The Tribocorrosion Behavior of High-Nitrogen Bearing Stainless Steel in Acetic Acid at Various Applied Loads
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Solutions
2.2. Tribocorrosion Tests
2.3. Characterization
3. Results
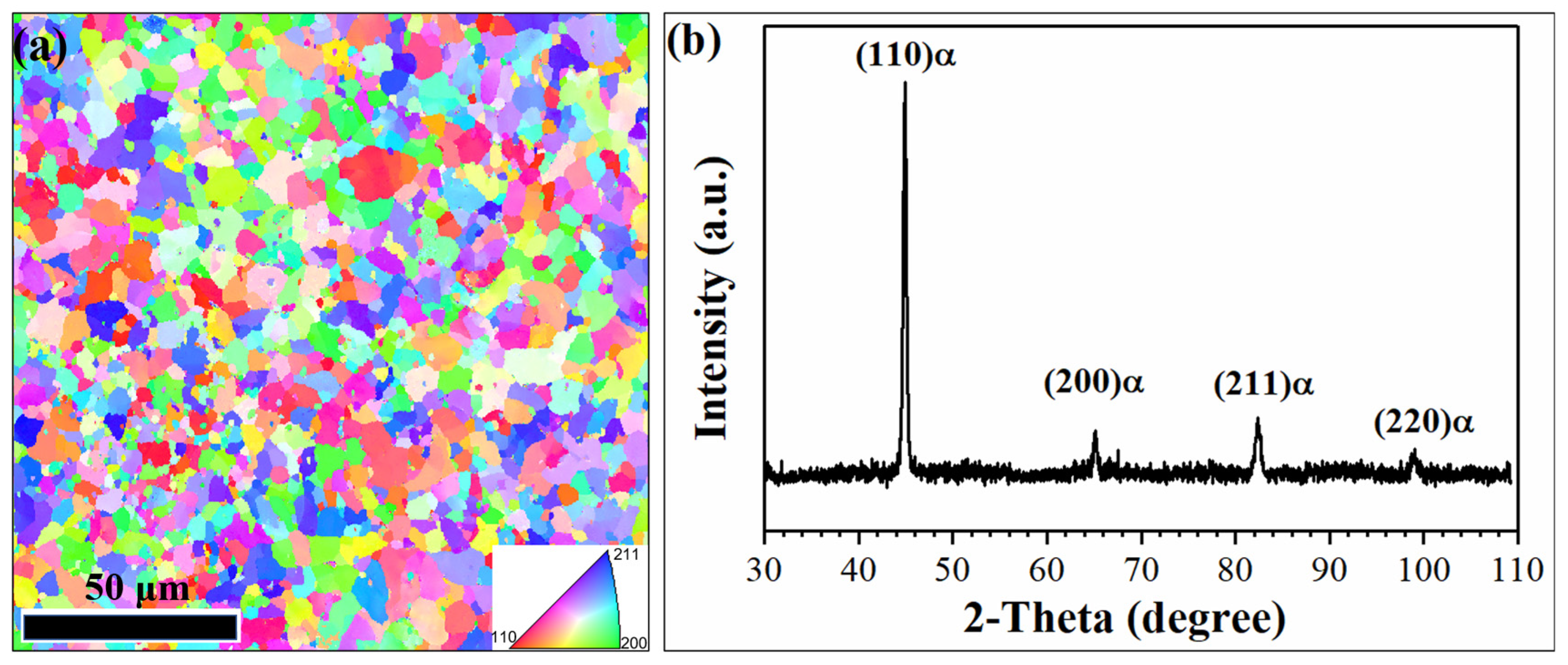
3.1. Microstructure
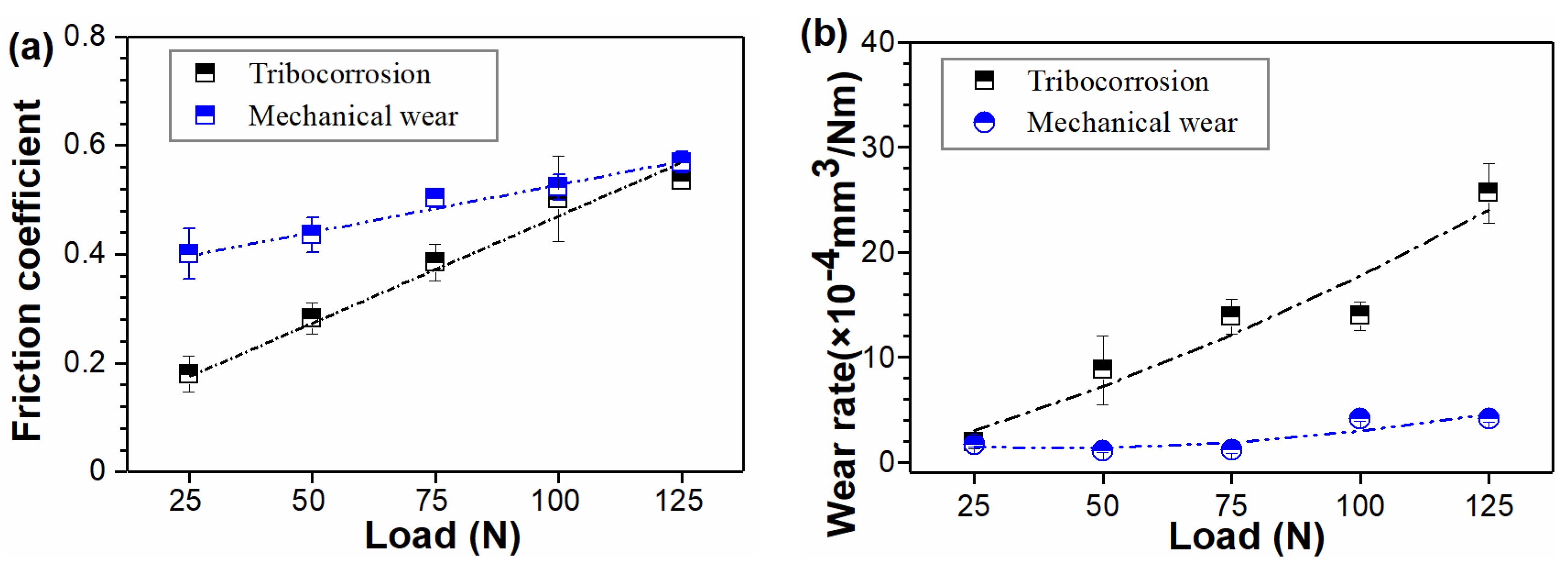
3.2. Tribocorrosion Behavior
3.3. Electrochemical Tests
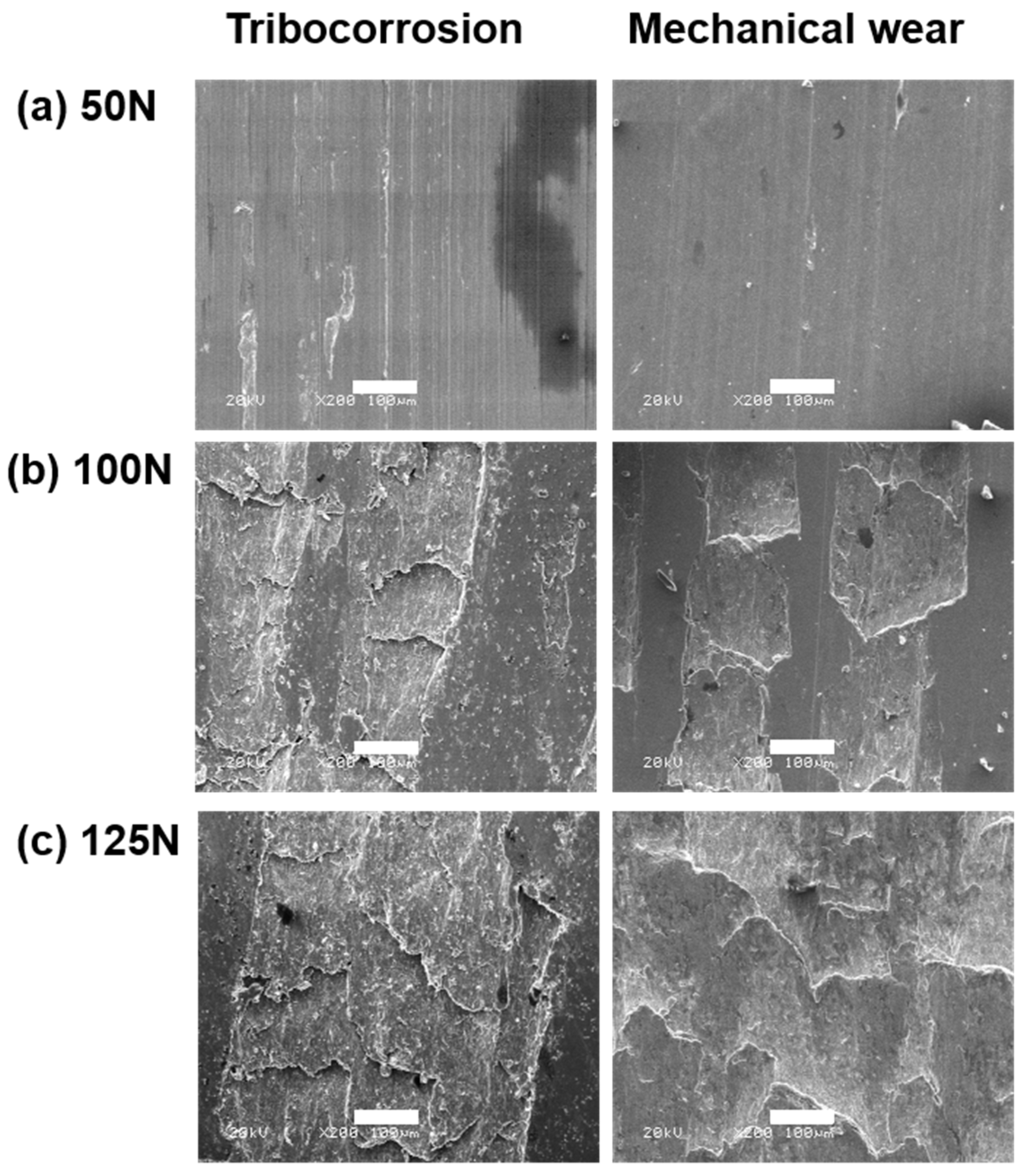
3.4. Damage Surface Morphologies
4. Conclusions
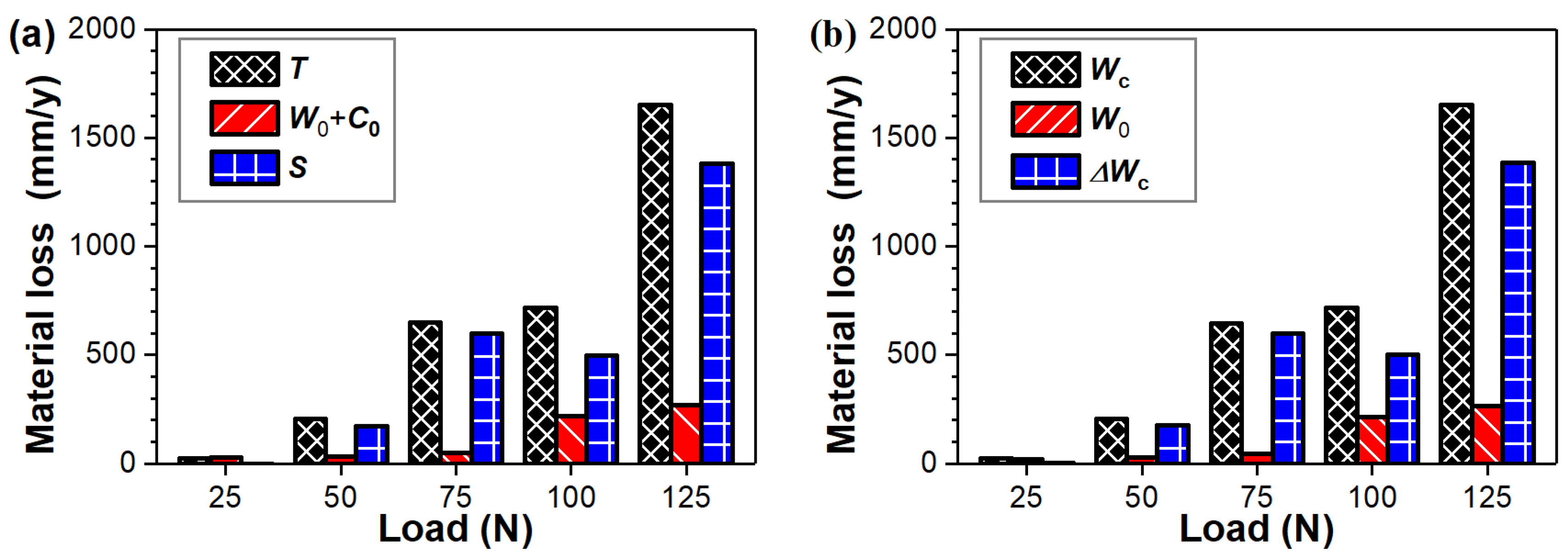
- A positive synergy between corrosion and wear was quantitatively determined. Simultaneous corrosion significantly promoted mechanical wear during the tribocorrosion process.
- The material loss induced by the tribocorrosion process in acetic acid solution primarily consisted of mechanical wear and corrosion-accelerated wear. The contribution of corrosion–wear synergism increased from approximately 6% at 25 N to over 83% at the highest load of 125 N.
- The material loss induced by mechanical wear increased with increasing load. The failure of 40Cr15Mo2VN was dominated by typical delamination wear.
- The corrosion mechanism of 40Cr15Mo2VN in acetic acid solution was mainly induced by general anodic dissolution, which promotes wear damage at surfaces.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davies, D.P. Survey of Fatigue Failures in Helicopter Components and Some Lessons Learnt. Eng. Fail. Anal. 2013, 32, 134–151. [Google Scholar] [CrossRef]
- Bayoumi, F.M.; Ghanem, W.A. Effect of Nitrogen on the Corrosion Behavior of Austenitic Stainless Steel in Chloride Solutions. Mater. Lett. 2005, 59, 3311–3314. [Google Scholar] [CrossRef] [Green Version]
- Berns, H. Manutacture and Application of High Nitrogen Steels. ISIJ Int. 1996, 36, 909–914. [Google Scholar] [CrossRef]
- Al-Shelkamy, S.A.; Abu Hashish, H.M.; Mahdy, A.A. Structural and Tribological Properties of Heat-Treated Stainless Steels against Abrasive and Lubricant Wear Conditions. Coatings 2021, 11, 1473. [Google Scholar] [CrossRef]
- Qiao, Y. Effect of Aging Treatment on Microstructure and Corrosion Behavior of a Fe-18Cr-15Mn-0.66N Stainless Steel. J. Mater. Sci. 2022, 107, 197–206. [Google Scholar] [CrossRef]
- Abreu, D.; Silva, W.M., Jr.; Ardila, M.A.N.; De Mello, J.D.B. Tribocorrosion in Ferritic Stainless Steels: An Improved Methodological Approach. Mat. Res. 2022, 25, e20210179. [Google Scholar] [CrossRef]
- Sastry, S.D.; Rohatgi, P.K.; Abraham, K.P.; Prasad, Y.V.R.K. Influence of Heat Treatment on the Strength and Fracture Behaviour of Fe-12Cr-6Al Ferritic Stainless Steel. J. Mater. Sci. 1982, 17, 3009–3016. [Google Scholar] [CrossRef]
- Reed, R.P. Nitrogen in Austenitic Stainless Steels. JOM 1989, 41, 16–21. [Google Scholar] [CrossRef]
- Simmons, J.W. Overview: High-Nitrogen Alloying of Stainless Steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Metikoš-Hukovic, M.; Babic, R.; Grubač, Z.; Petrovic, Z.; Lajçi, N. High Corrosion Resistance of Austenitic Stainless Steel Alloyed with Nitrogen in an Acid Solution. Corros. Sci. 2011, 53, 2176–2183. [Google Scholar] [CrossRef]
- Pujar, M.G. Electrochemical Noise Studies of the Effect of Nitrogen on Pitting Corrosion Resistance of High Nitrogen Austenitic Stainless Steels. Corros. Sci. 2011, 53, 4178–4186. [Google Scholar] [CrossRef]
- Xia, L.; Li, H.; Feng, H.; Jiang, Z.; Zhu, H.; Zhang, S.; Wang, X. Enhanced Strength and Toughness of High Nitrogen Stainless Bearing Steel by Controlling Interstitial Partitioning via V-Microalloying. J. Mater. Sci. Technol. 2023, 151, 204–218. [Google Scholar] [CrossRef]
- Gavriljuk, V. On the Correlation between Electron Structure and Short Range Atomic Order in Iron-Based Alloys. Acta Mater. 2000, 48, 3879–3893. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Shanina, B.D.; Berns, H. A Physical Concept for Alloying Steels with Carbon + nitrogen. Mater. Sci. Eng. A 2008, 481–482, 707–712. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.-B.; Jiang, Z.-H.; Zhang, T.; Dong, N.; Zhang, S.-C.; Han, P.-D.; Zhao, S.; Chen, Z.-G. Designing for High Corrosion-Resistant High Nitrogen Martensitic Stainless Steel Based on DFT Calculation and Pressurized Metallurgy Method. Corros. Sci. 2019, 158, 108081. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Ren, Y.; Yang, K. Eliminating Detrimental Effect of Cold Working on Pitting Corrosion Resistance in High Nitrogen Austenitic Stainless Steels. Corros. Sci. 2017, 123, 351–355. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, H.; Wang, X.; Pan, Z.; Jiang, Y.; Li, X. Electrochemical Corrosion and Passive Behavior of a New High-Nitrogen Austenitic Stainless Steel in Chloride Environment. Mater. Chem. Phys. 2022, 292, 126837. [Google Scholar] [CrossRef]
- Clayton, C.R.; Halada, G.P.; Kearns, J.R. Passivity of High-Nitrogen Stainless Alloys: The Role of Metal Oxyanions and Salt Films. Mater. Sci. Eng. A 1995, 198, 135–144. [Google Scholar] [CrossRef]
- Olefjord, I.; Wegrelius, L. The Influence of Nitrogen on the Passivation of Stainless Steels. Corros. Sci. 1996, 38, 1203–1220. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Zheng, Y.G.; Okafor, P.C.; Ke, W. Electrochemical Behaviour of High Nitrogen Bearing Stainless Steel in Acidic Chloride Solution: Effects of Oxygen, Acid Concentration and Surface Roughness. Electrochim. Acta 2009, 54, 2298–2304. [Google Scholar] [CrossRef]
- Zhu, Y.; Ning, L.; Liu, E.; Zhou, Y.; Tan, Z.; Tong, J.; Li, H.; Zheng, Z. A Novel Precipitation Mechanism of Laves Phase in Fe-30Cr-2Mo Super Ferritic Stainless Steel: In-Situ Phase Transformation. Mater. Lett. 2023, 338, 134022. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Wang, J.; Yan, F. Antagonistic Effect of Electrochemical Corrosion on the Mechanical Wear of Monel 400 Alloy in Seawater. Corros. Sci. 2022, 198, 110120. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yan, F. Load-Dependent Tribocorrosion Behaviour of Nickel-Aluminium Bronze in Artificial Seawater. Corros. Sci. 2018, 131, 252–263. [Google Scholar] [CrossRef]
- Liu, M.; Duan, D.-L.; Jiang, S.-L.; Li, M.-Y.; Li, S. Tribocorrosion Behavior of 304 Stainless Steel in 0.5 Mol/L Sulfuric Acid. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Maher, M.; Iraola-Arregui, I.; Ben Youcef, H.; Rhouta, B.; Trabadelo, V. The Synergistic Effect of Wear-Corrosion in Stainless Steels: A Review. Mater. Today Proc. 2022, 51, 1975–1990. [Google Scholar] [CrossRef]
- Chai, G.; Forsman, T.; Gustavsson, F.; Wang, C. Formation of Fine Grained Area in Martensitic Steel during Very High Cycle Fatigue: Formation of Fine Grained Area in Martensitic Steel during Very High Cycle Fatigue. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 1315–1323. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Lu, J.; Chen, R.; Chen, D.; Cui, G. A Review of Progress on High Nitrogen Austenitic Stainless-Steel Research. Mater. Express 2021, 11, 1901–1925. [Google Scholar] [CrossRef]
- Shen, H. Effects of Nitrogen on Predominant Sintering Mechanism during the Initial Stage of High Nitrogen Nickel-Free Stainless Steel Powder. J. Alloys Compd. 2023, 945, 169230. [Google Scholar] [CrossRef]
- Zhou, R.; Northwood, D.O.; Liu, C. On Nitrogen Diffusion during Solution Treatment in a High Nitrogen Austenitic Stainless Steel. J. Mater. Res. Technol. 2020, 9, 2331–2337. [Google Scholar] [CrossRef]
- Singh, M.M.; Gupta, A. Corrosion Behavior of Mild Steel in Acetic Acid Solutions. Corrosion 2000, 56, 371–379. [Google Scholar] [CrossRef]
- Kahyarian, A. Acidic Corrosion of Mild Steel in the Presence of Acetic Acid: Mechanism and Prediction. Electrochim. Acta 2017, 258, 639–652. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Wongpaiboon, C.; Sritha, N.; Anukulkich, N. Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract. Metals 2023, 13, 262. [Google Scholar] [CrossRef]




| Element | C | Cr | Mo | V | Mn | Si | Ni | N | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.42 | 14.6 | 1.9 | 0.33 | 0.4 | 0.21 | 0.41 | 0.18 | Bal. |
| Load(N) | 0 | 25 | 50 | 75 | 100 | 125 |
|---|---|---|---|---|---|---|
| Ecorr (V) | −0.418 | −0.471 | −0.494 | −0.511 | −0.527 | −0.548 |
| icorr (μA/cm2) | 3.6 | 64.6 | 114.8 | 134.9 | 229.1 | 363.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Wang, X.; Wang, H.; Huang, Y.; Wang, Y.; Li, Z. The Tribocorrosion Behavior of High-Nitrogen Bearing Stainless Steel in Acetic Acid at Various Applied Loads. Metals 2023, 13, 1287. https://doi.org/10.3390/met13071287
Su Q, Wang X, Wang H, Huang Y, Wang Y, Li Z. The Tribocorrosion Behavior of High-Nitrogen Bearing Stainless Steel in Acetic Acid at Various Applied Loads. Metals. 2023; 13(7):1287. https://doi.org/10.3390/met13071287
Chicago/Turabian StyleSu, Qiong, Xuhui Wang, Hongling Wang, Yaqi Huang, Yanbin Wang, and Zhenhua Li. 2023. "The Tribocorrosion Behavior of High-Nitrogen Bearing Stainless Steel in Acetic Acid at Various Applied Loads" Metals 13, no. 7: 1287. https://doi.org/10.3390/met13071287




