Study on the Microstructure and Properties of Flash-Butt Welding Joints of High Nitrogen Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Basic Equation of the Finite Element Calculation
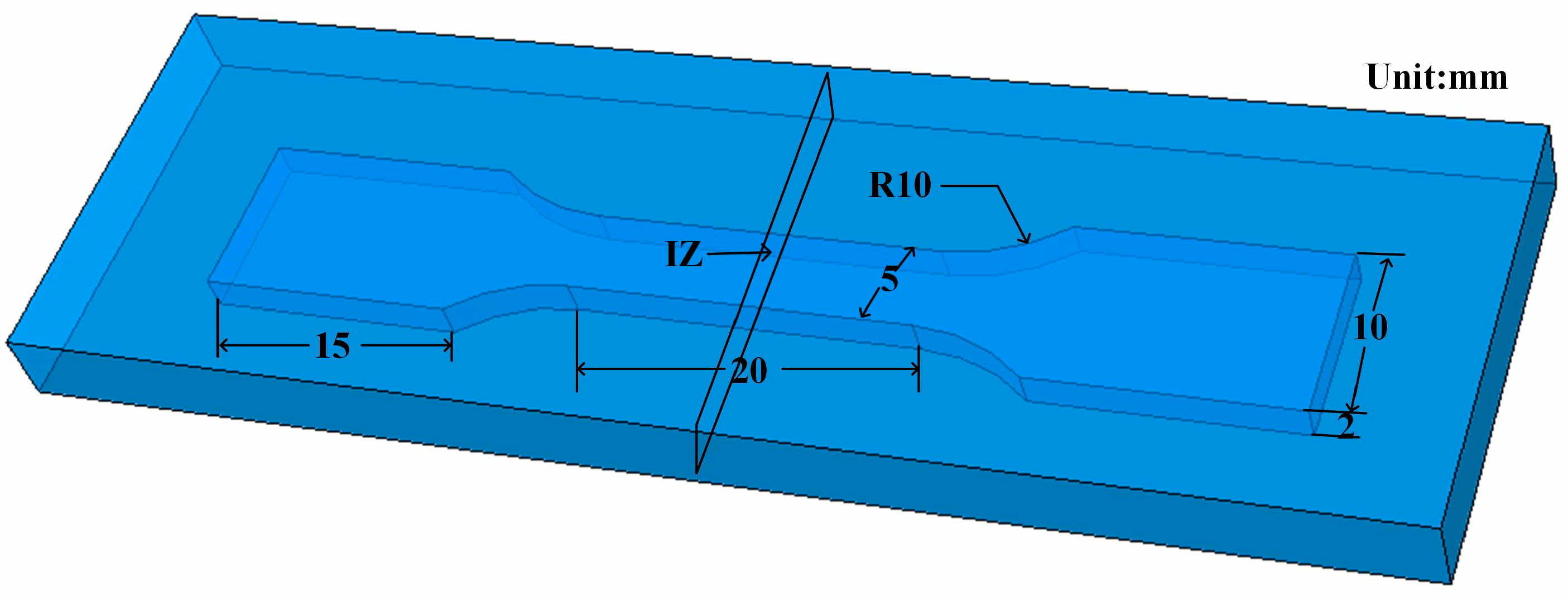
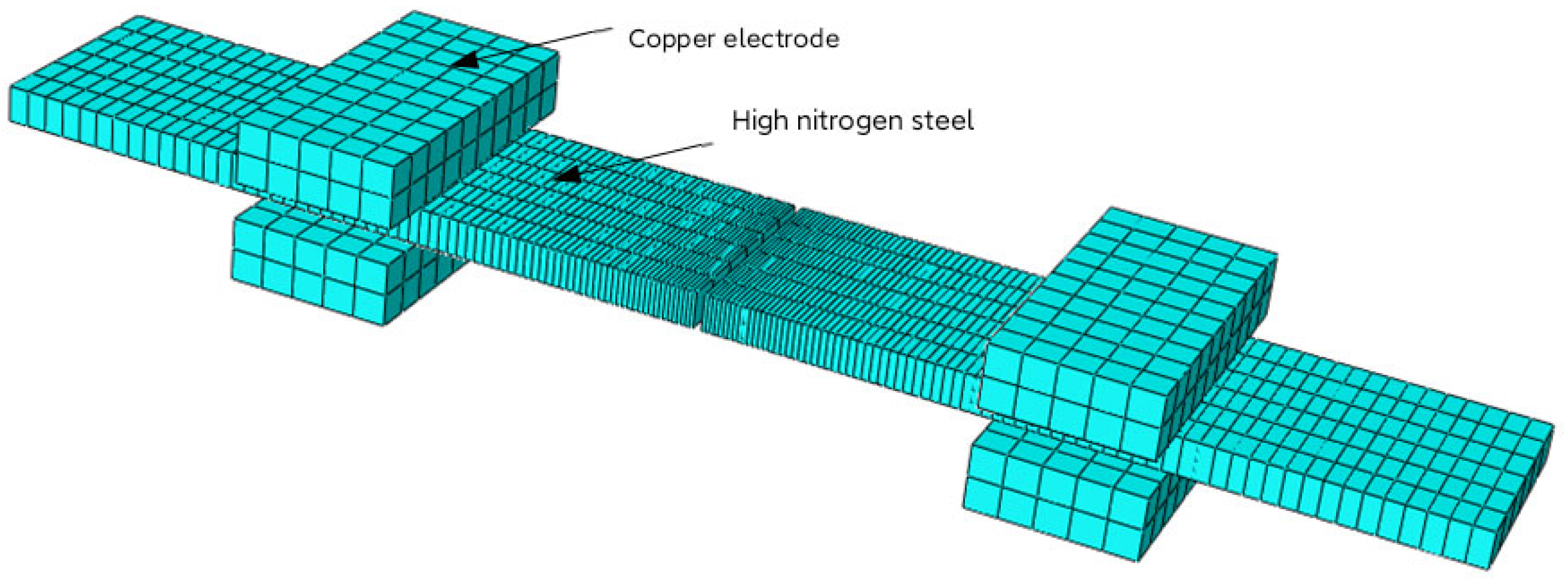
2.4. Three-Dimensional Model
2.5. Boundary Conditions and Contact Conditions
2.6. Temperature Measurement Verification
3. Results and Discussion
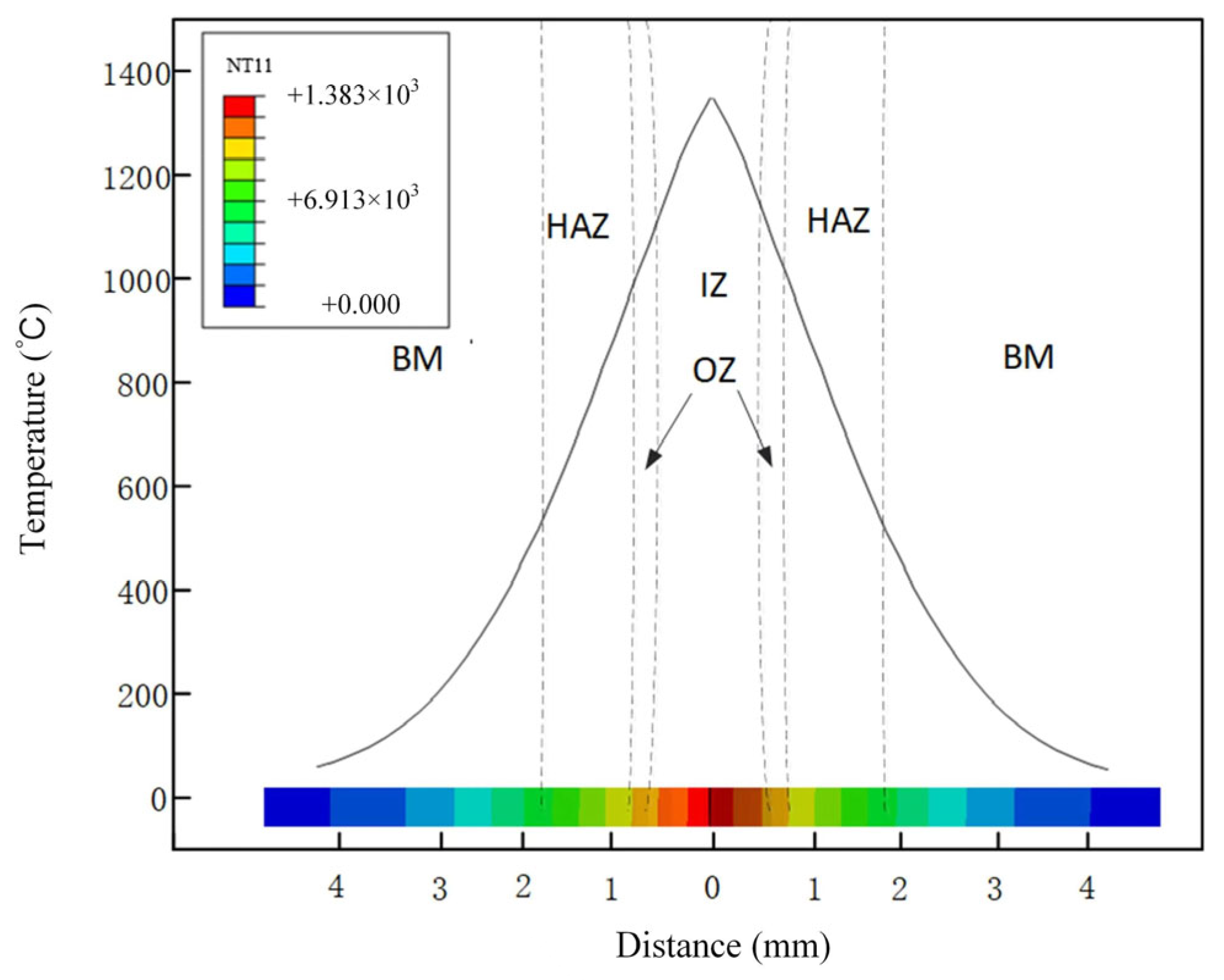
3.1. Thermodynamic Analysis
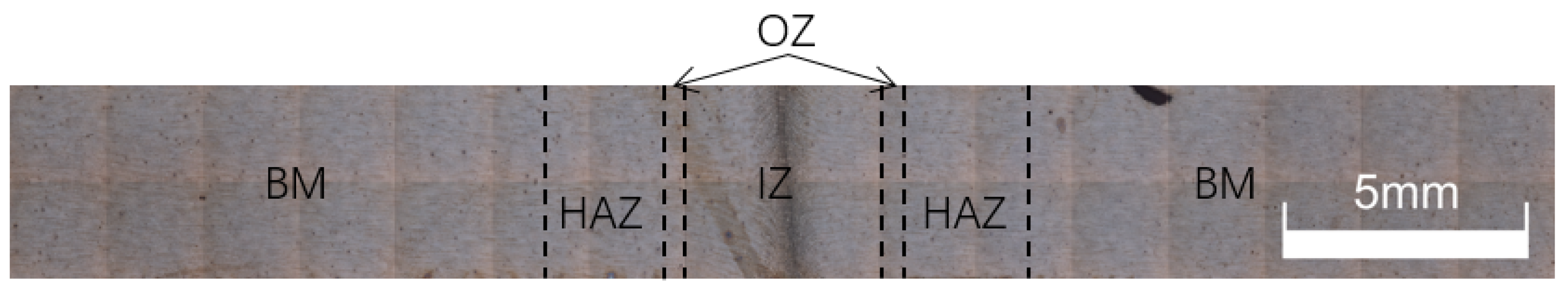
3.2. Joint Organization
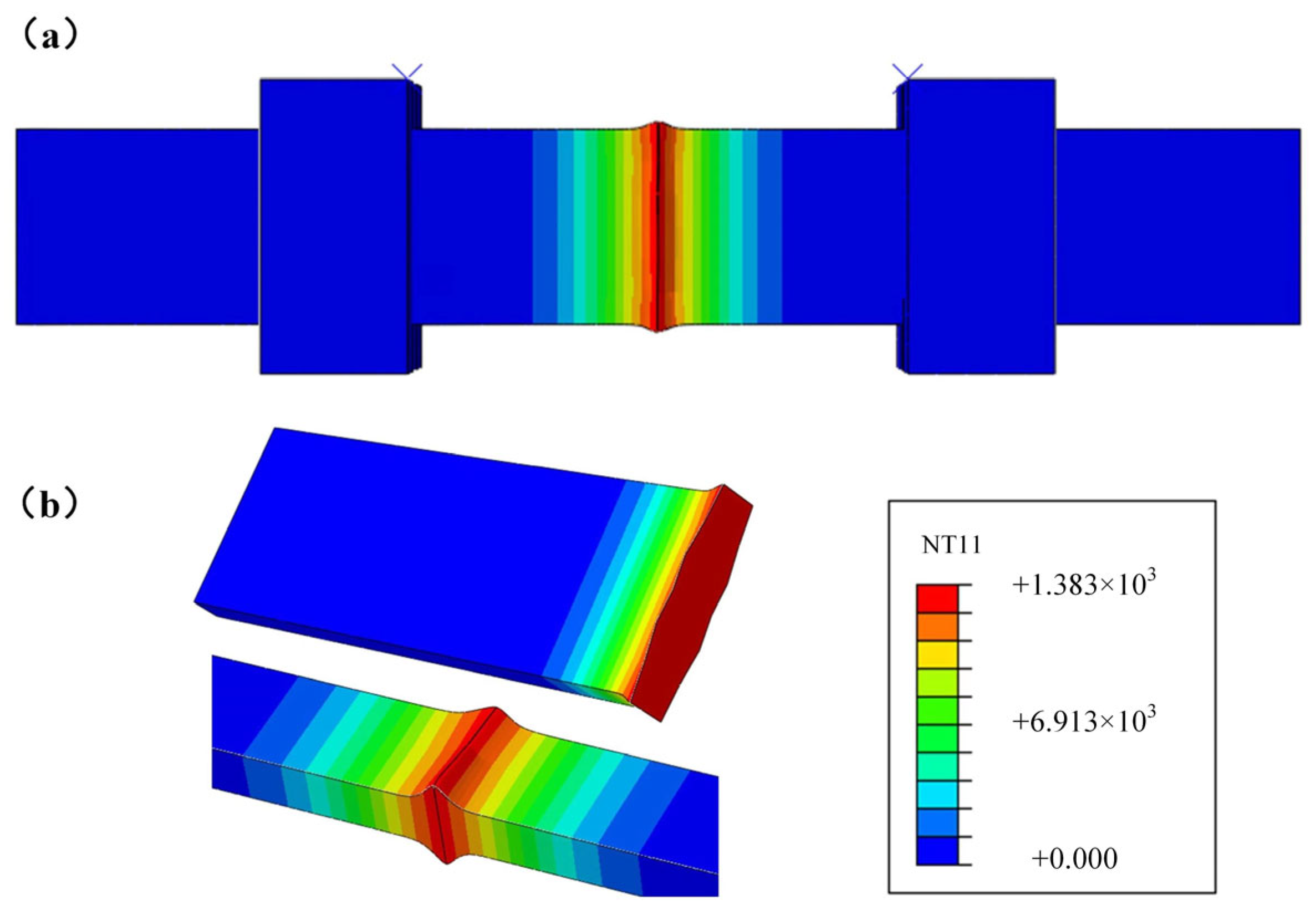
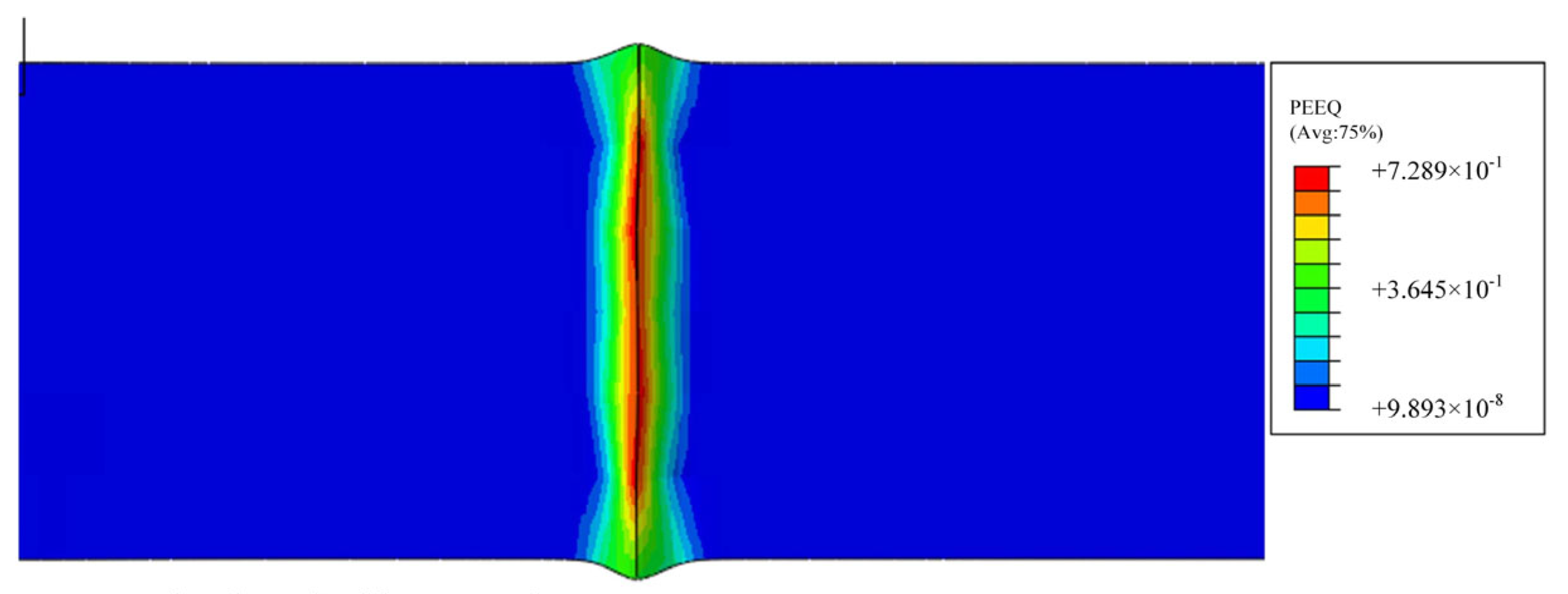
3.3. Simulation Results and Analysis
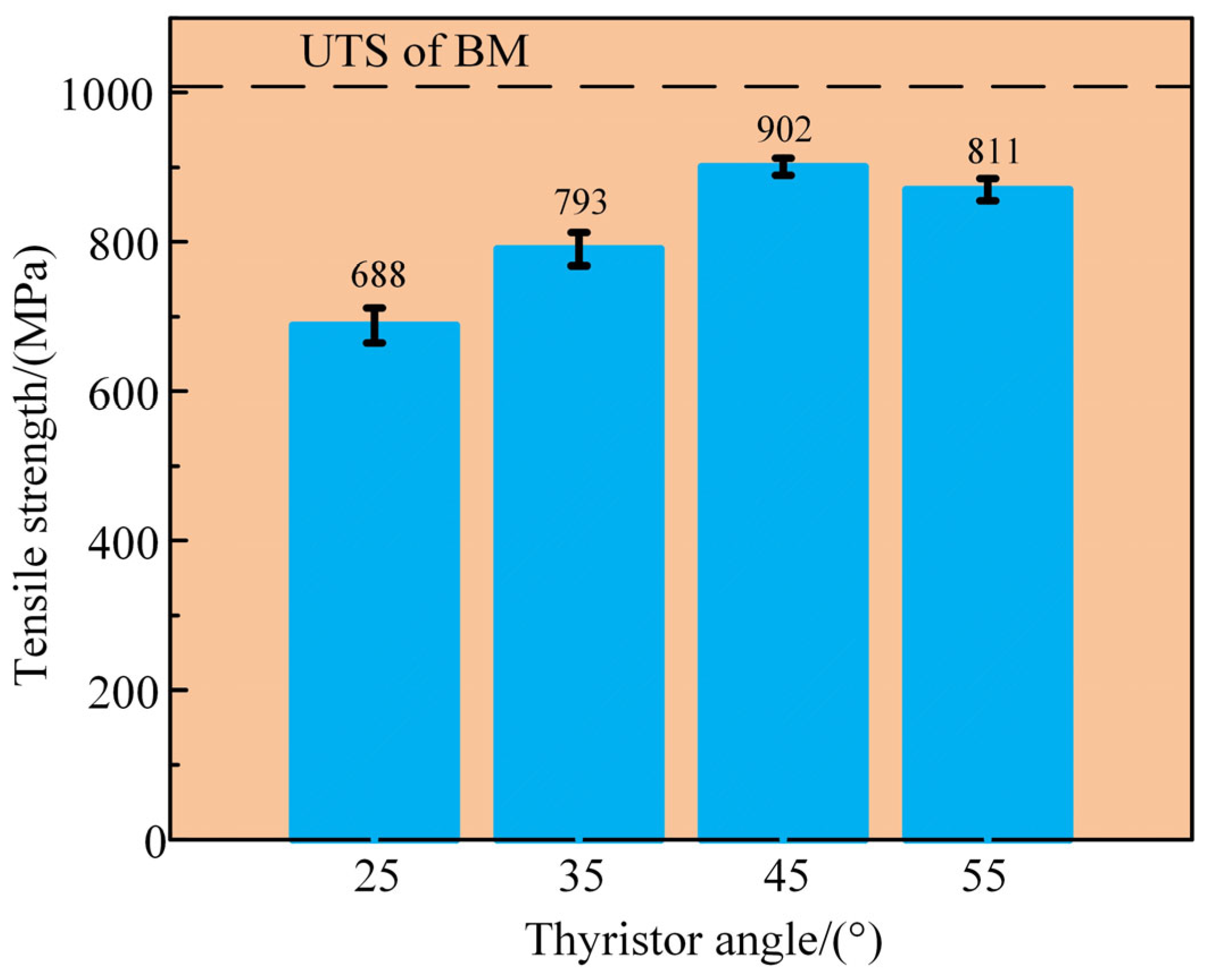
3.4. Mechanical Properties
3.5. Full Immersion Corrosion Test
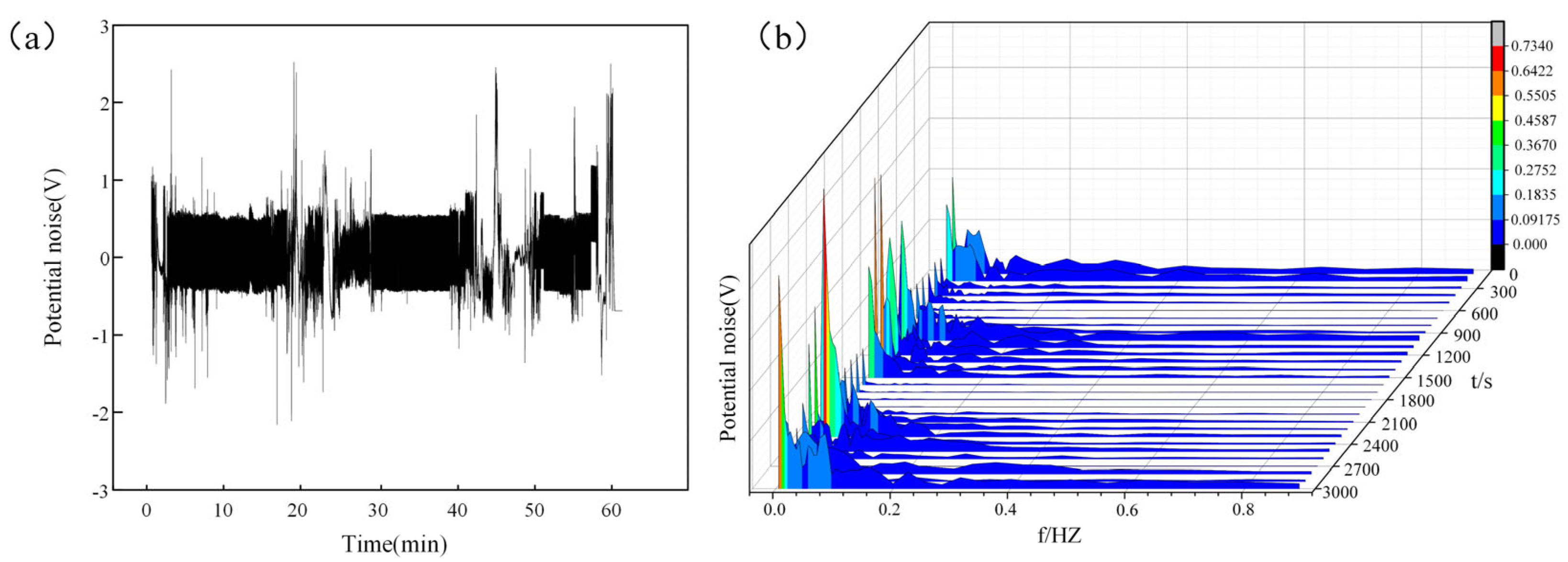
3.6. In Situ Detection
4. Conclusions
- The numerical simulation results of the FBW process of high nitrogen steel were consistent with the experimental results. The maximum temperature for the welded joints of high nitrogen steel using flash-butt welding with typical parameters was 1383 °C.
- When the thyristor angle was 45°, the interface was composed of austenite and high-temperature ferrite, while the superheated zone consisted of austenite, ferrite and a small amount of the M2 phase. The heat-affected zone was mainly single-phase austenite.
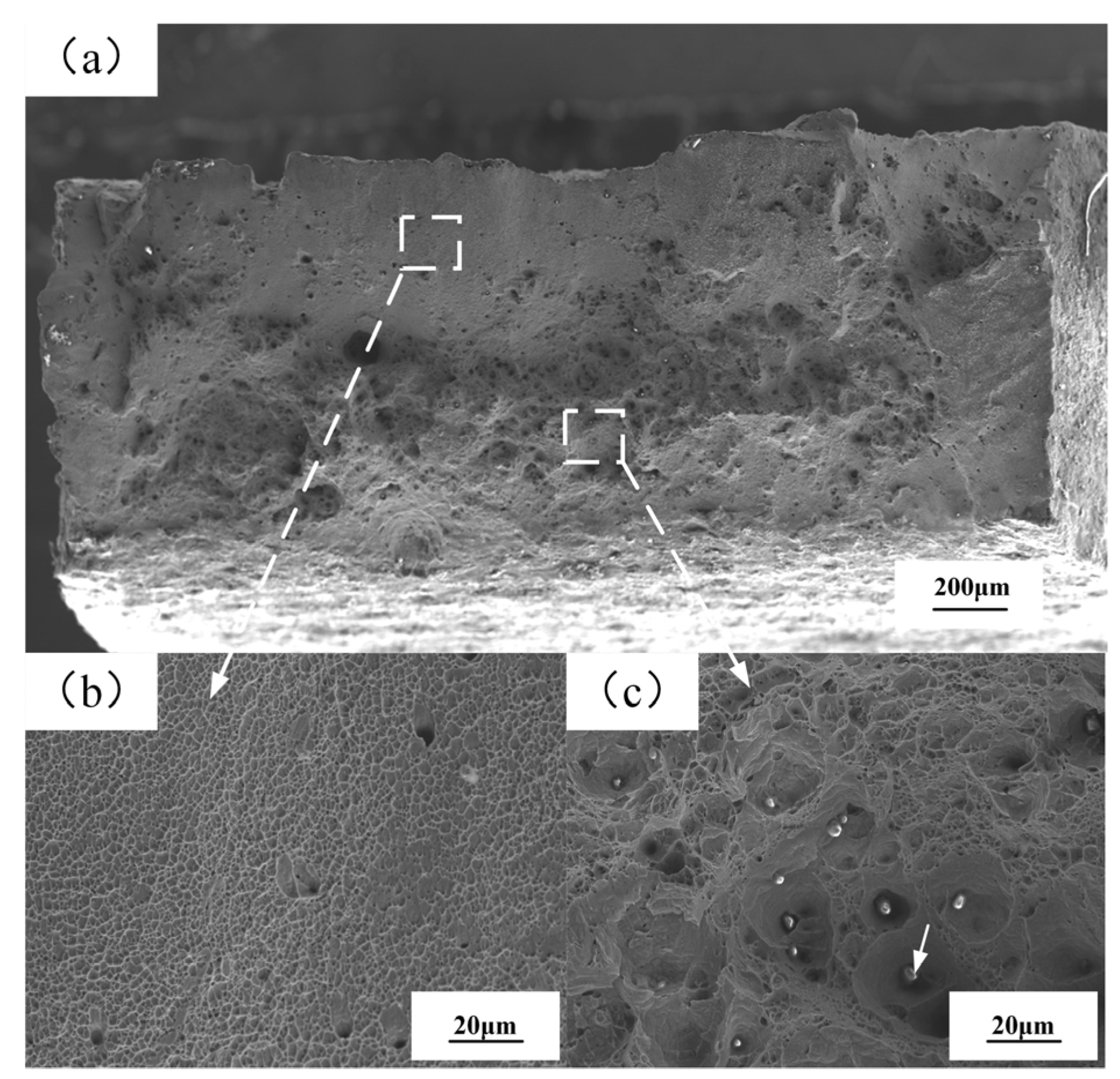
- The joint hardness showed a V-shaped distribution with the lowest hardness at the interface. The hardness and tensile strength of the interface area of the joint increased first and then decreased with increasing flash current. The maximum tensile strength reached 902 MPa at 45°, showing the best mechanical property. The fracture mode of the joint was ductile fracture.
- The total erosion experiment suggested that the interface center presented the most serious corrosion and the corrosion occurred first in the interface area of the joint.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, R.; Reddy, G.M.; Rao, K.S. Welding of nickel free high nitrogen stainless steel: Microstructure and mechanical properties. Def. Technol. 2017, 13, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, C.S.; Lu, J.P.; Chen, R.D.; Chen, D.Z. A review of progress on high nitrogen austenitic stainless-steel research. Mater. Express 2021, 11, 1901–1925. [Google Scholar] [CrossRef]
- Mohammed, R.; Rao, K.S.; Reddy, G.M. Studies on Fusion Welding of High Nitrogen Stainless Steel: Microstructure, Mechanical and corrosion Behaviour. Mater. Sci. Eng. 2018, 330, 012035. [Google Scholar] [CrossRef]
- Kiellerup, B.V.; Olesen, B.H.; Nielsen, J.L.; Sowers, K.R.; Nielsen, P.H. In situ detection of bacteria involved in cathodic depolarization and stainless steel surface corrosion using microautoradiography. J. Appl. Microbiol. 2008, 105, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Jiang, Z.H.; Feng, H. Microstructure, mechanical and corrosion properties of friction stir welded high nitrogen nickel-free austenitic stainless steel. Mater. Des. 2015, 84, 291–299. [Google Scholar] [CrossRef]
- Zhao, L.; Tian, Z.L.; Peng, Y. Porosity and nitrogen content of weld metal in laser welding of high nitrogen austenitic stainless steel. ISIJ Int. 2007, 12, 1772–1775. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Kokawa, H.; Sato, Y.S. Nitrogen desorption by high-nitrogen steel weld metal during CO2 laser welding. Metall. Mater. Trans. B Vol. 2005, 36, 677–681. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, C.L.; Ming, C.L.; Chen, C.; Liu, A.; Yang, C.L. Gas metal arc welding of high nitrogen stainless steel with Ar-N-2-O-2 ternary shielding gas. Def. Technol. 2021, 17, 923–931. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, C.L.; Ming, Z.; Chen, C.; Yang, C.L.; Lin, S.B. Optimization of shielding gas composition in high nitrogen stainless steel gas metal arc welding. J. Manuf. Process. 2020, 58, 19–29. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, C.L.; Chen, C.; Ming, Z.; Yang, C.L.; Lin, S.B. Design and evaluation of nitrogen-rich welding wires for high nitrogen stainless steel. J. Mater. Process. Technol. 2021, 288, 116885. [Google Scholar] [CrossRef]
- Chausov, M.G.; Maruschak, P.O.; Hutsaylyuk, V.; Śnieżek, L.; Pylypenko, A.P. Effect of complex combined loading mode on the fracture toughness of titanium alloys. Vacuum 2018, 147, 51–57. [Google Scholar] [CrossRef]
- Li, B.W.; Lei, Z.L.; Zhang, X.R.; Chen, Y.B.; Chen, X. Effect of mixed powder proportion on microstructure and defects of high-nitrogen steel welded joints. Opt. Laser Technol. 2023, 163, 109430. [Google Scholar] [CrossRef]
- Hafez, K.M. The effect of welding atmosphere on the pitting corrosion of AISI 304L resistance spot welds. Int. J. Adv. Manuf. Technol. 2018, 97, 243–251. [Google Scholar] [CrossRef]
- Fukumoto, S.; Matsuo, T.; Kuroda, D.; Yamamoto, A. Weldability of Nickel-Free Austenitic Stainless Steel Thin Sheet by Small-Scale Resistance Spot Welding. Mater. Trans. 2008, 49, 2844–2849. [Google Scholar] [CrossRef] [Green Version]
- Somervuori, M.E.; Johansson, L.S.; Heinonen, M.H.; Akdut, N.; Hanninen, H.E. Characterisation and corrosion of spot welds of austenitic stainless steels. Mater. Corros. 2004, 55, 6. [Google Scholar] [CrossRef]
- Woo, I.; Kikuchi, Y. Weldability of High Nitrogen Stainless Steel. ISIJ Int. 2002, 42, 1334–1343. [Google Scholar] [CrossRef] [Green Version]
- Konovalov, N.A.; Oshkadyorov, S.P.; Poloskov, S.I. The Ways of Improvement of the Joints’ Quality under Resistance Butt Welding with a Flash-Off. Metallofiz. Noveishie Tekhnol. 2014, 35, 1539–1549. [Google Scholar]
- Wang, X.; Liu, X.; Deng, J.R. Numerical simulation of end face heating in alternating current flash butt welding based on electrical-thermal bidirectional coupling. Int. J. Adv. Manuf. Technol. 2022, 120, 173–183. [Google Scholar] [CrossRef]
- Ma, N.S.; Cai, Z.P.; Huang, H.; Deng, D.A.; Murakawa, H.; Pan, J.L. Investigation of welding residual stress in flash-butt joint of U71Mn rail steel by numerical simulation and experiment. Mater. Des. 2015, 88, 1296–1309. [Google Scholar] [CrossRef]
- Goldak, J.; Bibby, M.; Moore, J. Computer modeling of heat flow in welds. Metall. Trans. B 1986, 17, 587–600. [Google Scholar] [CrossRef]
- Katada, Y.; Washizu, N.; Baba, H. \Localized corrosion behavior of high nitrogen steel. Mater. Sci. Forum 2005, 475, 225–228. [Google Scholar]
- Speidel, M.O.; Pedrazzoli, R.M. High nitrogen stainless-steels in chloride solutions. Mater. Perform. 1992, 31, 59–61. [Google Scholar]
- Alvarez, K.; Hyun, S.K.; Tsuchiya, H.; Fujimoto, S.; Nakajima, H. Corrosion behaviour of Lotus-type porous high nitrogen nickel-free stainless steels. Corros. Sci. 2008, 50, 183–193. [Google Scholar] [CrossRef]
- Klyuev, A.L.; Davydov, A.D.; Grafov, B.M.; Dobrovolskii, Y.A.; Ukshe, A.E. Electrochemical noise spectroscopy: Method of secondary Chebyshev spectrum. Russ. J. Electrochem. 2016, 52, 1001–1005. [Google Scholar] [CrossRef]
- Stakenborghs, R.J.; Goitia, R.M. Detection and characterization of corrosion under coatings. In Proceedings of the ASME Pressure Vessels and Piping Conference, Anaheim, CA, USA, 20–24 July 2014; p. 5. [Google Scholar]
- Braasch, D.A.; Gillis, M.; Pramanik, M.; Ferguson, R.C.; Delatte, D.; Blanton, M.; Rawlins, J.W. Detection of in Situ Early Corrosion on Polymer-Coated Metal Substrates. ACS Appl. Mater. Interfaces 2019, 11, 37193–37208. [Google Scholar] [CrossRef]








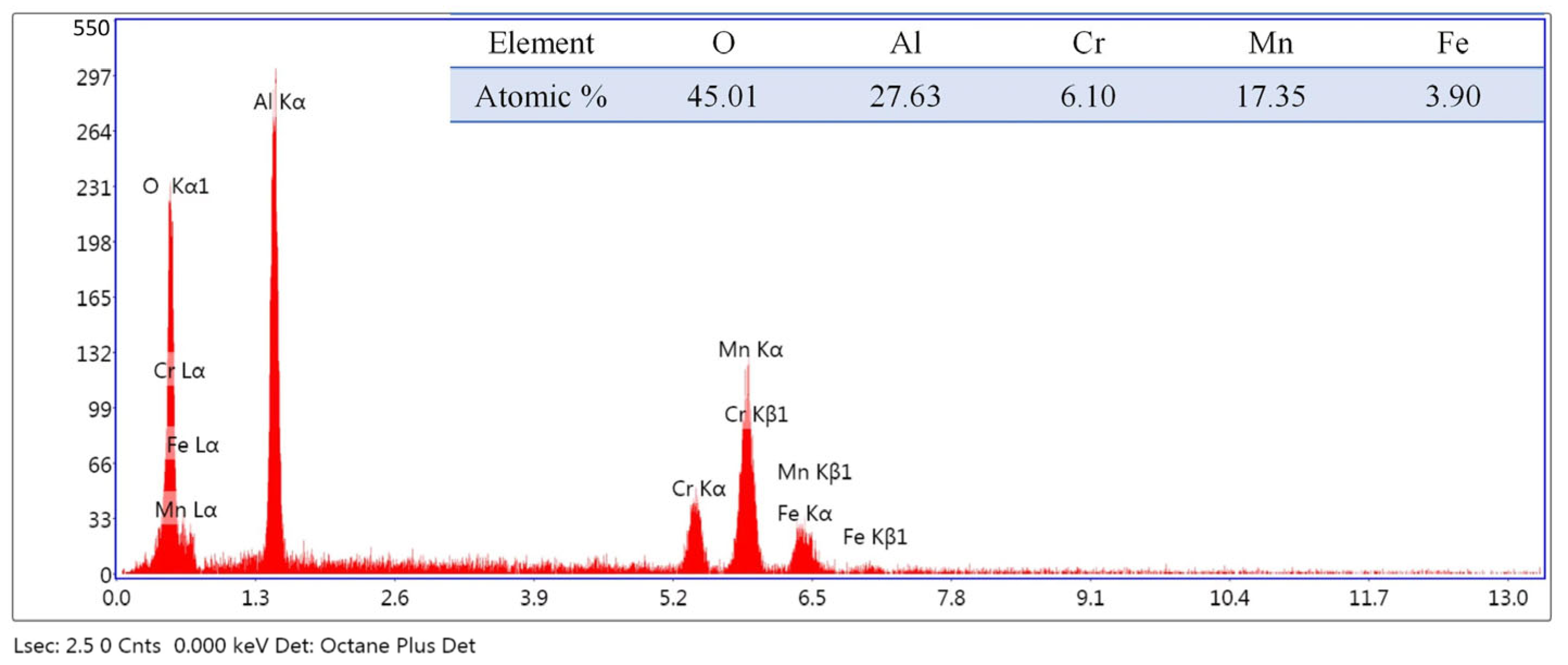


| C | Cr | Mn | N | Mo | P | S | Si | Nb |
|---|---|---|---|---|---|---|---|---|
| 0.02 | 21.1 | 16.8 | 0.83 | 2.34 | ≤0.012 | ≤0.05 | 0.48 | 0.14 |
| No. | Thyristor Angle | CAM Angle | Forging Force |
|---|---|---|---|
| 1 | 25° | 100° | 0.4 Mpa |
| 2 | 35° | 100° | 0.4 Mpa |
| 3 | 45° | 100° | 0.4 Mpa |
| 4 | 55° | 100° | 0.4 Mpa |
| Temperature/°C | Thermal Conductivity/[W/(m·°C)] | Specific Heat/[J/(kg·°C)] |
|---|---|---|
| 20 | 14.1 | 450 |
| 200 | 16.4 | 520 |
| 400 | 18.9 | 560 |
| 800 | 21.5 | 610 |
| 1000 | 27.2 | 640 |
| 1400 | 33.6 | 700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, T.; Ji, Q.; Yang, H.; Zhao, H. Study on the Microstructure and Properties of Flash-Butt Welding Joints of High Nitrogen Steel. Metals 2023, 13, 1200. https://doi.org/10.3390/met13071200
Zhao Y, Liu T, Ji Q, Yang H, Zhao H. Study on the Microstructure and Properties of Flash-Butt Welding Joints of High Nitrogen Steel. Metals. 2023; 13(7):1200. https://doi.org/10.3390/met13071200
Chicago/Turabian StyleZhao, Yixuan, Tongshun Liu, Qingjie Ji, Haifeng Yang, and Hongyun Zhao. 2023. "Study on the Microstructure and Properties of Flash-Butt Welding Joints of High Nitrogen Steel" Metals 13, no. 7: 1200. https://doi.org/10.3390/met13071200
APA StyleZhao, Y., Liu, T., Ji, Q., Yang, H., & Zhao, H. (2023). Study on the Microstructure and Properties of Flash-Butt Welding Joints of High Nitrogen Steel. Metals, 13(7), 1200. https://doi.org/10.3390/met13071200






