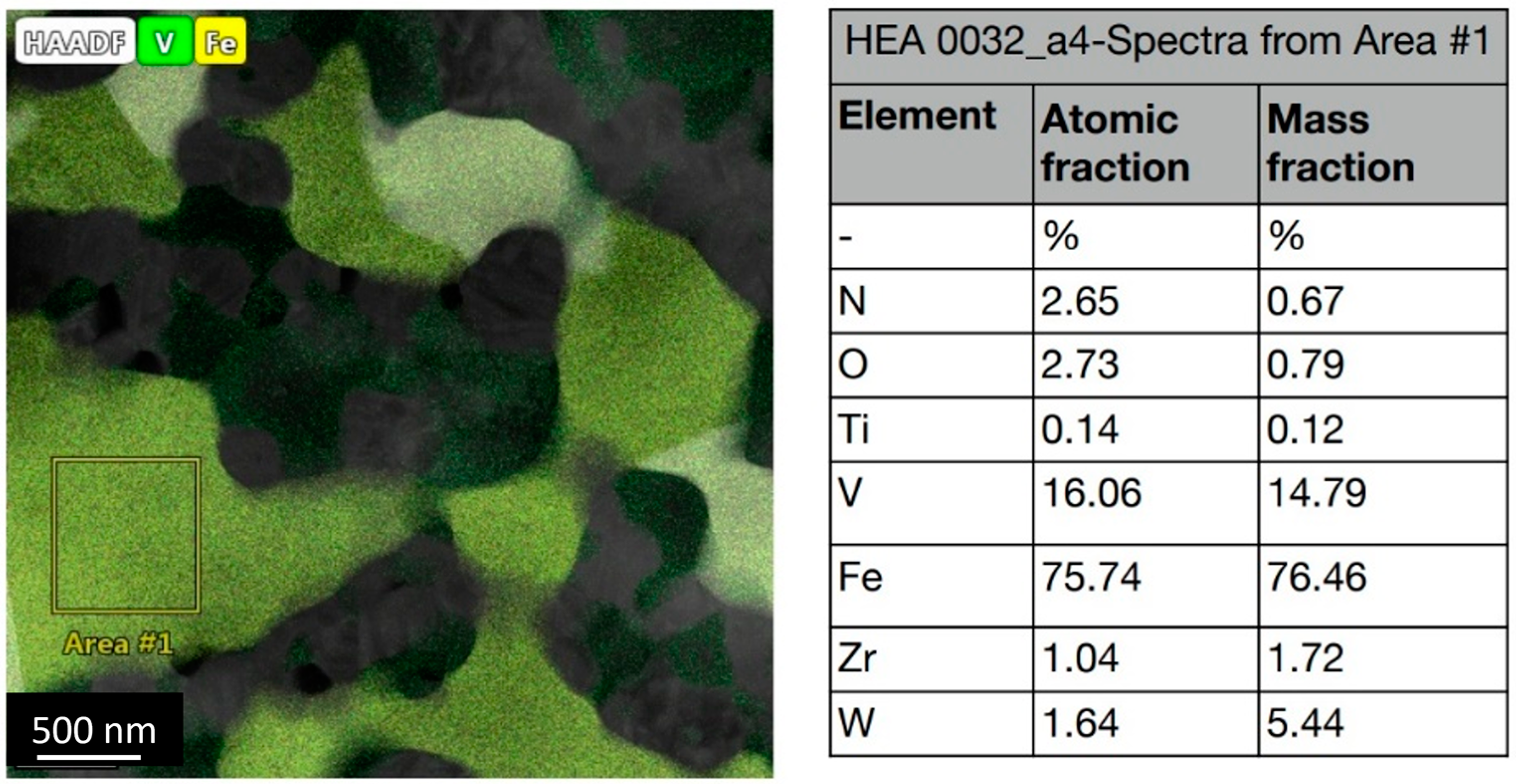
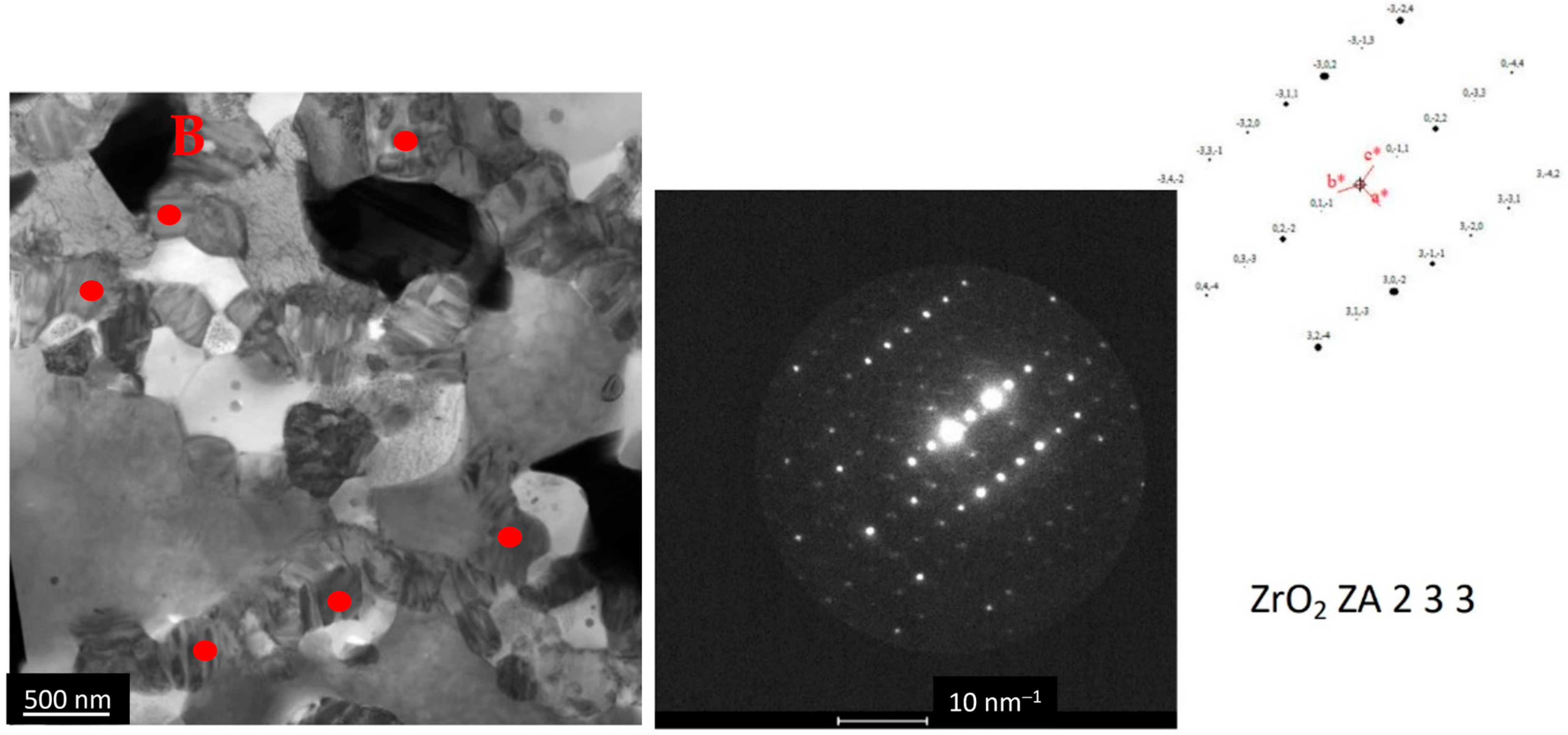
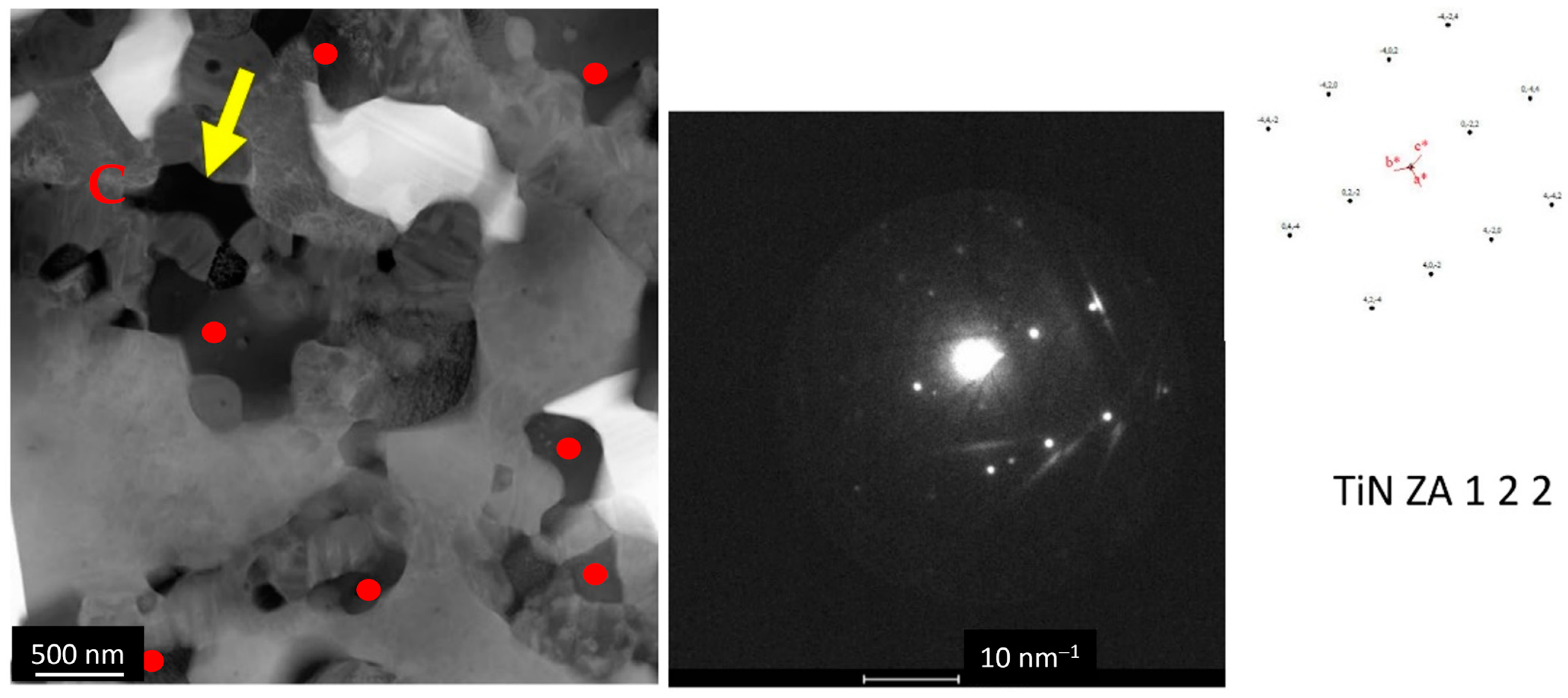
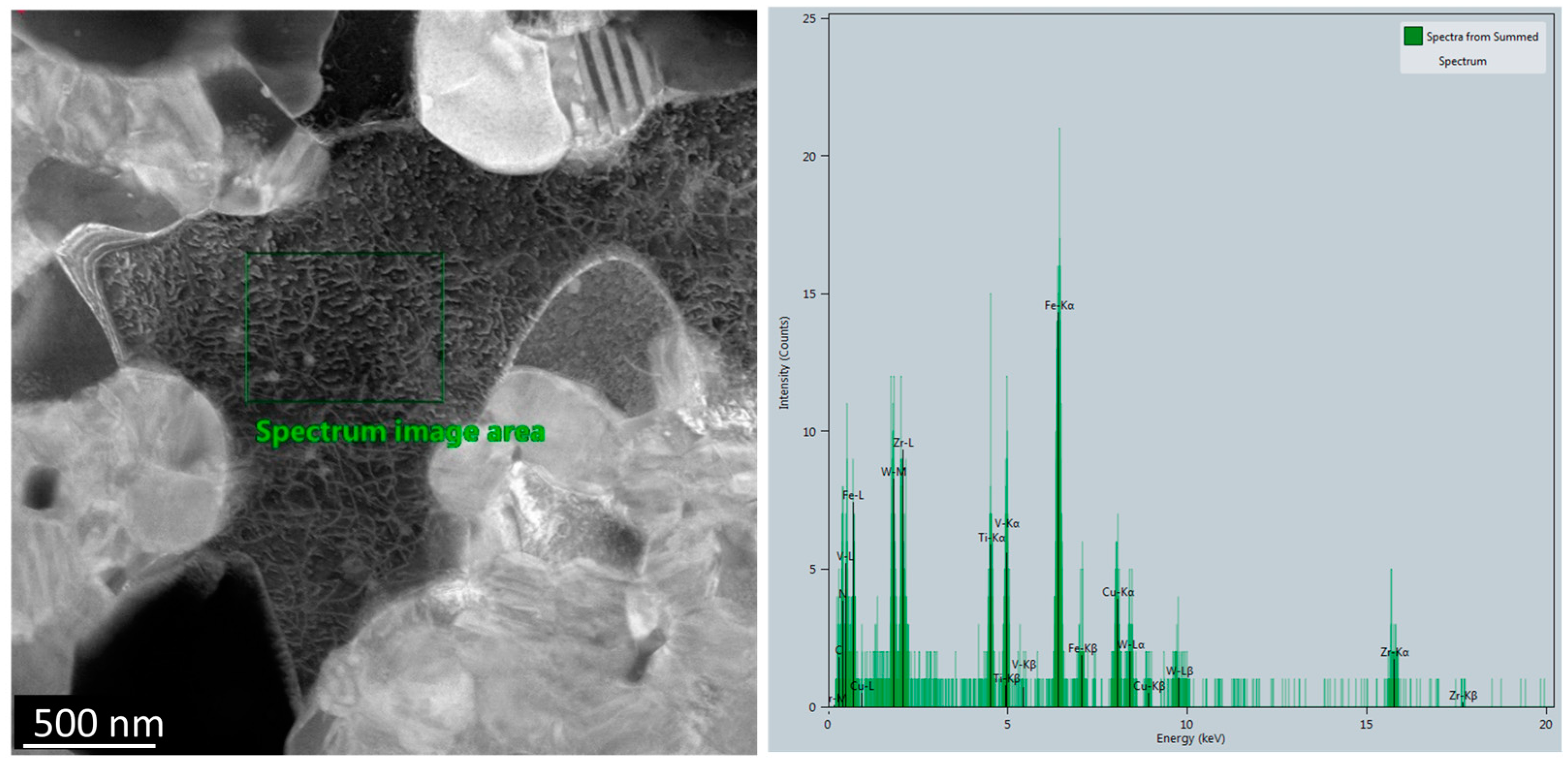
3.2.1. Microstructural Analysis
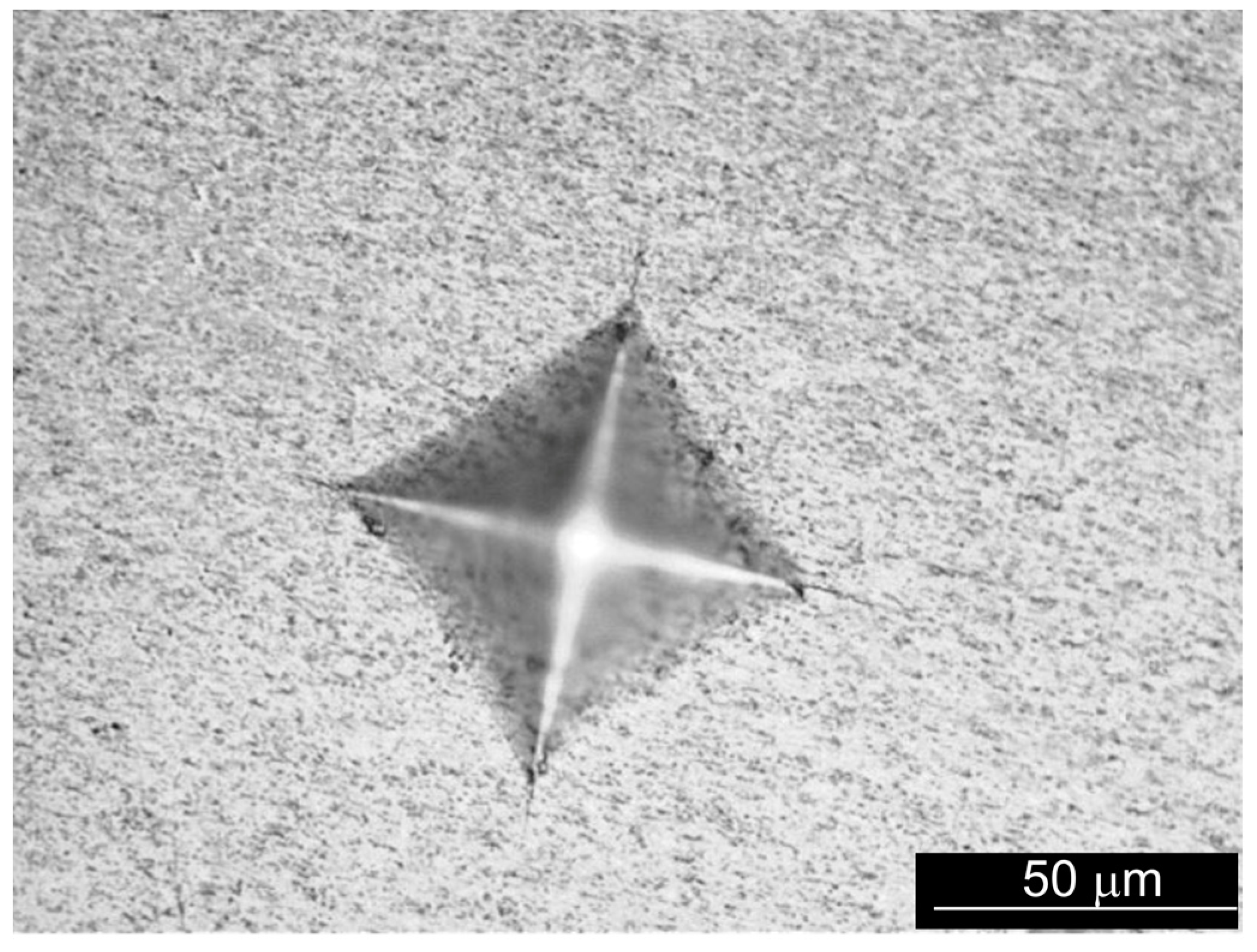
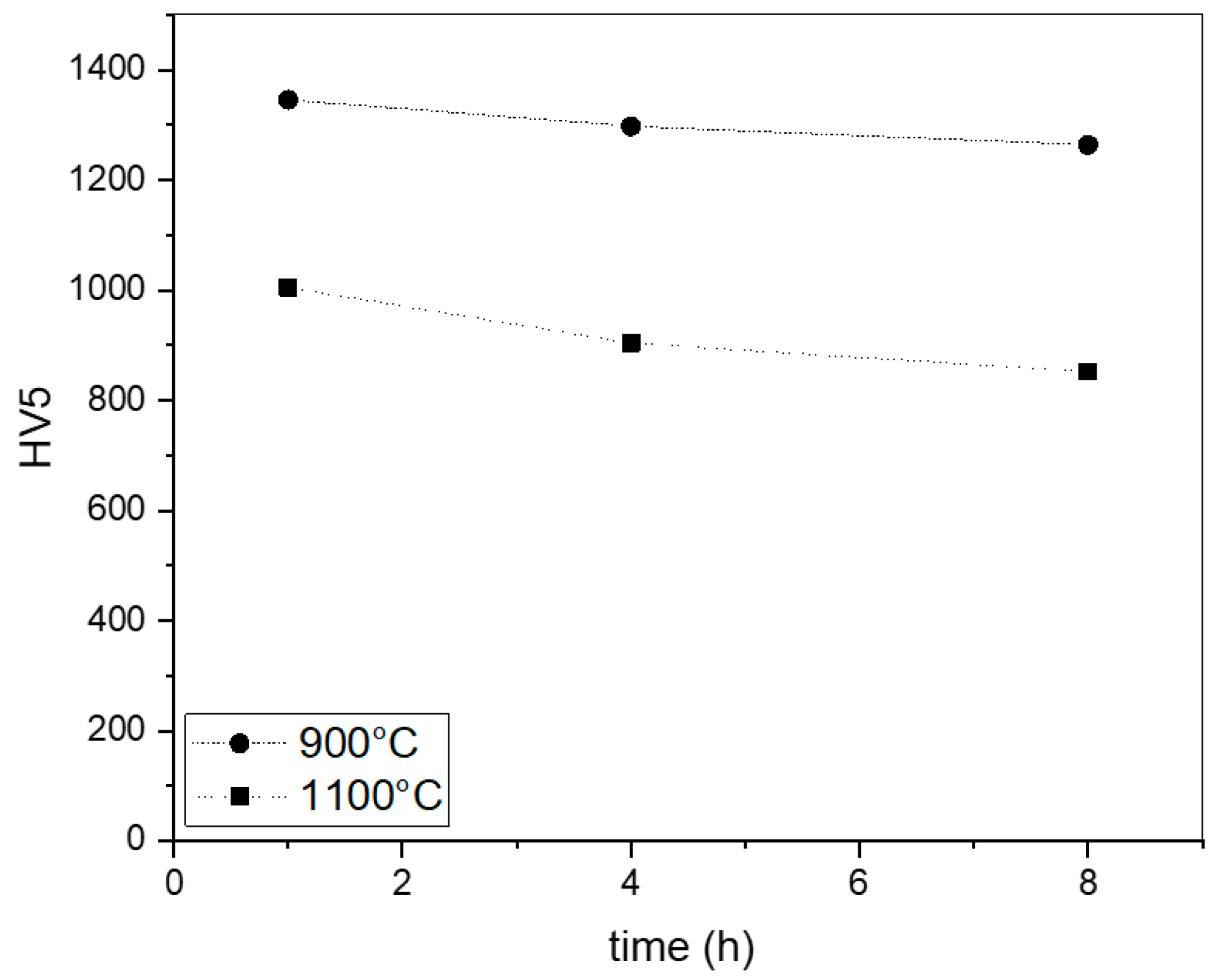
3.2.2. Hardness and Fracture Toughness
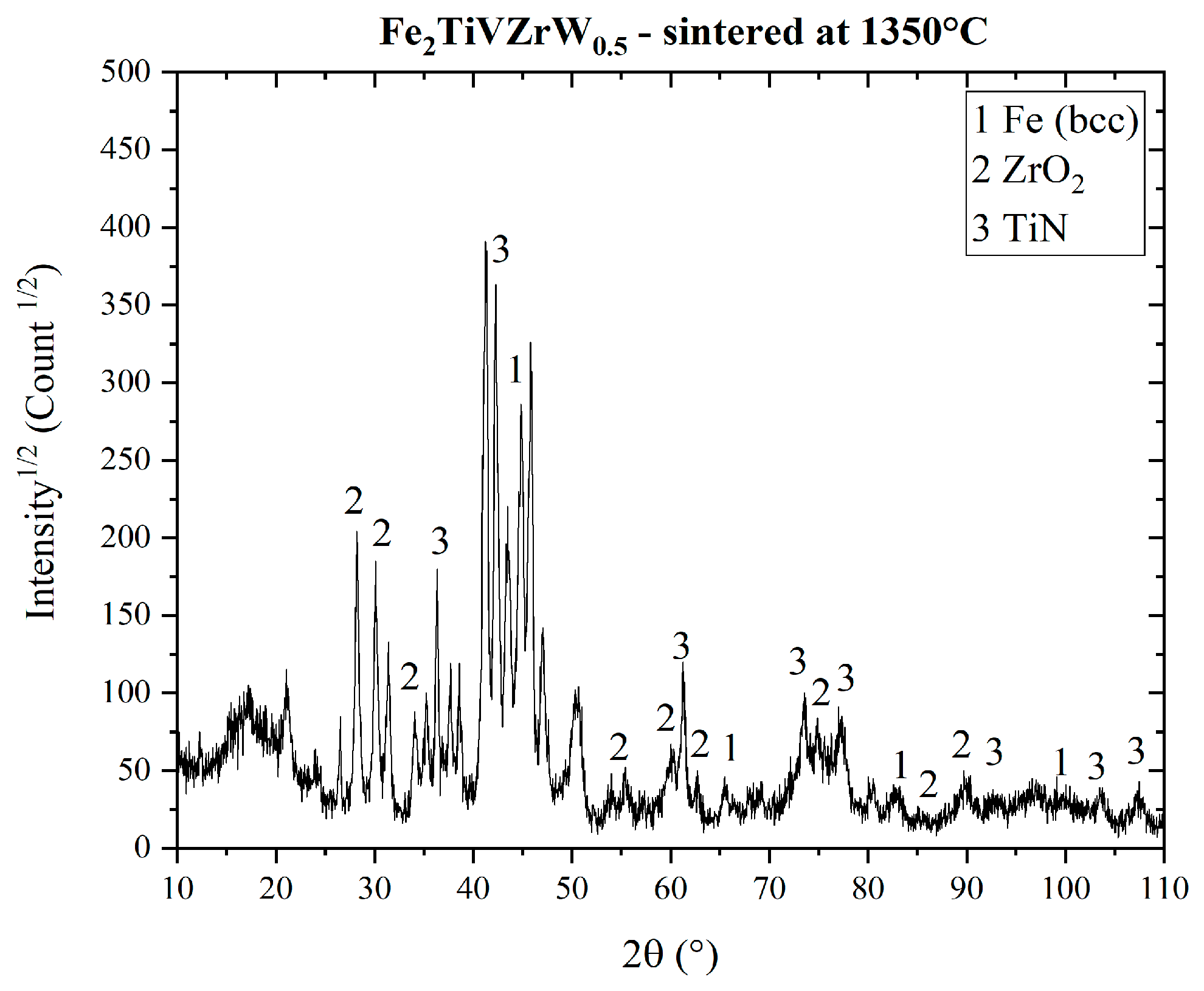
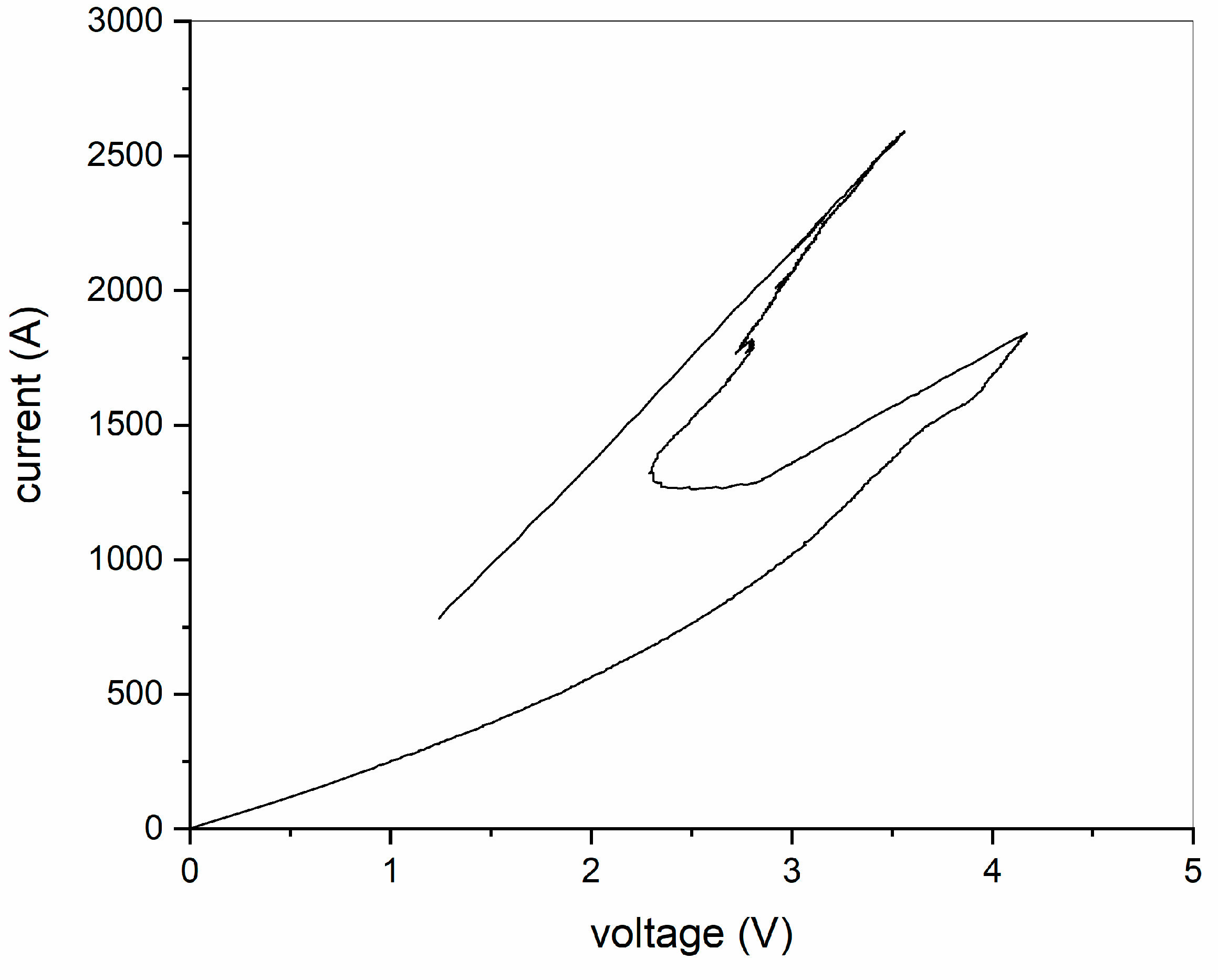
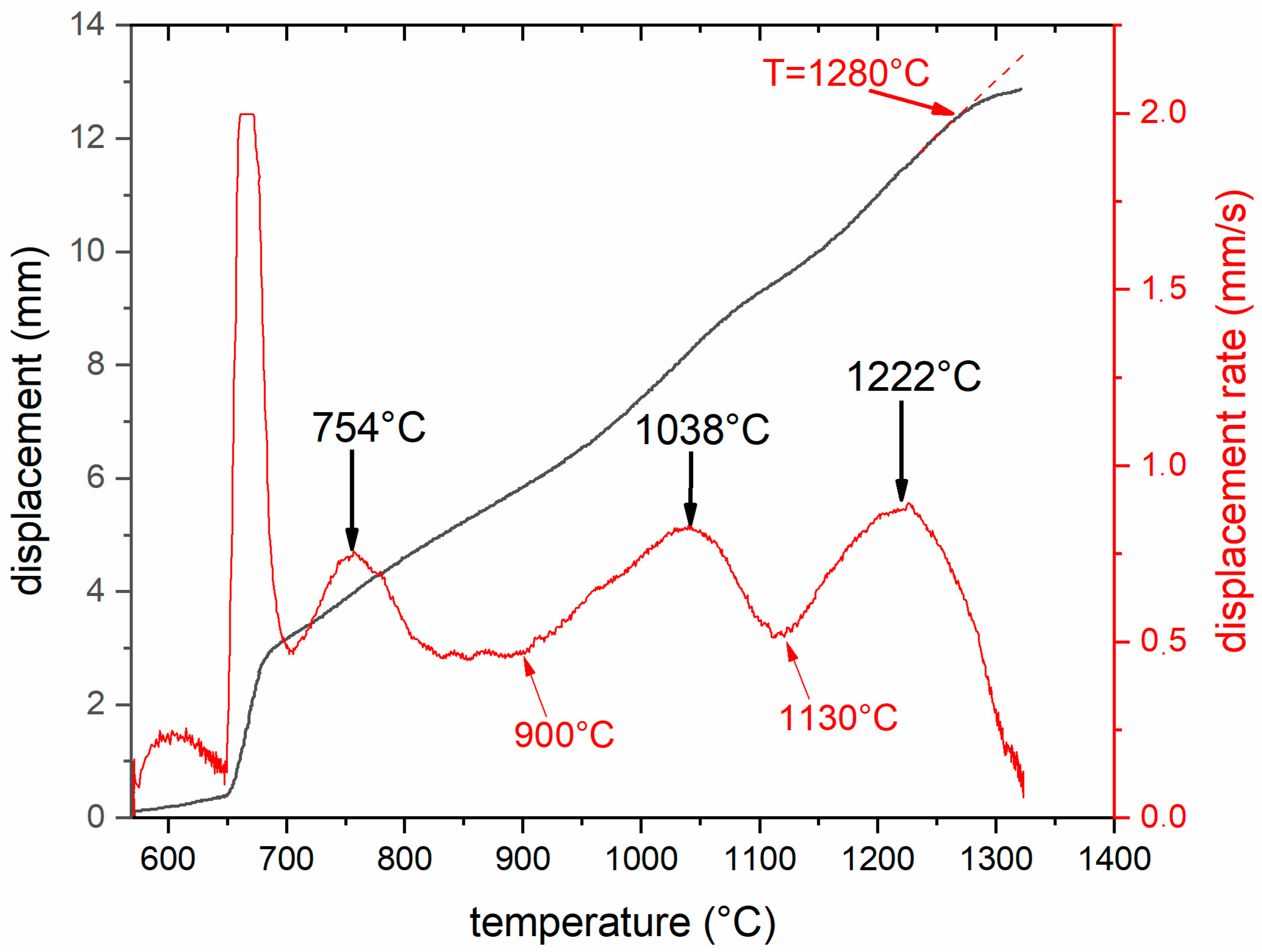
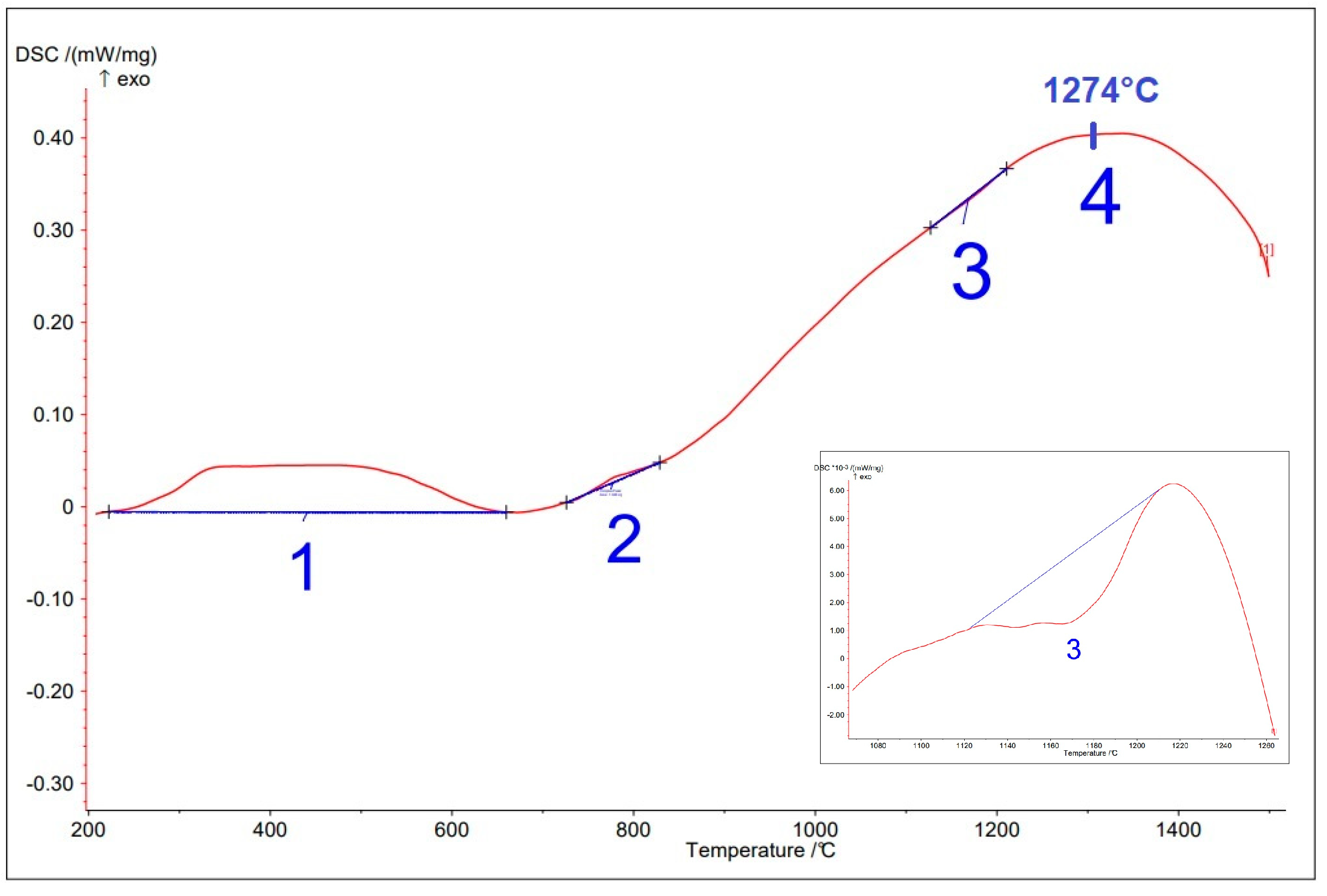
3.3. Sintering Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin, Germany, 2016. [Google Scholar]
- Liu, F.; Liaw, P.K. Recent Progress with BCC-Structured High-Entropy Alloys. Metals 2022, 12, 501. [Google Scholar] [CrossRef]
- Gao, X.; Wang, L.; Guo, N.; Luo, L.; Zhu, G.; Shi, C.; Su, Y.; Guo, J. Microstructure and Mechanical Properties of Multi-Phase Reinforced Hf-Mo-Nb-Ti-Zr Refractory High-Entropy Alloys. Int. J. Refract. Met. Hard Mater. 2022, 102, 105723. [Google Scholar] [CrossRef]
- Real, C.; Alcalá, M.D.; Trigo, I.; Fombella, I.; Córdoba, J.M. Fabrication and Characterization of FeCoNiCrMn,(Al) High Entropy Alloy Based (Ti,Ta,Nb)(C,N) Cermet. Int. J. Refract. Met. Hard Mater. 2021, 101, 105694. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Zhang, M.; Qiao, J. Development of Refractory High Entropy Alloys with Tensile Ductility at Room Temperature. Metals 2023, 13, 329. [Google Scholar] [CrossRef]
- Senkov, O.; Miracle, D.; Chaput, K.; Couzinie, J. Development and exploration of refractory high entropy alloys—A review. J. Mat. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98. [Google Scholar] [CrossRef]
- Tian, Y.-S.; Zhou, W.-Z.; Tan, Q.-B.; Wu, M.-X.; Qiao, S.; Zhu, G.-L.; Dong, A.-P.; Shu, D.; Sun, B.-D. A Review of Refractory High-Entropy Alloys. Trans. Nonferrous Met. Soc. China 2022, 32, 3487–3515. [Google Scholar] [CrossRef]
- Chen, S.; Qi, C.; Liu, J.; Zhang, J.; Wu, Y. Recent Advances in W-Containing Refractory High-Entropy Alloys—An Overview. Entropy 2022, 24, 1553. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory High-Entropy Alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Li, D.; Chen, Z.; Qi, Z. The Effect of Configurational Entropy on Mechanical Properties of Single BCC Structural Refractory High-Entropy Alloys Systems. Int. J. Refract. Met. Hard Mater. 2020, 93, 105370. [Google Scholar] [CrossRef]
- Vlasák, T.; Čížek, J.; Melikhova, O.; Lukáč, F.; Preisler, D.; Janeček, M.; Harcuba, P.; Zimina, M.; Srba, O. Thermal Stability of Microstructure of High-Entropy Alloys Based on Refractory Metals Hf, Nb, Ta, Ti, V, and Zr. Metals 2022, 12, 394. [Google Scholar] [CrossRef]
- Lu, S.; Li, X.; Liang, X.; Yang, W.; Chen, J. Effect of V and Ti on the Oxidation Resistance of Wmotanb Refractory High-Entropy Alloy at High Temperatures. Metals 2022, 12, 41. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Gridneva, A.O.; Mishunin, M.V.; Salishchev, G.A.; Zherebtsov, S.V. Effect of Cr and Zr on Phase Stability of Refractory Al-Cr-Nb-Ti-V-Zr High-Entropy Alloys. J. Alloys Compd. 2018, 757, 403–414. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P.; Yurchenko, N.Y.; Stepanov, N.D.; Gridneva, A.O.; Mishunin, M.V.; Salishchev, G.A.; Zherebtsov, S.V.; et al. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Powder Metall. 2018, 61, 115–122. [Google Scholar]
- Chen, H.; Kauffmann, A.; Seils, S.; Boll, T.; Liebscher, C.H.; Harding, I.; Kumar, K.S.; Szabó, D.V.; Schlabach, S.; Kauffmann-Weiss, S.; et al. Crystallographic Ordering in a Series of Al-Containing Refractory High Entropy Alloys Ta–Nb–Mo–Cr–Ti–Al. Acta Mater. 2019, 176, 123–133. [Google Scholar] [CrossRef]
- Moravcik, I.; Kubicek, A.; Moravcikova-Gouvea, L.; Adam, O.; Kana, V.; Pouchly, V.; Zadera, A.; Dlouhy, I. The Origins of High-Entropy Alloy Contamination Induced by Mechanical Alloying and Sintering. Metals 2020, 10, 1186. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, A.X.Y.; Zhan, S.; Liu, C.T.; Cao, S.C. Refractory High-Entropy Alloys: A Focused Review of Preparation Methods and Properties. J. Mater. Sci. Technol. 2023, 142, 196–215. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Y.; Wen, Y.; Zhang, Q.; Deng, D.; Nai, X. Microstructures and Properties of CoCrCuFeNiMox High-Entropy Alloys Fabricated by Mechanical Alloying and Spark Plasma Sintering. Powder Metall. 2018, 61, 115–122. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Liu, Y.; Liu, B.; Wang, J. FeCoCrNiMo High-Entropy Alloys Prepared by Powder Metallurgy Processing for Diamond Tool Applications. Powder Metall. 2018, 61, 123–130. [Google Scholar] [CrossRef]
- Shkodich, N.; Sedegov, A.; Kuskov, K.; Busurin, S.; Scheck, Y.; Vadchenko, S.; Moskovskikh, D. Refractory High-Entropy Hftatinbzr-Based Alloys by Combined Use of Ball Milling and Spark Plasma Sintering: Effect of Milling Intensity. Metals 2020, 10, 1268. [Google Scholar] [CrossRef]
- Diouf, S.; Menapace, C.; Molinari, A. Study of Effect of Particle Size on Densification of Copper during Spark Plasma Sintering. Powder Metall. 2012, 55, 228–234. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Xiao, H.; Zhou, L.; Zhu, D.; Yang, S. Fabrication and Properties of Nanocrystalline Co0.5FeNiCrTi0.5 High Entropy Alloy by MA–SPS Technique. Mater. Des. 2013, 44, 535–539. [Google Scholar] [CrossRef]
- Wang, C.; Ji, W.; Fu, Z. Mechanical Alloying and Spark Plasma Sintering of CoCrFeNiMnAl High-Entropy Alloy. Adv. Powder Technol. 2014, 25, 1334–1338. [Google Scholar] [CrossRef]
- Fang, S.; Chen, W.; Fu, Z. Microstructure and Mechanical Properties of Twinned Al0.5CrFeNiCo0.3C0.2 High Entropy Alloy Processed by Mechanical Alloying and Spark Plasma Sintering. Mater. Des. 2014, 54, 973–979. [Google Scholar] [CrossRef]
- Joo, S.H.; Kato, H.; Jang, M.J.; Moon, J.; Kim, E.B.; Hong, S.J.; Kim, H.S. Structure and Properties of Ultrafine-Grained CoCrFeMnNi High-Entropy Alloys Produced by Mechanical Alloying and Spark Plasma Sintering. J. Alloys Compd. 2017, 698, 591–604. [Google Scholar] [CrossRef]
- Diouf, S.; Menapace, C.; D’Incau, M.; Molinari, A.; Ischia, G. Spark Plasma Sintering of Cryomilled Copper Powder. Powder Metall. 2013, 56, 420–426. [Google Scholar] [CrossRef]
- Straffelini, G.; Nogueira, A.P.; Muterlle, P.; Menapace, C. Spark Plasma Sintering and Hot Compression Behaviour of AZ91 Mg Alloy. Mater. Sci. Technol. 2011, 27, 1582–1587. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Cuevas, F.G.; Cintas, J.; Montes, J.M.; Gallardo, J.M. Powder Metallurgy On Selection of Milling Atmosphere for Production of Al-10%Ti Powders On Selection of Milling Atmosphere for Production of Al-10%Ti Powders. Powder Metall. 2009, 52, 116–123. [Google Scholar] [CrossRef]
- Rajendrachari, S. An Overview of High-Entropy Alloys Prepared by Mechanical Alloying Followed by the Characterization of Their Microstructure and Various Properties. Alloys 2022, 1, 116–132. [Google Scholar] [CrossRef]
- Lu, C.J.; Zhang, J.; Li, Z.Q. Structural Evolution of Titanium Powder during Ball Milling in Different Atmospheres. J. Alloys Compd. 2004, 381, 278–283. [Google Scholar] [CrossRef]
- Olier, P.; Couvrat, M.; Cayron, C.; Lochet, N.; Chaffron, L. Incidence of Mechanical Alloying Contamination on Oxides and Carbides Formation in ODS Ferritic Steels. J. Nucl. Mater. 2013, 442, S106–S111. [Google Scholar] [CrossRef]
- Noh, S.; Choi, B.K.; Kang, S.H.; Kim, T.K. Influence of Mechanical Alloying Atmospheres on the Microstructures and Mechanical Properties of 15Cr Ods Steels. Nucl. Eng. Technol. 2014, 46, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.S. Dispersion Strengthened Superalloys by Mechanical Alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox High Entropy Alloys Strengthened by Hard Intermetallic Phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent Progress in High-Entropy Alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys Related Articles Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. Addit. Inf. J. Appl. Phys. J. Homepage 2011, 109, 144503. [Google Scholar]
- Senkov, O.N.; Gorsse, S.; Miracle, D.B. High Temperature Strength of Refractory Complex Concentrated Alloys. Acta Mater. 2019, 175, 394–405. [Google Scholar] [CrossRef]
- Senkov, O.N.; Jensen, J.K.; Pilchak, A.L.; Miracle, D.B.; Fraser, H.L. Compositional Variation Effects on the Microstructure and Properties of a Refractory High-Entropy Superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Srikanth, M.; Raja Annamalai, A.; Muthuchamy, A.; Jen, C.P. A Review of the Latest Developments in the Field of Refractory High-Entropy Alloys. Crystals 2021, 11, 612. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I. A Short Review on the Ultra-High Temperature Mechanical Properties of Refractory High Entropy Alloys. Front. Met. Alloy. 2023, 2, 1135826. [Google Scholar] [CrossRef]
- Rame, J.; Utada, S.; Bortoluci Ormastroni, L.M.; Mataveli-Suave, L.; Menou, E.; Després, L.; Kontis, P.; Cormier, J. Platinum-Containing New Generation Nickel-Based Superalloy for Single Crystalline Applications. In Proceedings of the Minerals, Metals and Materials Series, Seven Springs, PA, USA, 13–17 September 2020. [Google Scholar]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Aoki, K.; Memezawa, A.; Masumoto, T. Amorphization of Ti-Zr Powders by Mechanical Alloying in H2, N 2 and 02 Atmospheres. Mater. Sci. Eng. 1994, 181, 1263–1267. [Google Scholar] [CrossRef]
- Ustinovshikov, Y.; Pushkarev, B.; Sapegina, I. Phase Transformations in Alloys of the Fe–V System. J. Alloys Compd. 2005, 398, 133–138. [Google Scholar] [CrossRef]
- Hayakawa, M.; Kuntani, N.; Oka, M. Structural Study on the Tetragonal to Monoclinic Transformation in Arc-Melted ZrO2-2mol.%Y2O3—I. Experimental Observations. Acta Metall. 1989, 37, 2223–2228. [Google Scholar] [CrossRef]
- Leib, E.W.; Pasquarelli, R.M.; Blankenburg, M.; Müller, M.; Schreyer, A.; Janssen, R.; Weller, H.; Vossmeyer, T. High-Temperature Stable Zirconia Particles Doped with Yttrium, Lanthanum, and Gadolinium. Part. Part. Syst. Charact. 2016, 33, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Sathiyamoorthi, P.; Basu, J.; Kashyap, S.; Pradeep, K.G.; Kottada, R.S. Thermal Stability and Grain Boundary Strengthening in Ultrafine-Grained CoCrFeNi High Entropy Alloy Composite. Mater. Des. 2017, 134, 426–433. [Google Scholar] [CrossRef]
- Sheikh, S.; M’Saoubi, R.; Flasar, P.; Schwind, M.; Persson, T.; Yang, J.; Llanes, L. Fracture Toughness of Cemented Carbides: Testing Method and Microstructural Effects. Int. J. Refract. Met. Hard Mater. 2015, 49, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liaw, P.K.; Gao, Y. Fracture Resistance of High Entropy Alloys: A Review. Intermetallics 2018, 99, 69–83. [Google Scholar] [CrossRef]
- Menapace, C.; Cipolloni, G.; Hebda, M.; Ischia, G. Spark Plasma Sintering Behaviour of Copper Powders Having Different Particle Sizes and Oxygen Contents. Powder Technol. 2016, 291, 170–177. [Google Scholar] [CrossRef]
- Sundgren, J.-E. Formation and characterization of titanium nitride and titanium carbide films prepared by reactive sputtering. In Linkoping Stud. Sci. Technol. Diss. No. 79; Dep. Phys. Meas. Technol. Linkoping Inst. Technol.: Linkoping, Sweden, 1982; Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/13/693/13693374.pdf (accessed on 23 June 2023).
- Mary, C.; Le Mogne, T.; Beaugiraud, B.; Vacher, B.; Martin, J.M.; Fouvry, S. Tribochemistry of a Ti Alloy under Fretting in Air: Evidence of Titanium Nitride Formation. Tribol. Lett. 2009, 34, 211–222. [Google Scholar] [CrossRef]
- Ettmayer, P.; Kolaska, H. Ti (C, N) Cermets—Metallurgy and Properties. Int. J. 1995, 13, 343–351. [Google Scholar] [CrossRef]
- Taguchi, M.; Sumitomo, H.; Ishibashi, R.; Aono, Y. Effect of Zirconium Oxide Addition on Mechanical Properties in Ultrafine Grained Ferritic Stainless Steels. Mater. Trans. 2008, 49, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Gauna, M.R.; Conconi, M.S.; Gomez, S.; Suárez, G.; Aglietti, E.F.; Rendtorff, N.M. Monoclinic-Tetragonal Zirconia Quantification of Commercial Nanopowder Mixtures by XRD and DTA. Ceram. Silikaty 2015, 50, 318–325. [Google Scholar]
- Zhang, Y.; Chen, H.X.; Duan, L.; Fan, J.B.; Ni, L.; Ji, V. A Comparison Study of the Structural and Mechanical Properties of Cubic, Tetragonal, Monoclinic, and Three Orthorhombic Phases of ZrO2. J. Alloys Compd. 2018, 749, 283–292. [Google Scholar] [CrossRef]



| Element | Particle Size (μm) | Purity Level (%) | Weight (wt.%) | Atomic Fraction (-) | VEC (-) |
|---|---|---|---|---|---|
| Fe | <74 | 99.00 | 28.4 | 0.35 | 8 |
| Ti | <149 | 99.90 | 12.2 | 0.18 | 4 |
| V | <44 | 99.50 | 13.0 | 0.18 | 5 |
| Zr | <44 | 99.50 | 23.1 | 0.18 | 4 |
| W | 0.6–0.9 | 99.95 | 23.4 | 0.1 | 6 |
| KIC Measured through Palmqvist Method MPa m1/2 | ||
|---|---|---|
| treatment time | T = 900 °C | T = 1100 °C |
| 1 h | 4.10 | 5.05 |
| 4 h | 4.68 | 5.71 |
| 8 h | 4.87 | 5.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menapace, C.; Shaik, K.N.R.; Emanuelli, L.; Ischia, G. Production of a Reinforced Refractory Multielement Alloy via High-Energy Ball Milling and Spark Plasma Sintering. Metals 2023, 13, 1189. https://doi.org/10.3390/met13071189
Menapace C, Shaik KNR, Emanuelli L, Ischia G. Production of a Reinforced Refractory Multielement Alloy via High-Energy Ball Milling and Spark Plasma Sintering. Metals. 2023; 13(7):1189. https://doi.org/10.3390/met13071189
Chicago/Turabian StyleMenapace, Cinzia, Khaja Naib Rasool Shaik, Lorena Emanuelli, and Gloria Ischia. 2023. "Production of a Reinforced Refractory Multielement Alloy via High-Energy Ball Milling and Spark Plasma Sintering" Metals 13, no. 7: 1189. https://doi.org/10.3390/met13071189





