Study on High-Temperature Oxidation Behavior of Platinum-Clad Nickel Composite Wire
Abstract
1. Introduction
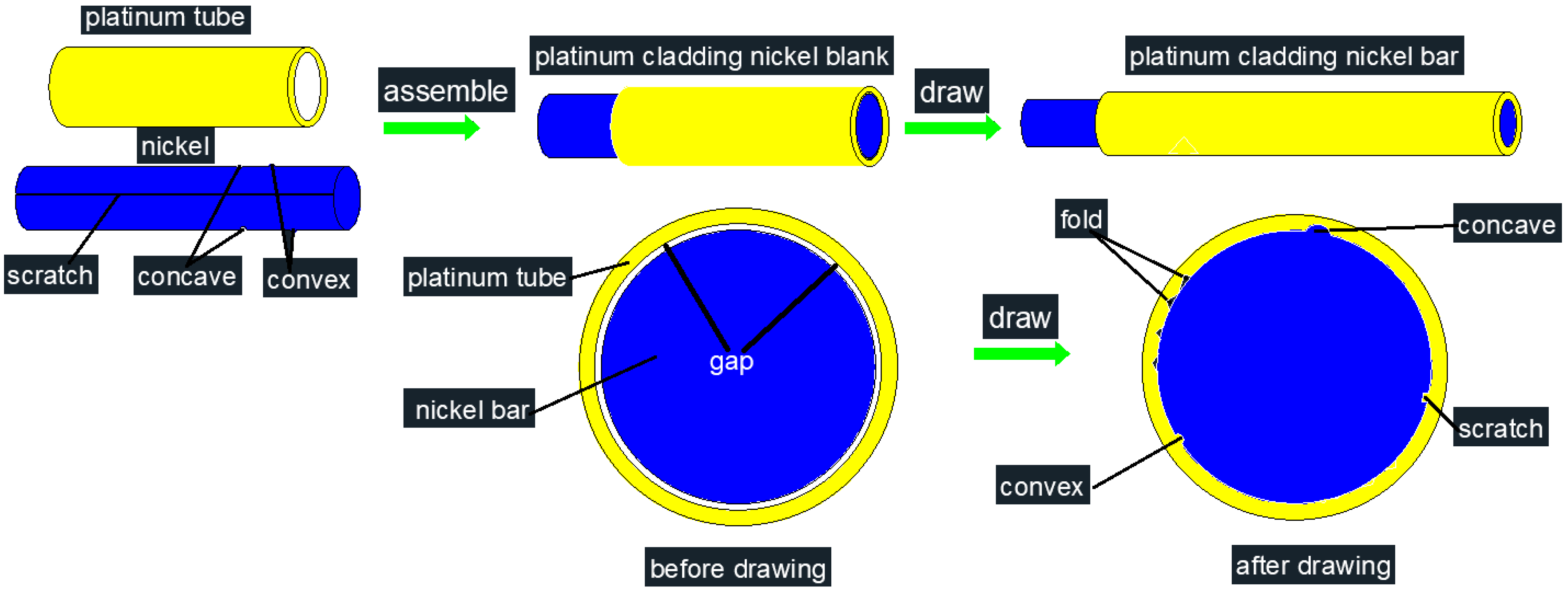
2. Materials and Methods
3. Results
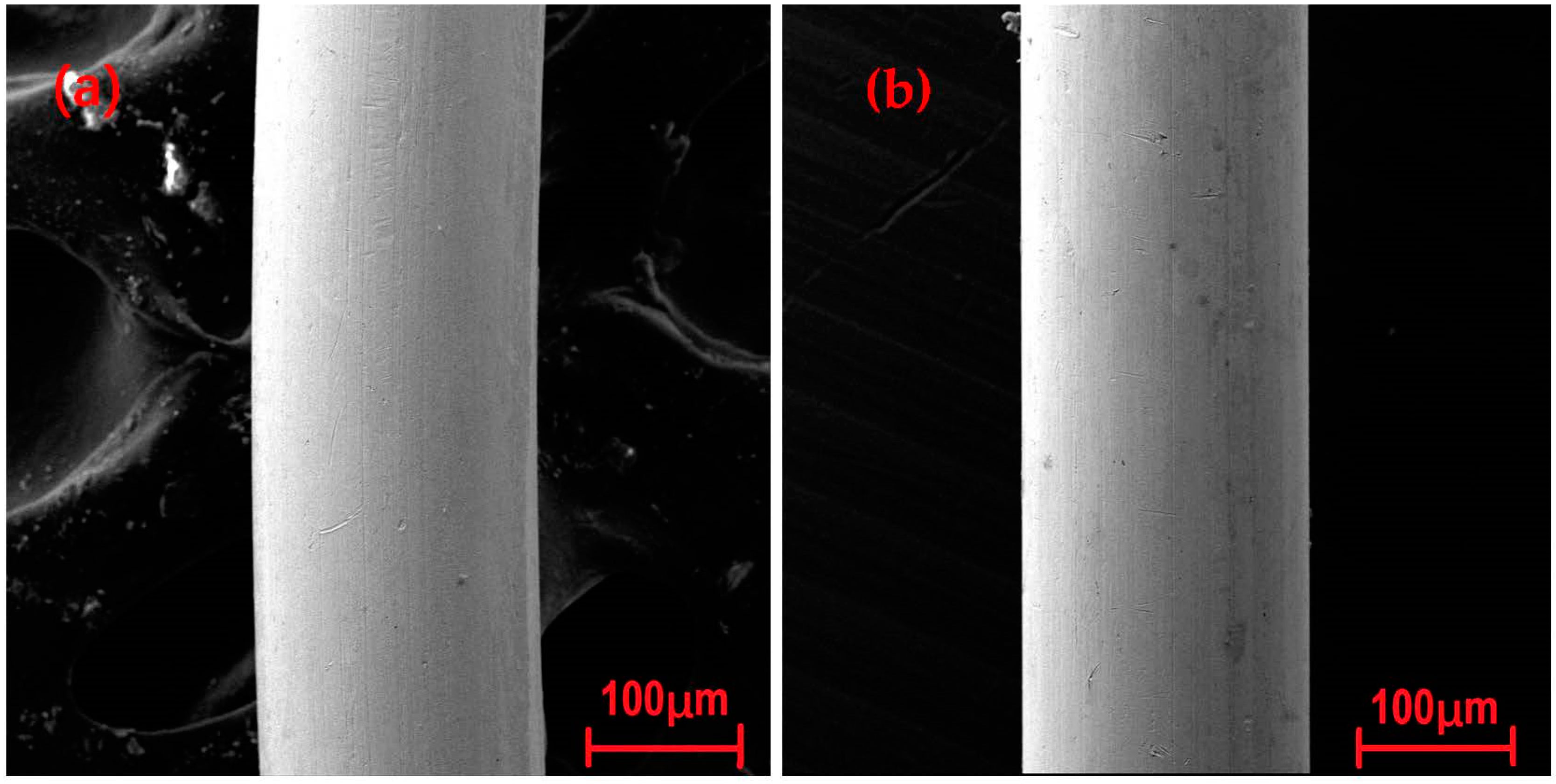
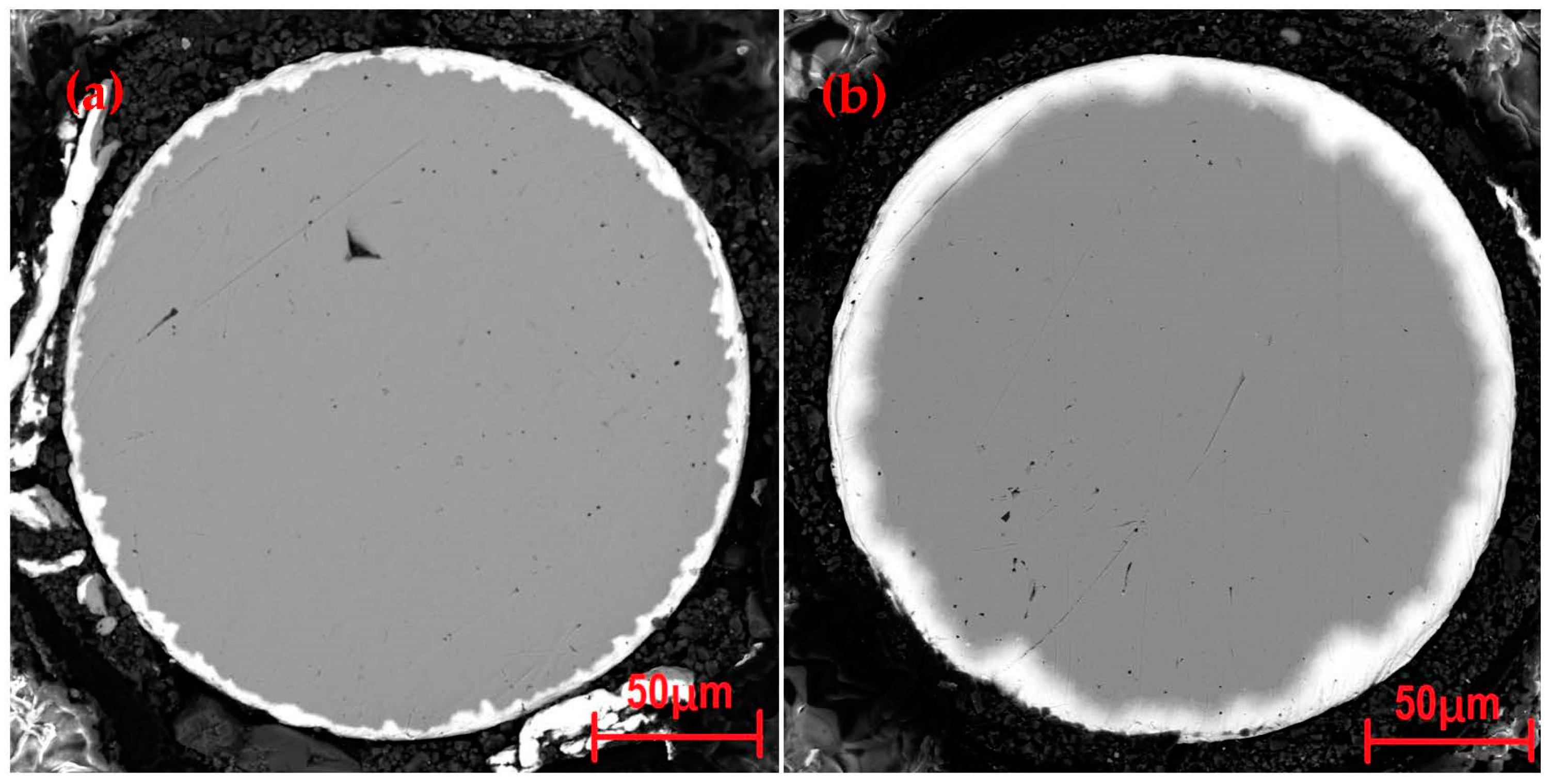
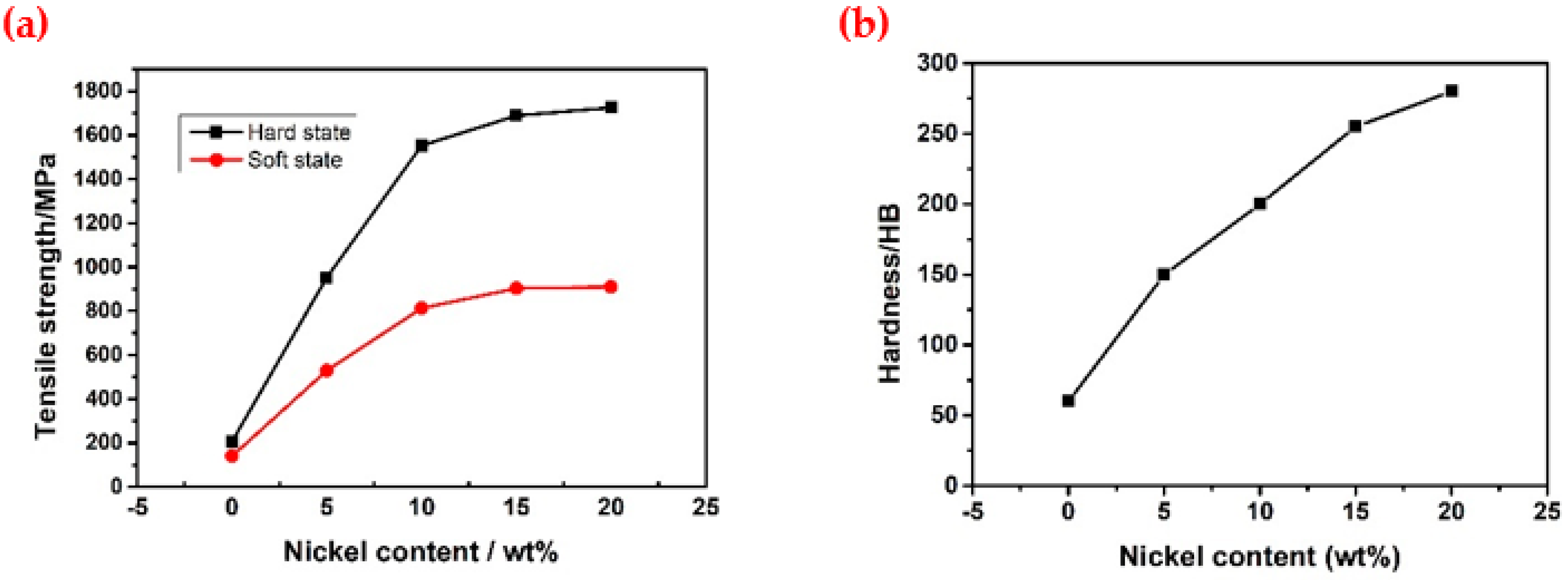
3.1. Raw Sample Analysis
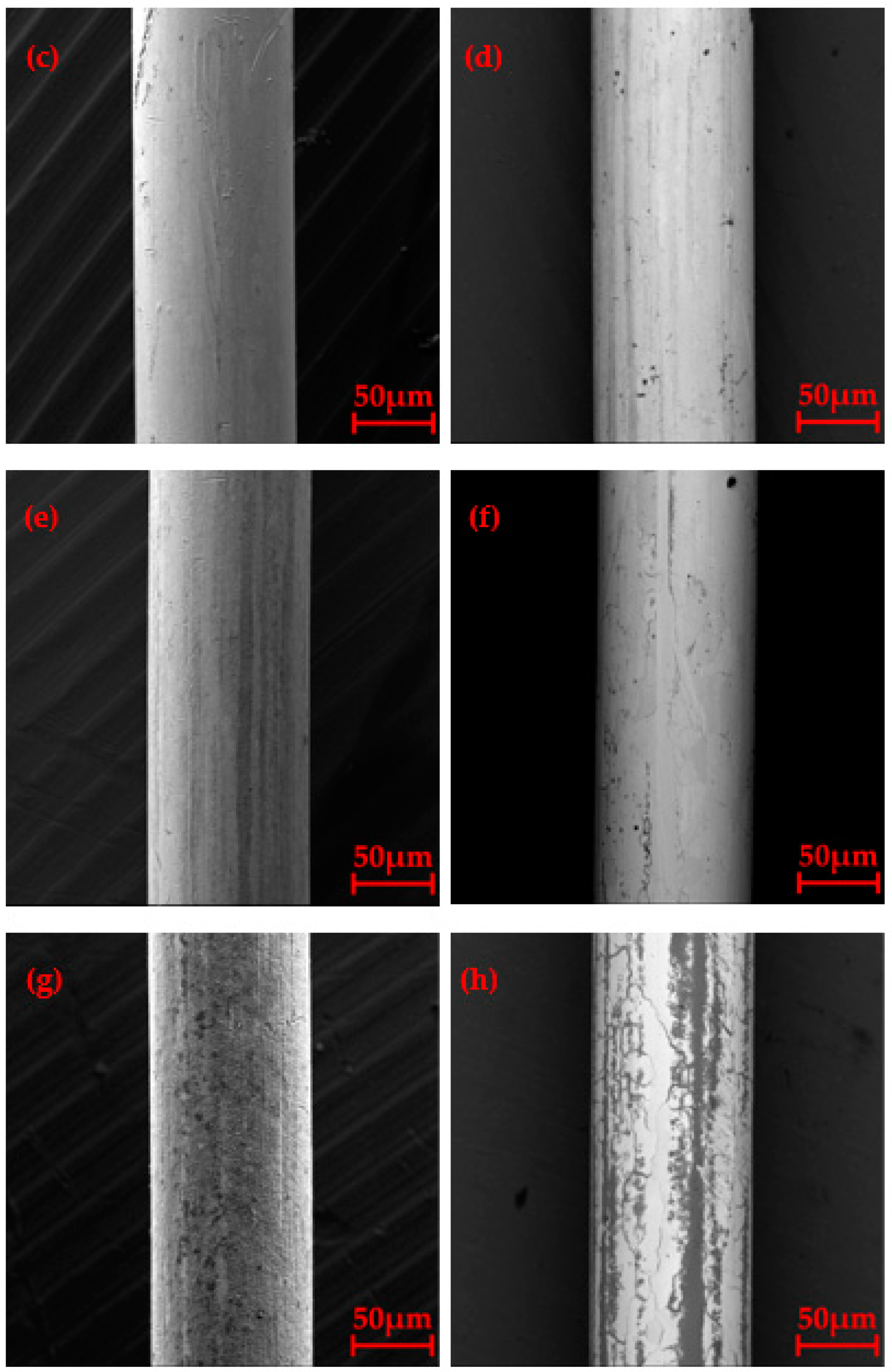
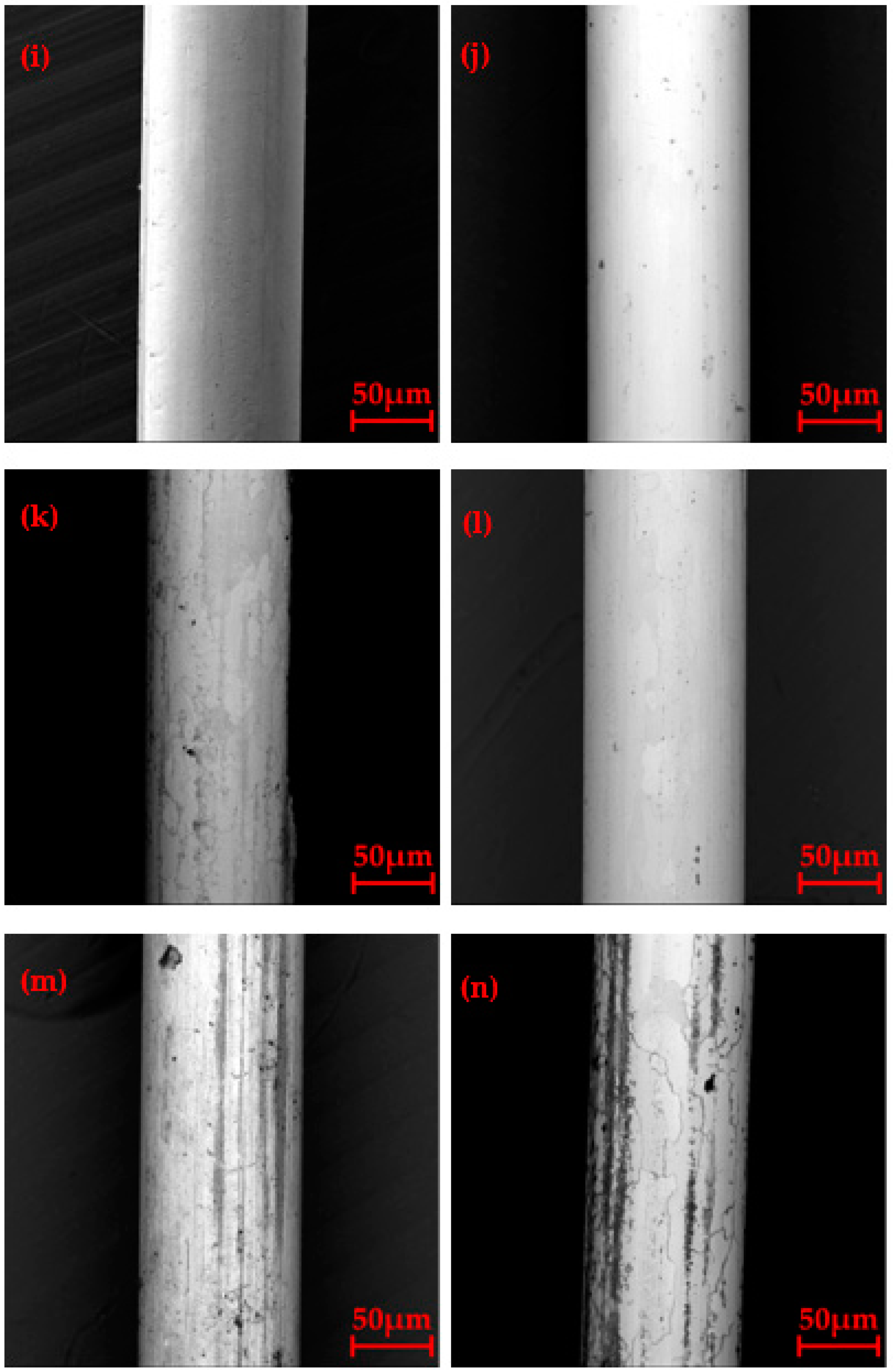
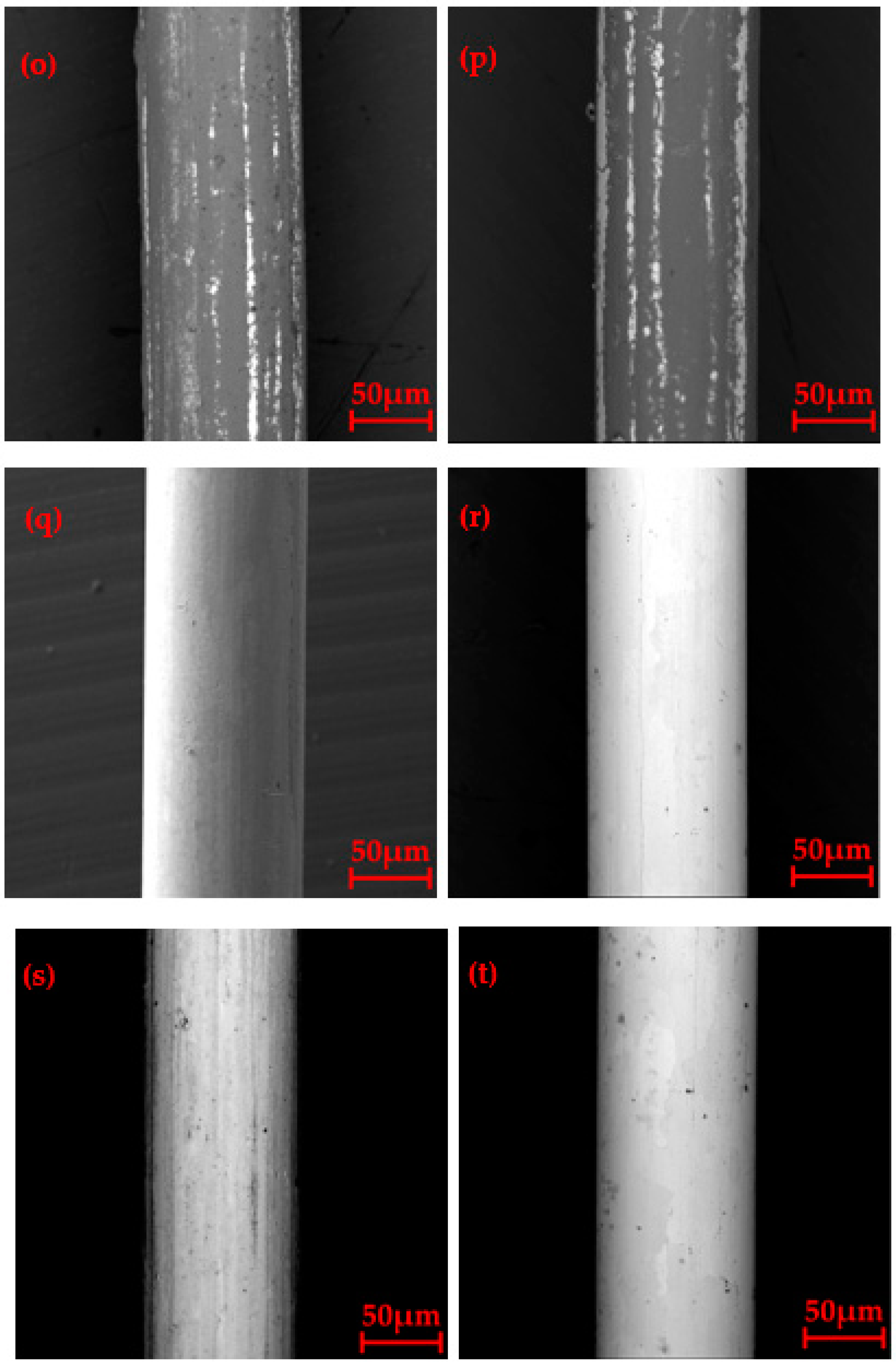
3.2. Surface Morphology after Oxidation
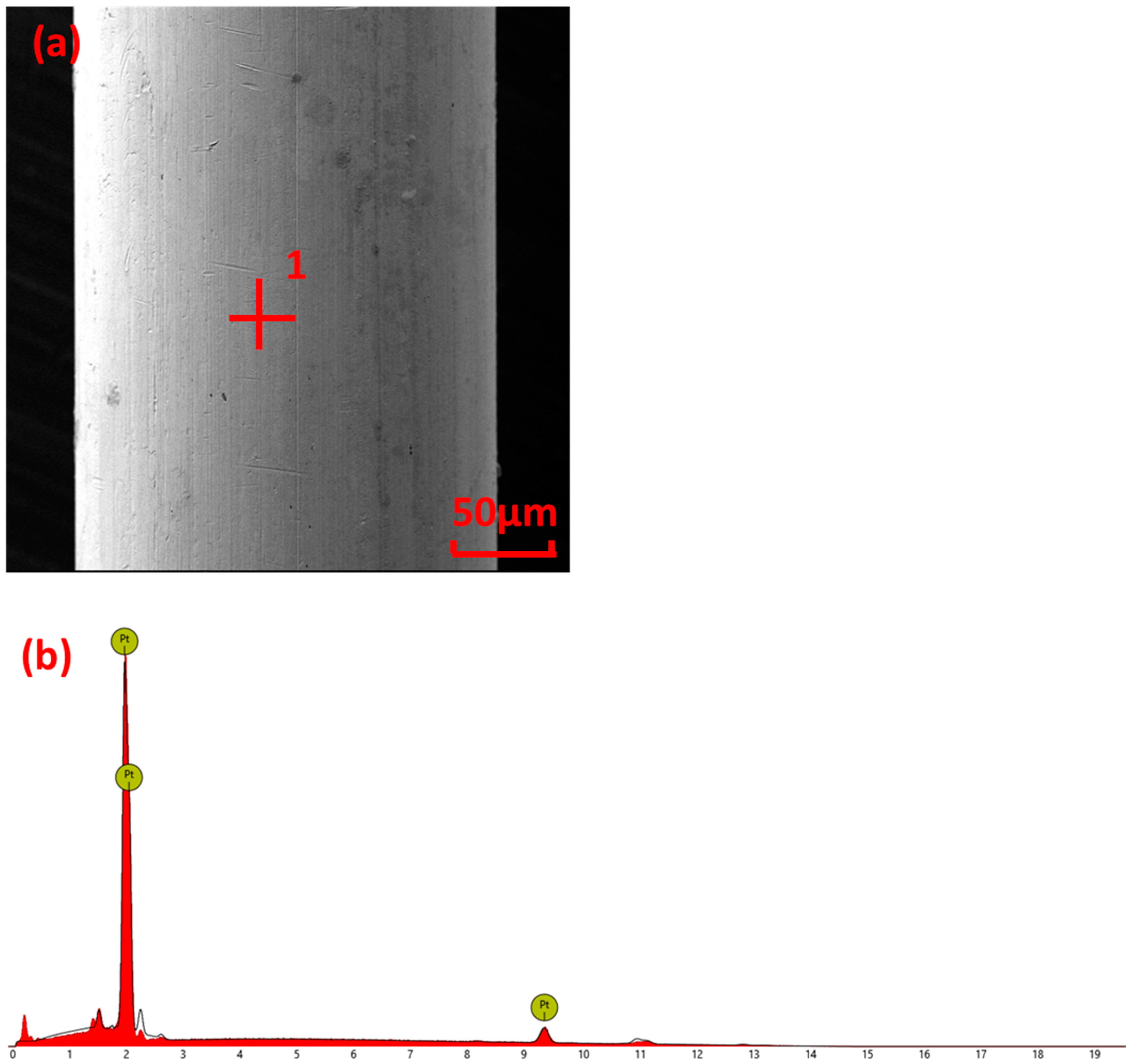
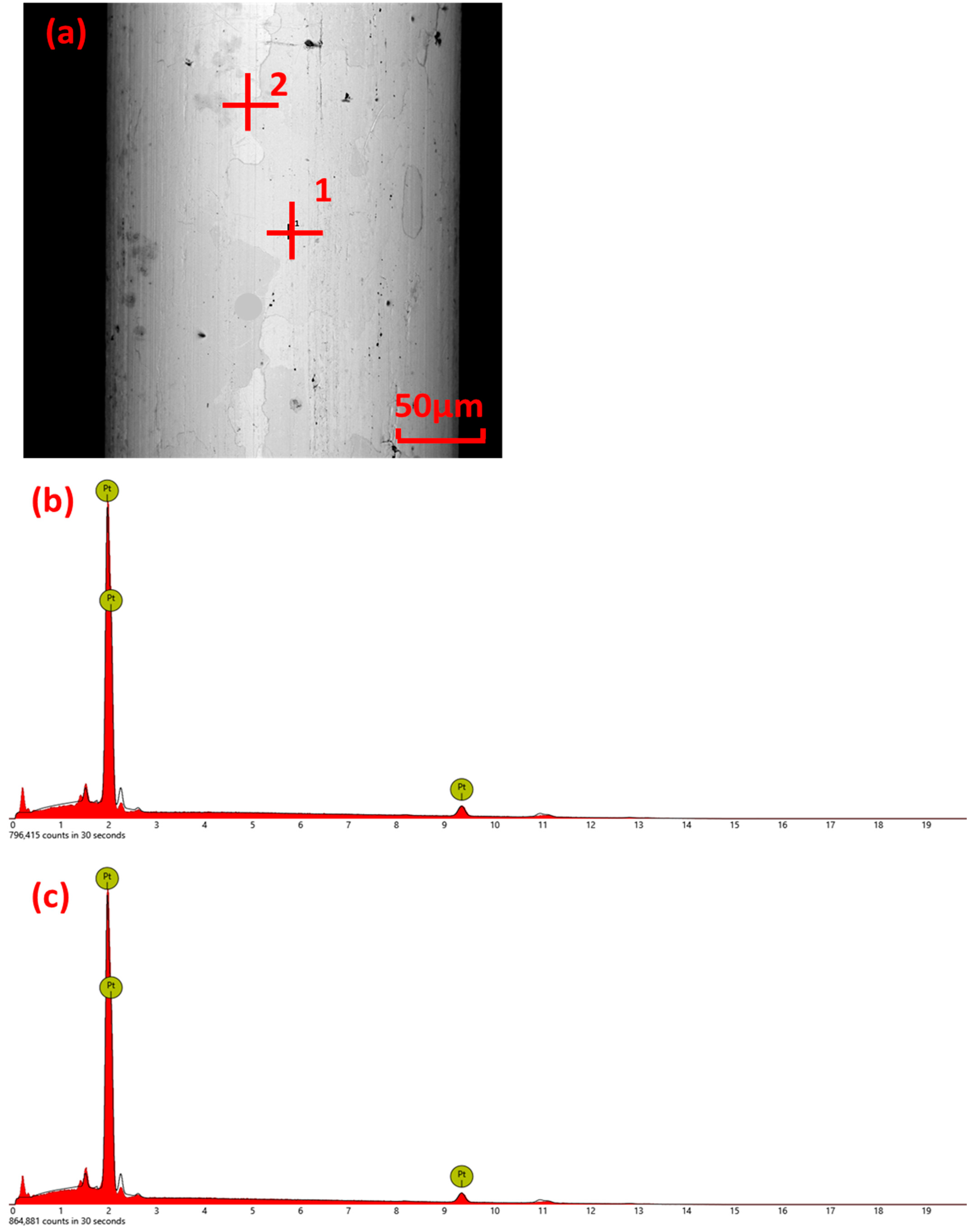
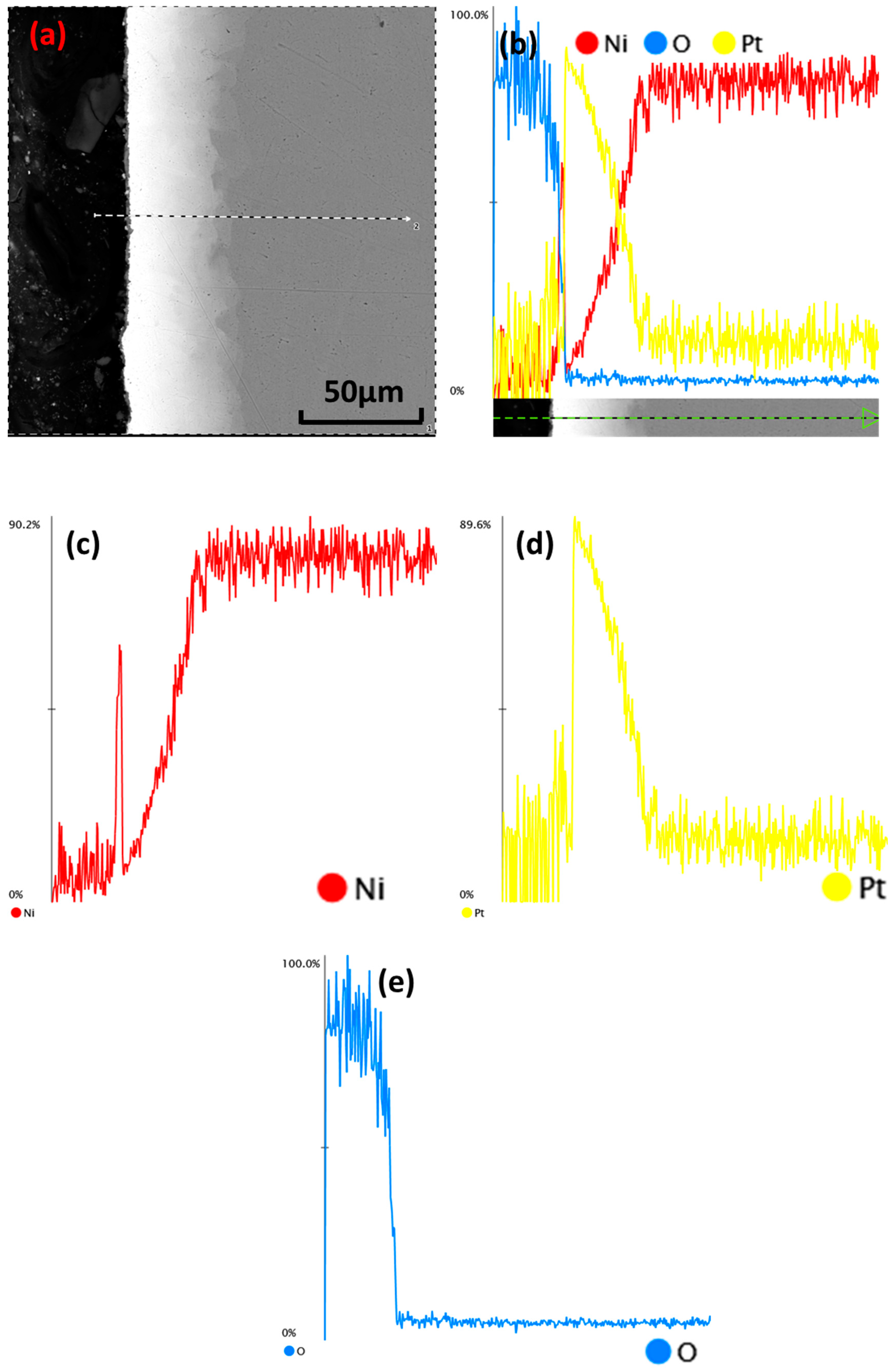
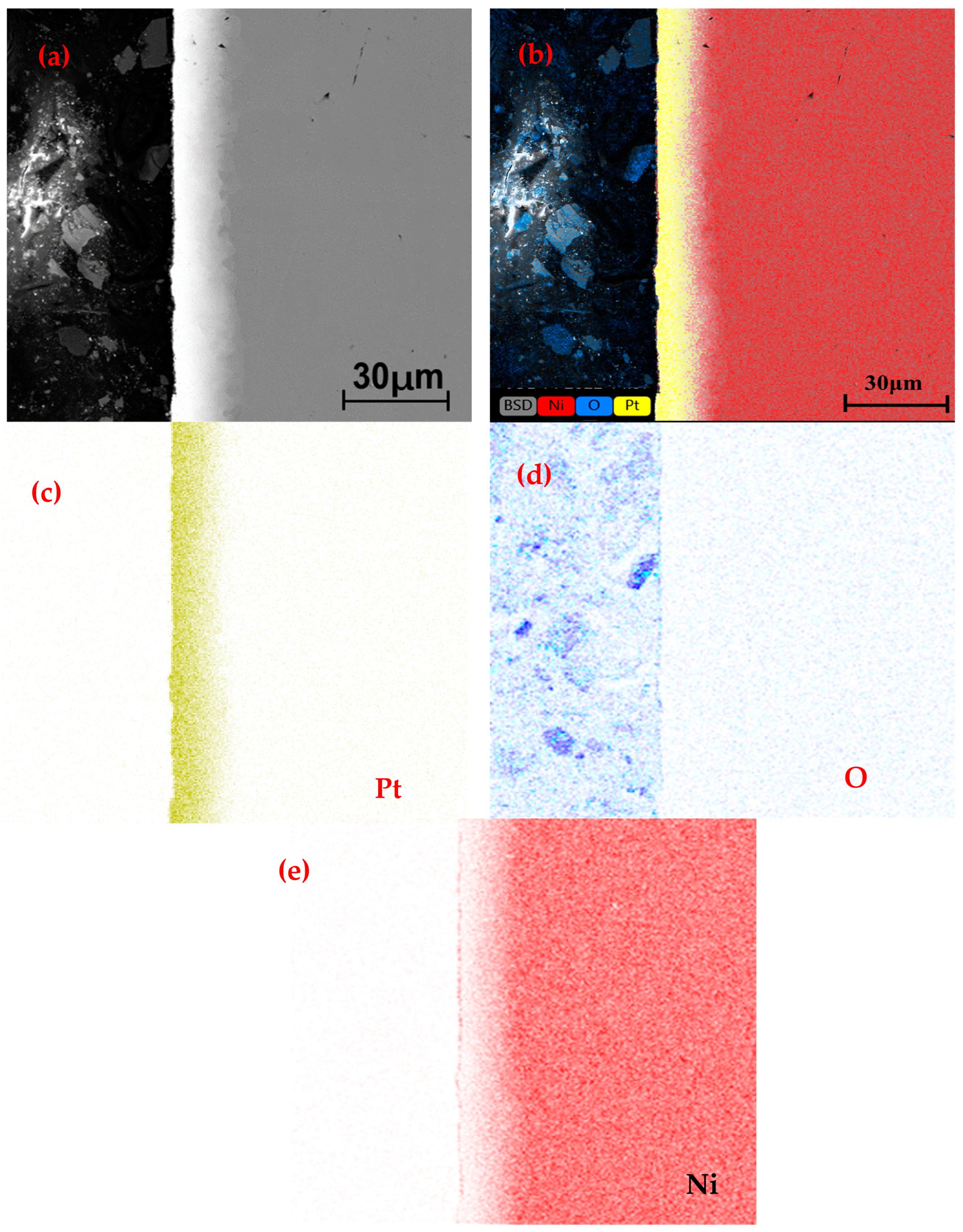
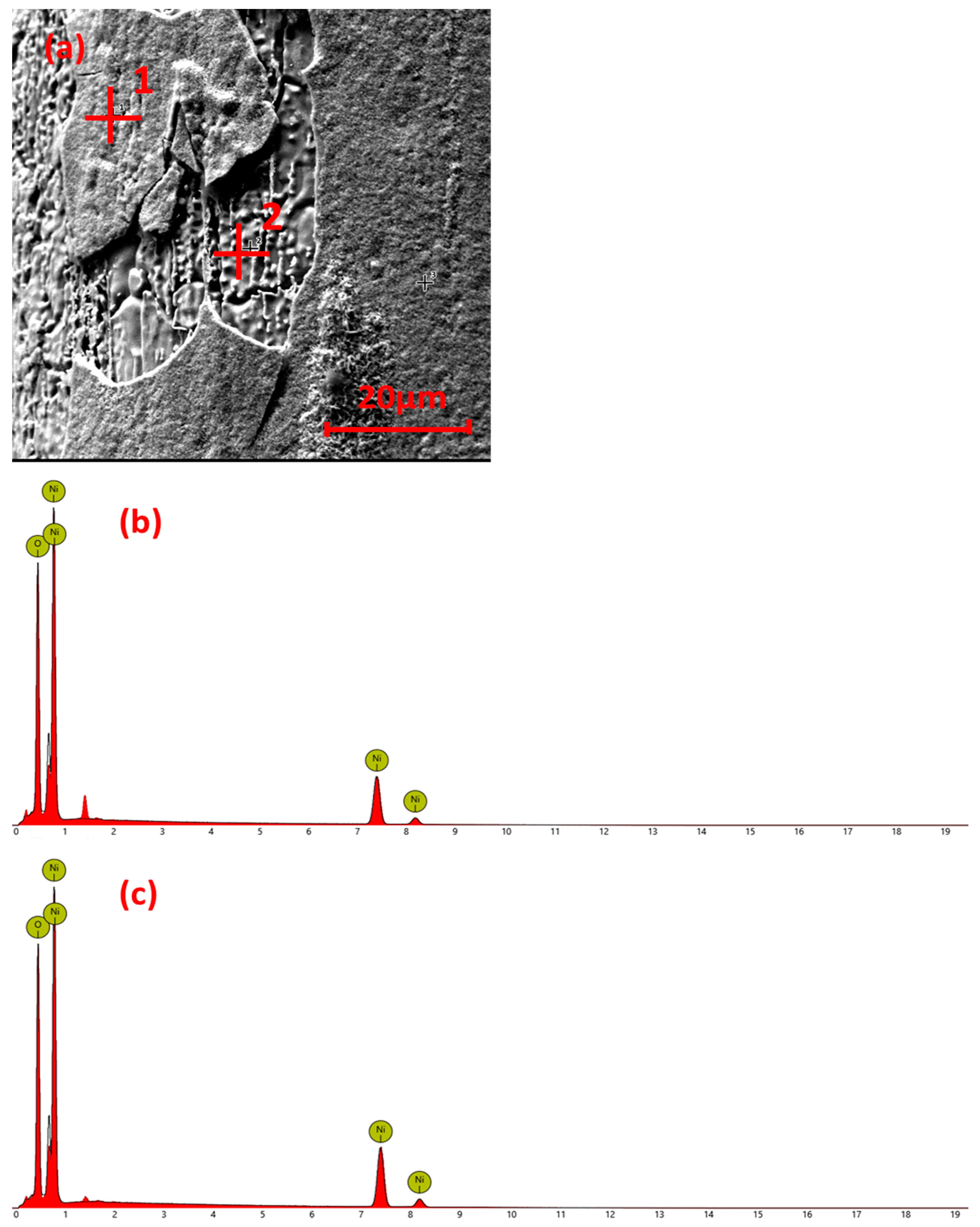
3.3. Surface Element Analysis
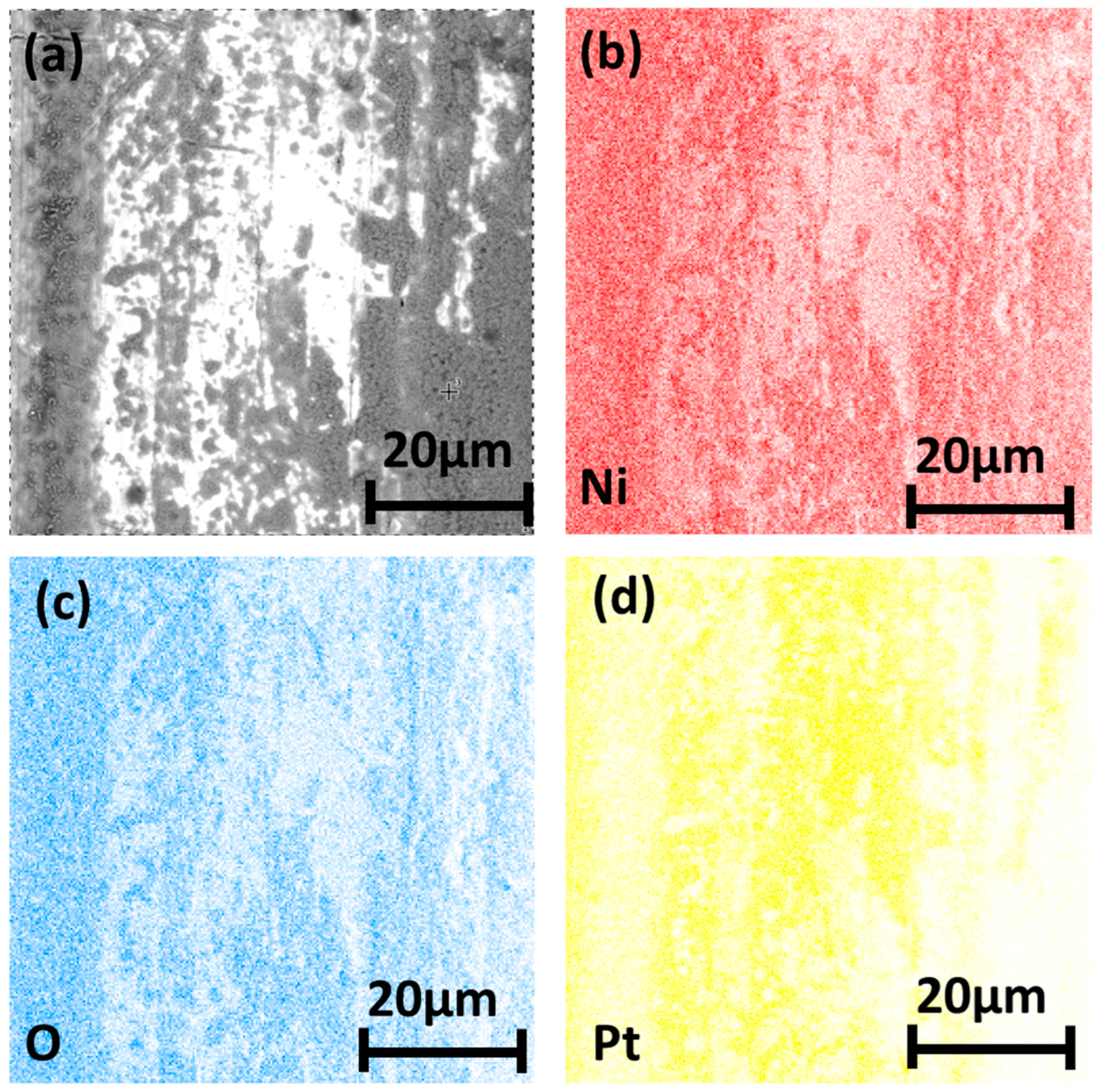
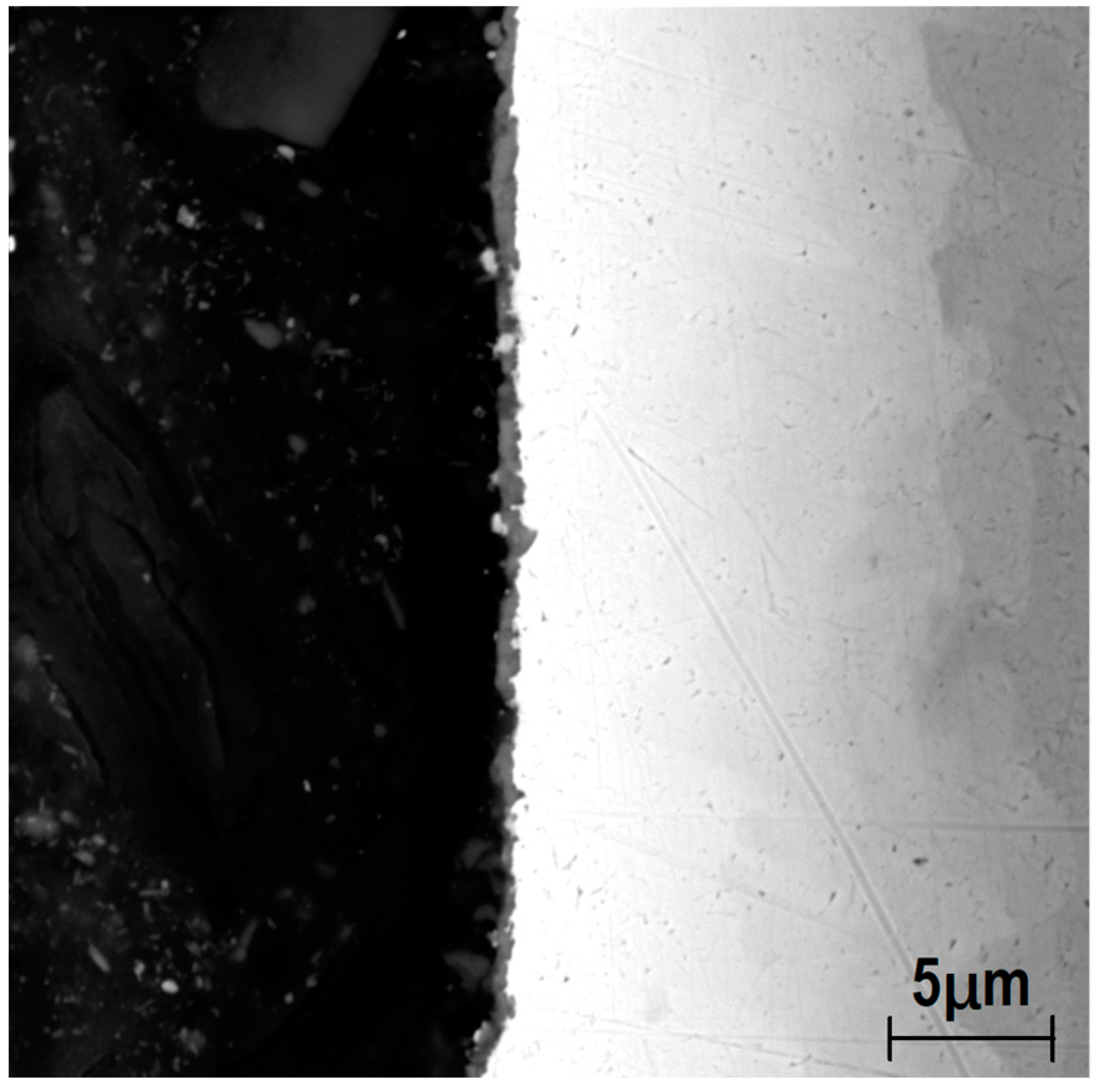
3.4. Microstructure of Longitudinal Sections
4. Discussion
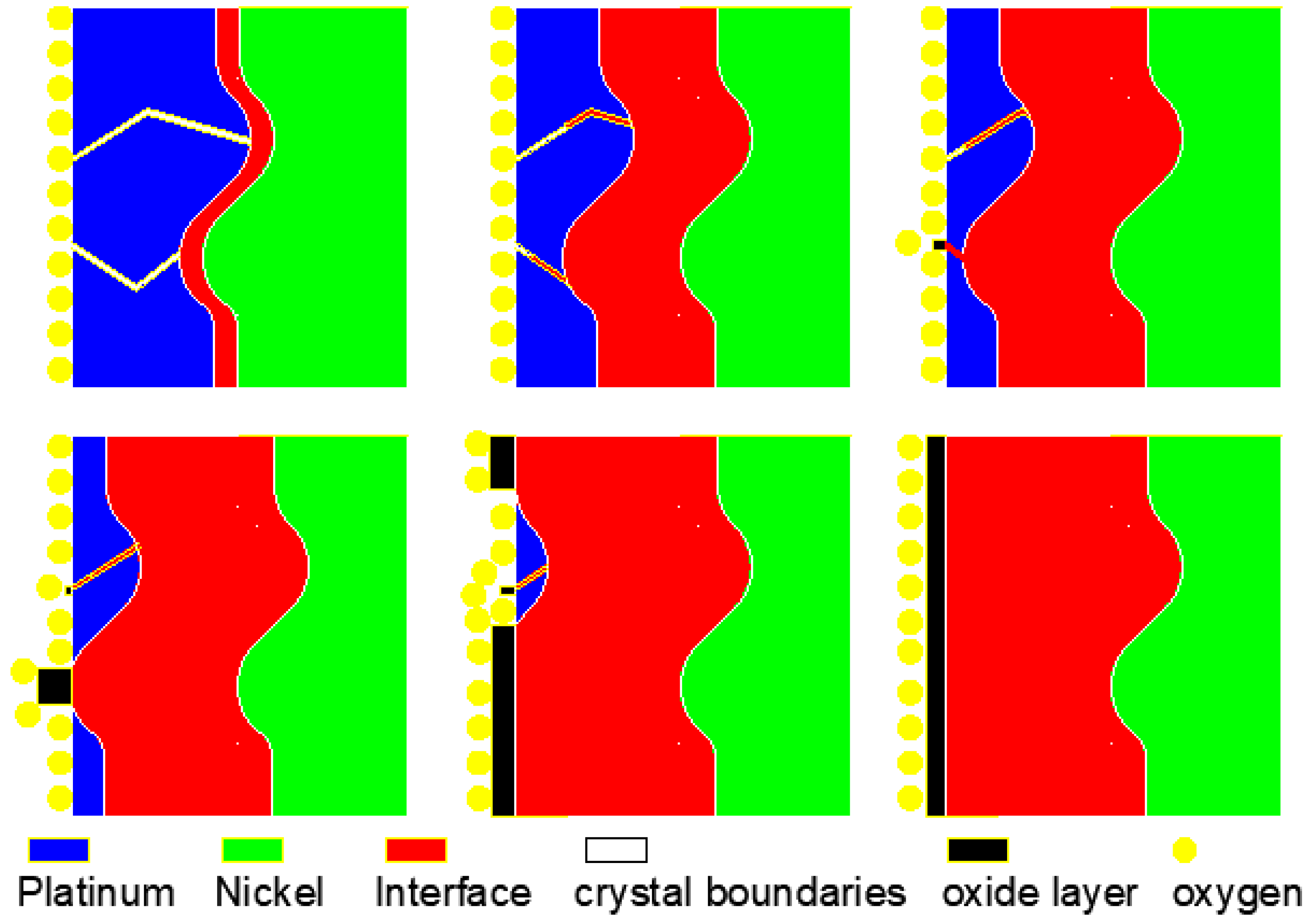
4.1. The Formation Mechanism of Serrations at the Interface
4.2. Diffusion and Oxidation Behavior of Nickel
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chauhan, A.K.; Tiwari, S.; Singh, S.; Singh, J.; Wadhwa, M. Experimental study of thermal annealing effects on evaporated platinum thin film with various substrate configurations. Microsyst. Technol. 2023, 29, 107–114. [Google Scholar] [CrossRef]
- Zhang, B.T.; Chen, X.; Yuan, J.F.; Zhu, H.D. Research on Temperature Measurement Technology of Platinum Film Thermistor. J. Phys. Conf. Ser. 2021, 1907, 012038. [Google Scholar] [CrossRef]
- Golub, V.V.; Zhilin, Y.V.; Rylov, S.A. A Platinum Thin-Film Resistance Thermometer. Instrum. Exp. Tech. 2018, 61, 138–145. [Google Scholar] [CrossRef]
- Wang, D.X.; Jiang, X.L.; Yang, Y.C.; Pi, Q.Q.; Liu, X. Development of high precision platinum thin film thermistors for wide temperature range. Sens. Microsyst. 2022, 41, 81–84. [Google Scholar]
- Lv, W.M.; Wang, Y.Q.; Shi, W.H.; Cheng, W.; Huang, R.; Zhong, R.F.; Zeng, Z.M.; Fan, Y.M.; Zhang, B.S. Role of micro-nano fabrication process on the temperature coefficient of resistance of platinum thin films resistance temperature detector. Mater. Lett. 2022, 309, 131313. [Google Scholar] [CrossRef]
- Han, J.; Cheng, P.; Wang, H.; Zhang, C.C.; Zhang, J.B.; Wang, Y.; Duan, L.; Ding, G.F. MEMS-based Pt film temperature sensor on an alumina substrate. Mater. Lett. 2014, 125, 224–226. [Google Scholar] [CrossRef]
- Mei, X.X.; Zhang, X.N.; Zhang, L.S.; Li, N.; Zhang, P.; Guo, Y.T.; Koval, N.N. Enhancing the Oxidation Resistance of NiCrAlY Bond Coat by High-Current Pulsed Electron Beam Irradiation. Coatings 2021, 11, 912. [Google Scholar] [CrossRef]
- Gurtaran, M.; Zhang, Z.X.; Li, X.Y.; Dong, H.S. High-Temperature Oxidation Behaviour of CrSi Coatings on 316 Austenitic Stainless Steel. Materials 2023, 16, 3533. [Google Scholar] [CrossRef]
- Fan, Q.X.; Yu, H.J.; Wang, T.Q.; Liu, Y.M. Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy. Coatings 2018, 8, 264. [Google Scholar] [CrossRef]
- Xu, Z.L.; Wang, F.T.; Peng, S.T.; Liu, W.W.; Guo, J.N. Effects of Process Parameters on Microstructure and High-Temperature Oxidation Resistance of Laser-Clad IN718 Coating on Cr5Mo Steel. Coatings 2023, 13, 197. [Google Scholar] [CrossRef]
- Lin, X.H.; Zhang, G.J.; Li, L.P.; Liu, Y.; Li, Y.C.; Li, B.; Zhang, W. Effect of Ti and Si Additions on Microstructure, Fracture Toughness and Oxidation Resistance of Nb-Mo5SiB2 Composite. JOM 2021, 73, 3476–3485. [Google Scholar] [CrossRef]
- Zhang, S.T.; Ouyang, P.X.; Yan, H.J.; Si, L.N. Study on Microstructure and Oxidation Resistance Mechanism of Y-Modified NiCrAlY Coating Prepared by Pack Cementation. Coatings 2023, 13, 63. [Google Scholar] [CrossRef]
- Kim, J.; Pyeon, J.; Kim, B.G.; Khadaa, T.; Choi, H.; Zhe, L.; Dube, T.; Zhang, J.; Yang, B.; Jung, Y.; et al. Oxidation Behavior of NiCoCrAlY Coatings Deposited by Vacuum Plasma Spraying and High-Velocity Oxygen Fuel Processes. Coatings 2023, 13, 319. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.H.; Ren, X.R.; Kang, X.Q.; Akhtar, F.; Feng, P.Z. Preparation and high-temperature oxidation resistance of multilayer MoSi2/MoB coating by spent MoSi2-based materials. J. Am. Ceram. Soc. 2021, 104, 3682–3694. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Z.H.; Yang, L.L.; Qiao, Y.X. The Oxidation Properties of a NiCrAlY Coating Fabricated by Arc Ion Plating. Coatings 2023, 13, 22. [Google Scholar] [CrossRef]
- Pan, Y.L.; Zhang, Z.D.; Wang, D.Y.; Guo, H.; Shi, Q.Y.; Lu, T.C. Sol–Gel-Derived Ni3Al Coating on Nickel Alloy for Oxidation Resistance in Supercritical Water Environments. Materials 2022, 15, 6566. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Yan, X.F.; Huan, C.H.; Bai, X.P. Manufacturing of Platinum-Nickel 30/70Compound Wires. Electrotech. Mater. 2006, 1, 6–9+13. [Google Scholar]
- Huang, B.F.; Yuan, H.M.; Luo, R.X. The Diffusion behaviour of Cu and Fe in Pt anode composite layer. Rare Met. Mater. Eng. 1992, 21, 67–71. [Google Scholar]
- Gelfond, N.V.; Krisyk, V.V.; Dorovskikh, S.I.; Kal’nyi, D.B.; Maksimovskii, E.A.; Shubin, Y.V.; Trubin, S.V.; Morozova, N.B. Structure of platinum coatings obtained by chemical vapor deposition. J. Struct. Chem. 2015, 56, 1215–1219. [Google Scholar] [CrossRef]
- Rooney, D.; Negrotti, D.; Byassee, T.; Macero, D.; Chaiken, J.; Vastag, B. Use of Laser-Directed Chemical Vapor Deposition to Fabricate Durable, Optically Transparent, Platinum Thin Film Electrodes. J. Electrochem. Soc. 2019, 137, 1162. [Google Scholar] [CrossRef]
- Arenas, L.F.; León, C.P.D.; Boardman, R.P.; Walsh, F.C. Characterisation of platinum electrodeposits on a titanium micromesh stack in a rectangular channel flow cell. Electrochim. Acta 2017, 247, 994–1005. [Google Scholar] [CrossRef]
- Bai, M.W.; Chen, Y.; Sun, Y.L.; Xiao, P. Mitigation of Platinum Depletion in Platinum Diffused Single Phase Bond Coat on CMSX-4 Superalloy. Coatings 2021, 11, 669. [Google Scholar] [CrossRef]
- Guan, J.Q.; Chen, Q.; Teng, H.T.; He, X. Preparation of catalytic platinum electrode paste for oxygen sensors and measurement of its electrical properties. Precious Met. 2017, 38 (Suppl. S1), 149–152. [Google Scholar]
- Xia, F.; Qian, X.L.; Yang, X.; Liu, G.K.; Sun, Y.Q. Process of fabricate Pt electrode in ZrO2 oxygen sensor. J. Sens. Sens. Technol. 2000, 19, 7–8+10. [Google Scholar]
- Zhang, S.H. Preparation and Performance of CVD Pure Tungsten Antioxidant Platinum Coatings. Ph.D. Thesis, Kunming University of Technology, Kunming, China, 2021. [Google Scholar]
- Igumenov, I.K.; Gelfond, N.V.; Galkin, P.S.; Morozova, N.B.; Fedotova, N.E.; Zharkova, G.I.; Shipachev, V.I.; Reznikova, E.F.; Ryabtsev, A.D.; Kotsupalo, N.P.; et al. Corrosion testing of platinum metals CVD coated titanium anodes in seawater-simulated solutions. Desalination 2001, 136, 273–280. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.L.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Tsampas, M.N.; Chatzitakis, A.; Sotiropoulos, S. Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules 2022, 27, 6382. [Google Scholar] [CrossRef] [PubMed]




| Material | Diameter/mm | Inner Diameter/mm | Outer Diameter/mm | Length/cm | Number |
|---|---|---|---|---|---|
| Nickel rod | 6.95 ± 0.01 mm | __ | __ | 50 cm | 2 |
| Platinum tube (No. 1) | __ | 7.00 ± 0.01 mm | 7.40 ± 0.01 mm | 40 cm | 1 |
| Platinum tube (No. 2) | __ | 7.00 ± 0.01 mm | 7.60 ± 0.01 mm | 40 cm | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, S.; Zhao, S.; Yang, Y.; Li, A.; Hu, J.; Fang, J.; Chong, X.; Xie, M. Study on High-Temperature Oxidation Behavior of Platinum-Clad Nickel Composite Wire. Metals 2023, 13, 1264. https://doi.org/10.3390/met13071264
Chen Y, Wang S, Zhao S, Yang Y, Li A, Hu J, Fang J, Chong X, Xie M. Study on High-Temperature Oxidation Behavior of Platinum-Clad Nickel Composite Wire. Metals. 2023; 13(7):1264. https://doi.org/10.3390/met13071264
Chicago/Turabian StyleChen, Yongtai, Saibei Wang, Shangqiang Zhao, Youcai Yang, Aikun Li, Jieqiong Hu, Jiheng Fang, Xiaoyu Chong, and Ming Xie. 2023. "Study on High-Temperature Oxidation Behavior of Platinum-Clad Nickel Composite Wire" Metals 13, no. 7: 1264. https://doi.org/10.3390/met13071264
APA StyleChen, Y., Wang, S., Zhao, S., Yang, Y., Li, A., Hu, J., Fang, J., Chong, X., & Xie, M. (2023). Study on High-Temperature Oxidation Behavior of Platinum-Clad Nickel Composite Wire. Metals, 13(7), 1264. https://doi.org/10.3390/met13071264







