Analysis of Phase Transformations in Fe-Ni-Al Alloys Using Diffusion Couples of Fe/Fe-33at.%Ni-33at.%Al Alloy/Ni
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Method
2.2. Experimental Method
3. Results and Discussion
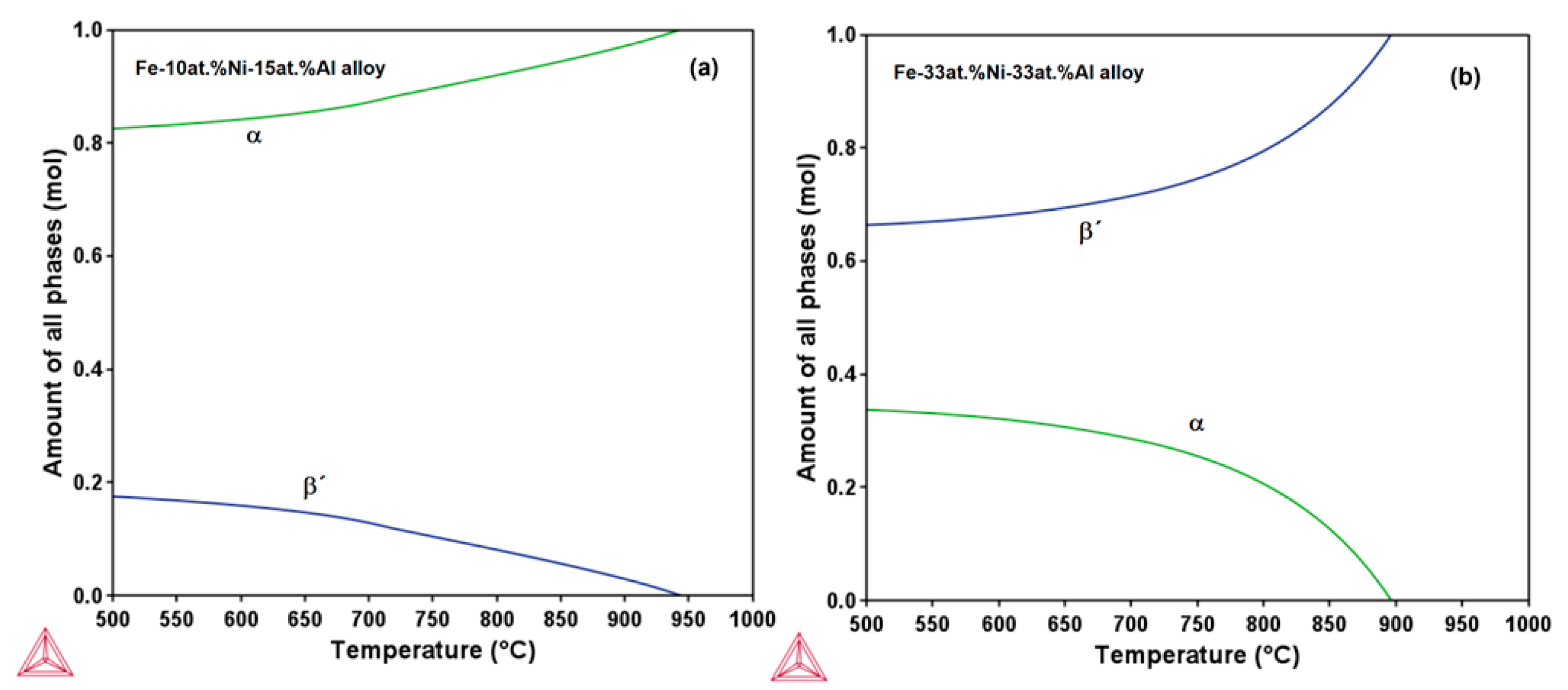
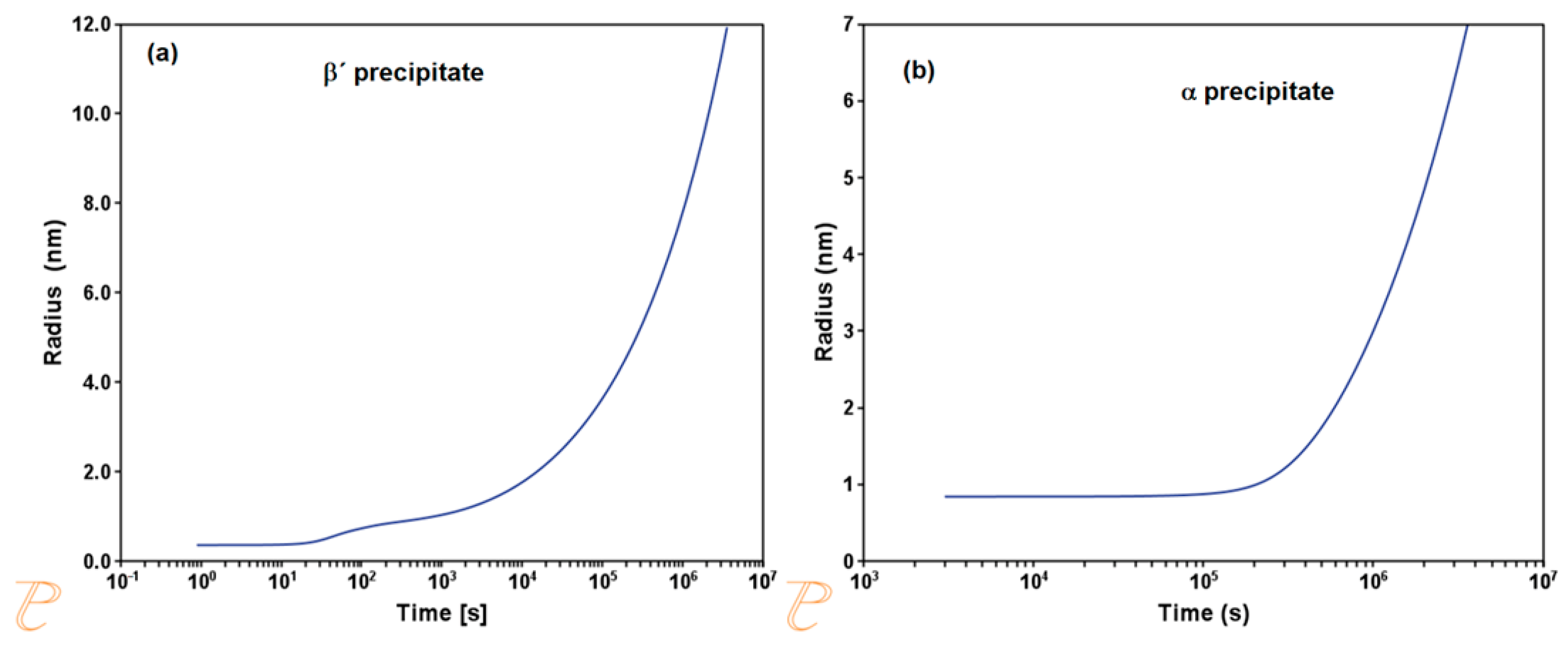
3.1. Phase Stability and TTT Diagram
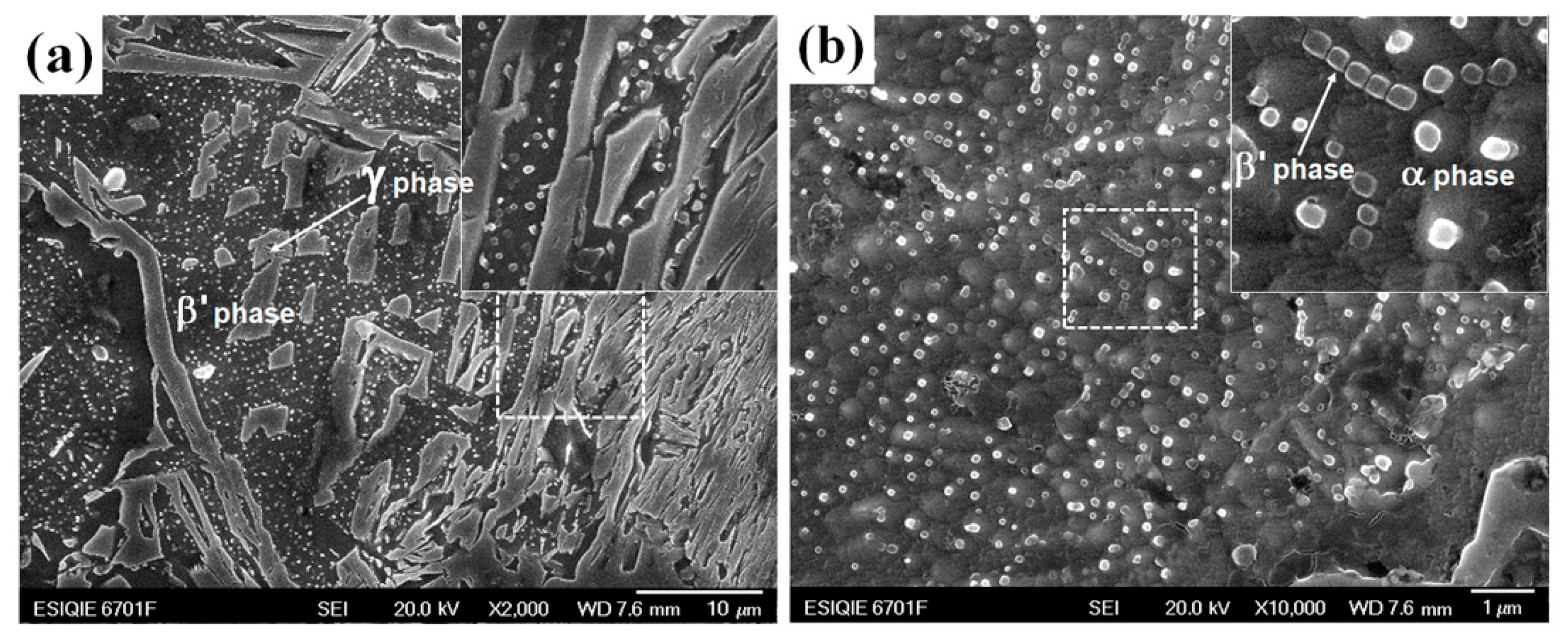
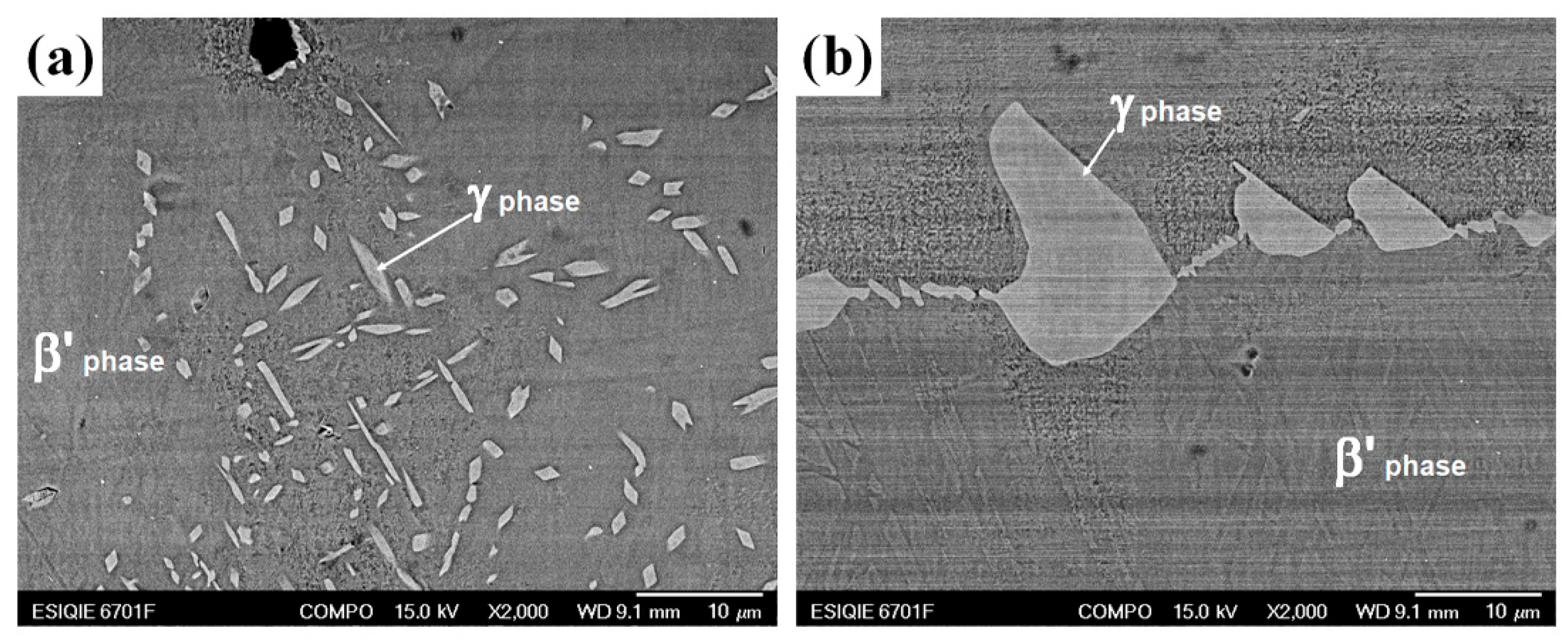
3.2. Microstructure Evolution of Aged Diffusion Couples
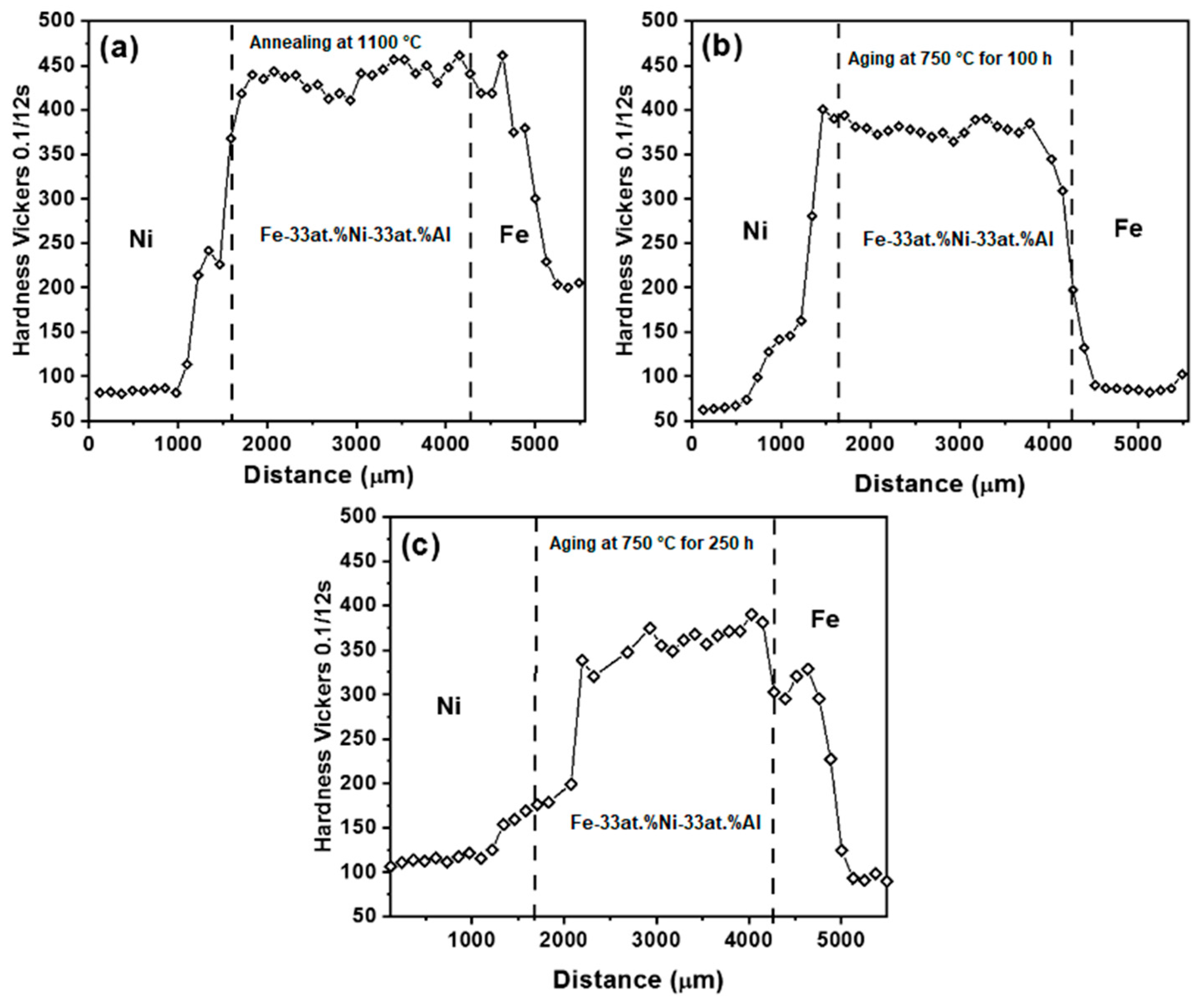
3.3. Effect of Microstructure on Hardness
3.4. Normal Precipitation versus Inverse Precipitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Prasath Babu, R.; Slater, T.J.; Bai, M.; Mitchell, R.; Ciuca, O.; Preuss, M.; Haigh, S.J. An investigation of diffusion-mediated cyclic coarsening and reversal coarsening in an advanced Ni-based superalloy. Acta Mater. 2016, 110, 295–305. [Google Scholar] [CrossRef]
- Liu, D.Y.; Zhao, M.J.; Tao, N.R. Precipitation and its hardening behavior in an austenitic steel with different strain-induced structures. Scr. Mater. 2023, 230, 115396. [Google Scholar] [CrossRef]
- Huang, Y.S.; Wang, X.G.; Cui, C.Y.; Li, J.G.; Ye, L.H.; Hou, G.C.; Yang, Y.H.; Liu, J.L.; Liu, J.G.; Zhou, Y.Z.; et al. The effect of coarsening of γ′ precipitate on creep properties of Ni-based single crystal superalloys during long-term aging. Mater. Sci. Eng. A 2020, 773, 138886. [Google Scholar] [CrossRef]
- Hardy, M.C.; Detrois, M.; McDevitt, E.T.; Argyrakis, C.; Saraf, V.; Jablonski, P.D.; Hawk, J.A.; Buckingham, R.C.; Kitaguchi, H.S.; Tin, S. Solving recent challenges for wrought Ni-based superalloys. Metall. Mater. Trans. A 2020, 51, 2626–2650. [Google Scholar] [CrossRef]
- Degeiter, M.; Le Bouar, Y.; Appolaire, B.; Perrut, M.; Finel, A. Instabilities in the periodic arrangement of elastically interacting precipitates in nickel-based superalloys. Acta Mater. 2020, 187, 41–50. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Qin, H.L.; Bi, Z.N.; Li, J.; Paul, S.; Lee, T.L.; Zhang, S.Y.; Zhang, J.; Dong, H.B. Evolution of lattice spacing of gamma double prime precipitates during aging of polycrystalline Ni-based superalloys: An in situ investigation. Metall. Mater. Trans. A 2020, 51, 574–585. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, Y.; Liu, Y.; Quan, F.; Han, J.; Yang, S.; Chen, X.; He, S.; Gorbatov, O.I.; Chen, X.; et al. Cr-promoted formation of B2+L21 composite nanoprecipitates and enhanced mechanical properties in ferritic alloy. Acta Mater. 2023, 243, 118506. [Google Scholar] [CrossRef]
- Sahoo, B.K.; Srivastava, V.C.; Chandan, K.A.; Chowdhury, S.G. Enhancing the properties of Al–Ni added medium Mn steel by tailoring B2–NiAl precipitates through aging treatment. Mater. Sci. Eng. A 2022, 837, 142757. [Google Scholar] [CrossRef]
- Muñoz-Morris, M.A.; Morris, D.G. Microstructure and mechanical behavior of a Fe-Ni-Al alloy. Mater. Sci. Eng. A 2007, 444, 236–241. [Google Scholar] [CrossRef]
- Stallybrass, C.; Schneide, A.; Sauthoff, G. The strengthening effect of (Ni,Fe)Al precipitates on the mechanical properties at high temperatures of ferritic Fe–Al–Ni–Cr alloys. Intermetallics 2005, 13, 1263–1268. [Google Scholar] [CrossRef]
- Stallybrass, C.; Sauthoff, G. Ferritic Fe–Al–Ni–Cr alloys with coherent precipitates for high-temperature applications. Mater. Sci. Eng. A 2004, 387, 985–990. [Google Scholar] [CrossRef]
- Vo, N.Q.; Liebscher, C.H.; Rawlings, M.J.S.; Asta, M.; Dunand, D.C. Creep properties and microstructure of a precipitation-strengthened ferritic Fe-Al-Ni-Cr alloy. Acta Mater. 2014, 71, 89–99. [Google Scholar] [CrossRef]
- Teng, Z.K.; Liu, C.T.; Ghosh, G.; Liaw, P.K.; Fine, M.E. Effects of Al on the microstructure and ductility of NiAl-strengthened ferritic steels at room temperature. Intermetallics 2010, 18, 1437–1443. [Google Scholar] [CrossRef]
- Sun, L.; Simm, T.H.; Martin, T.L.; McAdam, S.; Galvin, D.R.; Perkins, K.M.; Bagot, P.A.J.; Moody, M.P.; Ooi, S.W.; Hill, P.; et al. A novel ultra-high strength maraging steel with balanced ductility and creep resistance achieved by nanoscale β-NiAl and Laves phase precipitates. Acta Mater. 2018, 149, 285–301. [Google Scholar] [CrossRef]
- Yong, L.; Zou, Y.; Peng, H.; Chen, J.; Fan, Q.; Yang, Q.; Yan, J.; Huang, S.; Wen, Y. Precipitation behavior of coherent nano-ordered particles in Fe-Mn-Al-Ni shape memory alloys with different Ni contents. Mater. Charact. 2022, 188, 111912. [Google Scholar] [CrossRef]
- Ann, Y.F.; Chen, X.P.; Mei, L.; Ren, P.; Wei, D.; Cao, W.Q. Precipitation transformation pathway and mechanical behavior of nanoprecipitation strengthened Fe-Mn-Al-C-Ni austenitic low-density steel. J. Mater. Sci. 2023; in press. [Google Scholar]
- Baik, S.I.; Rawlings, M.J.S.; Dunand, D.C. Effect of aging on coarsening and creep resistance of a Ti-modified Fe-Ni-Al-Cr-Mo ferritic steel with L21/B2 composite precipitates. Mater. Sci. Eng. A 2020, 776, 138987. [Google Scholar] [CrossRef]
- Song, G.; Hong, S.J.; Song, S.H.; Hong, S.H.; Kim, K.B.; Liaw, P.K. Optimization of B2/L21 hierarchical precipitate structure to improve creep resistance of a ferritic Fe-Ni-Al-Cr-Ti superalloy via thermal treatments. Scr. Mater. 2019, 161, 18–22. [Google Scholar] [CrossRef]
- Basuki, E.A.; Nababan, D.C.; Muhammad, F.; Korda, A.A.; Prajitno, D.H. Isother mal Oxidation Behaviour of 69.5Fe-14Ni-9Al-7.5Cr Alloy at High Temperatures. Int. J. Corros. 2019, 2019, 8517648. [Google Scholar] [CrossRef]
- Rosales-Dorantes, H.J.; Cayetano-Castro, N.; Lopez-Hirata, V.M.; Saucedo-Muñoz, M.L.; Villegas-Cardenas, J.D.; Hernández-Santiago, F. Coarsening process of coherent β′ precipitates in Fe–10 wt-%Ni–15 wt-%Al and Fe–10 wt-%Ni–15 wt-%Al–1 wt-%Cu alloys. Mater. Sci. Technol. 2013, 29, 1492–1498. [Google Scholar] [CrossRef]
- Ferreira-Palma, C.; Contreras-Piedras, E.; Cayetano-Castro, N.; Saucedo-Muñoz, M.L.; Lopez-Hirata, V.M.; Gonzalez-Velazquez, J.L.; Dorantes-Rosales, H.J. Effect of temperature and composition on NiAl precipitation and morphology in Fe-Ni-Al alloys. Metall. Mater. Trans. A 2017, 48, 5285–5293. [Google Scholar] [CrossRef]
- Dorantes-Rosales, H.J.; Cayetano-Castro, N.; Cruz-Rivera, J.J.; López-Hirata, V.M.; González-Velázquez, J.L.; Moreno-Palmerín, J. Cinética de engrosamiento de precipitados coherentes en aleaciones base hierro. Rev. Latinoam. Metal. Mater. 2009, 2, 637–645. [Google Scholar]
- Zhou, X.; Dong, H.; Wang, Y.; Yuan, M. Microstructure characteristics and mechanical performance of Fe-Cr-Ni-Al-Ti superalloy fabricated by powder metallurgy. J. Alloys Compd. 2022, 918, 165612. [Google Scholar] [CrossRef]
- Xing, Z.; Pang, J.; Zhang, H.; Ji, Y.; Zhu, Z.; Wang, A.; Zhang, L.; Li, H.; Fu, H.; Zhang, H. Optimizing the microstructure and mechanical performance of Fe-Ni-Cr-Al high entropy alloys via Ti addition. J. Alloys Compd. 2020, 943, 169149. [Google Scholar] [CrossRef]
- Song, G.; Sun, Z.; Poplawsky, J.D.; Gao, Y.; Liaw, P.K. Microstructure evolution of single Ni2TiAl or hierarchical NiAl/Ni2TiAl precipitates in Fe-Ni-Al-Cr-Ti ferritic alloys during thermal treatment for elevated-temperature applications. Acta Mater. 2017, 127, 1–16. [Google Scholar] [CrossRef]
- Baldan, A. Progress in Ostwald ripening theories and their applications to nickel-base superalloys. J. Mater. Sci. 2002, 37, 2171–2202. [Google Scholar] [CrossRef]
- Shen, Q.; Xiong, X.; Li, T.; Cheng, Y.; Liu, W. Effect of co-addition of Ni and Al on precipitation evolution and mechanical properties of Fe-Cu alloy. Mater. Sci. Eng. A 2018, 723, 279–286. [Google Scholar] [CrossRef]
- Baik, S.I.; Rawlings, M.J.S.; Dunand, D.C. Effect of hafnium micro-addition on precipitate microstructure and creep properties of a Fe-Ni-Al-Cr-Ti ferritic superalloy. Acta Mater. 2018, 153, 126–135. [Google Scholar] [CrossRef]
- Rawlings, M.J.S.; Dunand, D.C. Dislocation dynamics modeling of precipitation strengthening in Fe-Ni-Al-Cr ferritic superalloys. J. Mater. Res. 2017, 32, 4241–4253. [Google Scholar] [CrossRef]
- Zeisl, S.; Landefeld, A.; Van Steenberge, N.; Chang, Y.; Schnitzer, R. The role of alloying elements in NiAl and Ni3Ti strengthened Co-free maraging steels. Mater. Sci. Eng. A 2022, 861, 144313. [Google Scholar] [CrossRef]
- Contreras-Piedras, E.; Dorantes-Rosales, H.J.; Lopez-Hirata, V.M.; Hernandez-Santiago, F.; Gonzalez-Velazquez, J.L.; Lopez-Monroy, F.I. Analysis of precipitation in Fe-rich Fe-Ni-Al alloys by diffusion couples. Mater. Sci. Eng. A 2012, 558, 366–370. [Google Scholar] [CrossRef]
- Dong, K.; Sun, L.; Zhang, Z.; Li, Z.; Li, J.; Liu, L.; Du, K.; Zhang, Y. Effects of V addition on microstructure and pseudoelastic response in Fe-Mn-Al-Ni alloys. Intermetallics 2023, 160, 107954. [Google Scholar] [CrossRef]
- Park, K.; Cho, B.; Hong, S.J.; Lim, K.R.; Lee, C.; Song, G. Outstanding high-temperature strength of novel Fe–Cr–Ni–Al–V ferritic alloys with hierarchical B2–NiAl precipitates. Mater. Sci. Eng. A 2022, 840, 142999. [Google Scholar] [CrossRef]
- Eris, R.; Akdeniz, M.V.; Mekhrabov, A.O. Unveiling the effect of Hf-(Mo,W) addition on the coarsening agglomeration, and dissolution behaviour of γ’-Ni3Al precipitates in model Ni-based superalloys. J. Alloys Compd. 2023, 936, 167869. [Google Scholar] [CrossRef]
- Baik, S.I.; Wang, S.Y.; Liaw, P.K.; Dunand, D.C. Increasing the creep resistance of Fe-Ni-Al-Cr superalloys via Ti additions by optimizing the B2/L21 ratio in composite nano-precipitates. Acta Mater. 2018, 157, 142–154. [Google Scholar] [CrossRef]
- Sohn, Y.H.; Puccio, A.; Dayananda, M.A. Interdiffusion structures and paths for multiphase Fe-Ni-Al diffusion couples at 1100 °C. Metall. Mater. Trans. A 2005, 36, 2361–2370. [Google Scholar] [CrossRef]
- Miyazaki, T.; Koyama, T.; Kobayashi, S. A new characterization method of the microstructure using microscopic gradients in alloys. Metall. Mater. Trans. A 1996, 27, 945–949. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, J.-C. Application of dual-anneal diffusion multiples to the effective study of phase diagrams and phase transformations in the Fe–Cr–Ni system. Acta Mater. 2015, 88, 196–206. [Google Scholar] [CrossRef]
- Shi, P.; Engstrom, A.; Hoglund, L.; Sundman, B.; Agren, J. Thermo-calc and DICTRA enhance materials design and processing. Mater. Sci. For. 2005, 475–479, 3339–3346. [Google Scholar]
- Borgestam, A.; Hoglund, L.; Agren, J.; Engstrorn, A. DICTRA, a tool for simulation of diffusional transformations in alloys. J. Phase Equil. 2000, 21, 269–280. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, G.; Chang, H.; Zhou, L.; Cui, Y. Assessment of diffusion mobility for bcc phase of Ti-Al-Ni ternary system. Calphad 2020, 71, 102203. [Google Scholar] [CrossRef]
- Dictra, Thermo-Calc Software Versión 2023a. Available online: https://thermocalc.com/products/add-on-modules/diffusion-module-dictra/ (accessed on 1 June 2023).
- Thermo-Calc, Thermo-Calc Software version 2023a, TCFE11 and MOBFE6 Steel/Fe Alloys Databases. Available online: https://thermocalc.com/products/databases/steel-and-fe-alloys/ (accessed on 1 June 2023).
- Sundman, B.; Ohnuma, I.; Dupin, N.; Kattner, U.R.; Fries, S.G. An assessment of the entire Al-Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Lindahl, B.B.; Burton, B.P.; Selleby, M. Ordering in ternary BCC alloys applied to the Al-Fe-Mn system. Calphad 2015, 51, 211–219. [Google Scholar] [CrossRef]
- Hu, X.X.; Lu, X.G.; He, Y.L. Assessments of impurity diffusion coefficients of selected pure metals in FCC Fe. Adv. Mater. Res. 2014, 936, 545–551. [Google Scholar] [CrossRef]
- Bokstein, B.S.; Bokstein, S.Z.; Spitsberg, I.T. Ni self-diffusion in alloyed Ni3Al. Intermetallics 1996, 4, 517–523. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.; Steinbach, I.; Chen, Q.; Huang, B. Diffusivities of an Al-Fe-Ni melt and their effects on the microstructure during solidification. Acta Mater. 2010, 10, 3664–3675. [Google Scholar] [CrossRef]
- Engström, A.; Agren, J. Interdiffusion in multiphase, Fe-Cr-Ni diffusion couples. Scand. J. Metall. 1995, 24, 12–20. [Google Scholar]
- Hood, G.M. The diffusion of iron in aluminium. J. Theor. Appl. Phys. 1970, 21, 305–328. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Ma, C.; Lv, M.; Ren, H. Segregation of alloying elements at the bcc-Fe/B2-NiAl interface and the corresponding effects on the interfacial energy. Intermetallics 2021, 131, 107096. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solid Metals and Alloys. In Landolt-Börnstein–Group III Condensed Matter; Springer: Berlin/Heidelberg, Germany, 1990; Volume 26, pp. 288–312. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 4th ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 96–98. [Google Scholar]
- Kostorz, K. Phase Transformations in Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 309–407. [Google Scholar]
- Pavarova, K.B.; Filin, S.A.; Maslenkov, S.B. Phase equilibria in the Ni-Al-Me (Me = Co, Fe, Mn, Cu) system in vicinity of β-phase at 900 and 1100 °C. Russ. Metall. (Engl. Transl.) 1993, 1, 156–169. [Google Scholar]
- Bramfitt, B.L.; Michael, J.R. Microanalysis of phase equilibria in Ni3Al intermetallic alloys containing iron. MRS Online Proc. Libr. 1985, 62, 201–208. [Google Scholar] [CrossRef]
- Looijenga-Vos, A.; Buerger, M.J. International Tables of Crystallography Space-Group Symmetry, 5th ed.; Hahn, T., Ed.; Springer: Dordrecht, The Netherlands, 2005; Volume A, pp. 52–53. [Google Scholar]
- Dubrovinskaia, N.A.; Dubrovinsky, L.S.; Karlsson, A.; Saxena, S.K. Experimental study of thermal expansion and phase transformations in iron-rich Fe-Al alloys. Calphad 1999, 23, 69–84. [Google Scholar] [CrossRef]
- Bitterlich, H.; Löser, W.; Schultz, L. Reassessment of Ni-Al and Ni-Fe-Al solidus temperatures. J. Phase Equilibria Diffus. 2002, 23, 301–304. [Google Scholar] [CrossRef]
- Tan, Y.; Shinoda, T.; Mishima, Y.; Suzuki, T. Stoichiometry splitting of β phase in Ni-Al-Mn, Ni-Al-Co and Ni-Al-Fe ternary systems. Mater. Trans. 2001, 42, 464–470. [Google Scholar] [CrossRef]
- Budberg, P.; Prince, A.; Cacciamani, G.; Ferro, R.; Grushko, B.; Perrot, P.; Schmid-Fetzer, R. Al-Fe-Ni (Aluminium–Iron–Nickel) Light Metal Systems Part 2. In Landolt-Börnstein–Group IV Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 11A2, pp. 345–346. [Google Scholar]
- Belan, J. GCP and TCP phases presented in nickel-base superalloys. Mater. Today 2016, 3, 936–941. [Google Scholar] [CrossRef]
- Alabbad, B.; Li, L.; Tin, S. Controlling the grain boundary morphology and secondary γ’ precipitate size distribution in Ni-based superalloys. J. Alloys Compd. 2019, 775, 931–941. [Google Scholar] [CrossRef]
- Turichin, G.A.; Klimova-Korsmik, O.G.; Valdaytseva, E.A.; Alekseev, A.V.; Rashkovets, M.V. Comparative analysis of the gamma prime phase formation in nickel alloys in additive manufacturing. Procedia CIRP 2020, 94, 320–323. [Google Scholar] [CrossRef]
- Vogel, F.; Ngai, S.; Fricke, K.; McKechnie, M.; Wanderka, N.; Hentrich, T.; Banhart, J.; Thompson, G.B. Tracing the three-dimensional nanochemistry of phase separation in an inverse Ni-based superalloy. Acta Mater. 2018, 157, 326–338. [Google Scholar] [CrossRef]
- Vogel, F.; Wanderka, N.; Balogh, Z.; Ibrahim, M.; Stender, P.; Schmitz, G.; Fedorova, T.; Banhart, J. Evolution of nanoscale clusters in γ’ precipitates of a Ni-Al-Ti model alloy. Ultramicroscopy 2015, 159, 278–284. [Google Scholar] [CrossRef]
- Kumar, A.S.D.; Bhaskar, M.S.; Sarkar, S.; Abinandanan, T.A. Phase field modelling of precipitation coarsening in binary alloys with respect to atomic mobility of solute in the precipitate phase. Trans. Indian Inst. Met. 2020, 73, 1469–1474. [Google Scholar] [CrossRef]




| Phase | at.% Fe | at.% Ni | at.% Al |
|---|---|---|---|
| α | 90.0 | 2.5 | 7.5 |
| β′ | 45.5 | 13.5 | 41.0 |
| γ | 23.4 | 70.0 | 6.6 |
| γ′ | 4.0 | 72.0 | 24.0 |
| Phase | Crystal Structure (Space Group) | Elements |
|---|---|---|
| α | A2 (229) [57,58] | Fe [61] |
| β′ | B2 (221) [57,59,60] | NiAl [60,61] |
| γ | A1 (225) [57,61,62] | Ni [62] |
| γ′ | L12 (221) [57,62,63,64] | Ni3Al [62] |
| Phase | at.% Fe | at.% Ni | at.% Al |
|---|---|---|---|
| α | 93.0 | 2.0 | 5.0 |
| β′ | 36.0 | 16.0 | 48.0 |
| γ | 15.0 | 80.0 | 5.0 |
| γ′ | 19.0 | 66.0 | 15.0 |
| Alloy | Phase | at.% Fe | at.% Ni | at.% Al |
|---|---|---|---|---|
| Fe-10at.%Ni-15at.%Al | α | 89.5 | 1.5 | 9.0 |
| β′ | 9.5 | 48.5 | 42.0 | |
| Fe-33at.%Ni-33at.%Al | α | 88.6 | 1.2 | 10.2 |
| β′ | 8.3 | 48.0 | 43.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Badillo, E.; Dorantes-Rosales, H.J.; Saucedo-Muñoz, M.L.; Lopez-Hirata, V.M. Analysis of Phase Transformations in Fe-Ni-Al Alloys Using Diffusion Couples of Fe/Fe-33at.%Ni-33at.%Al Alloy/Ni. Metals 2023, 13, 1221. https://doi.org/10.3390/met13071221
Perez-Badillo E, Dorantes-Rosales HJ, Saucedo-Muñoz ML, Lopez-Hirata VM. Analysis of Phase Transformations in Fe-Ni-Al Alloys Using Diffusion Couples of Fe/Fe-33at.%Ni-33at.%Al Alloy/Ni. Metals. 2023; 13(7):1221. https://doi.org/10.3390/met13071221
Chicago/Turabian StylePerez-Badillo, Eduardo, Hector J. Dorantes-Rosales, Maribel L. Saucedo-Muñoz, and Victor M. Lopez-Hirata. 2023. "Analysis of Phase Transformations in Fe-Ni-Al Alloys Using Diffusion Couples of Fe/Fe-33at.%Ni-33at.%Al Alloy/Ni" Metals 13, no. 7: 1221. https://doi.org/10.3390/met13071221
APA StylePerez-Badillo, E., Dorantes-Rosales, H. J., Saucedo-Muñoz, M. L., & Lopez-Hirata, V. M. (2023). Analysis of Phase Transformations in Fe-Ni-Al Alloys Using Diffusion Couples of Fe/Fe-33at.%Ni-33at.%Al Alloy/Ni. Metals, 13(7), 1221. https://doi.org/10.3390/met13071221






