Research Status and Prospective Properties of the Al-Zn-Mg-Cu Series Aluminum Alloys
Abstract
:1. Introduction
2. Main Alloying Elements
2.1. Cu
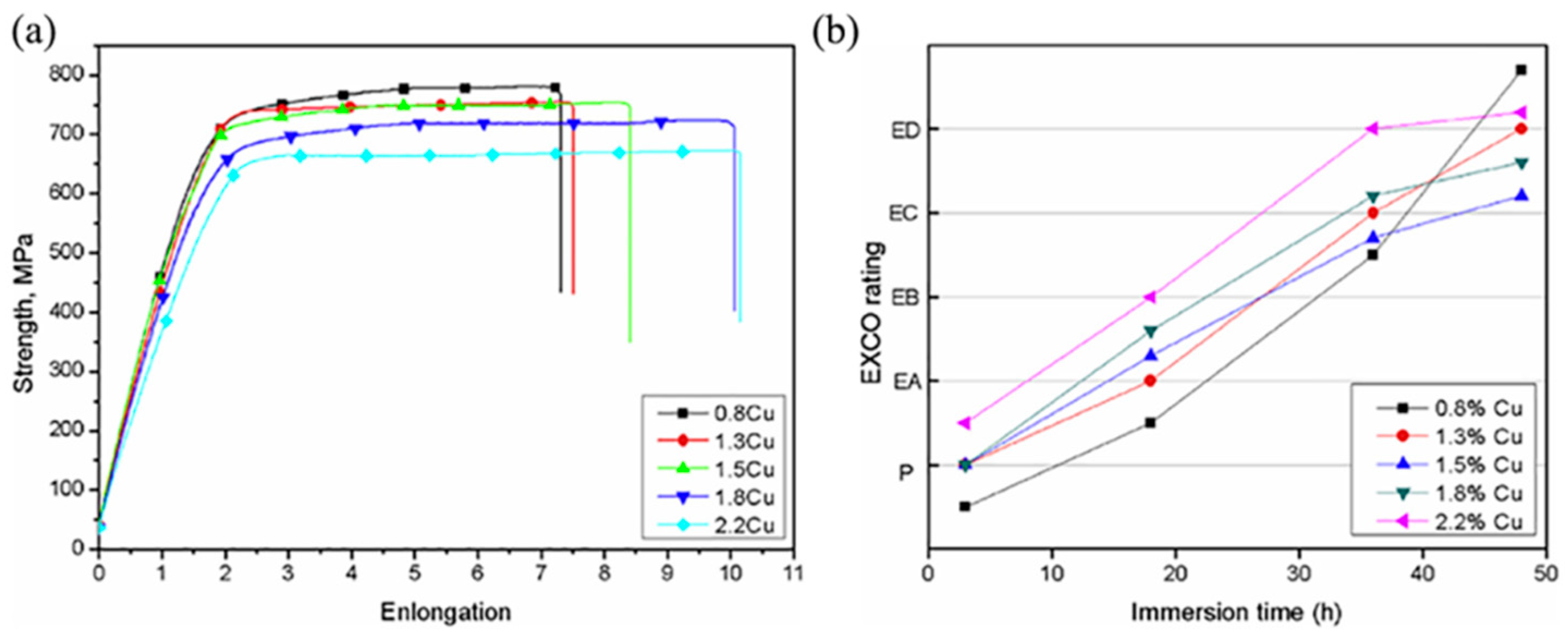
2.1.1. Effect of Different Cu Contents on an Al-Zn-Mg-Cu Alloy
2.1.2. Effect of Cu on Alloys at Different Zn/Mg Ratios
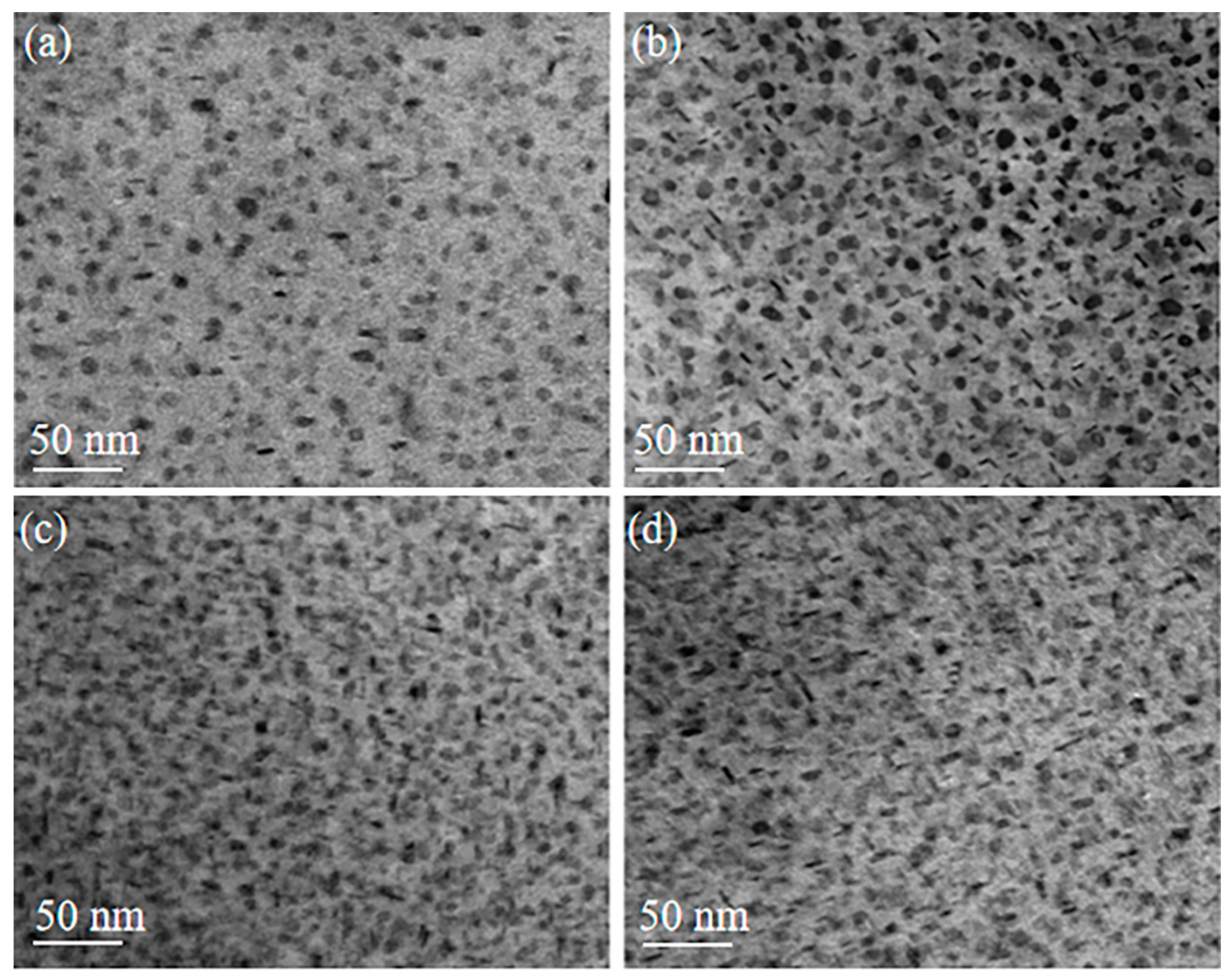
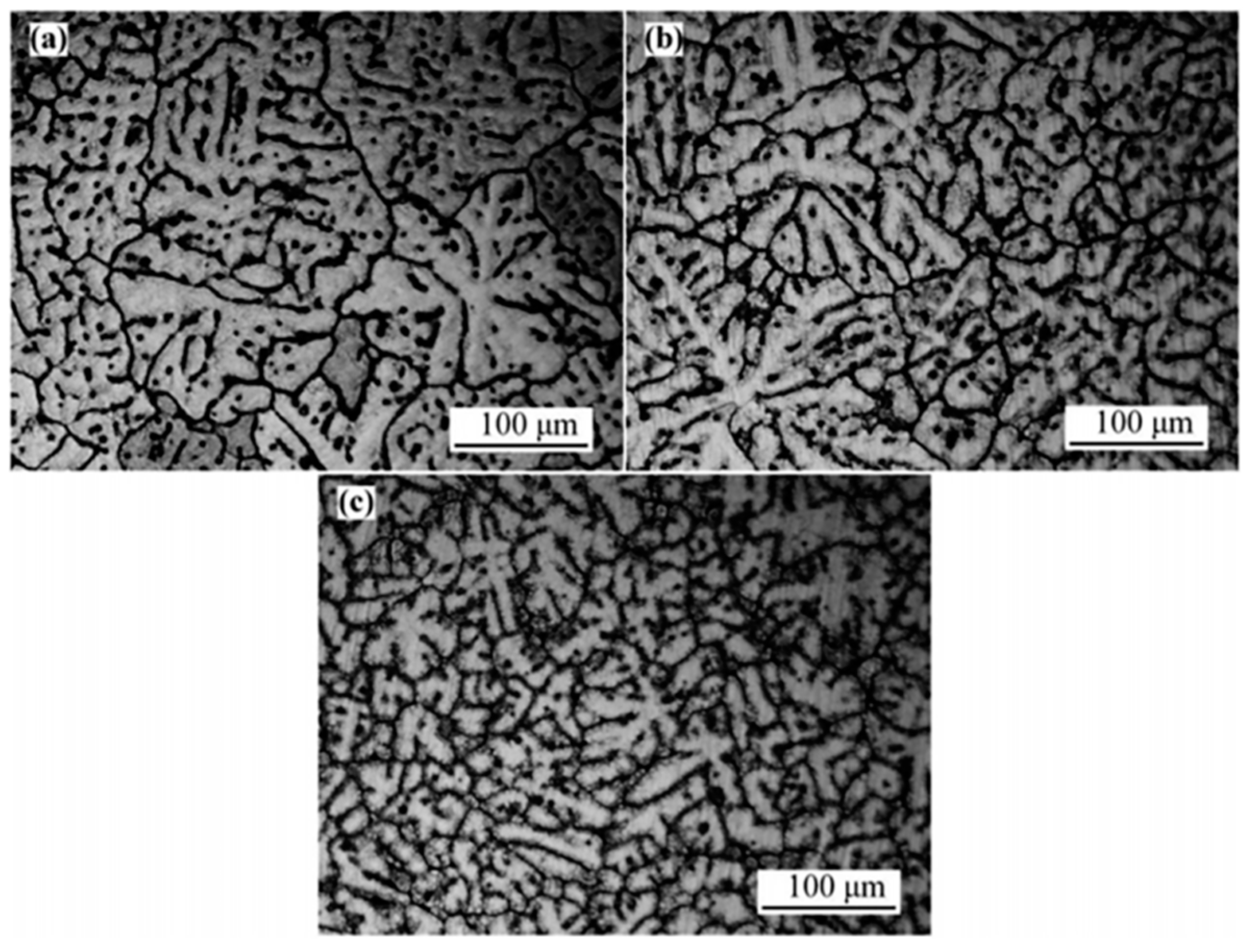
2.1.3. Effect of Cu in a Highly Alloyed Al-Zn-Mg-Cu Alloy
2.2. Mg
2.3. Zn
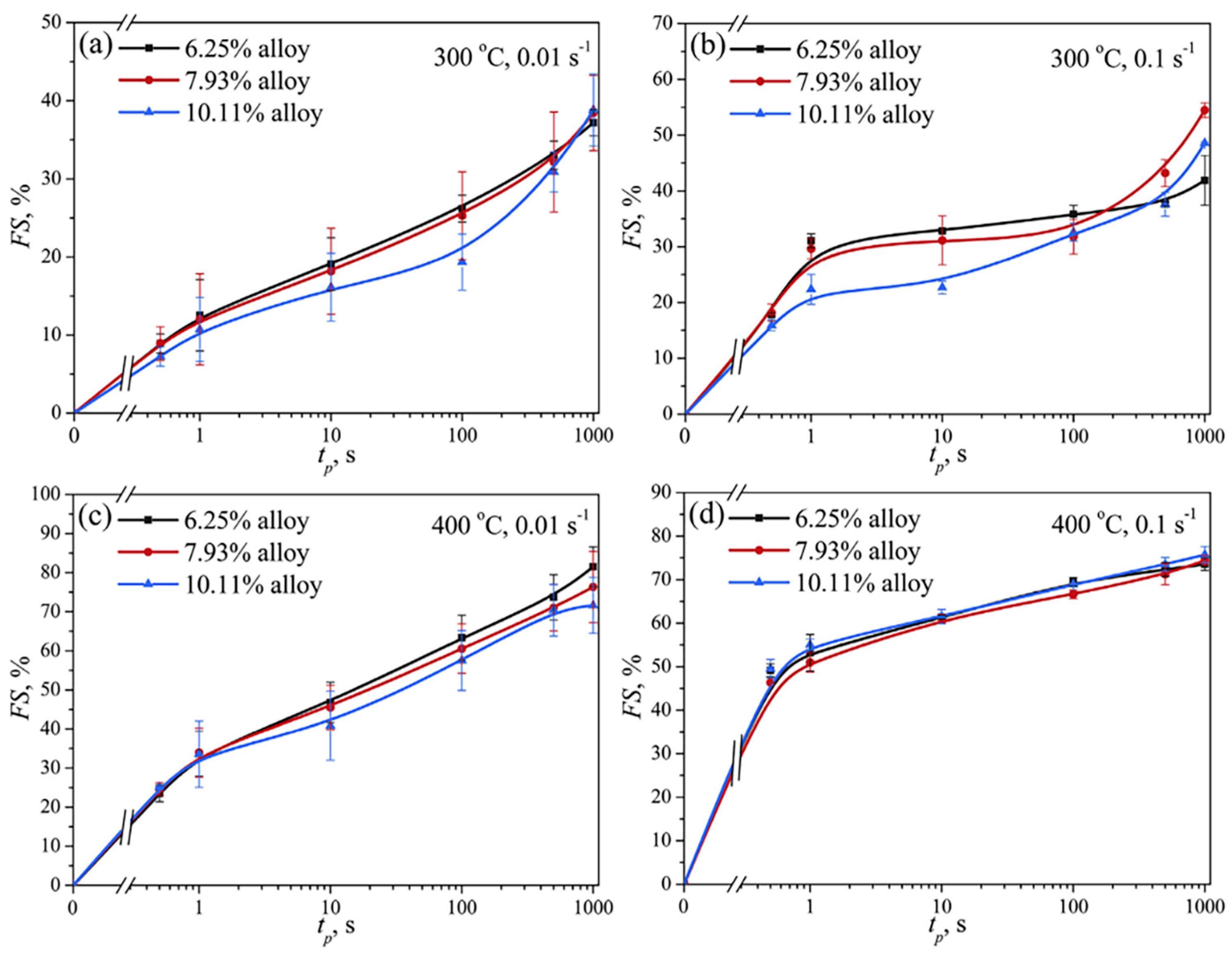
2.3.1. Effect of Different Zn Contents on Al-Zn-Mg-Cu Alloys
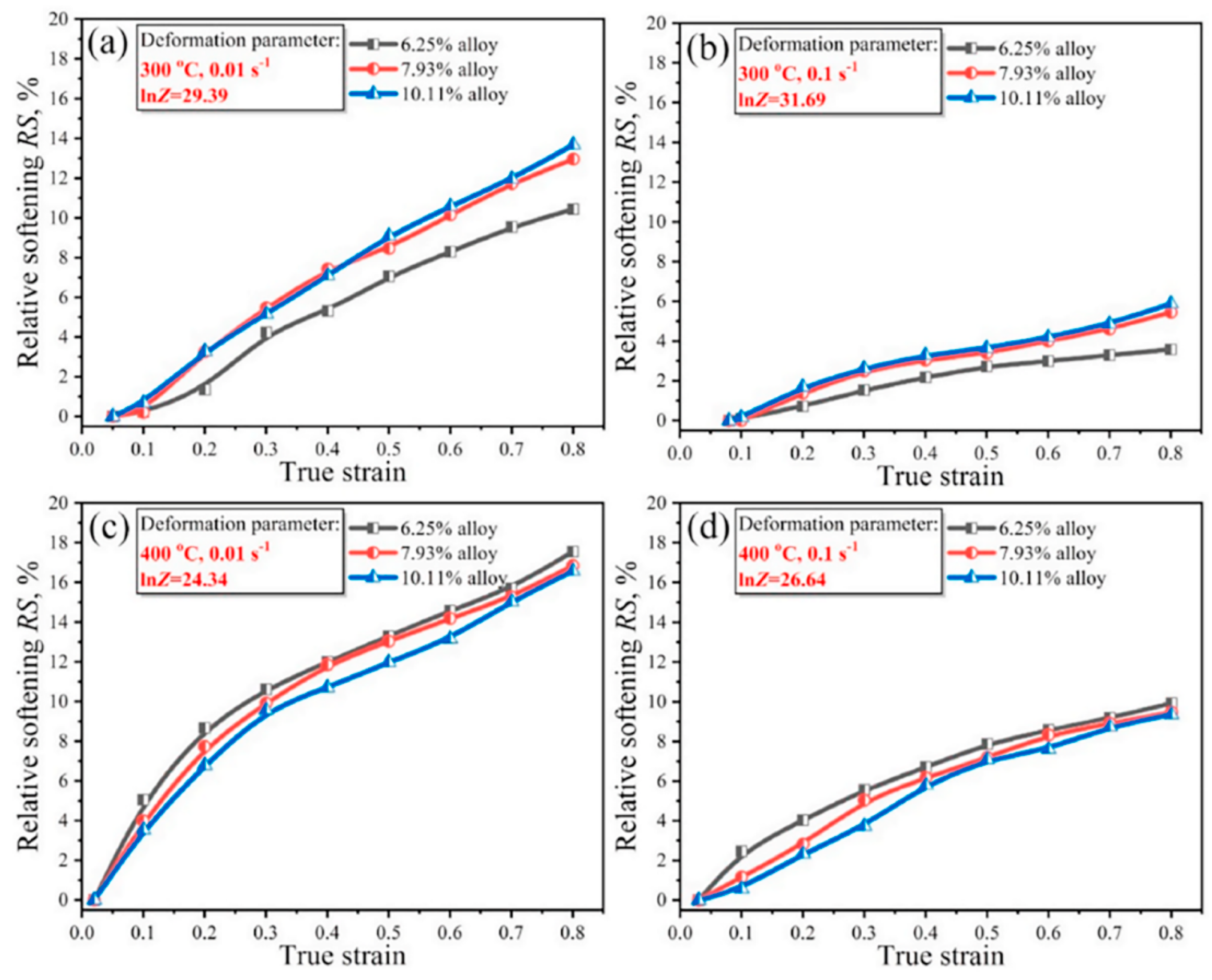
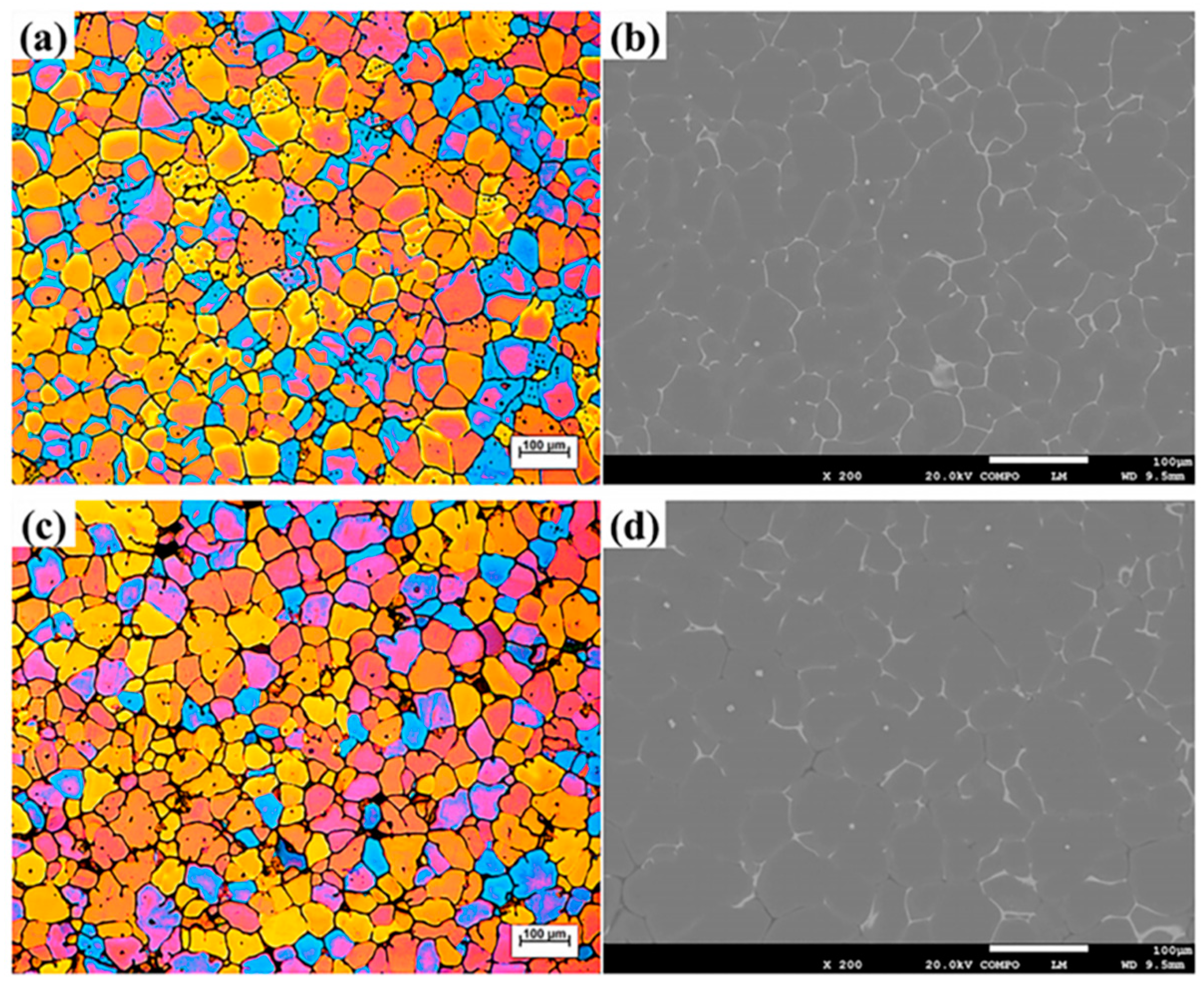
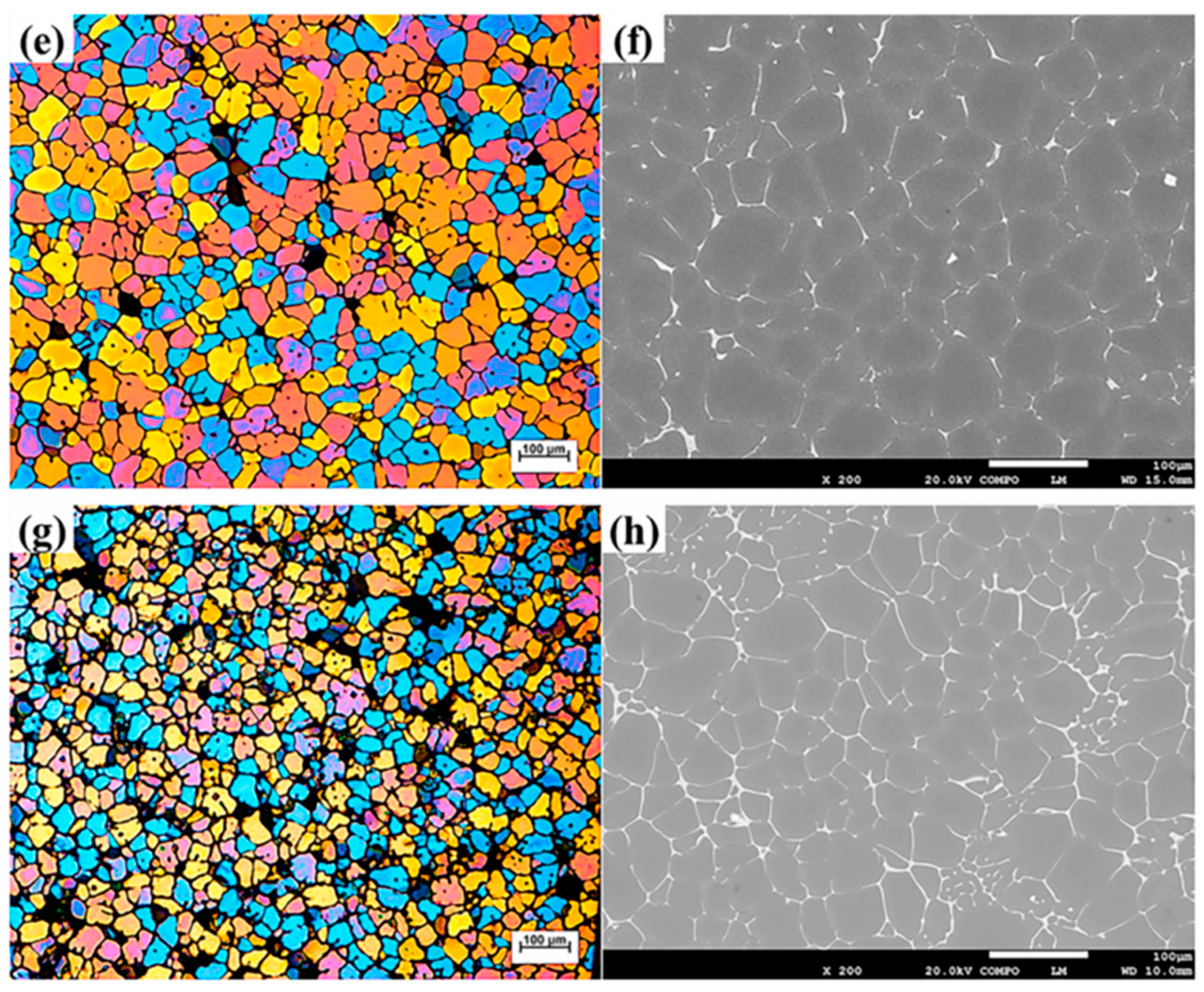
2.3.2. Effect of Zn on Softening Behavior of Al-Zn-Mg-Cu Alloys
2.3.3. Effect of Zn on Hot-Cracking Sensitivity of Al-Zn-Mg-Cu Alloys
2.3.4. Effect of Zn on Thermal Stability of Al-Zn-Mg-Cu Alloys
2.4. Cu/Mg Ratio
2.4.1. The Synergistic Action of Cu and Mg
2.4.2. Effect of Different Cu/Mg Ratio on Al-Zn-Mg-Cu Alloy
2.5. Zn/Mg Ratio
2.5.1. Effect of Zn/Mg Ratio on the Precipitation Sequence and Phase of the Alloy
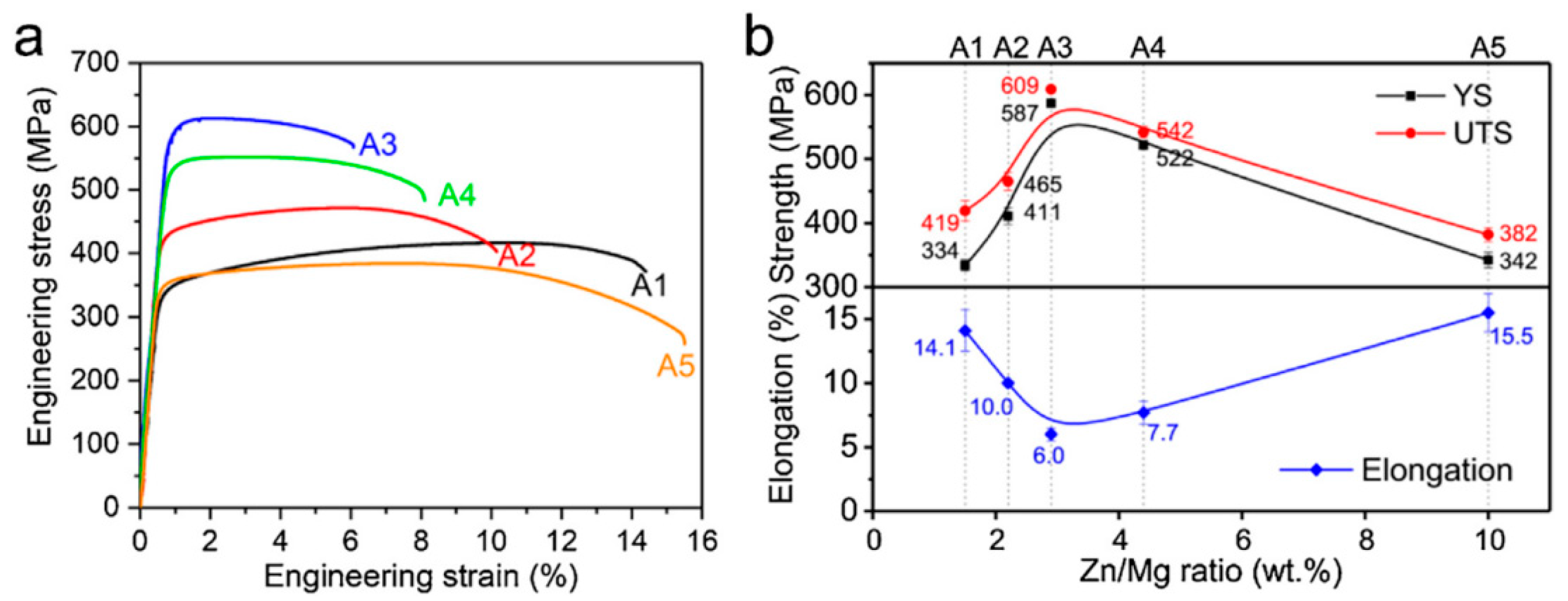
2.5.2. Effect of Different Zn/Mg Ratio on Al-Zn-Mg-Cu Alloy
2.5.3. Effect of Different Zn/Mg Ratio on Corrosion Resistance of Al-Zn-Mg-Cu Alloy
2.6. Brief Summary
3. Microalloy Elements
3.1. Fe, Si
3.1.1. Effect of Fe and Si on the Properties of Al-Zn-Mg-Cu Alloys
3.1.2. Effect of Fe and Si on the Recrystallization Process of Al-Zn-Mg-Cu Series Alloys
3.2. Ag
3.2.1. Effect of Ag on the Hardness and Yield Strength of Al-Zn-Mg-Cu Alloy
3.2.2. Effect of Ag on the Corrosion Resistance of Al-Zn-Mg-Cu Alloy
3.2.3. Effect of Ag on the Fracture of Al-Zn-Mg-Cu Alloy
3.3. Ni
3.3.1. Effect of Ni on the Thermal Cracking Sensitivity of Al-Zn-Mg-Cu Alloy
3.3.2. Effect of Ni on the Strength and Elongation of Al-Zn-Mg-Cu Alloy
3.4. Ti
3.4.1. Effect of Ti on the Thermal Cracking Sensitivity of Al-Zn-Mg-Cu Alloy
3.4.2. Effect of Ti on the Strength and Plasticity of Al-Zn-Mg-Cu Alloy
3.5. Brief Summary
4. Rare Earth Element
4.1. Sc
4.1.1. Refinement Effect of Sc on Al-Zn-Mg-Cu Alloy
4.1.2. Effect of Sc on the η’ Phase Distribution and Corrosion Resistance of Al-Zn-Mg-Cu Alloys
4.1.3. Effect of Sc on the Damping Properties of Al-Zn-Mg-Cu Alloy
4.1.4. Effect of Sc on the Plastic Properties of Al-Zn-Mg-Cu Alloy
4.2. Zr
4.2.1. Refinement Effect of Zr on Al-Zn-Mg-Cu Alloy
4.2.2. Effect of Zr on the Quenching Sensitivity of Al-Zn-Mg-Cu Alloy
4.3. Sc + Zr
4.3.1. Effect of Different Sc + Zr Contents on Al-Zn-Mg-Cu Alloy
4.3.2. Effect of Sc + Zr on the Thermal Stability of Al-Zn-Mg-Cu Alloy
4.3.3. Effect of Sc + Zr on the Yield Strength of Al-Zn-Mg-Cu Alloy
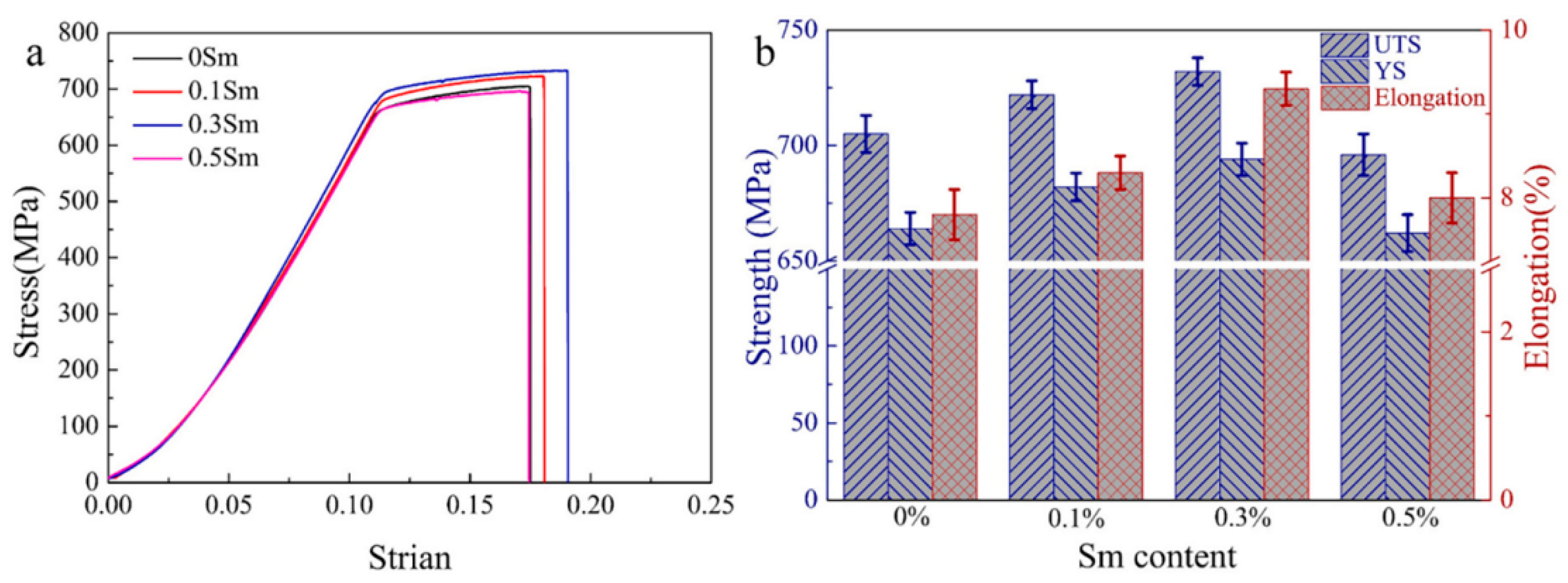
4.4. Sm
4.4.1. Effect of Sm on the Peak Hardness of Al-Zn-Mg-Cu Alloy
4.4.2. Effect of Sm on the Yield Strength and Elongation of Al-Zn-Mg-Cu Alloy
4.5. Er
4.6. La
4.7. Ce
4.7.1. The Effect of Ce on the Second Phase in Al-Zn-Mg-Cu Alloy
4.7.2. The Effect of Ce on the Corrosion Resistance on Al-Zn-Mg-Cu Alloy
4.8. Y
4.8.1. Effect of Y on the Effect of Al-Zn-Mg-Cu Alloy Refinement and Dislocation Density
4.8.2. Effect of the Phase of the Y on the Al-Zn-Mg-Cu Alloy
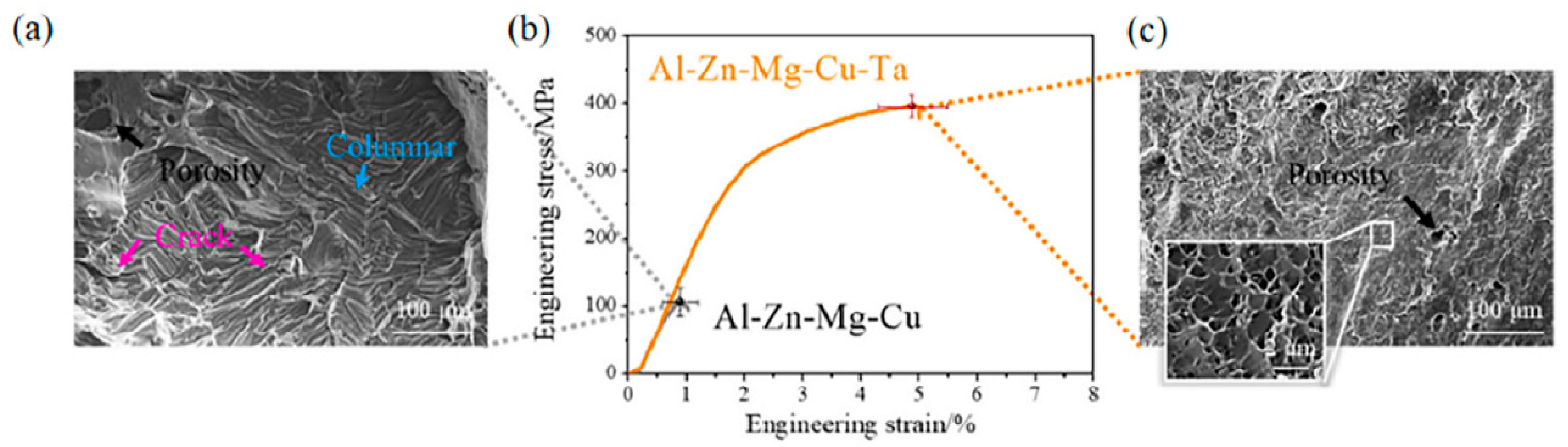
4.9. Ta
4.10. Brief Summary
5. Forecast
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsiao, T.-J.; Chiu, P.-H.; Tai, C.-L.; Tsao, T.-C.; Tseng, C.-Y.; Lin, Y.-X.; Chen, H.-R.; Chung, T.-F.; Chen, C.-Y.; Wang, S.-H. Effect of Cu Additions on the Evolution of Eta-prime Precipitates in Aged AA 7075 Al-Mg-Zn-Cu Alloys. Metals 2022, 12, 2120. [Google Scholar] [CrossRef]
- Jiao, H.; Chen, K.; Chen, S.; Yang, Z.; Xie, P.; Chen, S. Effect of Cu on the Fracture and Exfoliation Corrosion Behavior of Al-Zn-Mg-xCu Alloy. Metals 2018, 8, 1048. [Google Scholar] [CrossRef] [Green Version]
- Chinh, N.; Lendvai, J.; Ping, D.; Hono, K. The effect of Cu on mechanical and precipitation properties of Al-Zn-Mg alloys. J. Alloys Compd. 2004, 378, 52–60. [Google Scholar] [CrossRef]
- Mondal, C.; Mukhopadhyay, A. On the nature of T(Al2Mg3Zn3) and S(Al2CuMg) phases present in as-cast and annealed 7055 aluminum alloy. Mater. Sci. Eng. A 2005, 391, 367–376. [Google Scholar] [CrossRef]
- Dong, P.; Chen, S.; Chen, K. Effects of Cu content on microstructure and properties of super-high-strength Al-9.3Zn-2.4Mg-xCu-Zr alloy. J. Alloys Compd. 2019, 788, 329–337. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Liu, Y.; Huang, Y. The microstructures and mechanical properties of a highly alloyed Al-Zn-Mg-Cu alloy: The role of Cu concentration. J. Mater. Res. Technol. 2022, 18, 122–137. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Yang, H.; Lu, S.; Zuo, H.; Zheng, S.; Duan, Y.; Yuan, X. Effects of Mg contents on microstructures and second phases of as-cast Al-Mg-Zn-Cu alloys. J. Mater. Sci. Technol. 2022, 21, 2105–2117. [Google Scholar] [CrossRef]
- Li, H.; Cao, F.; Guo, S.; Jia, Y.; Zhang, D.; Liu, Z.; Wang, P.; Scudino, S.; Sun, J. Effects of Mg and Cu on microstructures and properties of spray-deposited Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2017, 719, 89–96. [Google Scholar] [CrossRef]
- Shu, W.; Hou, L.; Zhang, C.; Zhang, F.; Liu, J.; Zhuang, L.; Zhang, J. Tailored Mg and Cu contents affecting the microstructures and mechanical properties of high-strength Al-Mg-Zn-Cu alloys. Mater. Sci. Eng. A 2016, 657, 269–283. [Google Scholar] [CrossRef]
- Tang, J.; Liu, M.; Bo, G.; Jiang, F.; Luo, C.; Teng, J.; Fu, D.; Zhang, H. Unraveling precipitation evolution and strengthening function of the Al-Zn-Mg-Cu alloys with various Zn contents: Multiple experiments and integrated internal-state-variable modeling. J. Mater. Sci. Technol. 2022, 116, 130–150. [Google Scholar] [CrossRef]
- Tan, P.; Sui, Y.; Jin, H.; Zhu, S.; Jiang, Y.; Han, L. Effect of Zn content on the microstructure and mechanical properties of as-cast Al-Mg-Zn-Cu alloy with medium Zn content. J. Mater. Res. Technol. 2022, 18, 2620–2630. [Google Scholar] [CrossRef]
- Curle, U.; Cornish, L.; Govender, G. Predicting yield strengths of Al-Zn-Mg-Cu-(Zr) aluminium alloys based on alloy composition or hardness. Mater. Des. 2016, 99, 211–218. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, H.; Teng, J.; Fu, D.; Jiang, F. Effect of Zn content on the static softening behavior and kinetics of Al-Mg-Zn-Cu alloys during double-stage hot deformation. J. Alloys Compd. 2019, 806, 1081–1096. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Teng, J.; Wang, G.; Fu, D.; Zhang, H.; Jiang, F. Effect of Zn content on the dynamic softening of Al-Mg-Zn-Cu alloys during hot compression deformation. Vacuum 2021, 184, 109941. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Gao, Z.; Bai, Y.; Zhao, P.; Mao, W. Effect of main elements (Zn-Mg and Cu) on the microstructure, castability and mechanical properties of 7xxx series aluminum alloys with Zr and Sc. Mater. Charact. 2021, 182, 111559. [Google Scholar] [CrossRef]
- Mao, J.; Wen, S.; Liang, S.; Wu, X.; Wei, W.; Huang, H.; Gao, K.; Nie, Z. Precipitation behaviors and thermal stability of Al-3.5Mg-1.0Cu alloy with co-addition of Zn and Si. J. Alloys Compd. 2023, 946, 169401. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Li, H.; Zhang, D.; Zhang, J. Effect of high Cu concentration on the mechanical property and precipitation behavior of Al-Mg-Zn-(Cu) crossover alloys. J. Mater. Sci. Technol. 2022, 20, 4585–4596. [Google Scholar] [CrossRef]
- Wei, S.; Wang, R.; Zhang, H.; Xu, C.; Wu, Y.; Feng, Y. Influence of Cu/Mg ratio on microstructure and mechanical properties of Al-Mg-Zn-Cu alloys. J. Mater. Sci. 2021, 56, 3472–3487. [Google Scholar] [CrossRef]
- Chung, T.-F.; Yang, Y.-L.; Huang, B.-M.; Shi, Z.; Lin, J.; Ohmura, T.; Yang, J.-R. Transmission electron microscopy investigation of separated nucleation and in-situ nucleation in AA7050 aluminium alloy. Acta Mater. 2018, 149, 377–387. [Google Scholar] [CrossRef]
- Hou, S.; Liu, P.; Zhang, D.; Zhang, J.; Zhuang, L. Precipitation hardening behavior and microstructure evolution of Al-5.1 Mg-0.15Cu alloy with 3.0Zn (wt %) addition. J. Mater. Sci. 2018, 53, 3846–3861. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Guo, M.; Cao, L. Investigation on microstructure and mechanical properties of Al-Zn-Mg-Cu alloys with various Zn/Mg ratios. J. Mater. Sci. Technol. 2021, 85, 106–117. [Google Scholar] [CrossRef]
- Liu, S.; Xu, G.; Li, Y.; Huang, L.; Tang, L.; Peng, X. The Influence of the Zn/Mg ratio on the quench sensitivity of Al-Zn-Mg-Cu alloys. J. Mater. Eng. Perform. 2020, 29, 5787. [Google Scholar] [CrossRef]
- Graf, G.; Spoerk-Erdely, P.; Staron, P.; Stark, A.; Martin, F.M.; Clemens, H.; Klein, T. Quench rate sensitivity of age-hardenable Al-Zn-Mg-Cu alloys with respect to the Zn/Mg ratio: An in situ SAXS and HEXRD study. Acta Mater. 2022, 227, 117727. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, P.; Cai, J.; Zhang, Y.; Xia, H.; Guan, Q. Enhanced corrosion property of W-Al coatings fabricated on aluminum using surface alloying under high-current pulsed electron beam. J. Alloys Compd. 2017, 723, 258–265. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Chen, H.; Tabie, V.; Cai, J.; Liu, Y.; Liu, Z.; Peng, C.-T. Effect of Zn/Mg Ratio on Microstructure and Properties of Cold Extruded Al-xZn-2.4Mg-0.84Cu-0.2Zr-0.25Ti Aluminum Alloy. J. Mater. Eng. Perform. 2020, 29, 5787–5795. [Google Scholar] [CrossRef]
- Li, X.; Starink, M. Identification and analysis of intermetallic phases in overaged Zr-containing and Cr-containing Al-Mg-Zn-Cu alloys. J. Alloys Compd. 2011, 509, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Guo, E.; Li, Z.; Wang, G. Research on microstructure in as-cast 7A55 aluminum alloy and its evolution during homogenization. Rare Met. 2011, 30, 664–668. [Google Scholar] [CrossRef]
- Morgeneyer, T.; Starink, M.; Sinclair, I. Evolution of voids during ductile crack propagation in an aluminium alloy sheet toughness test studied by synchrotron radiation computed tomography. Acta Mater. 2008, 56, 1671–1679. [Google Scholar] [CrossRef] [Green Version]
- Vratnica, M.; Pluvinage, G.; Jodin, P.; Cvijović, Z.; Rakin, M.; Burzić, Z. Influence of notch radius and microstructure on the fracture behavior of Al-Mg-Zn-Cu alloys of different purity. Mater. Des. 2010, 31, 1790–1798. [Google Scholar] [CrossRef]
- Ohira, T.; Kishi, T. Effect of iron content on fracture toughness and cracking processes in high strength Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. 1986, 78, 9–19. [Google Scholar] [CrossRef]
- Hu, K.; Lin, C.; Xia, S.; Zheng, C.; Lin, B. Effect of Fe content on low cycle fatigue behavior of squeeze cast Al-Zn-Mg-Cu alloys. Mater. Charact. 2020, 170, 110680. [Google Scholar] [CrossRef]
- Kovarik, L.; Court, S.; Fraser, H.; Mills, M. GPB zones and composite GPB/GPBII zones in Al–Cu–Mg alloys. Acta Mater. 2008, 56, 4804–4815. [Google Scholar] [CrossRef]
- Guo, K.; Liang, S.; Wen, S.; Wei, W.; Hu, J.; Wu, X.; Gao, K.; Huang, H.; Nie, Z. GPB-Ⅱ zone in Si microalloyed Al-Mg-Zn-Cu alloy and its strengthening effect. Mater. Sci. Eng. A 2022, 852, 143721. [Google Scholar] [CrossRef]
- Zeng, X.H.; Xue, P.; Wu, L.H.; Ni, D.R.; Xiao, B.L.; Wang, K.S.; Ma, Z.Y. Microstructural evolution of aluminum alloy during friction stir welding under different tool rotation rates and cooling conditions. J. Mater. Sci. Technol. 2019, 35, 972. [Google Scholar] [CrossRef]
- She, H.; Shu, D.; Dong, A.; Wang, J.; Sun, B.; Lai, H. Relationship of particle stimulated nucleation, recrystallization and mechanical properties responding to Fe and Si contents in hot-extruded 7055 aluminum alloys. J. Mater. Sci. Technol. 2019, 35, 2570–2581. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, L.; Wu, X.; Zou, Y.; Couper, M.J. Effect of Ag on age-hardening response of Al-Zn-Mg-Cu alloys. Mater. Sci. Eng. A 2019, 754, 265–268. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Cao, L.; Tong, X.; Zou, Y.; Zhu, Q.; Tang, S.; Song, H.; Guo, M. Effect of Ag on aging precipitation behavior and mechanical properties of aluminum alloy 7075. Mater. Sci. Eng. A 2021, 804, 140515. [Google Scholar] [CrossRef]
- Wang, S.; He, C.; Luo, B.; Bai, Z.; Jiang, G. The role of trace Ag in controlling the precipitation and stress corrosion properties of aluminium alloy 7N01. Vacuum 2021, 184, 109948. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Z.; Bai, S.; Zhou, Y.; Liu, W.; Lv, Q. Effects of Ge and Ag additions on quench sensitivity and mechanical properties of an Al-Mg-Zn-Cu alloy. Mater. Sci. Eng. A 2017, 682, 640–647. [Google Scholar] [CrossRef]
- Naeem, H.T.; Mohammed, K.S.; Ahmad, K.R.; Rahmat, A. The Influence of Nickel and Tin Additives on the Microstructural and Mechanical Properties of Al-Zn-Mg-Cu Alloys. Adv. Mater. Sci. Eng. 2014, 2014, 686474. [Google Scholar] [CrossRef] [Green Version]
- Akopyan, T.K.; Padalko, A.G.; Belov, N.A.; Karpova, Z.A. Effect of Barothermal Treatment on the Structure and the Mechanical Properties of a High-Strength Eutectic Al-Mg-Zn-Cu–Ni Aluminum Alloy. Russ. Met. (Met.) 2017, 2017, 922–927. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, X.; Ji, S. Effects of Ni on the microstructure, hot tear and mechanical properties of Al-Mg-Zn-Cu alloys under as-cast condition. J. Alloys Compd. 2020, 821, 153458. [Google Scholar] [CrossRef]
- Zeng, X.C.; Ferguson, C.; Shankar, S. Effect of Titanium levels on the hot tearing sensitivity and abnormal grain growth after T4 treatment of Al-Zn-Mg-Cu alloys. Int. J. Met. 2018, 12, 457. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jung, J.-G.; Baik, S.-I.; Park, S.H.; Kim, M.-S.; Lee, Y.-K.; Euh, K. Effects of Ti addition on the microstructure and mechanical properties of Al-Mg-Zn-Cu-Zr alloy. Mater. Sci. Eng. A 2021, 801, 140437. [Google Scholar] [CrossRef]
- Kayani, S.H.; Jung, J.-G.; Kim, M.-S.; Euh, K. Effect of Cooling Rate on Precipitation Behavior of Al-7.65Zn-2.59Mg-1.95Cu Alloy with Minor Elements of Zr and Ti. Met. Mater. Int. 2020, 26, 1079–1086. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kayani, S.H.; Jung, J.-G.; Baik, S.-I.; Kim, M.-S.; Lee, Y.-K.; Euh, K. Crystallographic characterization of Al18Mg3Ti2 intermetallic phase in Al-Mg-Zn-Cu-Zr-Ti alloy. J. Alloys Compd. 2020, 844, 156173. [Google Scholar] [CrossRef]
- Ying, T.; Gu, L.; Tang, X.; Wang, J.; Zeng, X. Effect of Sc microalloying on microstructure evolution and mechanical properties of extruded Al-Mg-Zn-Cu alloys. Mater. Sci. Eng. A 2022, 831, 142197. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, Q.; Luo, Y.; Liu, Y.; Sun, Y.; Long, L.; Li, M.; Wang, X.; Liu, S. Study on the primary Al3Sc phase and the structure heredity of Al-Zn-Mg-Cu-Sc-Zr alloy. Mater. Charact. 2020, 169, 110601. [Google Scholar] [CrossRef]
- Teng, G.; Liu, C.; Ma, Z.; Zhou, W.; Wei, L.; Chen, Y.; Li, J.; Mo, Y. Effects of minor Sc addition on the microstructure and mechanical properties of 7055 Al alloy during aging. Mater. Sci. Eng. A 2018, 713, 61–66. [Google Scholar] [CrossRef]
- Mondol, S.; Alam, T.; Banerjee, R.; Kumar, S.; Chattopadhyay, K. Development of a high temperature high strength Al alloy by addition of small amounts of Sc and Mg to 2219 alloy. Mater. Sci. Eng. A 2017, 687, 221–231. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Pan, Q.; Liu, B.; Long, L.; Wang, W.; Ye, J.; Huang, Z.; Xiang, S. Effect of Sc content on microstructure and properties of Al-Zn-Mg-Cu-Zr alloy. Mater. Today Commun. 2021, 26, 101899. [Google Scholar] [CrossRef]
- Luo, B.; Bai, Z.; Xie, Y. The effects of trace Sc and Zr on microstructure and internal friction of Zn–Al eutectoid alloy. Mater. Sci. Eng. A 2004, 370, 172–176. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, C.; Ma, Z.; Zhang, X.; Yu, L.; Ma, M.; Liu, R. Fabrication of Al-35Zn alloys with excellent damping capacity and mechanical properties. J. Alloys Compd. 2017, 722, 138–144. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Zhang, B.; Ma, Z.; Zhou, W.; Jiang, H.; Huang, H.; Wei, L. Effects of friction stir processing and minor Sc addition on the microstructure, mechanical properties, and damping capacity of 7055 Al alloy. Mater. Charact. 2018, 135, 25–31. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, G.; Zhou, L.; Xiao, D.; Deng, Y.; Yin, Z.; Peng, B.; Pan, Q.; Wang, Y.; Lu, L. Achieving high superplasticity of a traditional thermal–mechanical processed non-superplastic Al-Zn-Mg alloy sheet by low Sc additions. J. Alloys Compd. 2015, 638, 364–373. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, P.; Sui, Y.; Jiang, Y.; Zhou, R. Influence of Zr content on microstructure and mechanical properties of As-cast Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2021, 867, 158920. [Google Scholar] [CrossRef]
- Cassell, A.; Robson, J.; Race, C.; Eggeman, A.; Hashimoto, T.; Besel, M. Dispersoid composition in zirconium containing Al-Zn-Mg-Cu (AA7010) aluminium alloy. Acta Mater. 2019, 169, 135–146. [Google Scholar] [CrossRef]
- Pan, T.-A.; Tzeng, Y.-C.; Bor, H.-Y.; Liu, K.-H.; Lee, S.-L. Effects of the coherency of Al3Zr on the microstructures and quench sensitivity of Al-Mg-Zn-Cu alloys. Mater. Today Commun. 2021, 28, 102611. [Google Scholar] [CrossRef]
- Xiao, Q.-F.; Huang, J.-W.; Jiang, Y.-G.; Jiang, F.-Q.; Wu, Y.-F.; Xu, G.-F. Effects of minor Sc and Zr additions on mechanical properties and microstructure evolution of Al-Zn-Mg-Cu alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 1429–1438. [Google Scholar] [CrossRef]
- Liu, J.; Yao, P.; Zhao, N.; Shi, C.; Li, H.; Li, X.; Xi, D.; Yang, S. Effect of minor Sc and Zr on recrystallization behavior and mechanical properties of novel Al-Mg-Zn-Cu alloys. J. Alloys Compd. 2016, 657, 717–725. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, B.; Li, Z.; Zhang, Y.; Teng, H. Precipitation Behavior of Al3(Sc,Zr) Particles in High-Alloyed Al-Mg-Zn-Cu-Zr-Sc Alloy During Homogenization. Arab. J. Sci. Eng. 2021, 46, 6027–6037. [Google Scholar] [CrossRef]
- Jia, M.; Zheng, Z.; Gong, Z. Microstructure evolution of the 1469 Al-Cu-Li-Sc alloy during homogenization. J. Alloys Compd. 2014, 614, 131–139. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Teng, G.-B.; Ma, Z.-Y.; Wei, L.-L.; Zhang, B.; Chen, Y. Effects of Sc and Zr microalloying on the microstructure and mechanical properties of high Cu content 7xxx Al alloy. Int. J. Miner. Met. Mater. 2019, 26, 1559–1569. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Z.; Yan, H. Effect of samarium (Sm) addition on microstructure and mechanical properties of Al-5Cu alloys. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 624–629. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, L.; Gao, X.; Feng, Y.; Zhao, S.; Wang, L. Phase evolution of a novel Al-Mg-Zn-Cu–Zr–Sm alloy during homogenization annealing treatment. Mater. Res. Express 2020, 7, 076518. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, L.; Gao, X.; Zhao, S.; Feng, Y.; Ma, T.; Fan, R. Effect of samarium on the high temperature tensile properties and fracture behaviors of Al-Mg-Zn-Cu–Zr alloy. Mater. Res. Express 2021, 8, 016521. [Google Scholar] [CrossRef]
- Zhai, F.; Fan, R.; Feng, Y.; Wang, L. Effect of samarium modification on the microstructures evolution and mechanical properties of high strength aluminum alloy. Mater. Charact. 2022, 194, 112349. [Google Scholar] [CrossRef]
- Fang, H.; Chao, H.; Chen, K. Effect of Zr, Er and Cr additions on microstructures and properties of Al-Mg-Zn-Cu alloys. Mater. Sci. Eng. A 2014, 610, 10–16. [Google Scholar] [CrossRef]
- Zhong, H.; Li, S.; Zhang, Z.; Li, D.; Deng, H.; Chen, J.; Qi, L.; Ojo, O.A. Precipitation behavior, mechanical properties, and corrosion resistance of rare earth–modified Al-Zn-Mg-Cu alloys. Mater. Today Commun. 2022, 31, 103732. [Google Scholar] [CrossRef]
- Krishnan, A.; Raja, V.; Mukhopadhyay, A. Influence of Retrogression and Re-aging on the Exfoliation Corrosion Behavior of AA 7085 Sheets. Corros. Sci. Technol. 2016, 15, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, X.; Cao, L.; Tong, X.; Couper, M.J.; Liu, Q. Effect of trace Er on the microstructure and properties of Al-Mg-Zn-Cu-Zr alloys during heat treatments. Mater. Sci. Eng. A 2020, 792, 139807. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Hu, W.-X.; Shi, L.; Wang, W. Effect of rare earth on morphology and dispersion of TiB2 phase in Al-Ti-B alloy refiner. China Foundry 2023, 20, 115–124. [Google Scholar] [CrossRef]
- Yin, D.S.; Zhang, N.; Chen, K.J.; Zhang, Y.L. Effect of Gd on microstructure and refinement performance of Al-5Ti-B alloy. China Foundry 2021, 18, 223. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, H.; Ma, L.; Fan, T.; Tang, P. Effects of rare-earth elements on twinning deformation of Al alloys from first-principles calculations. Solid State Commun. 2022, 356, 114946. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, L.; Gao, X.; Feng, Y.; Zhao, S.; Wang, L. Study on Phases Formation and Modification Ability of Rare Earth Elements La, Ce, Sm and Er in Al-Mg-Zn-Cu–Zr Alloy. Trans. Indian Inst. Met. 2021, 74, 2639–2649. [Google Scholar] [CrossRef]
- Yu, X.-X.; Sun, J.; Li, Z.-T.; Dai, H.; Fang, H.-J.; Zhao, J.-F.; Yin, D.-F. Solidification behavior and elimination of undissolved Al2CuMg phase during homogenization in Ce-modified Al-Mg-Zn-Cu alloy. Rare Met. 2020, 39, 1279–1287. [Google Scholar] [CrossRef]
- Michalik, R.; Woźnica, H. The Effect of Ti and REE Addition on the Corrosion Resistance of the AlZn12Mg3.5Cu2.5 Alloy in “Acid Rain” Environment. Solid State Phenom. 2015, 227, 111–114. [Google Scholar] [CrossRef]
- Hu, G.; Zhu, C.; Xu, D.; Dong, P.; Chen, K. Effect of cerium on microstructure, mechanical properties and corrosion properties of Al-Zn-Mg alloy. J. Rare Earths 2021, 39, 208–216. [Google Scholar] [CrossRef]
- Wang, M.; Knezevic, M.; Chen, M.; Li, J.; Liu, T.; Wang, G.; Zhao, Y.; Wang, M.; Liu, Q.; Huang, Z.; et al. Microstructure design to achieve optimal strength, thermal stability, and electrical conductivity of Al-7.5wt.%Y alloy. Mater. Sci. Eng. A 2022, 852, 143700. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, X.; Chen, T.; Zhang, H.; Liu, X.; Cheng, Y.; Lei, D. Effect of rare earth Y and Al–Ti–B master alloy on the microstructure and mechanical properties of 6063 aluminum alloy. J. Alloys Compd. 2020, 830, 154685. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Li, M.; Hu, Y.; Zeng, Q.; Zhang, P. Effect of combined addition of Zr, Ti and Y on microstructure and tensile properties of an Al-Zn-Mg-Cu alloy. Mater. Des. 2022, 223, 111129. [Google Scholar] [CrossRef]
- Yang, W.; Pang, M.; Tan, Y.; Zhan, Y. A comparative first-principles study on electronic structures and mechanical properties of ternary intermetallic compounds Al8Cr4Y and Al8Cu4Y: Pressure and tension effects. J. Phys. Chem. Solids 2016, 98, 298–308. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, D.; Gibson, M.A.; Zheng, Y.; Fraser, H.L.; StJohn, D.H.; Easton, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Yahata, B.; Mayer, J.; Mone, R.; Stonkevitch, E.; Miller, J.; O’Masta, M.R.; Schaedler, T.; Hundley, J.; Callahan, P.; et al. Grain refinement mechanisms in additively manufactured nano-functionalized aluminum. Acta Mater. 2020, 200, 1022–1037. [Google Scholar] [CrossRef]
- Straumal, B.; Baretzky, B.; Mazilkin, A.; Phillipp, F.; Kogtenkova, O.; Volkov, M.; Valiev, R. Formation of nanograined structure and decomposition of supersaturated solid solution during high pressure torsion of Al-Zn and Al-Mg alloys. Acta Mater. 2004, 52, 4469–4478. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Li, G.; Cai, Q. Microstructure, mechanical properties, aging behavior, and corrosion resistance of a laser powder bed fusion fabricated Al-Mg-Zn-Cu–Ta alloy. Mater. Sci. Eng. A 2022, 832, 142364. [Google Scholar] [CrossRef]
- Won, S.-J.; So, H.; Han, J.-W.; Oh, S.J.; Kang, L.; Kim, K.-H. Effects of Sc and Be Microalloying Elements on Mechanical Properties of Al-Zn-Mg-Cu (Al7xxx) Alloy. Metals 2023, 13, 340. [Google Scholar] [CrossRef]
- Won, S.-J.; So, H.; Han, J.-W.; Oh, S.J.; Kim, K.-H. Roles of Sc and Ag Microalloying Elements in the Mechanical Properties of Al-Zn-Mg-Cu (Al7xxx) Alloy. Metals 2023, 13, 244. [Google Scholar] [CrossRef]


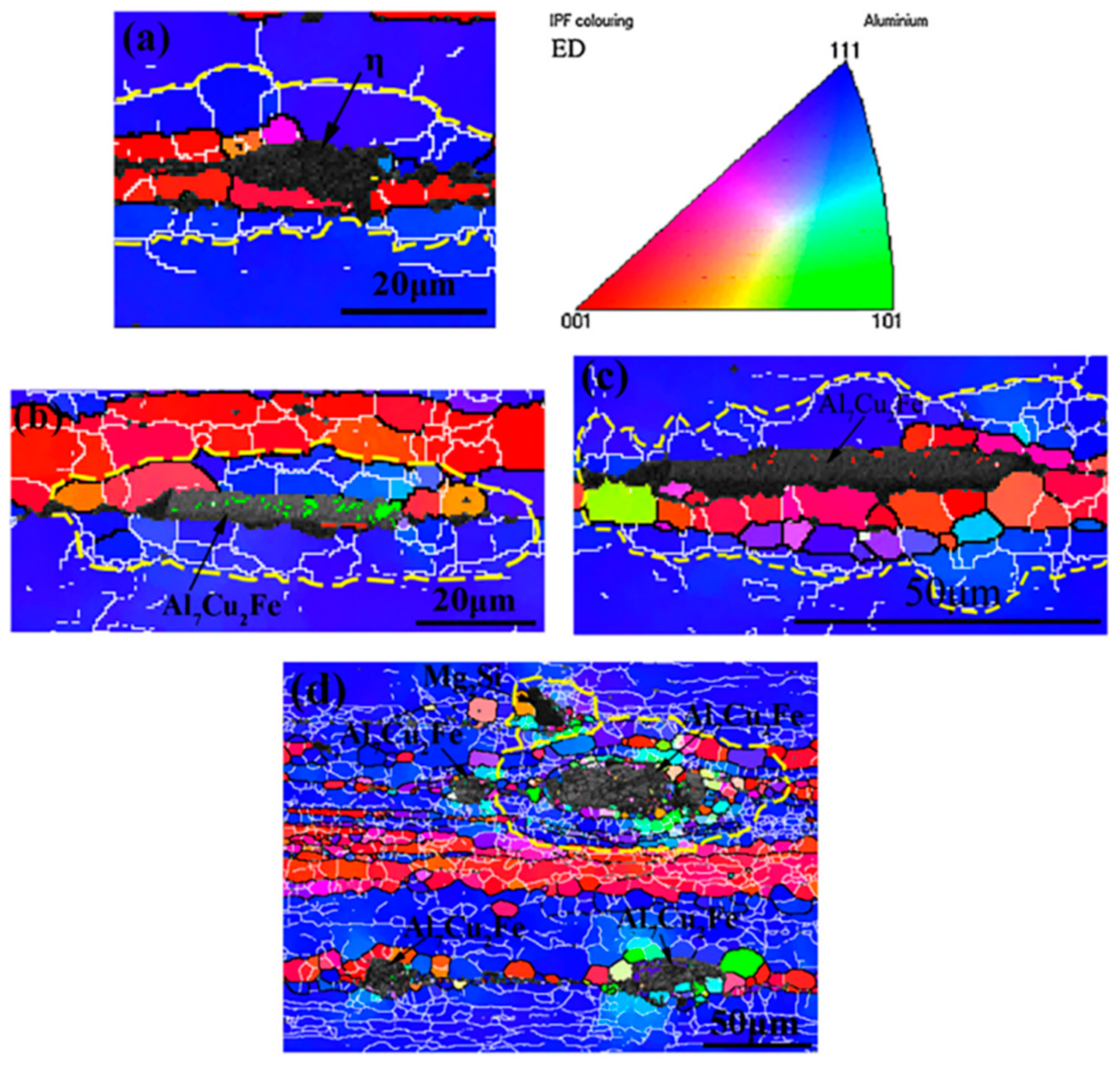
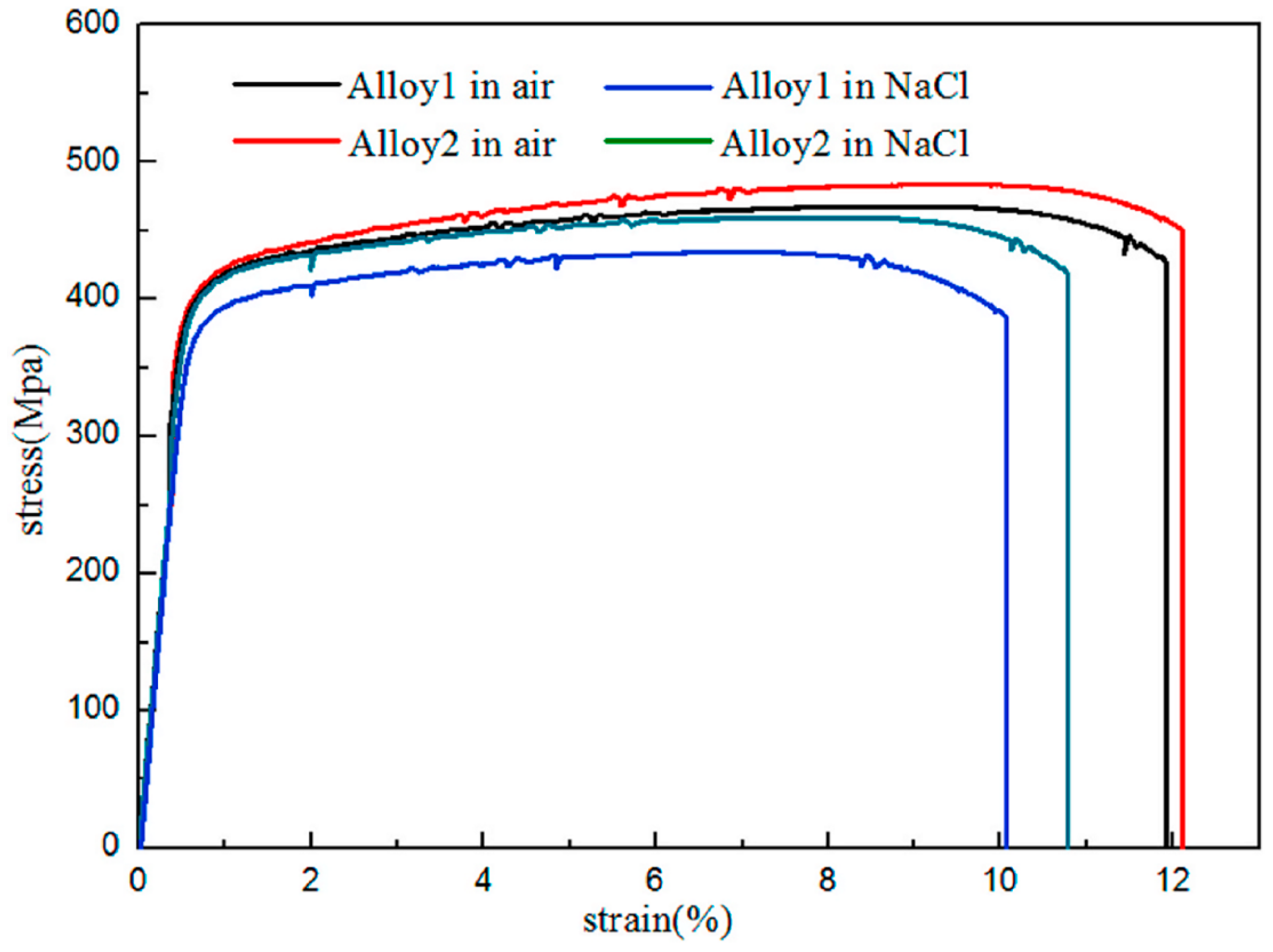
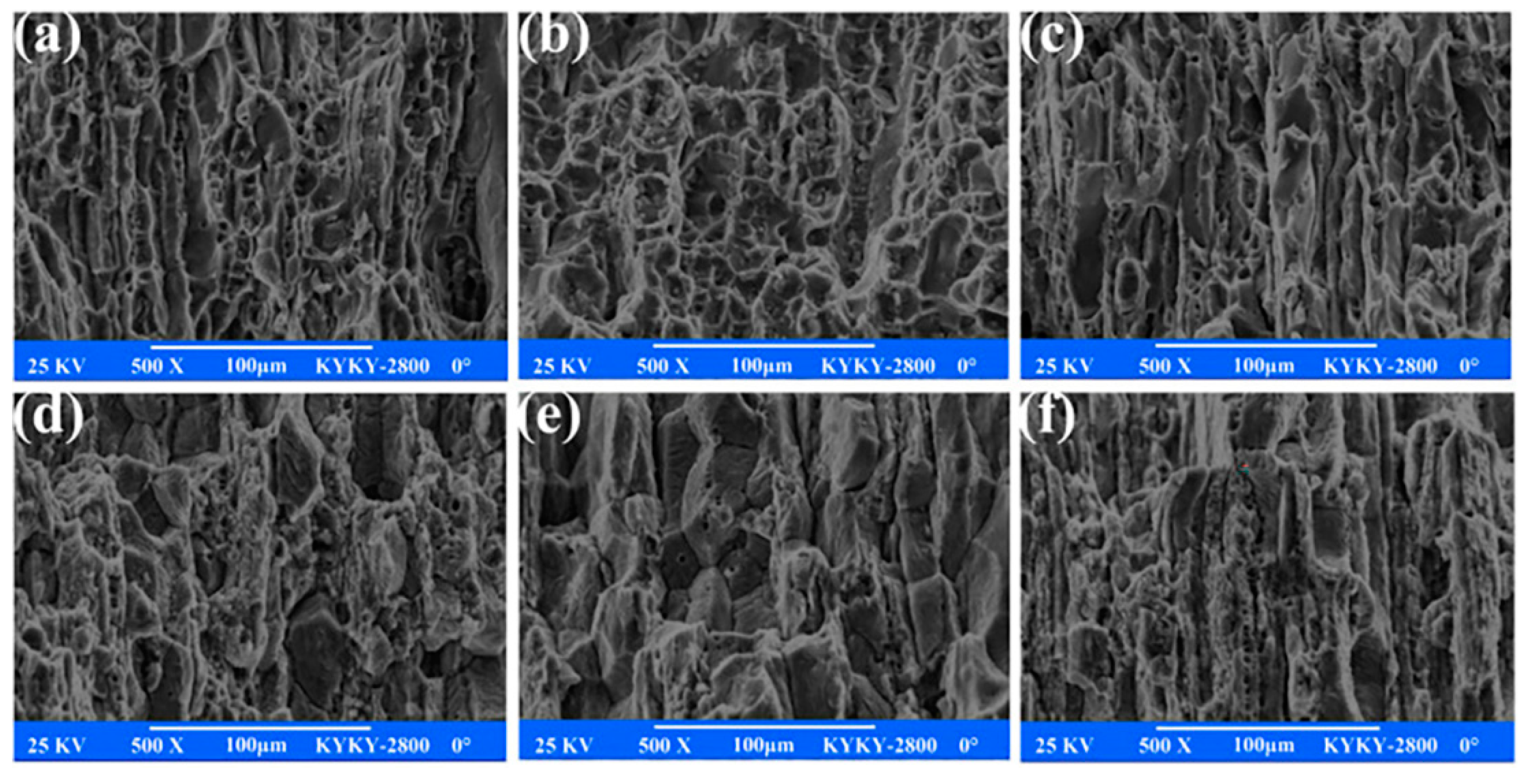
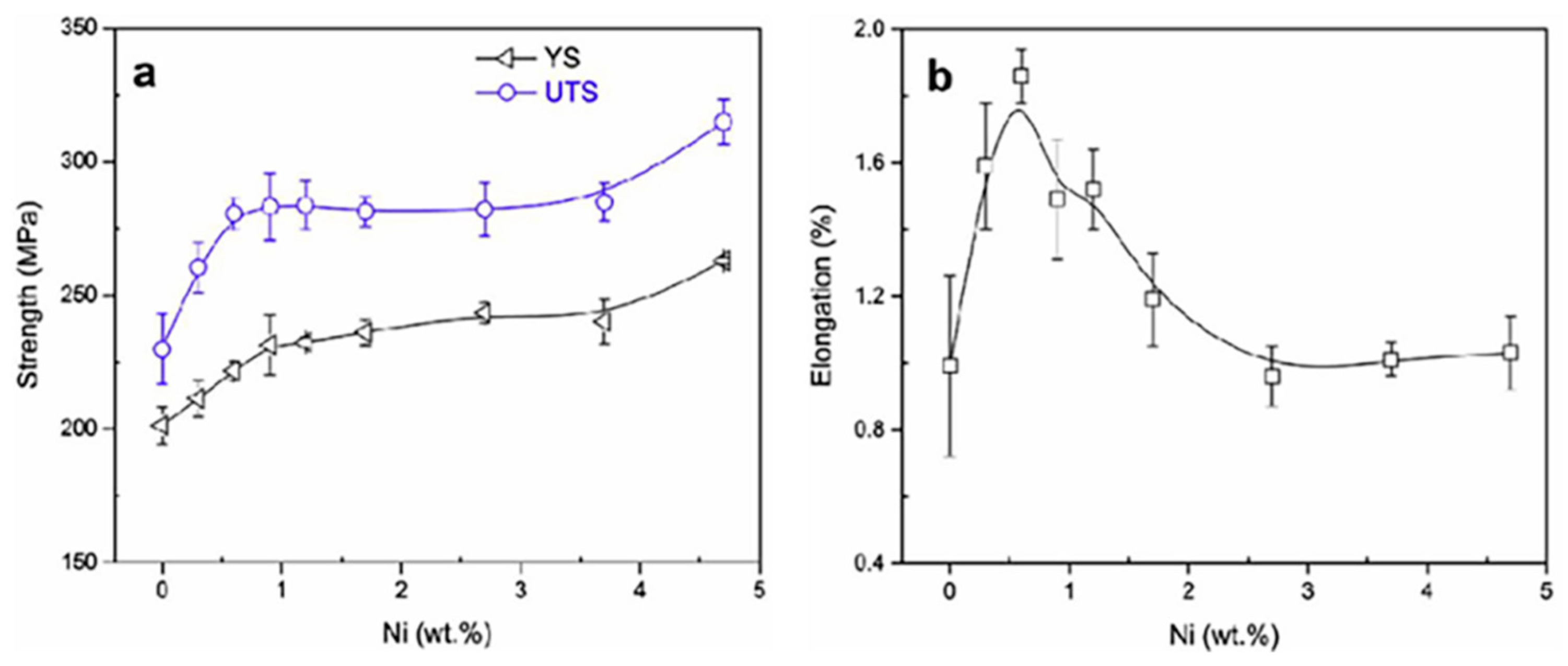
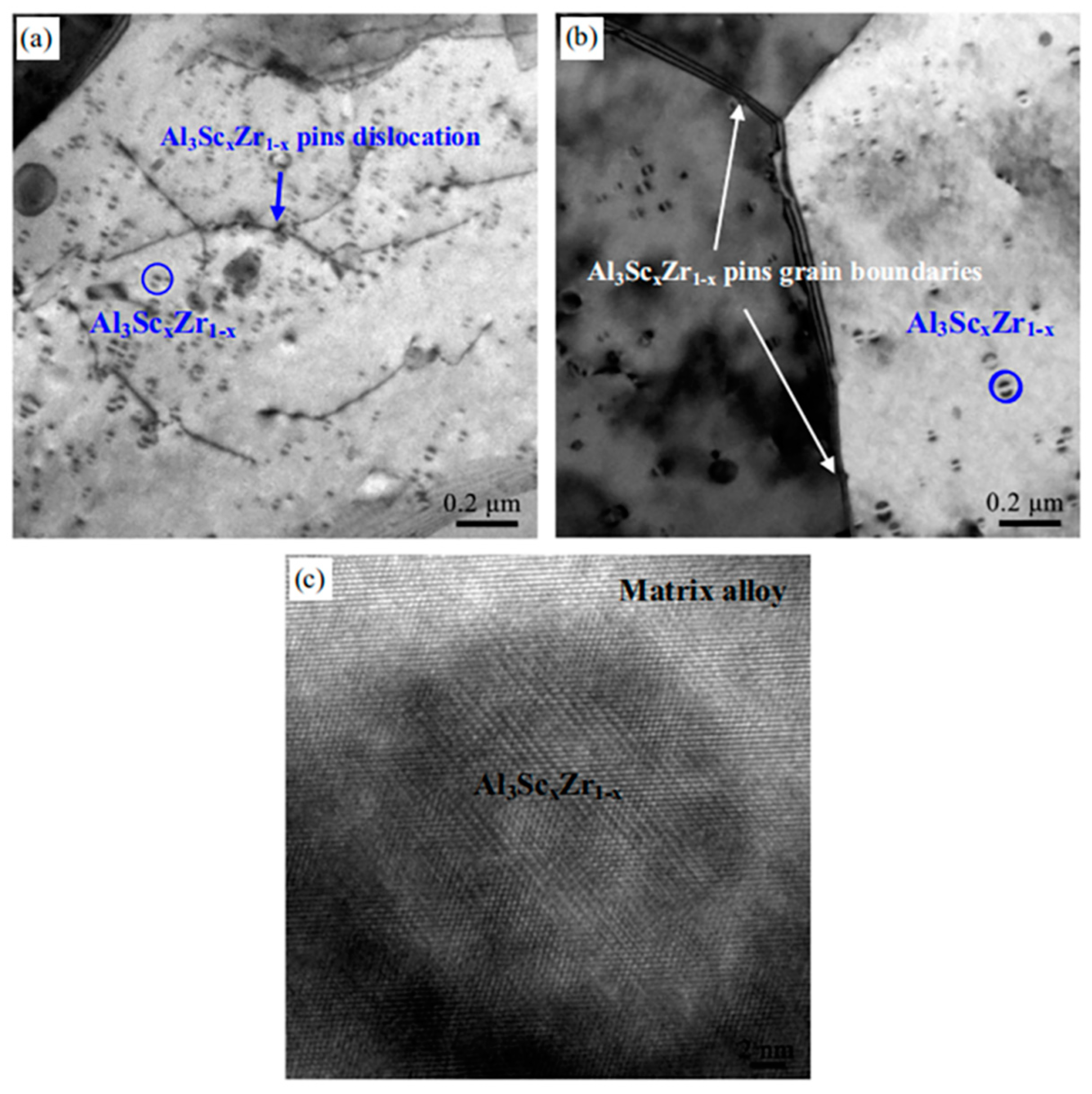
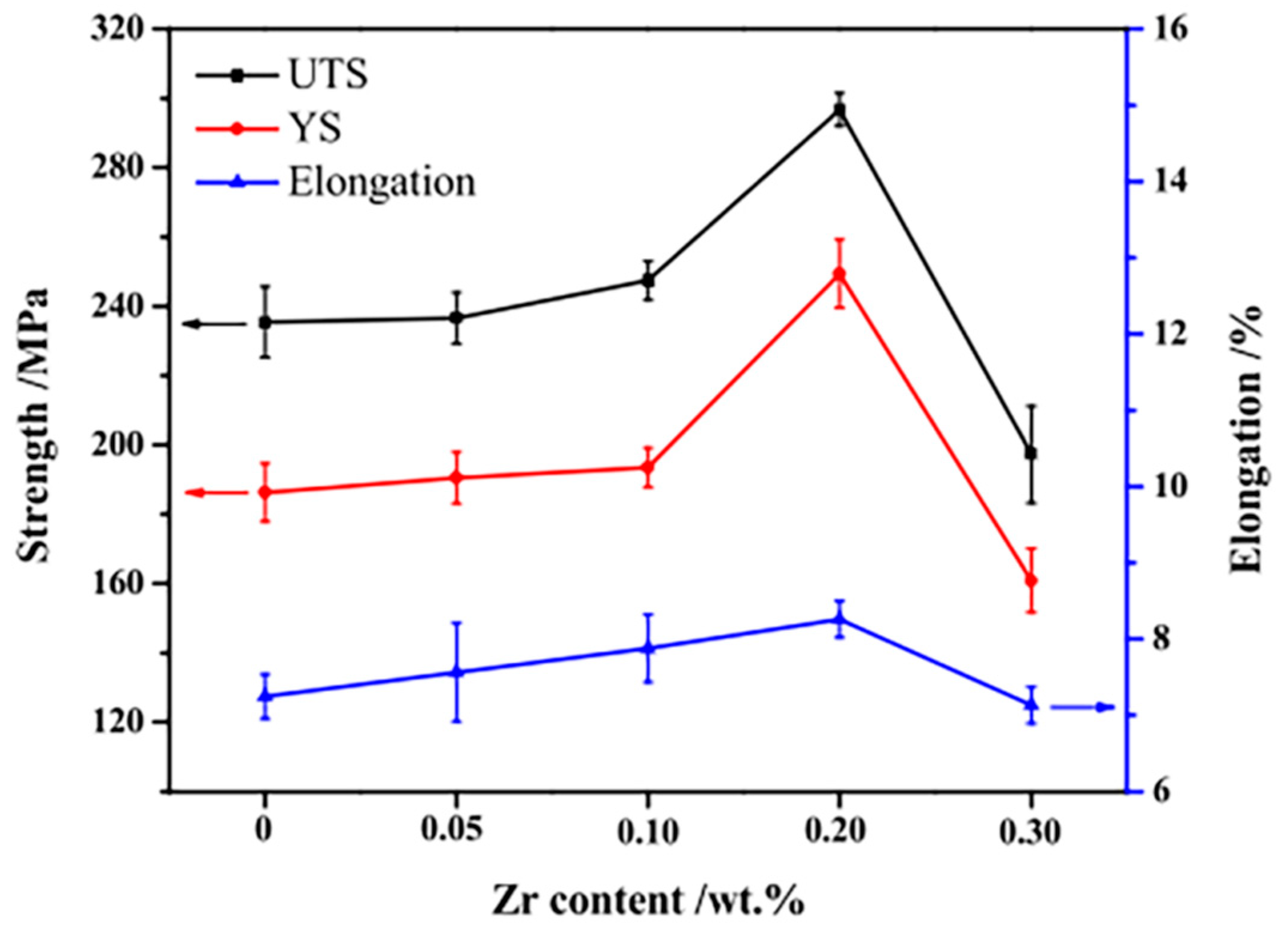
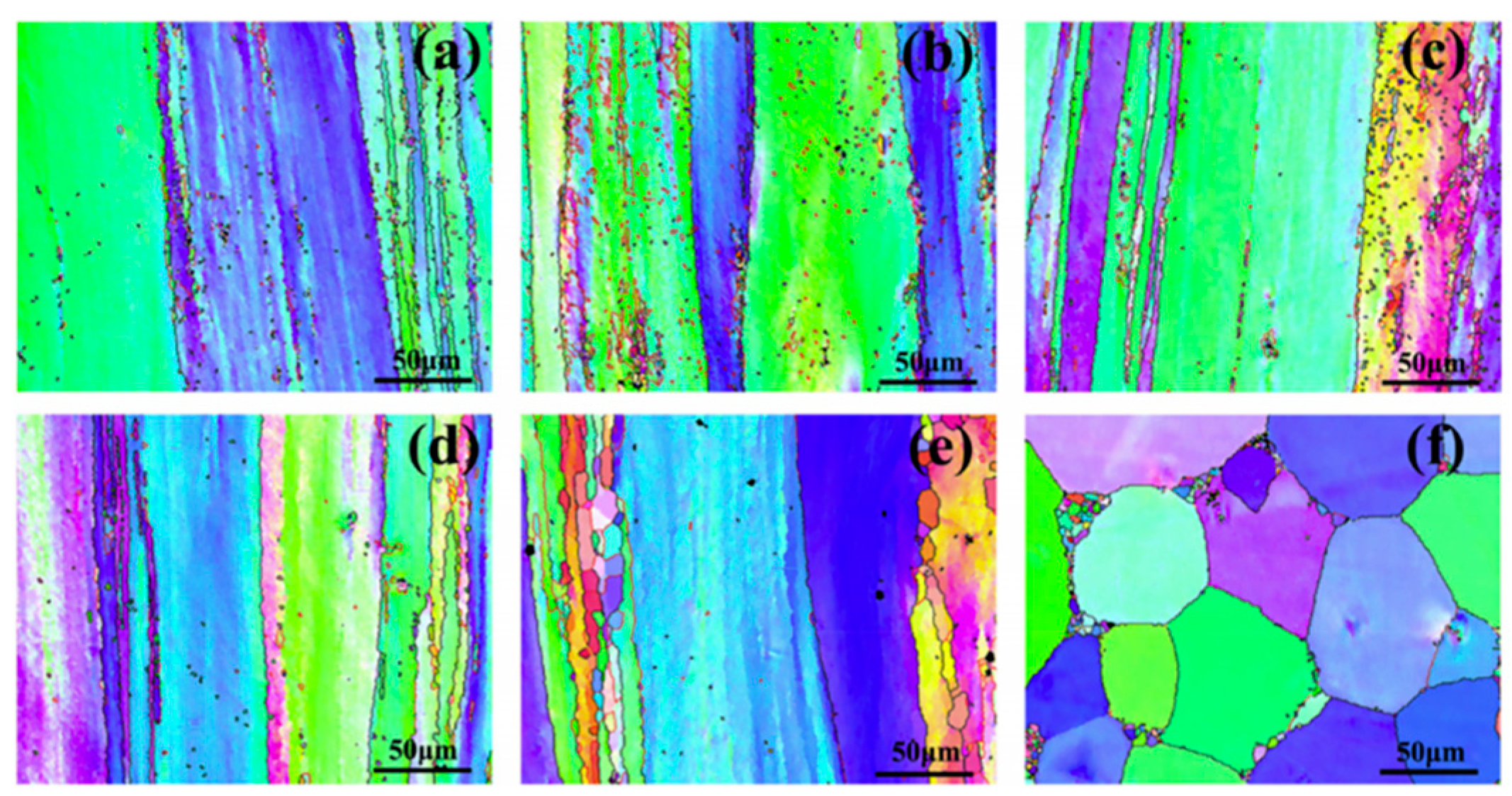
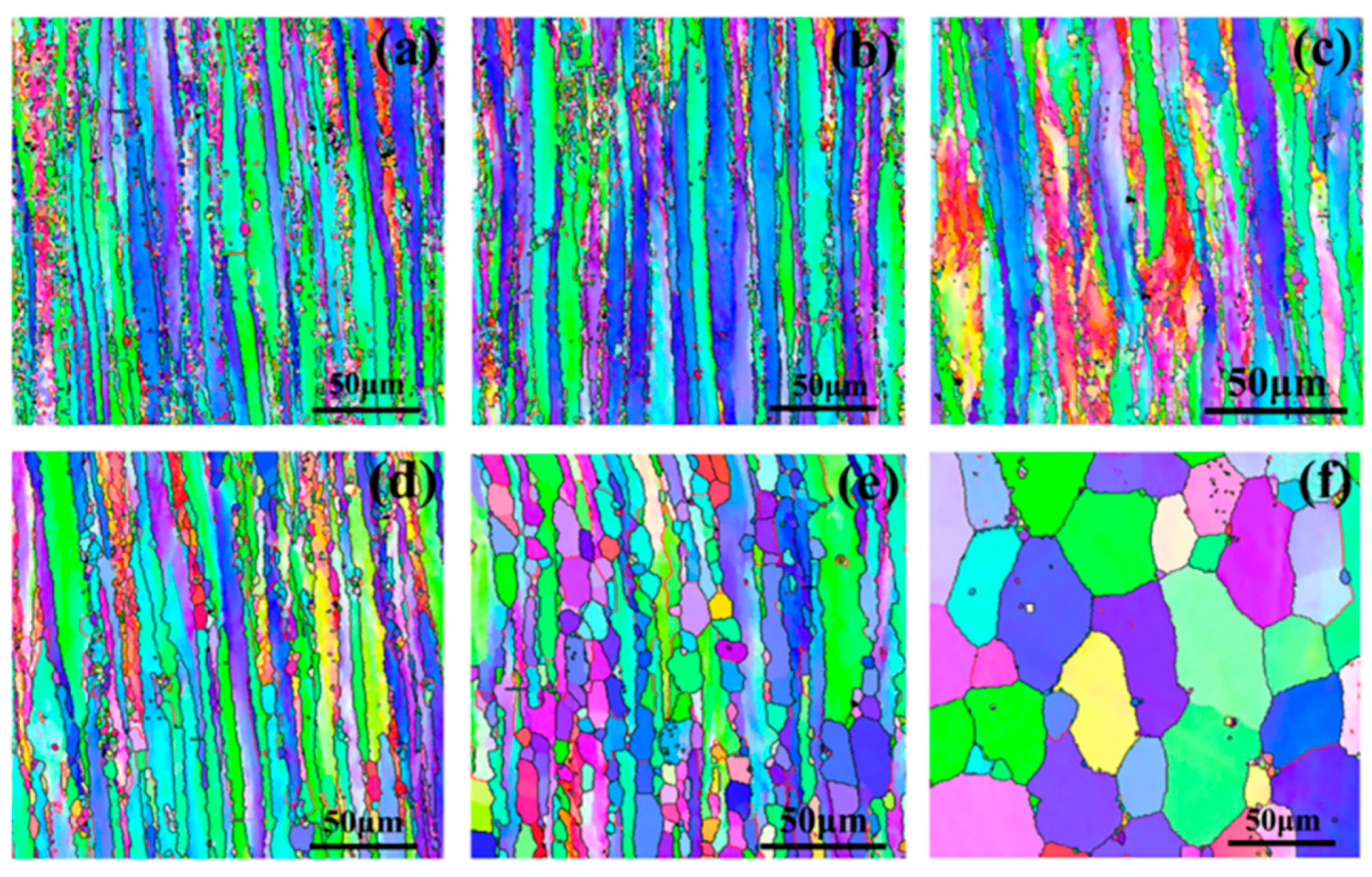
| Alloy Element Content | Dissolving Process |
|---|---|
| low Zn/Mg ratio | α(supersaturated solid solution) → GP region → η′(MgZn2) → η(MgZn2) |
| high Zn/Mg ratio | α(supersaturated solid solution) → GP region → T′(Al2Mg3Zn2) → T(MgZn2) |
| excessive Cu | α(supersaturated solid solution) → GP region → η′(MgZn2) → S(MgZn2) |
| 7055-xZr-ySc | Sc + Zr/wt.% | YS/Mpa | UTS/Mpa | EL/% |
|---|---|---|---|---|
| 7055 | 0.16 | 577 ± 2 | 654 ± 2 | 12.8 ± 0.4 |
| 7055-0.2Sc | 0.36 | 609 ± 2 | 649 ± 3 | 13 ± 0.5 |
| 7055-0.25Sc | 0.41 | 600 ± 2 | 679 ± 2 | 14.3 ± 0.5 |
| 7055-0.14Zr-0.15Sc | 0.45 | 602 ± 3 | 657 ± 4 | 13.7 ± 0.8 |
| 7055-0.14Zr-0.2Sc | 0.5 | 593 ± 3 | 635 ± 5 | 10.3 ± 0.6 |
| 7055-0.24Zr-0.15Sc | 0.55 | 580 ± 2 | 616 ± 3 | 7.3 ± 0.3 |
| Rare Earth Element | Rare Earth Phase | Crystal Structure |
|---|---|---|
| Sc | Al3Sc | FCC |
| Sm | Al10Cu7Sm2 | Hexagonal structure |
| Er | Al8Cu4Er | Hexagonal structure |
| Ce | Al4Ce | FCC |
| La | Al11La3 | FCC |
| Y | Al8Cu4Y | Hexagonal structure |
| Ta | Al3Ta | FCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, F. Research Status and Prospective Properties of the Al-Zn-Mg-Cu Series Aluminum Alloys. Metals 2023, 13, 1329. https://doi.org/10.3390/met13081329
Wang J, Li F. Research Status and Prospective Properties of the Al-Zn-Mg-Cu Series Aluminum Alloys. Metals. 2023; 13(8):1329. https://doi.org/10.3390/met13081329
Chicago/Turabian StyleWang, Jue, and Faguo Li. 2023. "Research Status and Prospective Properties of the Al-Zn-Mg-Cu Series Aluminum Alloys" Metals 13, no. 8: 1329. https://doi.org/10.3390/met13081329
APA StyleWang, J., & Li, F. (2023). Research Status and Prospective Properties of the Al-Zn-Mg-Cu Series Aluminum Alloys. Metals, 13(8), 1329. https://doi.org/10.3390/met13081329






