Microstructure and Phase Transition of Ag50.5Cu33.3Sn16.2-xInx Alloys through Experimental Study and Thermodynamic Calculation
Abstract
:1. Introduction
2. Experimental Procedure
3. Thermodynamic Calculation
3.1. Solution Phases
3.2. Intermetallic Compounds
4. Results and Discussion
4.1. Microstructure Characterization
4.2. Phase Transition
4.3. Solidification Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Illes, B.; Choi, H.; Hurtony, T.; Dusek, K.; Busek, D.; Skwarek, A. Suppression of Sn whisker growth from SnAgCu solder alloy with TiO2 and ZnO reinforcement nano-particles by increasing the corrosion resistance of the composite alloy. J. Mater. Res. Technol. 2022, 20, 4231–4240. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, M.; Lin, J.; Lu, S. Effect of temperature on the corrosion behavior of lead-free solders under polyvinyl chloride fire smoke atmosphere. J. Mater. Res. Technol. 2021, 15, 3088–3098. [Google Scholar] [CrossRef]
- Hardwick, L.; Webb, P.; Goodall, R. Design of higher temperature copper brazing filler metals with reduced brittle phase content. Mater. Today Commun. 2023, 35, 105524. [Google Scholar] [CrossRef]
- Way, M.; Willingham, J.; Goodall, R. Brazing filler metals. Int. Mater. Rev. 2020, 65, 257–285. [Google Scholar] [CrossRef]
- Wu, J.; Xue, S.B.; Liu, L.; Zhang, P.; Luo, Q.C. Influence of Ga Content on the Microstructure and Mechanical Properties of Cadmium-Free Filler Metal. Metals 2022, 12, 1151. [Google Scholar] [CrossRef]
- Vidyatharran, K.; Hanim, M.A.; Dele-Afolabi, T.T.; Matori, K.A.; Azlina, O.S. Microstructural and shear strength properties of GNSs-reinforced Sn-1.0Ag-0.5Cu (SAC105) composite solder interconnects on plain Cu and ENIAg surface finish. J. Mater. Res. Technol. 2021, 15, 2497–2506. [Google Scholar] [CrossRef]
- Rashidi, R.; Naffakh-Moosavy, H. Metallurgical, physical, mechanical and oxidation behavior of lead-free chromium dissolved Sn-Cu-Bi solders. J. Mater. Res. Technol. 2021, 13, 1805–1825. [Google Scholar] [CrossRef]
- Wang, H.; Xue, S.B. Effect of Ag on the properties of solders and brazing filler metals. J. Mater. Sci. Mater. Electron. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Li, J.J.; Zhang, P.; Wu, S.D.; Zhang, Z.F.; Kawasaki, M.; Langdon, T.G. Microstructures, strengthening mechanisms and fracture behavior of Cu-Ag alloys processed by high-pressure torsion. Acta Mater. 2012, 60, 269–281. [Google Scholar] [CrossRef]
- Mu, G.Q.; Qu, W.Q.; Zhang, Y.H.; Zhuang, H.S. Effect of Ni on the wetting and brazing characterization of 304 stainless steel by Ag–Cu alloy. J. Mater. Sci. 2023, 58, 6297–6312. [Google Scholar] [CrossRef]
- Basri, D.K.; Sisamouth, L.; Farazila, Y.; Miyazawa, Y.; Ariga, T. Brazeability and mechanical properties of Ag-Cu-Sn brazing filler metals on copper-brazed joint. Mater. Res. Innov. 2015, 18, 429–432. [Google Scholar] [CrossRef]
- Tong, Q.S.; Ge, J.; Rong, M.H.; Li, J.L.; Jiao, J.; Zhang, L.; Wang, J. Thermodynamic modeling of the Ag-Cu-Sn ternary system. Metals 2022, 12, 1557. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, M.; Wang, X.R.; Fan, Z.H.; Yu, D.K.; Chen, R.; Shen, H.Y.; Guo, J.Y.; Guo, B.; He, P. Processing and Characterization of Ag-Cu-Sn Brazing Alloy Prepared by a Mechanical Alloying Method. J. Mater. Eng. Perform. 2018, 27, 1147–1158. [Google Scholar] [CrossRef]
- Ma, C.L.; Xue, S.B.; Wang, B. Study on novel Ag-Cu-Zn-Sn brazing filler metal bearing Ga. J. Alloys Compd. 2016, 688, 854–862. [Google Scholar] [CrossRef]
- Luktuke, A.; Singaravelu, A.S.S.; Mannodi-Kanakkithodi, A.; Chawla, N. Influence of Indium addition on microstructural and mechanical behavior of Sn solder alloys: Experiments and first principles calculations. Acta Mater. 2023, 249, 118853. [Google Scholar] [CrossRef]
- Kanlayasiri, K.; Mongkolwongrojn, M.; Ariga, T. Influence of indium addition on characteristics of Sn-0.3Ag-0.7Cu solder alloy. J. Alloys Compd. 2009, 485, 225–230. [Google Scholar] [CrossRef]
- Leong, Y.M.; Haseeb, A.S.M.A.; Nishikawa, H.; Mokhtari, O. Microstructure and mechanical properties of Sn-1.0Ag-0.5Cu solder with minor Zn additions. J. Mater. Sci. Mater. Electron. 2019, 30, 11914–11922. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, C.Q.; Qi, L.; Chi, C.Y. Kinetics of intermetallic compound layers and shear strength in Bi-bearing Sn-Ag-Cu/Cu soldering couples. J. Alloys Compd. 2009, 473, 382–388. [Google Scholar] [CrossRef]
- Wang, Y.W.; Lin, Y.W.; Tu, C.T.; Kao, C.R. Effects of minor Fe, Co, and Ni additions on the reaction between Sn-Ag-Cu solder and Cu. J. Alloys Compd. 2009, 478, 121–127. [Google Scholar] [CrossRef]
- Shi, Y.W.; Tian, J.; Hao, H.; Xia, Z.D.; Lei, Y.P.; Guo, F. Effects of small amount addition of rare earth Er on microstructure and property of Sn-Ag-Cu solder. J. Alloys Compd. 2008, 453, 180–184. [Google Scholar] [CrossRef]
- Ren, X.L.; Wang, Y.P.; Lai, Y.Q.; Shi, S.Y.; Liu, X.Y.; Zou, L.J.; Zhao, N. Effects of In addition on microstructure and properties of SAC305 solder. Trans. Nonferrous Met. Soc. China 2022. Available online: https://kns.cnki.net/kcms/detail/43.1239.TG.20220809.1614.008.html (accessed on 15 June 2023).
- Tang, Y.; Li, Y.; Zhao, W.; Roslyakova, I.; Zhang, L. Thermodynamic descriptions of quaternary Mg-Al-Zn-Bi system supported by experiments and their application in descriptions of solidification behavior in Bi-additional AZ casting alloys. J. Magnes. Alloys. 2020, 8, 1238–1252. [Google Scholar] [CrossRef]
- Sopousek, J.; Palcut, M.; Hodulova, E.; Janovec, J. Thermal analysis of the Sn-Ag-Cu-In solder alloy. J. Electron. Mater. 2010, 39, 312–317. [Google Scholar] [CrossRef]
- Liu, Z.K. Computational thermodynamics and its applications. Acta Mater. 2020, 200, 745–792. [Google Scholar] [CrossRef]
- Li, X.F.; Li, Z.; Chen, C.; Ren, Z.F.; Wang, C.P.; Liu, X.J.; Zhang, Q.; Chen, S. CALPHAD as a powerful technique for design and fabrication of thermoelectric materials. J. Mater. Chem. A 2021, 9, 6634–6649. [Google Scholar] [CrossRef]
- Luo, X.B.; Peng, J.B.; Zhang, W.B.; Wang, S.; Cai, S.S.; Wang, X.J. CALPHAD-guided alloy design of Sn–In based solder joints with multiphase structure and their mechanical properties. Mater. Sci. Eng. A 2022, 860, 144284. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- He, X.C.; Wang, H.; Liu, H.S.; Jin, Z.P. Thermodynamic description of the Cu-Ag-Zr system. Calphad 2006, 30, 367–374. [Google Scholar] [CrossRef]
- Muzzillo, C.P.; Anderson, T. Thermodynamic assessment of Ag-Cu-In. J. Mater. Sci. 2018, 53, 6893–6910. [Google Scholar] [CrossRef]
- Du, J.Y.; Zemanova, A.; Hutabalian, Y.; Kroupa, A.; Chen, S.W. Phase diagram of Ag-Pb-Sn system. Calphad 2020, 71, 101997. [Google Scholar] [CrossRef]
- Liu, H.S.; Cui, Y.; Ishida, K.; Liu, X.J.; Wang, C.P.; Ohnuma, I.; Kainuma, R.; Jin, Z.P. Thermodynamic Assessment of the Cu-In Binary System. J. Phase Equilib. 2002, 23, 409–415. [Google Scholar] [CrossRef]
- Liu, X.J.; Liu, H.S.; Ohnuma, I.; Kainuma, R.; Ishida, K.; Itabashi, S.; Kameda, K.; Yamaguchi, K. Experimental determination and thermodynamic calculation of the phase equilibria in the Cu-In-Sn system. J. Electron. Mater. 2001, 30, 1093–1103. [Google Scholar] [CrossRef]
- Moelans, N.; Hari, K.C.; Wollants, P. Thermodynamic optimization of the lead-free solder system Bi-In-Sn-Zn. J. Alloys Compd. 2003, 360, 98–106. [Google Scholar] [CrossRef]
- Tong, Q.S. Thermodynamic Calculation of Phase Equilibrium in Ag-Cu-Sn-X(X=In, Sb) Alloy Systems. Master’s Thesis, Guilin University of Electronic and Technology, Guilin, China, 2023. [Google Scholar]
- Redlich, O.; Kister, A.T. Thermodynamics of nonelectrolyte solutions. Ind. Eng. Chem. 1948, 40, 341–345. [Google Scholar] [CrossRef]
- Muggianu, Y.M.; Gambino, M.; Bros, J.P. Enthalpies of formation of liquid alloys bismuth-gallium-tin at 723 K. Choice of an analytical representation of integral and partial excess functions of mixing. J. Chim. Phys. 1975, 75, 83–88. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, L.J. Thermodynamic description of the quaternary Al-Si-Mg-Sc system and its application to the design of novel Sc-additional A356 alloys. Mater. Des. 2017, 116, 427–437. [Google Scholar] [CrossRef]
- Su, D.; Rong, M.H.; Yang, K.C.; Yao, Q.R.; Wang, J.; Rao, G.H.; Zhou, H.Y. Thermodynamic calculation and solidification behavior of the La-Pr-Fe and Ce-Pr-Fe ternary systems. Calphad 2021, 74, 102285. [Google Scholar] [CrossRef]
- Jung, J.G.; Cho, Y.H.; Lee, J.M.; Kim, H.W.; Kwangjun, E. Designing the composition and processing route of aluminum alloys using CALPHAD: Case studies. Calphad 2019, 64, 236–247. [Google Scholar] [CrossRef]
- Scheil, E. Remarks on layer crystal formation. Z. Metallkd. 1942, 34, 70–72. [Google Scholar]
- Wang, Y.H.; Gao, K.; Tao, X.M.; Ko, W.S.; Wang, J.; Chen, F.W.; Xu, G.L.; Cui, Y.W. Experimental investigation and thermodynamic description of Mg-Sc-Zn ternary system. Calphad 2022, 77, 102406. [Google Scholar] [CrossRef]
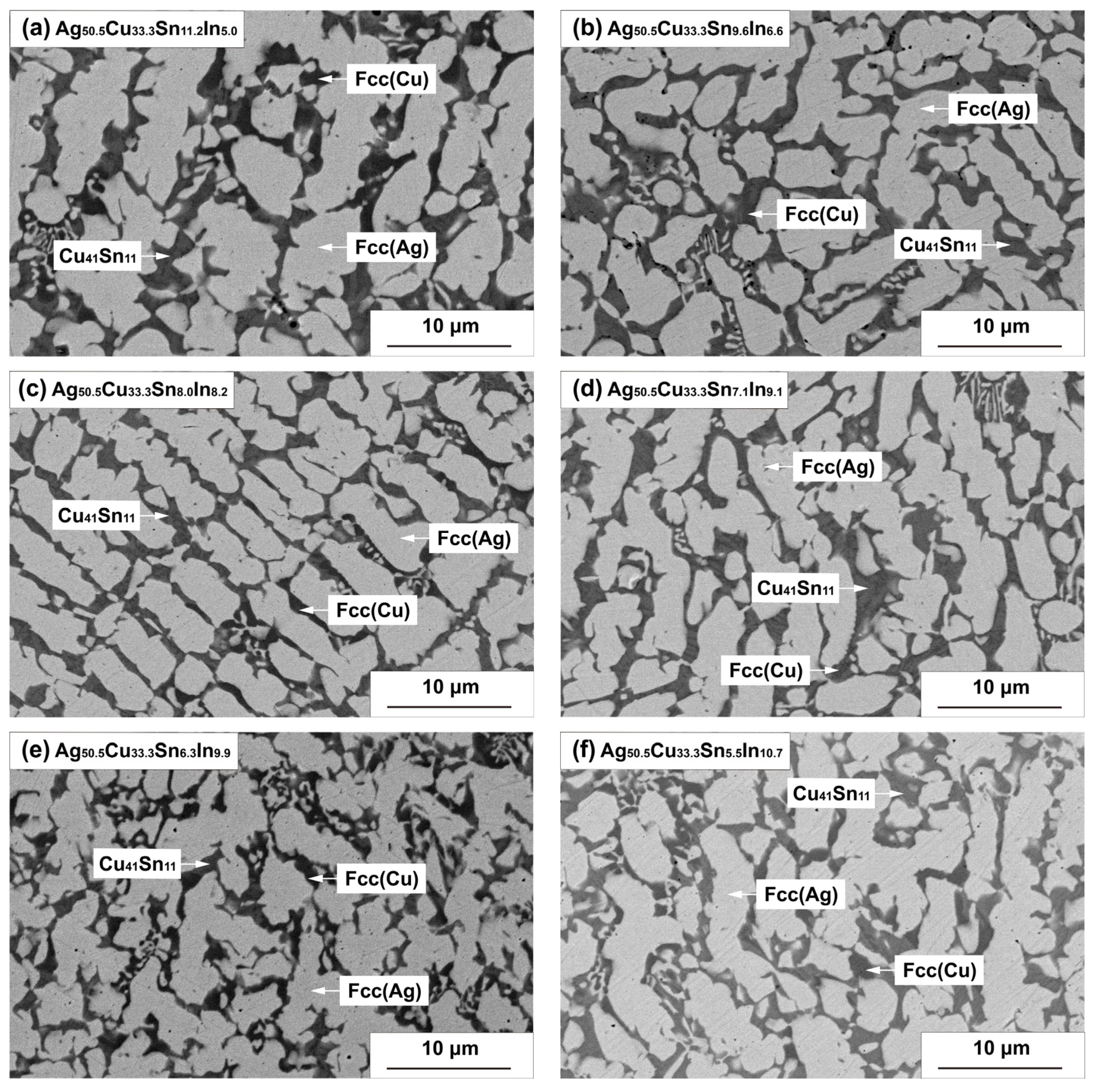






| Alloy Composition (at.%) | EDS Measurement (at.%) | Phase Identification | |||
|---|---|---|---|---|---|
| Ag | Cu | Sn | In | ||
| A1—Ag50.5Cu33.3Sn11.2In5.0 | 82.23 | 12.00 | 3.00 | 2.77 | Fcc(Ag) |
| 4.70 | 74.74 | 18.97 | 1.59 | Cu41Sn11 | |
| 6.12 | 85.96 | 7.52 | 0.40 | Fcc(Cu) | |
| A2—Ag50.5Cu33.3Sn9.6In6.6 | 79.89 | 11.78 | 2.75 | 5.58 | Fcc(Ag) |
| 2.84 | 76.50 | 17.40 | 2.26 | Cu41Sn11 | |
| 2.83 | 80.46 | 14.57 | 2.14 | Fcc(Cu) | |
| A3—Ag50.5Cu33.3Sn8.0In8.2 | 79.19 | 11.89 | 1.66 | 7.26 | Fcc(Ag) |
| 5.44 | 75.61 | 13.35 | 5.60 | Cu41Sn11 | |
| 7.92 | 84.29 | 5.84 | 1.95 | Fcc(Cu) | |
| A4—Ag50.5Cu33.3Sn7.1In9.1 | 79.16 | 11.54 | 2.16 | 7.14 | Fcc(Ag) |
| 2.75 | 75.74 | 16.09 | 5.42 | Cu41Sn11 | |
| 3.21 | 79.74 | 13.66 | 3.39 | Fcc(Cu) | |
| A5—Ag50.5Cu33.3Sn6.3In9.9 | 80.48 | 9.02 | 3.58 | 6.92 | Fcc(Ag) |
| 4.06 | 78.41 | 15.57 | 1.96 | Cu41Sn11 | |
| 6.68 | 85.43 | 6.95 | 0.94 | Fcc(Cu) | |
| A6—Ag50.5Cu33.3Sn5.5In10.7 | 79.76 | 12.44 | 0.79 | 7.01 | Fcc(Ag) |
| 4.11 | 74.80 | 13.99 | 7.10 | Cu41Sn11 | |
| 6.12 | 84.57 | 6.33 | 2.98 | Fcc(Cu) | |
| A7—Ag50.5Cu33.3Sn4.7In11.5 | 77.91 | 10.52 | 2.00 | 9.57 | Fcc(Ag) |
| 5.53 | 75.69 | 12.35 | 6.43 | Cu41Sn11 | |
| 5.73 | 79.40 | 10.46 | 4.41 | Fcc(Cu) | |
| A8—Ag50.5Cu33.3Sn3.9In12.3 | 79.14 | 9.98 | 0.85 | 10.03 | Fcc(Ag) |
| 5.31 | 75.96 | 9.37 | 9.36 | Cu41Sn11 | |
| 5.27 | 87.78 | 3.66 | 3.29 | Fcc(Cu) | |
| Alloy Composition (at.%) | Phase Transformation Temperatures (K) | Liquidus | |||||
|---|---|---|---|---|---|---|---|
| A1—Ag50.5Cu33.3Sn11.2In5.0 | 763.9 | — | — | — | 873.4 | 904.2 | 969.2 |
| A2—Ag50.5Cu33.3Sn9.6In6.6 | 764.4 | 783.4 | — | 854.6 | 869.2 | 901.4 | 956.2 |
| A3—Ag50.3Cu33.3Sn8.0In8.2 | 764.3 | — | — | 854.9 | 876.3 | 903.4 | 959.3 |
| A4—Ag50.5Cu33.3Sn7.1In9.1 | 764.3 | 783.4 | — | — | 873.2 | 902.2 | 971.3 |
| A5—Ag50.5Cu33.3Sn6.3In9.9 | 762.0 | 784.2 | — | 854.2 | 872.3 | 903.4 | 957.3 |
| A6—Ag50.5Cu33.3Sn5.5In10.7 | 766.3 | 784.6 | — | — | 860.1 | 908.5 | 955.7 |
| A7—Ag50.5Cu33.3Sn4.7In11.5 | 766.1 | — | 817.2 | 842.2 | 869.2 | 903.4 | 947.1 |
| A8—Ag50.5Cu33.3Sn3.9In12.3 | 764.6 | 786.5 | 833.8 | 851.1 | 858.1 | 885.7 | 945.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Q.; Rong, M.; Wang, J. Microstructure and Phase Transition of Ag50.5Cu33.3Sn16.2-xInx Alloys through Experimental Study and Thermodynamic Calculation. Metals 2023, 13, 1296. https://doi.org/10.3390/met13071296
Tong Q, Rong M, Wang J. Microstructure and Phase Transition of Ag50.5Cu33.3Sn16.2-xInx Alloys through Experimental Study and Thermodynamic Calculation. Metals. 2023; 13(7):1296. https://doi.org/10.3390/met13071296
Chicago/Turabian StyleTong, Qingsong, Maohua Rong, and Jiang Wang. 2023. "Microstructure and Phase Transition of Ag50.5Cu33.3Sn16.2-xInx Alloys through Experimental Study and Thermodynamic Calculation" Metals 13, no. 7: 1296. https://doi.org/10.3390/met13071296
APA StyleTong, Q., Rong, M., & Wang, J. (2023). Microstructure and Phase Transition of Ag50.5Cu33.3Sn16.2-xInx Alloys through Experimental Study and Thermodynamic Calculation. Metals, 13(7), 1296. https://doi.org/10.3390/met13071296





