A Pretreatment of Refractory Gold Ores Containing Sulfide Minerals to Improve Gold Leaching by Ammonium Thiosulfate: A Model Experiment Using Gold Powder and Arsenic-Bearing Sulfide Minerals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaching Experiments
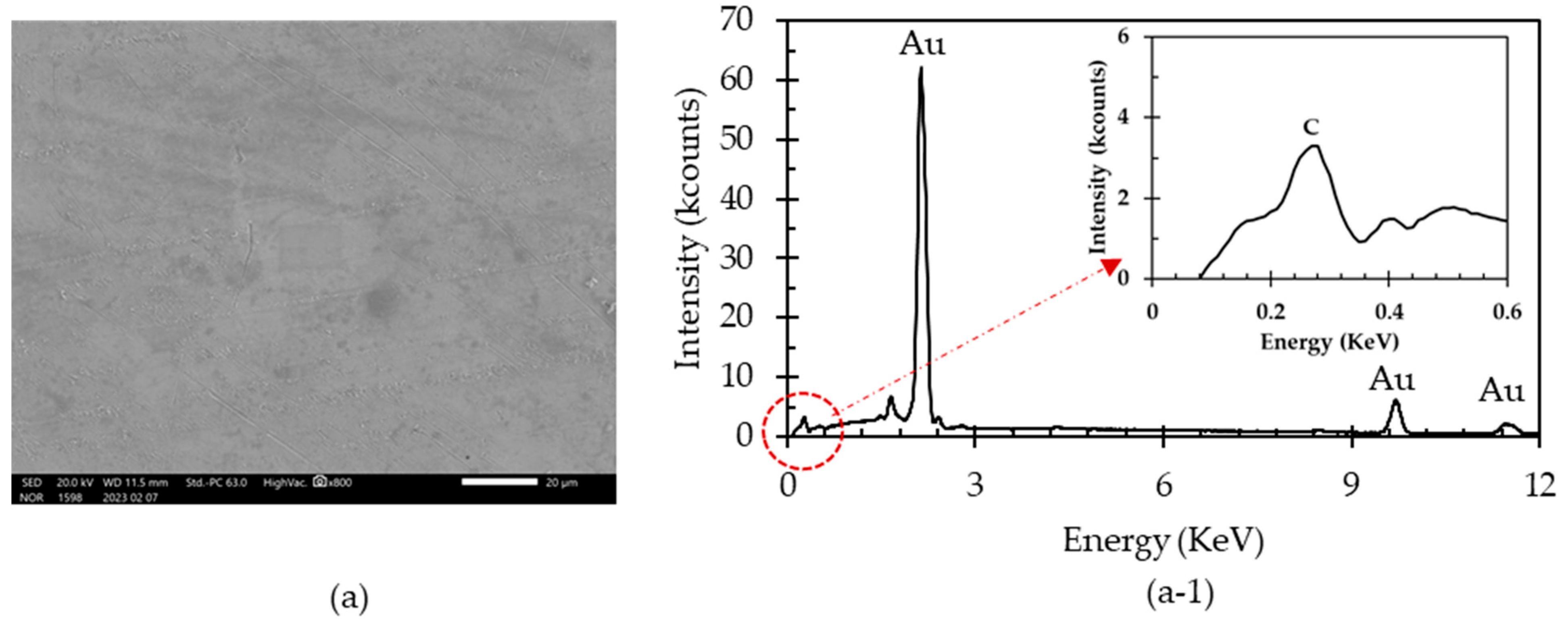
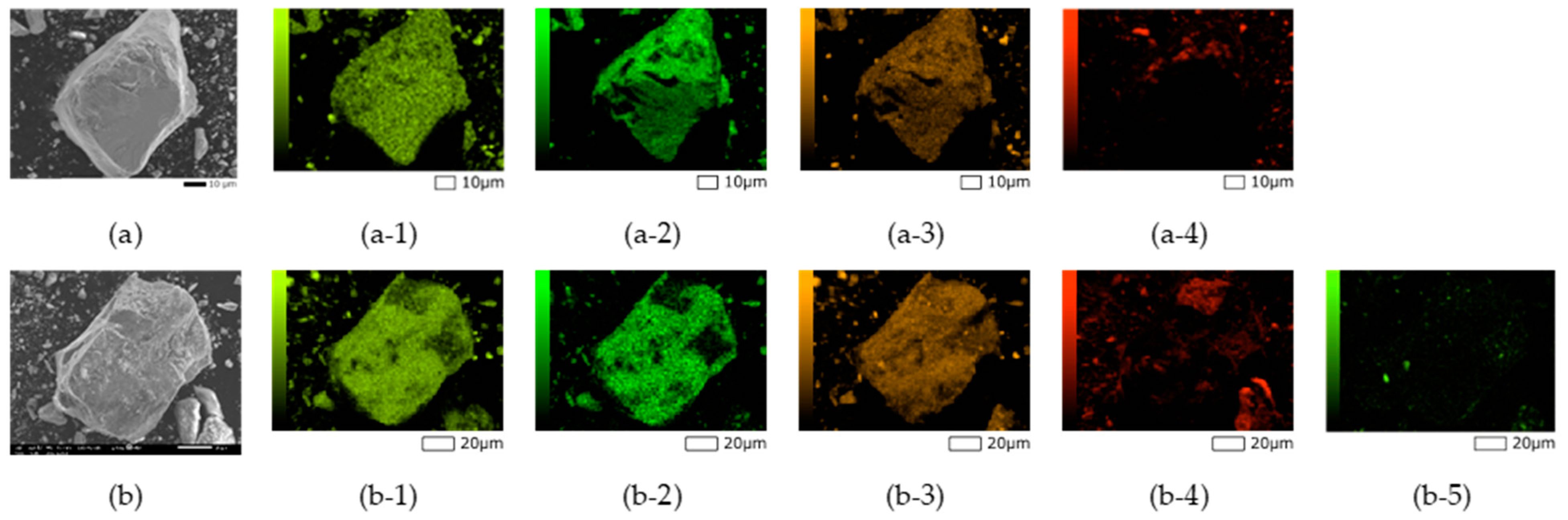
2.3. Characterization of the Passivation Layer on Gold Surface
2.4. Pretreatment Experiments Using Ammonium Solution Containing Cupric Ions
3. Results and Discussion
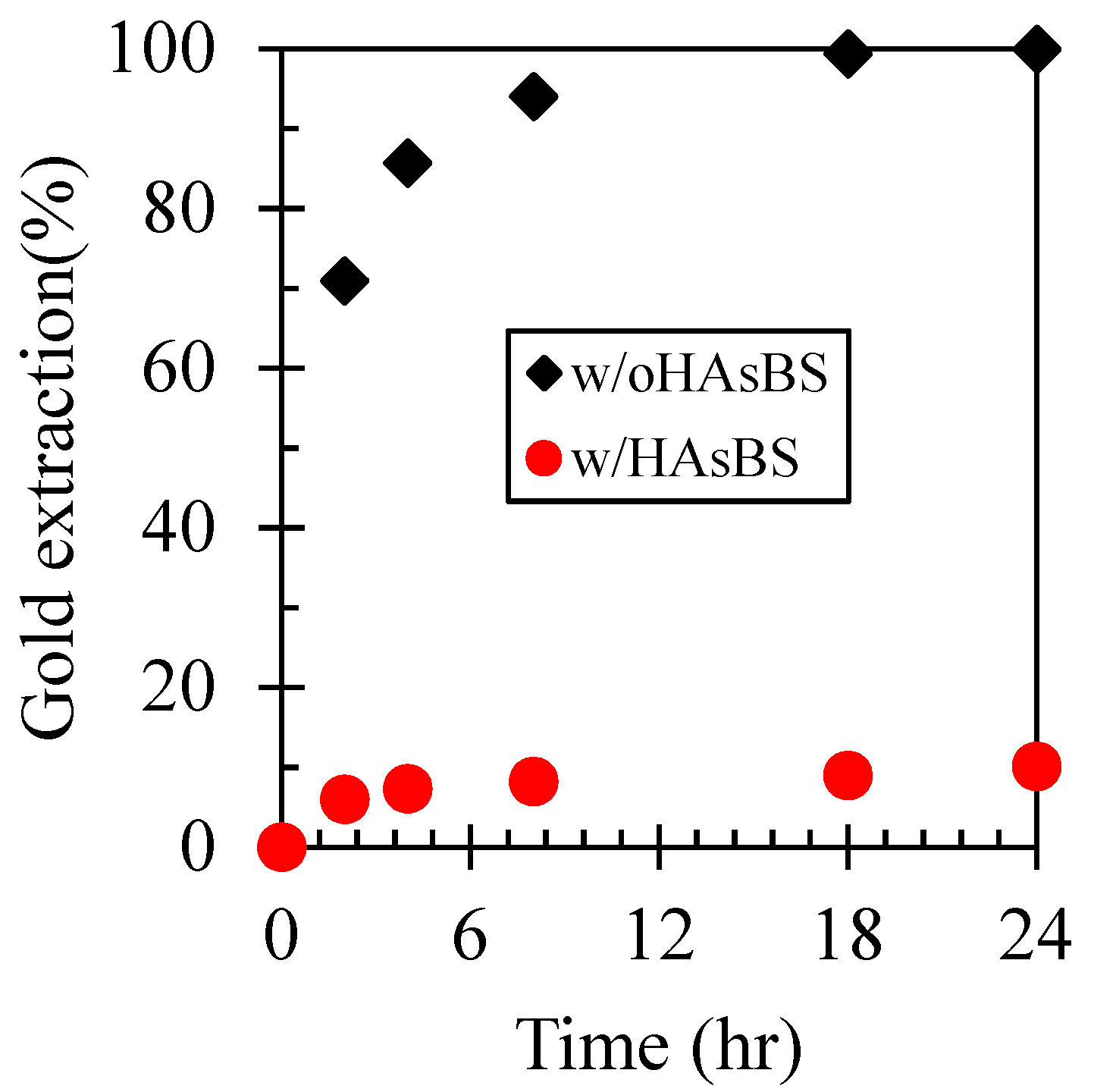
3.1. Effect of HAsBS Ore on Gold Dissolution
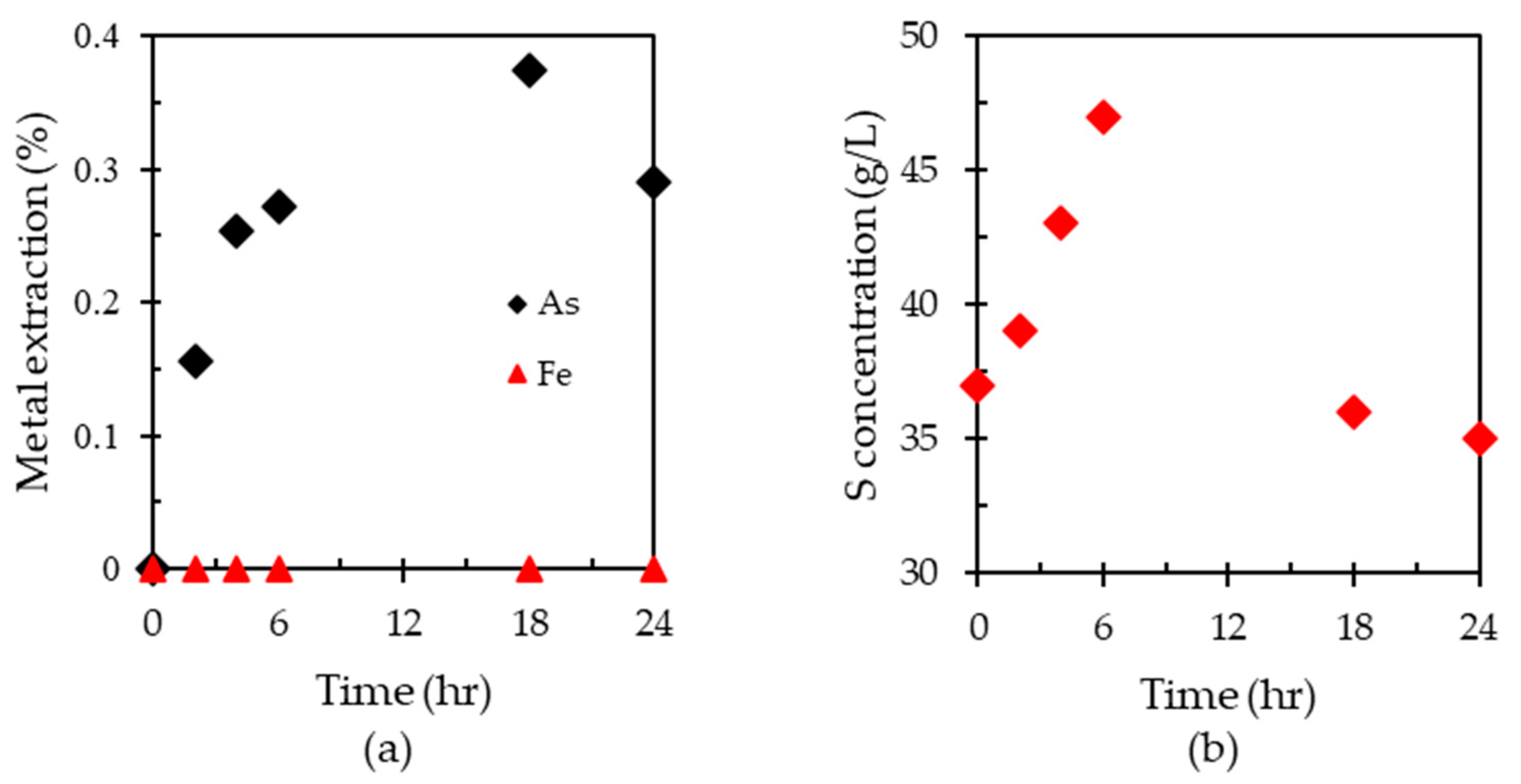
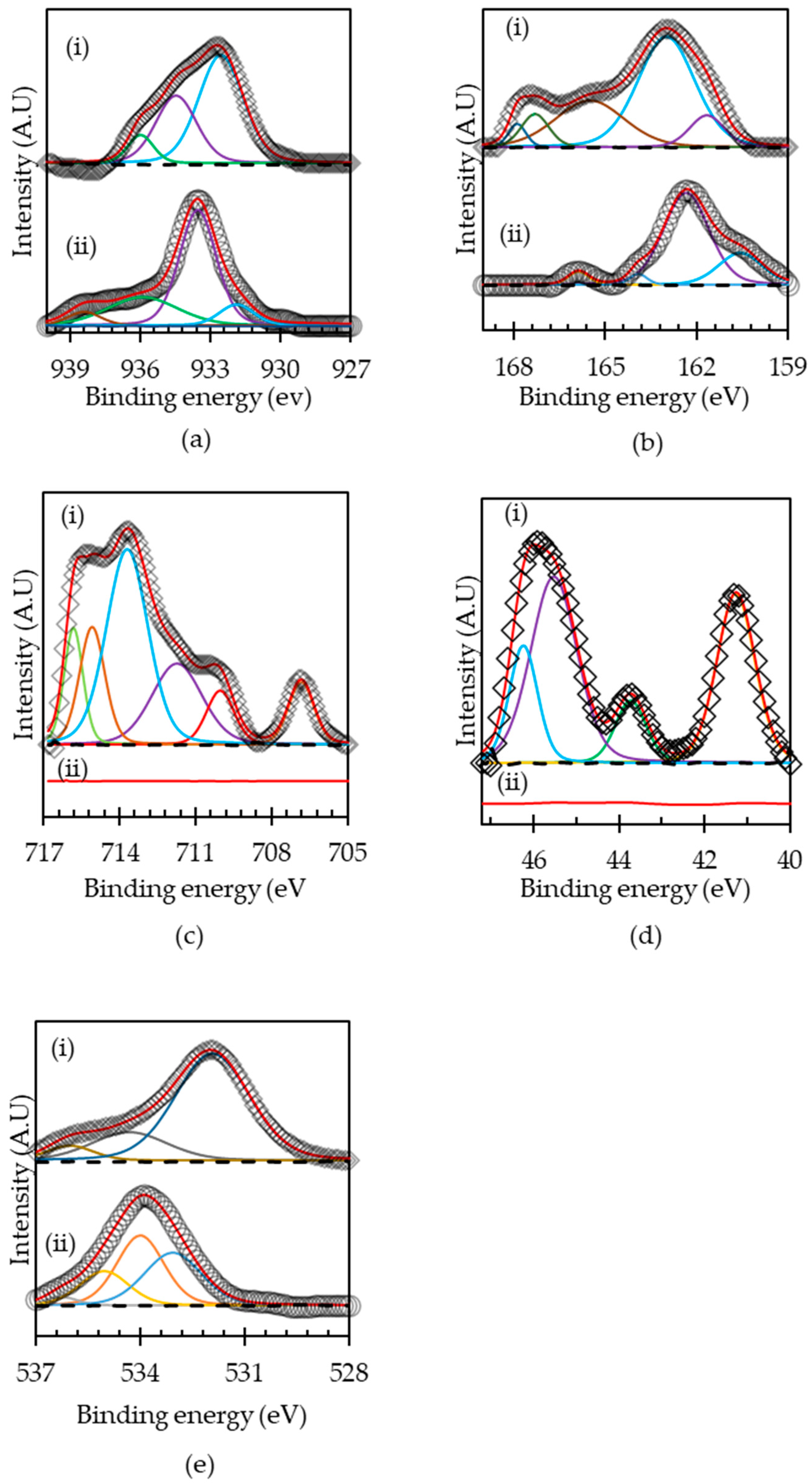
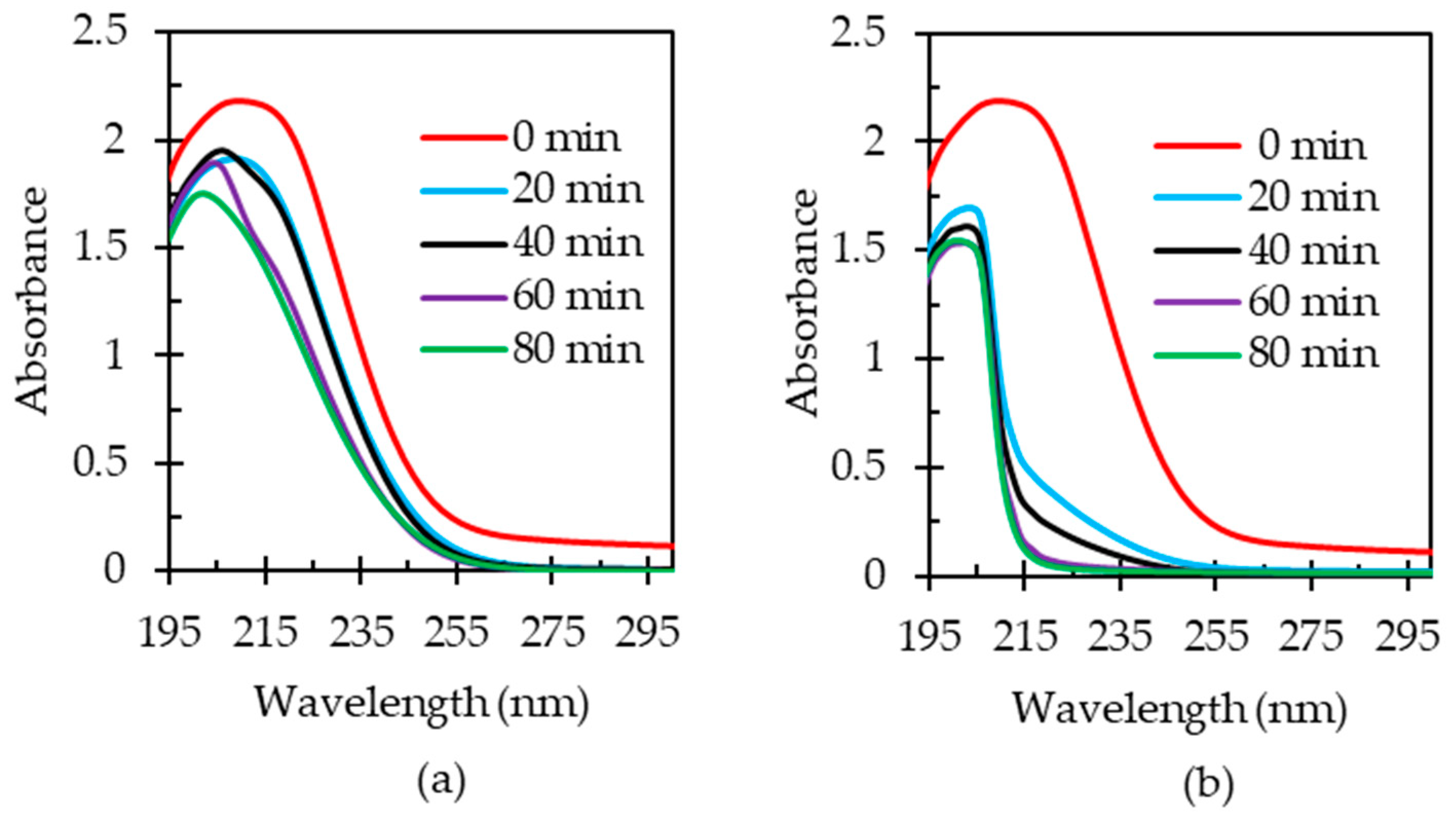
3.2. Oxidation of HAsBS Ore in Ammonium Thiosulfate Solution
3.3. Effects of the Passivation Layer on Gold Extraction
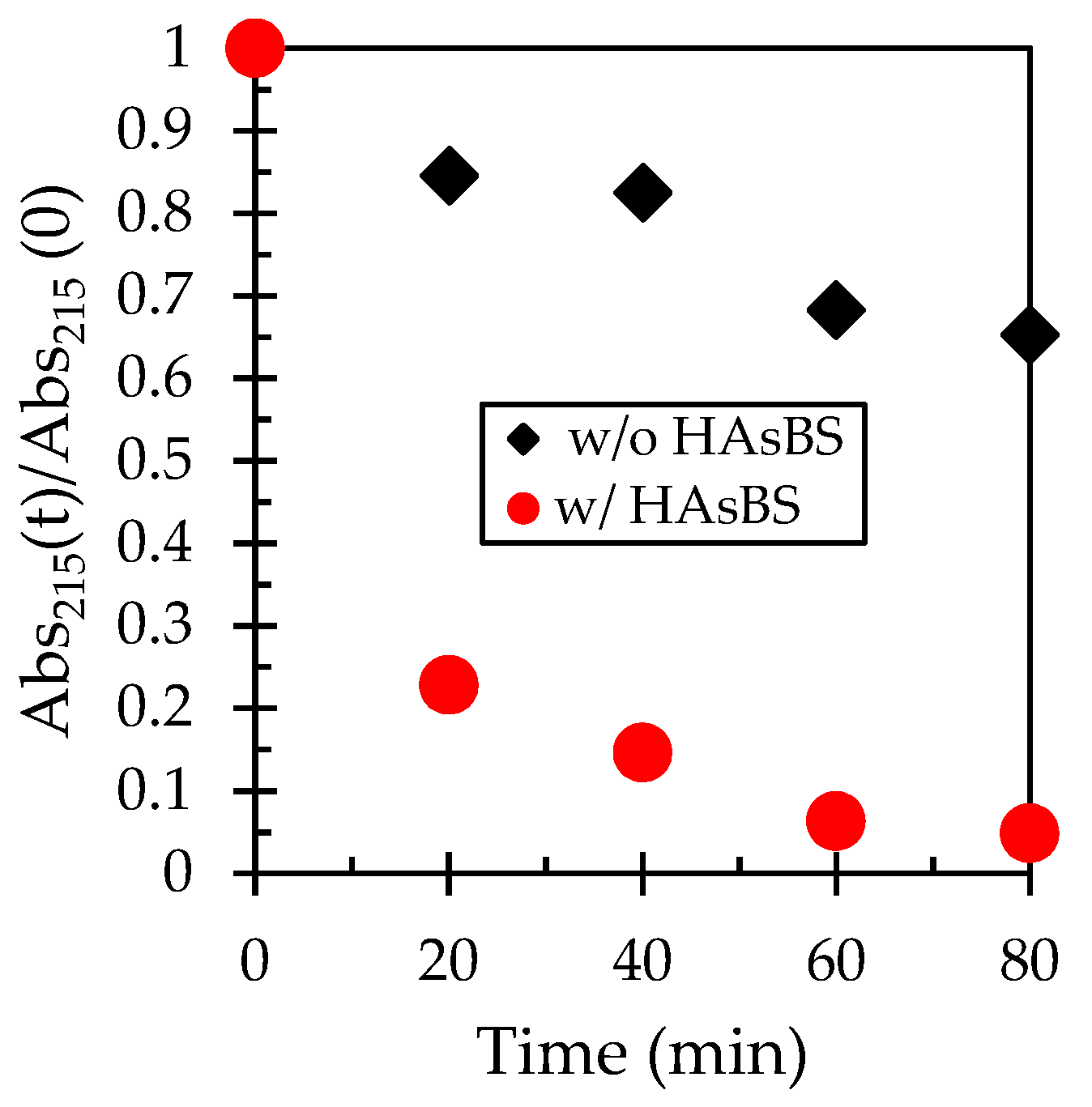
3.4. Effect of HAsBS on Thiosulfate Consumption
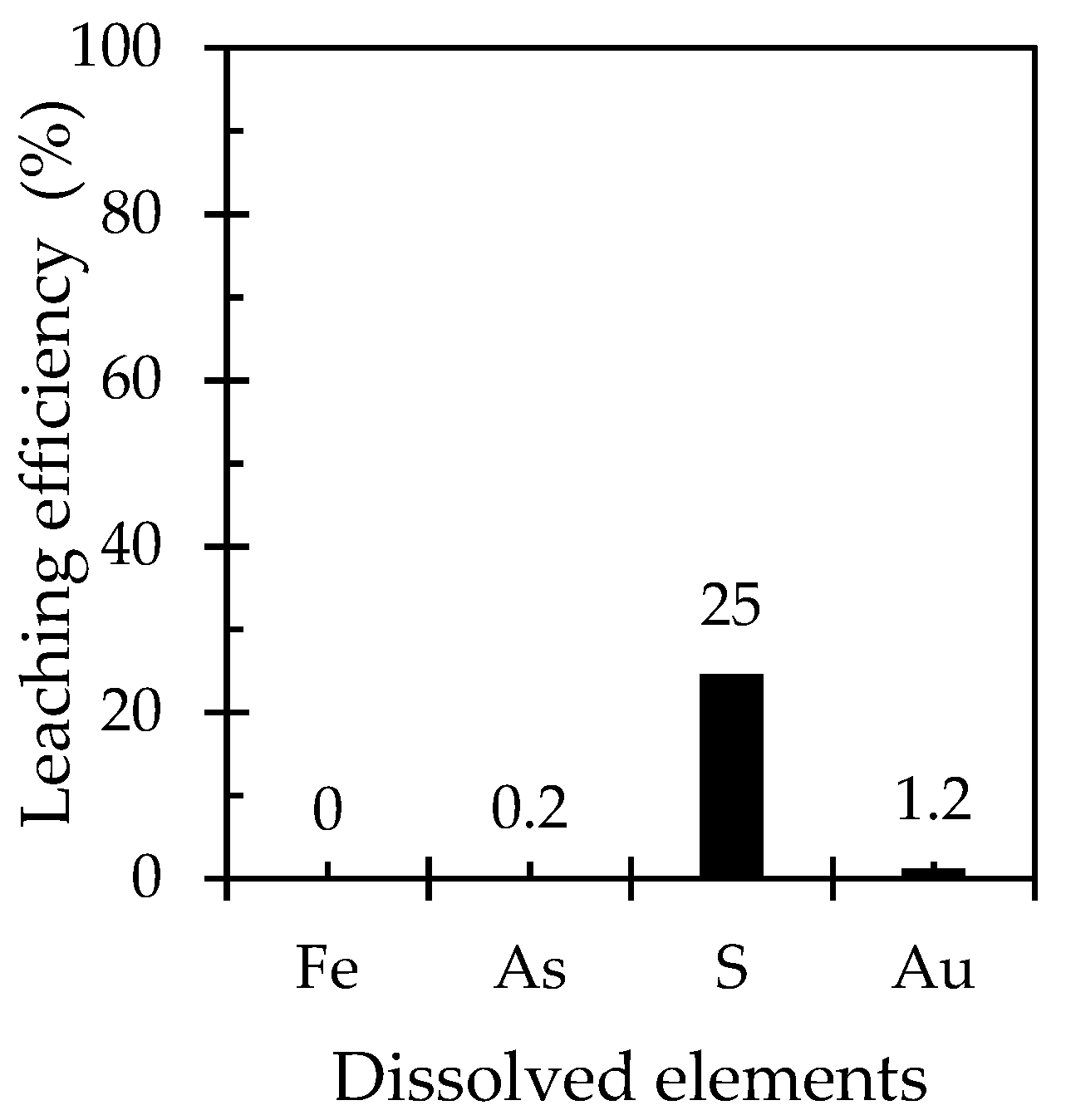
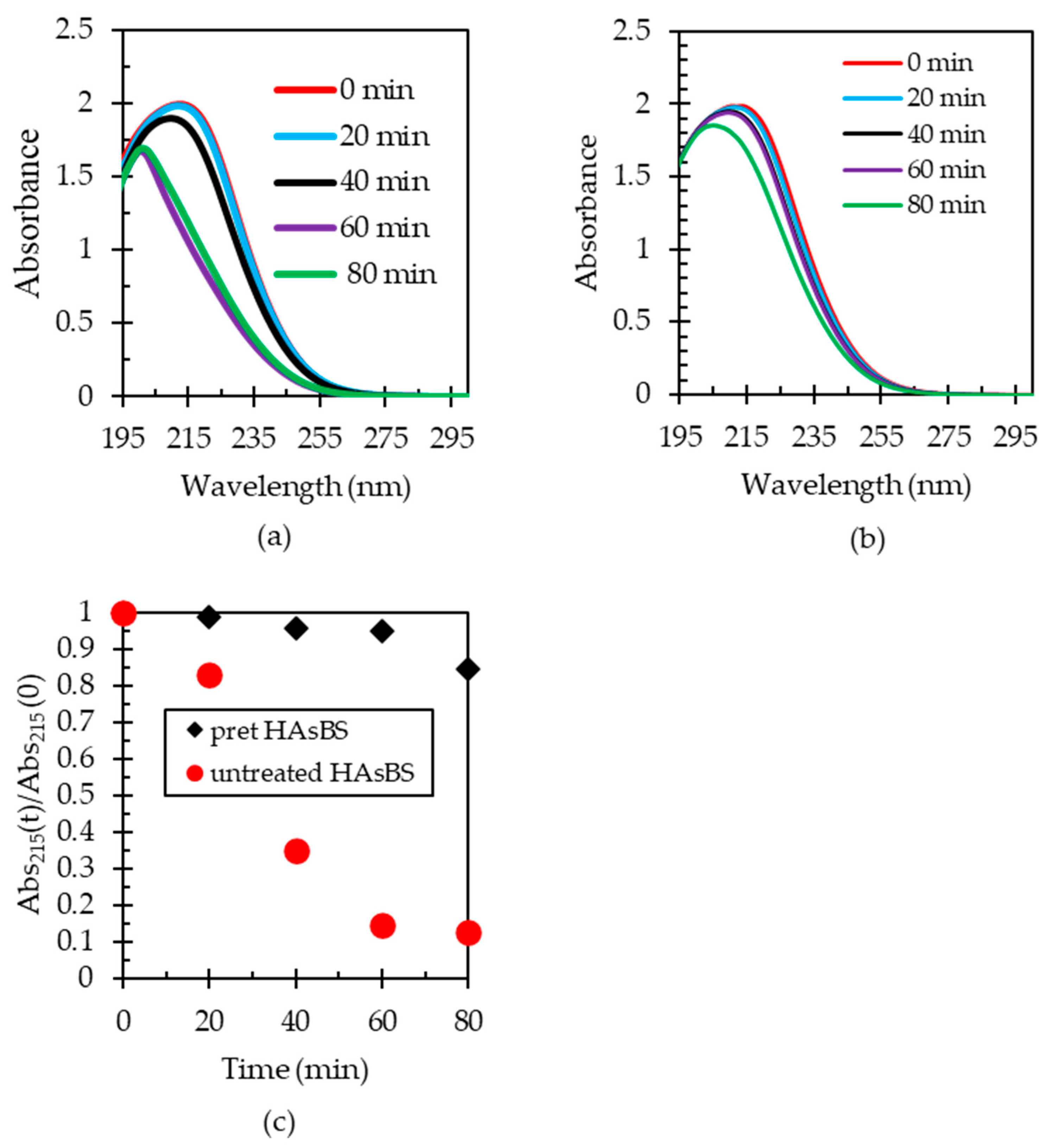
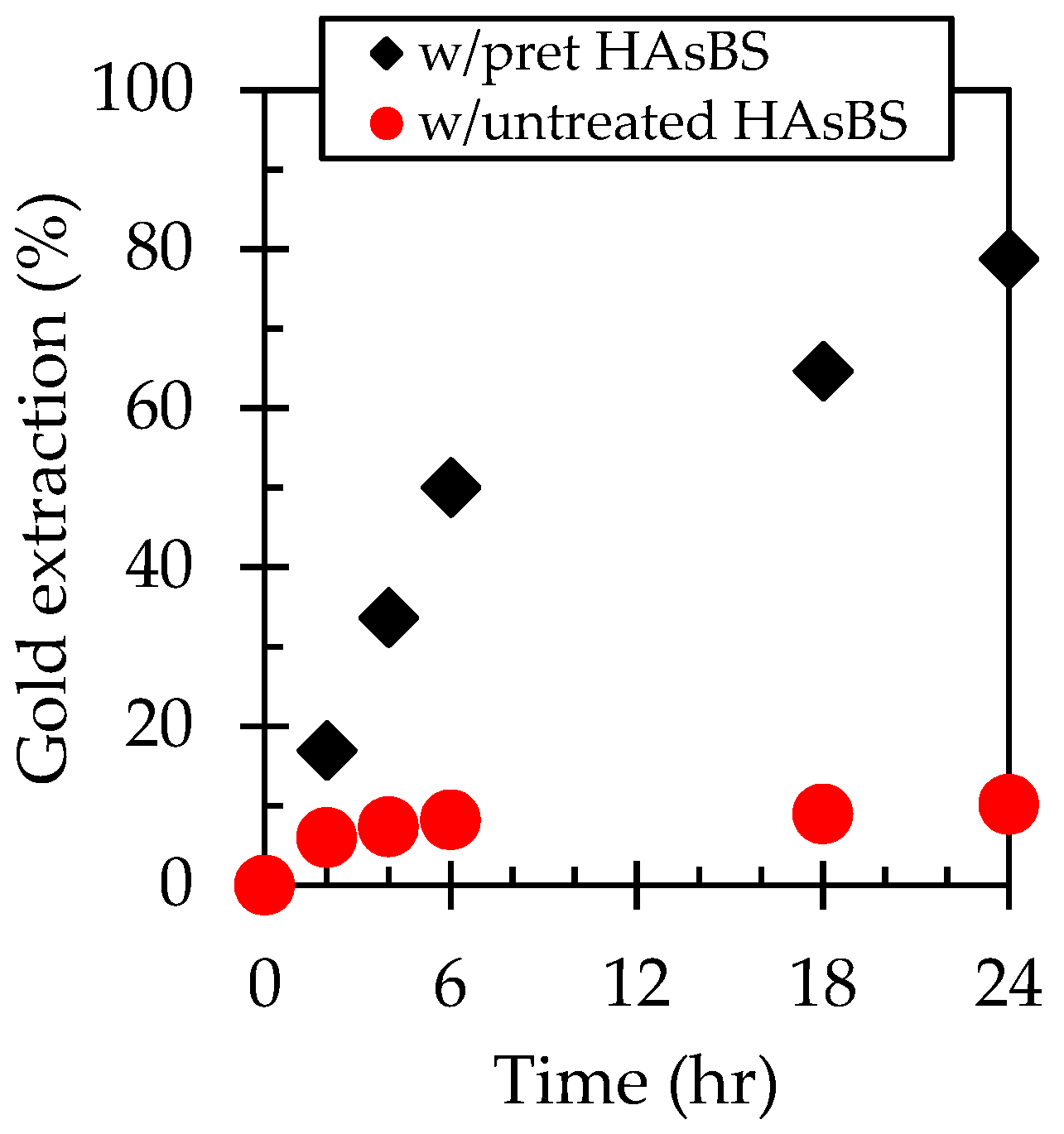
3.5. Pre-Oxidation of HAsBS and Its Effect on Gold Leaching
3.5.1. Pre-Oxidation of HAsBS Using Ammonia Solution Containing Cupric Ions
3.5.2. Effect of Pre-Oxidation of HAsBS on Thiosulfate Decomposition
3.5.3. Effect of Pre-Oxidation of HAsBS on Gold Extraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ortego, A.; Calvo, G.; Valero, A.; Iglesias-Émbil, M.; Valero, A.; Villacampa, M. Assessment of strategic raw materials in the automobile sector. Resour. Conserv. Recycl. 2020, 161, 104968. [Google Scholar] [CrossRef]
- Moore, J.; James, C. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- WorldGoldCouncil. World Gold Council, GOLDHUB. 1 November 2022. Available online: https://www.gold.org/goldhub/data/gold-supply-and-demand-statistics (accessed on 23 January 2023).
- Schodde, R. Recent trends in gold discovery. In Proceedings of the 2011 NewGenGold Conference, Perth, Australia, 22–23 November 2011. [Google Scholar]
- MetalsFocus. Gold Focus 2020. MetalsFocus. 25 June 2020. Available online: https://www.metalsfocus.com/wp-content/uploads/2020/11/GOLD-FOCUS-2020.pdf (accessed on 14 February 2023).
- Mudd, G.; Giurco, D.; Mohr, S.; Mason, L. Gold Resources and Production: Australia in a Global Context. The Department of Civil Engineering (Monash University) and the Institute for Sustainable Futures. University of Technology Sydney, Sydney, Australia, 2012. 1 November 2012. Available online: http://cfsites1.uts.edu.au/find/isf/publications/muddetal2012goldresourcesproduction (accessed on 4 December 2022).
- Tabelin, C.; Park, I.; Jeon, S.; Phengsaart, T.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar]
- Vikentyev, I.; Vikent, O.; Tyukova, E.; Nikolsky, M.; Ivanova, J.; Sidorova, N.; Tonkacheev, D.; Abramova, V.; Blokov, V.; Spirina, A. Noble Metal Speciations in Hydrothermal Sulphides. Minerals 2021, 11, 488. [Google Scholar] [CrossRef]
- Donoghue, S.; Young, M. Environmental Assessment Report; Dargues Reef Gold Mine Modification 3 (10_0054 MOD 3); NSW Government, Department of Planning & Environment: New South Wales, Australia, 2016.
- Xiao, L.; Wang, Y.; Qian, P. Advances in non-cyanide process for gold smelting. Gold Sci. Technol. 2019, 27, 292–301. [Google Scholar]
- Adams, C.R.; Porter, C.P.; Robshaw, T.J.; Bezzina, J.P.; Shields, V.R.; Hides, A.; Bruca, R.; Ogden, M.D. An alternative to cyanide leaching of waste activated carbon ash for gold and silver recovery via synergistic dual-lixiviant treatment. J. Ind. Eng. Chem. 2020, 92, 120–130. [Google Scholar]
- Zhang, Y.; Cui, M.; Wang, J.; Liu, X.; Lyu, X. A review of gold extraction using alternatives to cyanide: Focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviants. Miner. Eng. 2022, 176, 107336. [Google Scholar] [CrossRef]
- Brooy, S.; Linge, H.; Walker, G. Review of gold extraction from ores. Miner. Eng. 1994, 70, 1231–1241. [Google Scholar] [CrossRef]
- Celep, O.; Altinkaya, E.; Yazici, Y.; Deveci, H. Thiosulfate leaching of silver from arsenical refractory ore. Miner. Eng. 2018, 122, 63–70. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsmrankul, R.; Kitajima, N.; Park, I.; Hiroyoshi, N. Gold recovery from shredder light fraction of E-waste recycling plant by flotation-ammonium thiosulfate leaching. Waste Manag. 2018, 77, 195–202. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.; Wang, J. Review of gold leaching in thiosulfate-based solutions. Trans. Nonferrous Met. Soc. China 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Xu, B.; Wenhao, K.; Qian, L.; Yongbin, Y.; Tao, J.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from refractory ores. Minerals 2017, 7, 222. [Google Scholar]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Ilhwan, P.; Ito, M.; Hiroyoshi, N. Enhanced cementation of gold via galvanic interactions using activated arbon and zero-valent aluminum: A novel approach to recover gold ions from ammonium thiosulfate medium. Hydrometallurgy 2020, 191, 105165. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J. Leaching behaviour of sulphides in ammoniacal thiosulphate systems. Hydrometallurgy 2002, 63, 5–13. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J. The role of heavy metal ions in gold dissolution in the ammoniacal thiosulfate system. Hydrometallurgy 2002, 64, 231–246. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Xu, B.; Li, Q.; Jiang, T.; Wang, Y. Effect of arsenopyrite on thiosulfate leaching of gold. Trans. Nonferrous Met. Soc. China 2015, 25, 3454–3460. [Google Scholar] [CrossRef]
- Park, I.; Higuchi, K.; Tabelin, C.; Jeon, S.; Ito, M.; Hiroyoshi, N. Suppression of arsenopyrite oxidation by microencapsulation using ferric-catecholate complexes and phosphate. Chemosphere 2021, 269, 129413. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, J.; Wang, Q.; Zhang, C.; Shi, C.; Zhao, J. Use of dry grinding process to increase the leaching of gold from a roasted concentrate containing hematite in the thiosulfate system. Hydrometallurgy 2021, 201, 105582. [Google Scholar] [CrossRef]
- Bowell, R.; Alpers, C.; Jamieson, H.; Nordstrom, D.; Majzlan, J. The environmental geochemistry of arsenic- an overview. Rev. Mineral. Geochem. 2014, 79, 1–16. [Google Scholar] [CrossRef]
- Yang, D.; Shi, M.; Zhang, J.; Sasaki, A.; Endo, M. Reductive roasting of arsenic-contaminated red mud for Fe resources recovery driven by johnbaumite-based arsenic thermostabilization strategy. J. Harzadous Mater. 2023, 452, 131255. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. The Global Biogeochemical Cycle of Arsenic. Glob. Biogeochem. Cycles 2022, 36, e2022GB007515. [Google Scholar] [CrossRef]
- Duan, L.; Li, X.; Jiang, Y.; Lei, M.; Dong, Z. Arsenic transformation behaviour during thermal decomposition of P. vittata, an arsenic hyperaccumulator. J. Anal. Appl. Pyrolysis 2017, 124, 584–591. [Google Scholar] [CrossRef]
- Fleming, C.; McMullen, J.; Thomas, K.; Wells, J. Recent advances in the development of an alternative to the cyanidation process: Thiosulfate leaching and resin in pulp. Min. Met. Explor. 2001, 20, 1–9. [Google Scholar] [CrossRef]
- Grosse, A.; Dicinoski, G.; Shaw, M.; Haddad, P. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors. Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Zhang, X.; Senanayake, G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in Non-cyanide gold lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Ter-Arakelyan, K. On technological expediency of sodium thiosulfate usage for gold extraction from raw material. Izv. V. U. Z. Tsvetn. Metall. 1984, 12, 72–76. [Google Scholar]
- Alguacil, F. The Chemistry of Gold Extraction, 2nd ed.; John O Marsden and CIain House SME: Littleton, CO, USA, 2006. [Google Scholar]
- Salinas-Rodríguez, E.; Hernández-Ávila, J.; Cerecedo-Sáenz, E.; Arenas-Flores, A.; Veloz-Rodríguez, M.; Toro, N.; Gutiérrez-Amador, M.; Acevedo-Sandoval, O.A. Leaching of Copper Contained in Waste Printed Circuit Boards, Using the Thiosulfate—Oxygen System: A Kinetic Approach. Materials 2022, 15, 2354. [Google Scholar] [CrossRef]
- Aylmore, M.; Muir, D. Thiosulfate leaching of gold-A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Robert, T.; Bartel, M.; Offergeld, G. Characterization of oxygen species adsorbed on copper and nickel oxides by X-ray photoelectron spectroscop. Surf. Sci. 1972, 33, 123–130. [Google Scholar] [CrossRef]
- Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. X-ray photoelectron spectroscopic study of copper minerals. J. Inorg. Nucl. Chem. 1978, 40, 789–791. [Google Scholar] [CrossRef]
- Mielczarski, J.; Minni, E. The adsorption of diethyldithiophosphate on cuprous sulphide. Surf. Interface Anal. 1984, 6, 221. [Google Scholar] [CrossRef]
- Wagner, C.; Riggs, W.; Davis, L.; Moulder, J.; Muilenberg, G. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MI, USA, 1979. [Google Scholar]
- Hitchcook, A.; Brion, C. K-shell excitation spectra of CO, N2 and O2. J. Electron Spectrosc. Relat. Phenom. 1980, 18, 1–21. [Google Scholar] [CrossRef]
- Nefedov, V.; Gati, D.; Dzhurinskii, B.; Sergushin, N.; Salyn, Y. A qualitative and quantitative study of the oxides of aluminum and silicon using AES and XPS. Russ. J. Inorg. Chem. 1975, 35, 1279. [Google Scholar]
- Can, F.; Demirci, O.; Dumoulin, F.; Erhan, E.; Arslan, L.; Ergenekon, P. Iron porphyrin-modified PVDF membrane as a biomimetic material and its effectiveness on nitric oxide binding. Appl. Surf. Sci. 2017, 420, 625–630. [Google Scholar] [CrossRef]
- Rajumon, M.; Prabhakaran, K.; Rao, C. Adsorption of oxygen on (100), (110) and (111) surfaces of Ag, Cu and Ni: An electron spectroscopic study. Surf. Sci. 1990, 233, 233–242. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex:nature of oxidation products, effects on anodic and cathodic reactions, andcoating stability under simulated weathering condition. Heliyon 2020, 6, 03189. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Hu, X.; Tian, J.; Wang, J.; Wan, R.; Zhou, X.; Shen, P.; Liu, D. Oxidation mechanism of the arsenopyrite surface by oxygen with and without water: Experimental and theoretical analysis. Appl. Surf. Sci. 2022, 573, 151574. [Google Scholar]
- Zhu, T.; Lu, X.; Liu, H.; Li, J.; Zhu, L.; Lu, J.; Wang, R. Quantitative X-ray photoelectron spectroscopy-based depth profiling of bioleached arsenopyrite surface by Acidithiobacillus ferrooxidans. Geochim. Et Cosmochim. Acta 2014, 127, 120–139. [Google Scholar] [CrossRef]
- Feng, X.; Liu, Q.; Wang, S.; Cen, L.; Li, H. Arsenopyrite weathering in acid rain: Arsenic transfer and environmental implications. J. Hazard. Mater. 2021, 420, 126612. [Google Scholar] [CrossRef]
- Hollinger, G.; Kabban, I.S.; Gendry, M. Oxides on GaAs and InAs surfaces: An x-ray-photoelectron-spectroscopy study of reference compounds and thin oxide layers. Phys. Rev. 1994, 49, 11159. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Akashino, R.; Bu, X.; Hassan, F.; Park, I.; Jeon, S.; Aikawa, K.; Hiroyoshi, N. Heterogenous carrier flotation technique for recovering finely ground chalcopyrite particles using coarse pyrite particles as a carrier. Miner. Eng. 2022, 180, 107518. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, X.; Tian, Q.; Liu, M.; Cai, N.; Xue, Y.; Chen, W.; Li, W.; Yu, F. Nitrogen-Doped Cu2S/MoS2 Heterojunction Nanorod Arrays on Copper Foam for Efficient Hydrogen Evolution Reaction. ChemCatChem 2018, 11, 1143–1373. [Google Scholar] [CrossRef]
- Wu, C.; Yin, M.; O’Brien, S.; Koberstein, J. Quantitative Analysis of Copper Oxide Nanoparticle Composition and Structure by X-ray Photoelectron Spectroscopy. Chem. Mater. 2006, 18, 6054–6058. [Google Scholar] [CrossRef]
- Biesinger, M.; Lau, L.; Gerson, A.; Smart, R. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar]
- Khan, S. UV-ATR Spectroscopy Study of the Speciation in Aqueous Polysulfide Electrolyte Solutions. Int. J. Electrochem. Sci. 2012, 7, 561–568. [Google Scholar] [CrossRef]
- Pappoe, M.; Bottaro, C. Systematic optimization of pyromellitic acid background electrolyte for capillary electrophoresis with indirect UV-Vis detection and online pre-concentration analysis of thiosalt anions in treated mine tailing. Anal. Methods 2014, 6, 9305–9312. [Google Scholar]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Electrochemical behaviour of the dissolution and passivation of arsenopyrite in 9K culture medium. Appl. Surf. Sci. 2020, 508, 145269. [Google Scholar] [CrossRef]
- de Jong, A.B.H.; Van Ijzendoorn, L.; Soudant, V.; de Beer, V. Preparation, Structure and Surface Chemical Properties of Hydrotreating Model Catalysts: A Surface Science Approach. J. Phys. Chem. 1993, 97, 6477. [Google Scholar]
- Yu, X.-R.; Liu, F.; Wang, Z.-Y.; Chen, Y. Auger parameters for sulfur-containing compounds using a mixed aluminum-silver excitation source. J. Electron Spectrosc. Relat. Phenom. 1990, 50, 159–166. [Google Scholar] [CrossRef]
- Contini, G.; Laajalehto, K.; Suoninen, E.; Marabini, A. 5-Methyl-2-mercaptobenzoxazole adsorbed onto chalcocite (Cu2S): An XPS and X-AES study. J. Colloid Interface Sci. 1995, 171, 234–239. [Google Scholar]
- Feng, D.; Van Deventer, J. The effect of sulfur species on thiosulfate leaching of gold. Miner. Eng. 2007, 20, 273–281. [Google Scholar] [CrossRef]





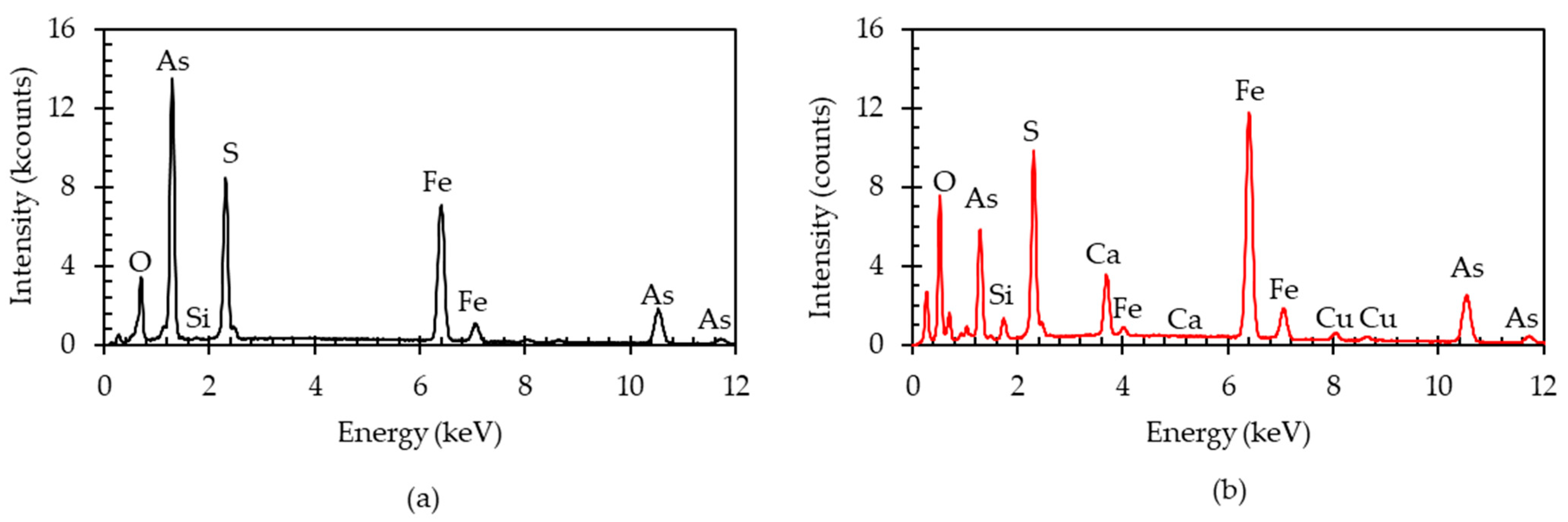
| Elements | Fe | As | S | O | Mg | Si | Cu | Br | Zn | Sb | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 29.2 | 25.1 | 15.4 | 14.5 | 11.5 | 3.9 | 0.24 | 0.1 | 0.03 | 0.01 | 0.02 |
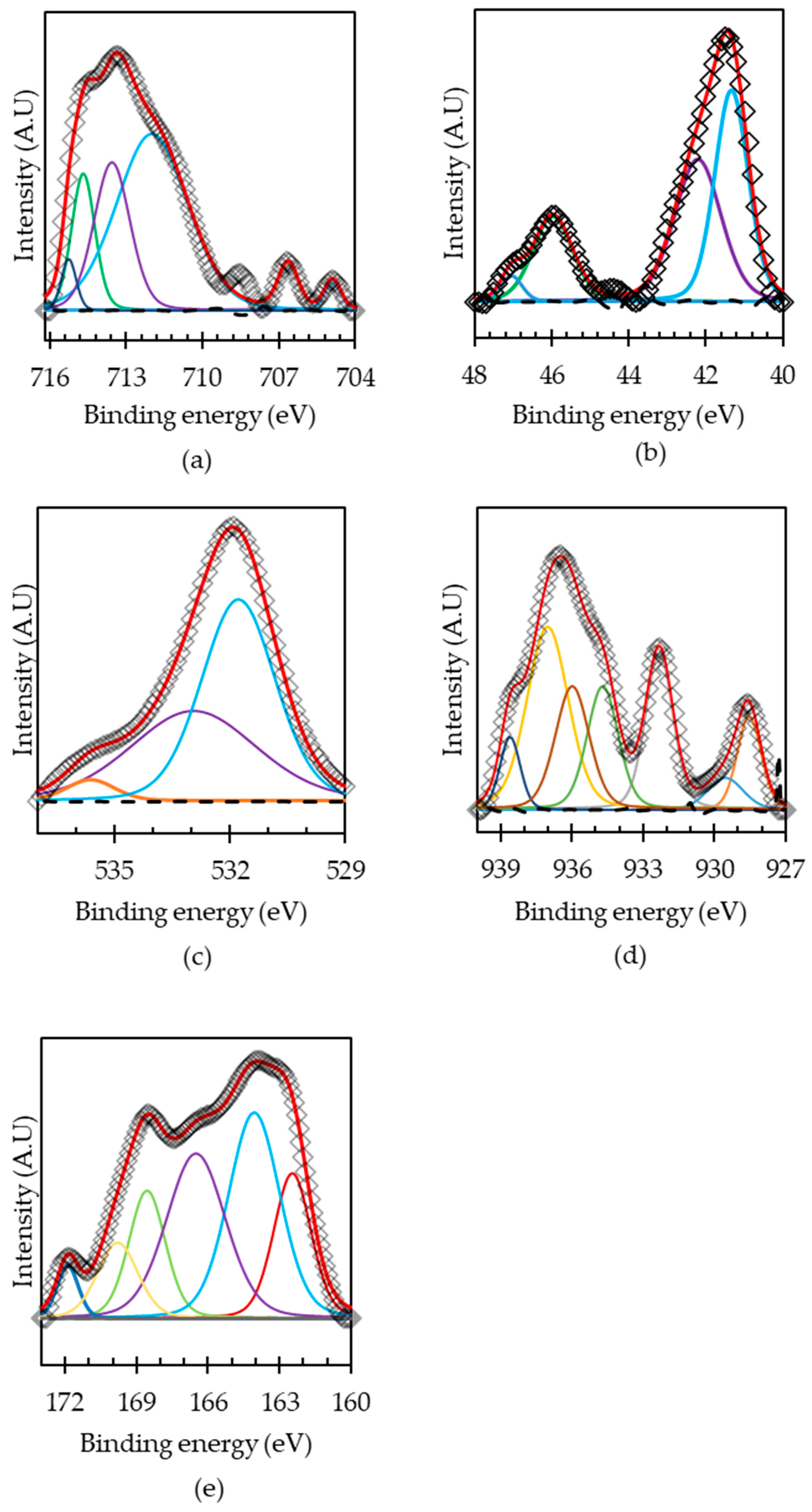
| Spectral Peak | Binding Energy | FWHM | Line Shape | Chemical Species | Content (at. %) | References |
|---|---|---|---|---|---|---|
| Fe 2p3/2 a | 706.8 | 1.2 | GL (20) | Fe(II)–AsS | 7.8 | [22] |
| Fe 2p3/2 a | 710.0 | 2.4 | GL (20) | Fe(III)–AsS | 7.3 | [40] |
| Fe 2p3/2 a | 711.7 | 0.9 | GL (20) | Fe(III)–O | 19.9 | [43,44] |
| Fe 2p3/2 a | 713.7 | 1.3 | GL (20) | Fe(III)–SO42− | 39.5 | [45,46] |
| As 3d5/2 a | 41.3 | 1.1 | GL (20) | As(O) | 31.6 | [43] |
| As 3d5/2 a | 43.7 | 0.9 | GL (20) | As(I)–O | 9.5 | [43] |
| As 3d5/2 a | 45.5 | 1.3 | GL (20) | As(III)–O | 42.6 | [47] |
| As 3d5/2 a | 46.2 | 0.8 | GL (20) | As(V)–O | 16.4 | [47] |
| S 2p3/2 a | 161.7 | 1.3 | GL (20) | Monosulfide (S2−) | 9.3 | [43] |
| S 2p3/2 a | 163.0 | 2.2 | GL (20) | Disulfide (S22−) | 52.7 | [43] |
| S 2p3/2 a | 165.5 | 2.7 | GL (20) | Elemental (S) | 27.0 | [43] |
| S 2p3/2 a | 167.9 | 0.7 | GL (20) | Thiosulfate (SO32−) | 3.5 | [43] |
| S 2p3/2 a | 167.3 | 1.1 | GL (20) | Thiosulfate (SO32−) | 7.6 | [43] |
| S 2p3/2 b | 160.6 | 1.7 | GL (20) | Monosulfide (S2−) | 22.8 | [43] |
| S 2p3/2 b | 162.3 | 1.77 | GL (20) | Disulfide (S22−) | 69.6 | [43] |
| S 2p3/2 b | 163.9 | 0.68 | GL (20) | Elemental sulfur (S0) | 3.0 | [45] |
| S 2p3/2 b | 165.9 | 0.81 | GL (20) | Sulfate (SO42−) | 4.52 | [43] |
| Cu 2p3/2 a | 932.5 | 2.3 | GL (20) | Cu(I)–S | 57.9 | [48] |
| Cu 2p3/2 a | 934.5 | 2.1 | GL (20) | Cu(II)–O | 33.5 | [35,49] |
| Cu 2p3/2 a | 936.0 | 1.3 | GL (20) | Cu(II)–sulfate. | 8.8 | [36] |
| Cu 2p3/2 b | 931.9 | 1.9 | GL (20) | Cu(I)–S | 57.5 | [48] |
| Cu 2p3/2 b | 933.5 | 3.6 | GL (20) | Cu(II)–O/Cu(OH)2 | 26.8 | [50,51] |
| Cu 2p3/2 b | 935.9 | 1.7 | GL (20) | Cu(OH)2 | 6.1 | [21,51] |
| Cu 2p3/2 b | 938.4 | 1.7 | GL (20) | Cu(II)–sulfate. | 9.5 | [36] |
| O 1s a | 531.9 | 2.5 | GL (20) | Hydroxyl oxygen (OH−) | 73.9 | [22] |
| O 1s a | 534.3 | 1.6 | GL (20) | (O)attached to water | 6.5 | [38] |
| O 1s a | 536.0 | 2.6 | GL (20) | (O)attached to water | 19.6 | [38] |
| O 1s b | 533.1 | 1.9 | GL (20 | Hydroxyl oxygen (OH−) | 36.6 | [51] |
| O 1s b | 534 | 1.6 | GL (20) | (O)attached to water | 39.7 | [51] |
| O 1s b | 535.0 | 1.7 | GL (20) | (O)attached to water | 20.1 | [51] |
| O 1s b | 536.3 | 1.1 | GL (20) | (O)attached to water | 3.5 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhandu, T.J.; Park, I.; Jeon, S.; Hamatsu, S.; Elakneswaran, Y.; Ito, M.; Hiroyoshi, N. A Pretreatment of Refractory Gold Ores Containing Sulfide Minerals to Improve Gold Leaching by Ammonium Thiosulfate: A Model Experiment Using Gold Powder and Arsenic-Bearing Sulfide Minerals. Metals 2023, 13, 1357. https://doi.org/10.3390/met13081357
Mhandu TJ, Park I, Jeon S, Hamatsu S, Elakneswaran Y, Ito M, Hiroyoshi N. A Pretreatment of Refractory Gold Ores Containing Sulfide Minerals to Improve Gold Leaching by Ammonium Thiosulfate: A Model Experiment Using Gold Powder and Arsenic-Bearing Sulfide Minerals. Metals. 2023; 13(8):1357. https://doi.org/10.3390/met13081357
Chicago/Turabian StyleMhandu, Takunda Joseph, Ilhwan Park, Sanghee Jeon, Sohta Hamatsu, Yogarajah Elakneswaran, Mayumi Ito, and Naoki Hiroyoshi. 2023. "A Pretreatment of Refractory Gold Ores Containing Sulfide Minerals to Improve Gold Leaching by Ammonium Thiosulfate: A Model Experiment Using Gold Powder and Arsenic-Bearing Sulfide Minerals" Metals 13, no. 8: 1357. https://doi.org/10.3390/met13081357
APA StyleMhandu, T. J., Park, I., Jeon, S., Hamatsu, S., Elakneswaran, Y., Ito, M., & Hiroyoshi, N. (2023). A Pretreatment of Refractory Gold Ores Containing Sulfide Minerals to Improve Gold Leaching by Ammonium Thiosulfate: A Model Experiment Using Gold Powder and Arsenic-Bearing Sulfide Minerals. Metals, 13(8), 1357. https://doi.org/10.3390/met13081357








