Investigation of Corrosion Properties and Composition of the Surface Formed on AISI 321 Stainless Steel by Ion Implantation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
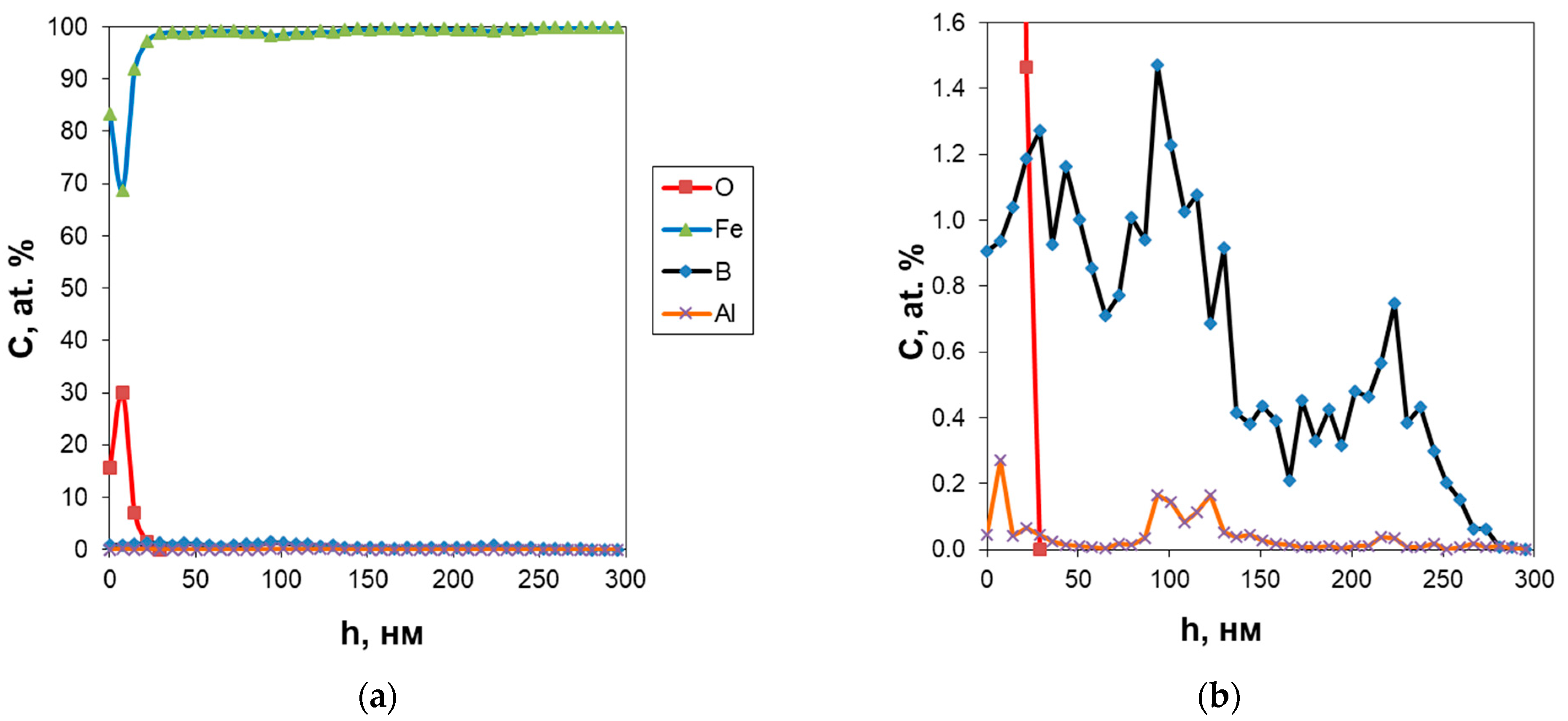
3.1. SIMS Analysis
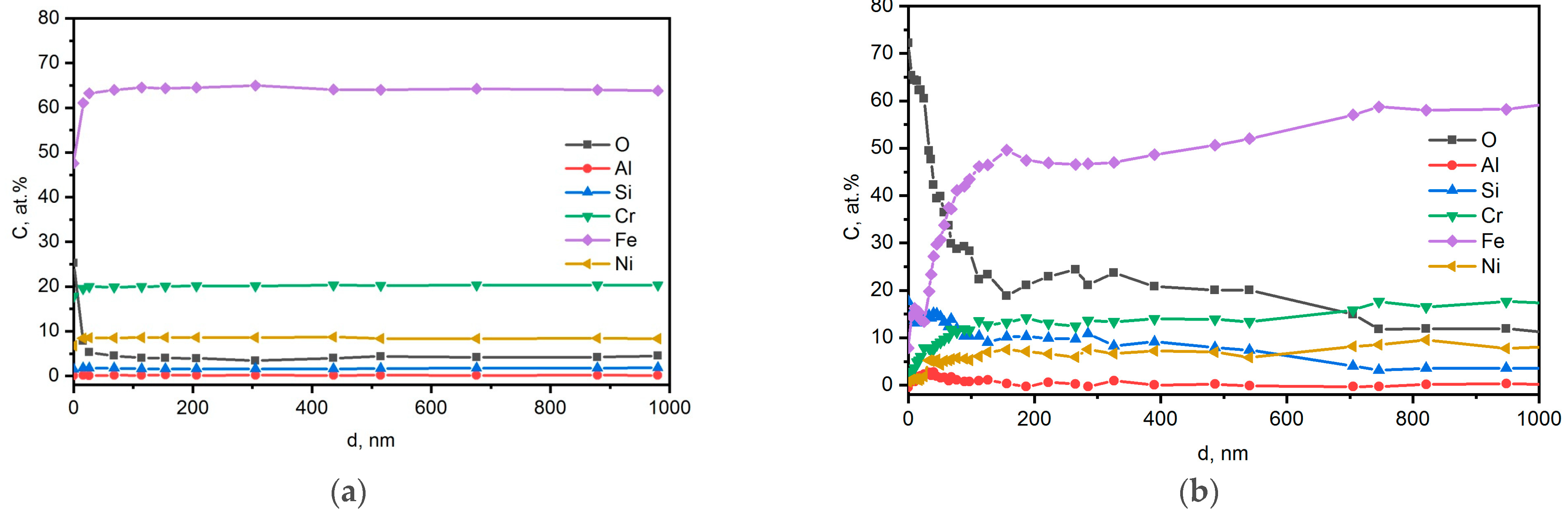
3.2. EDX Spectra and TEM Analysis
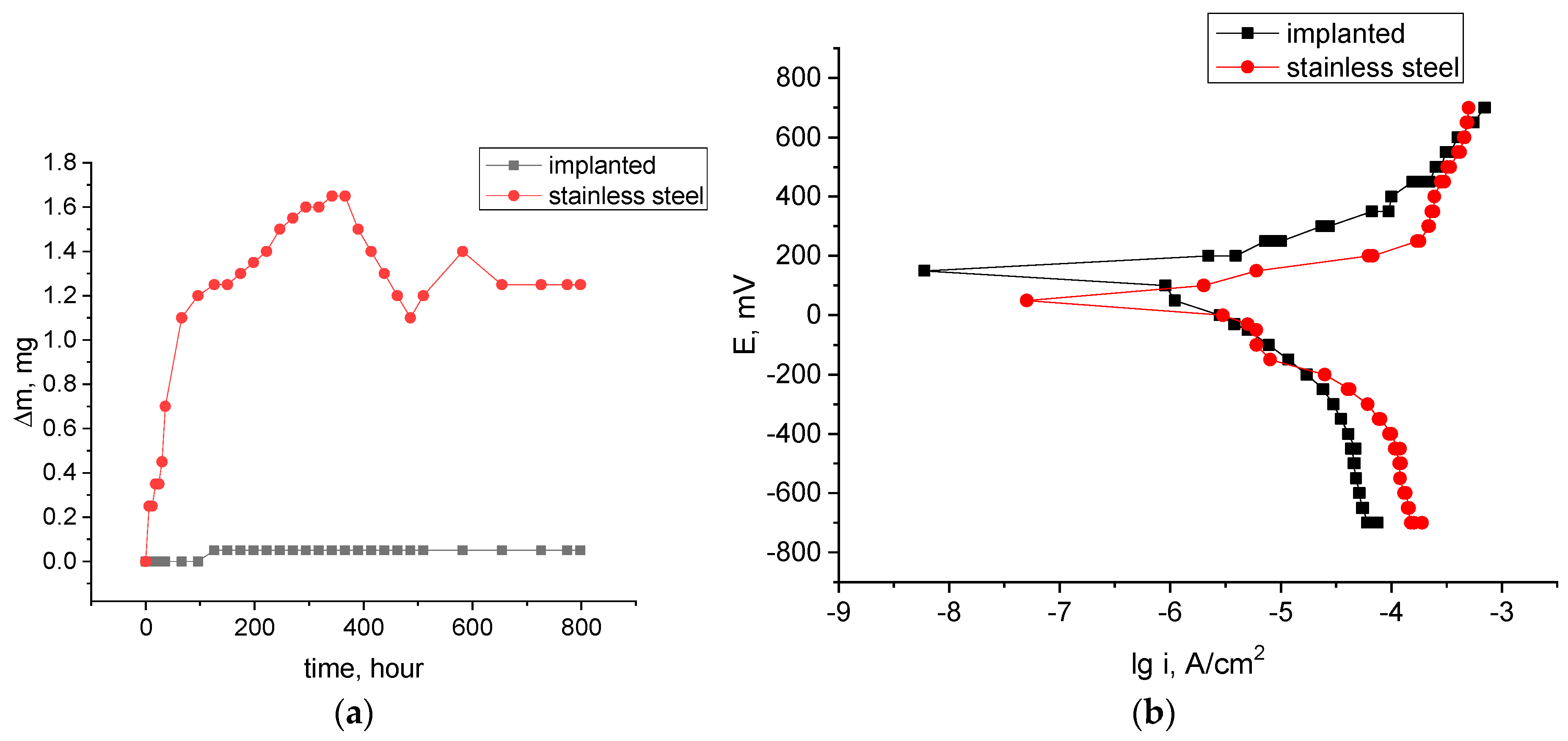
3.3. Corrosion Properties
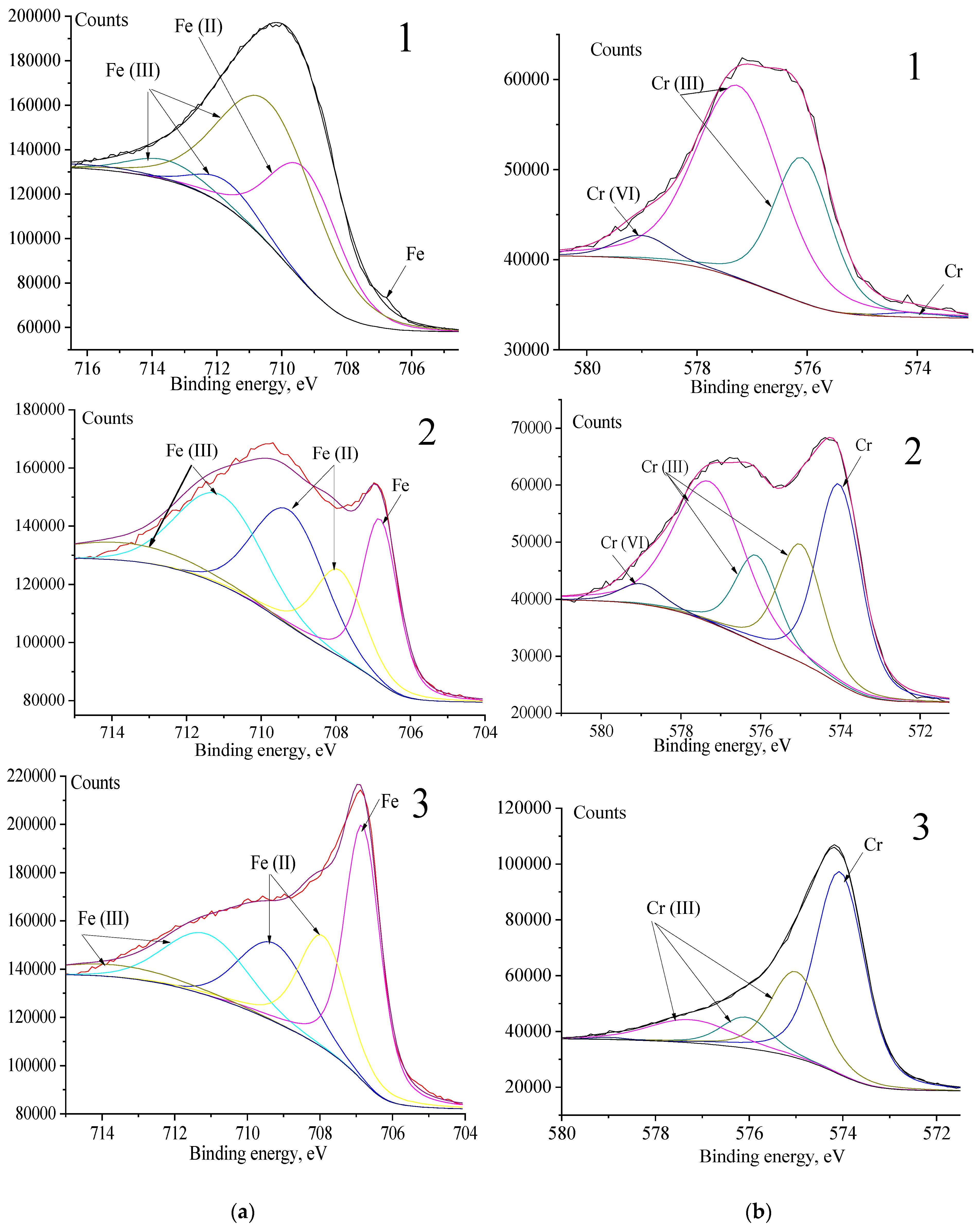
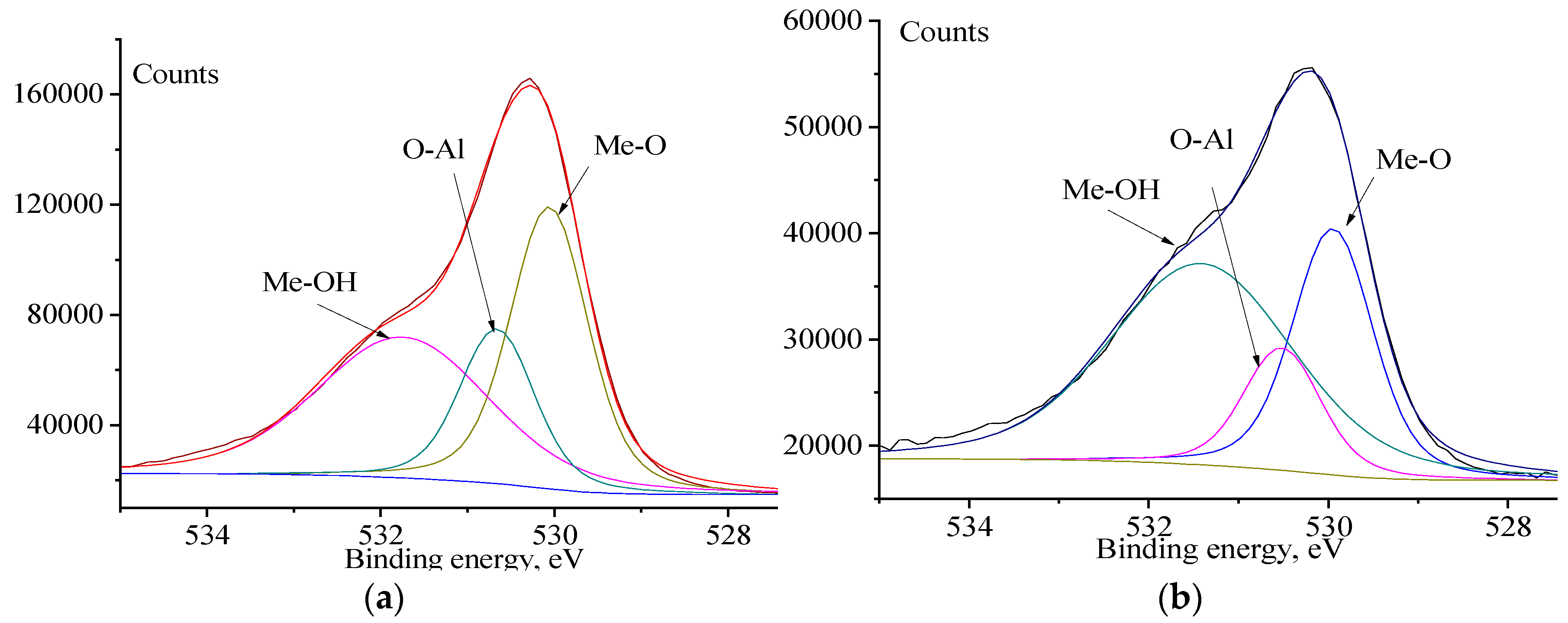
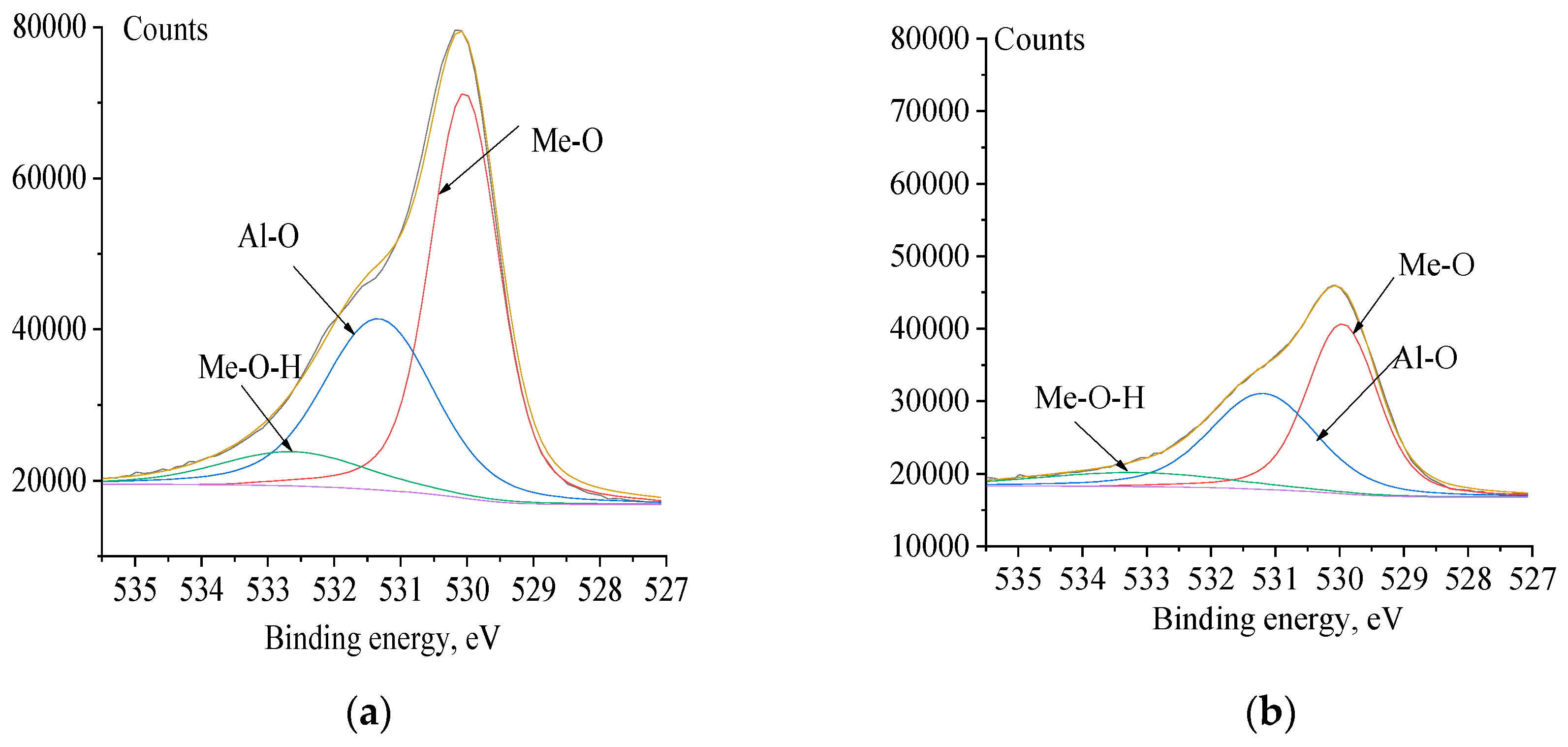
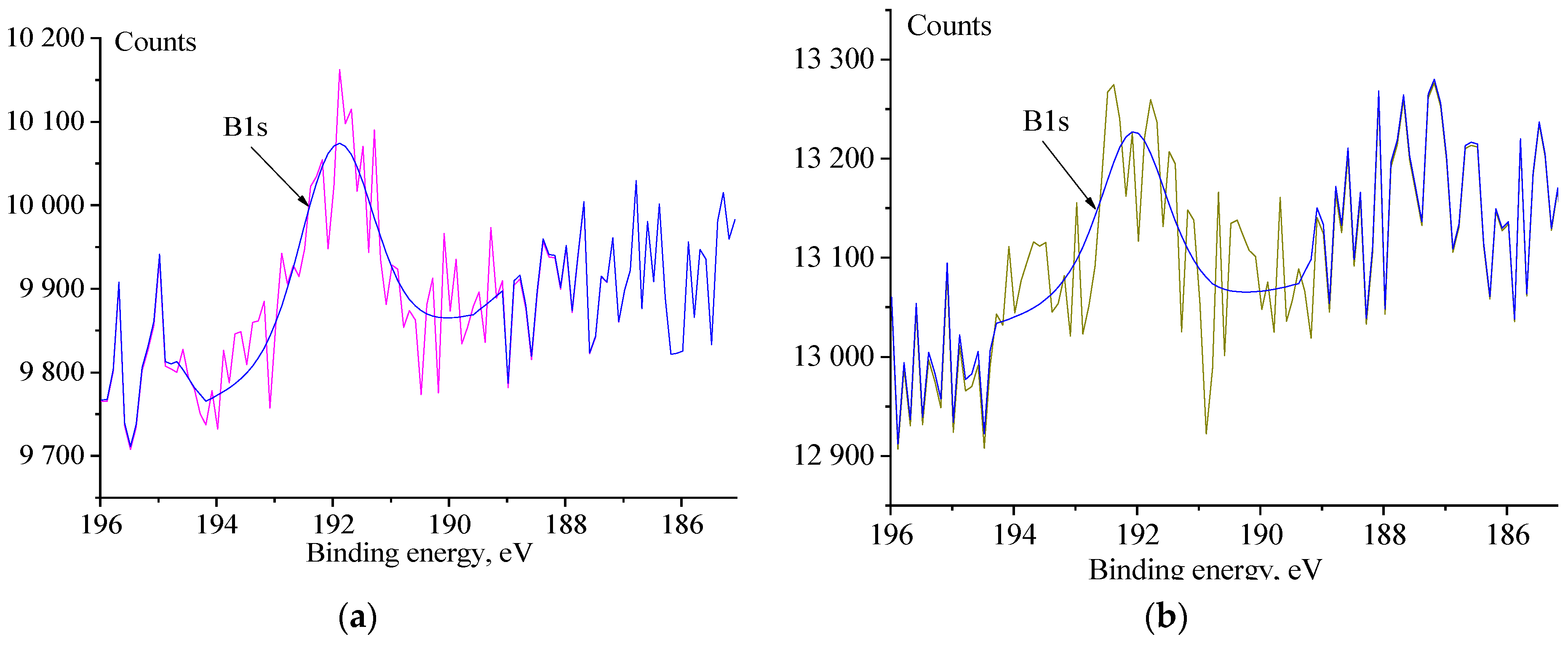
3.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, P.A. Fundamentals of Corrosion-Mechanisms, Causes, and Preventative Methods; CRC Press: Boca Raton, FL, USA, 2013; Volume 53, ISBN 9788578110796. [Google Scholar]
- Galal, A.; Amin, K.M.; Atta, N.F.; Abd El-Rehim, H.A. Protective Ability of Graphene Prepared by γ-Irradiation and Impregnated with Organic Inhibitor Applied on AISI 316 Stainless Steel. J. Alloys Compd. 2017, 695, 638–647. [Google Scholar] [CrossRef]
- Gaber, G.A.; Mohamed, L.Z.; Järvenpää, A.; Hamada, A. Enhancement of Corrosion Protection of AISI 201 Austenitic Stainless Steel in Acidic Chloride Solutions by Ce-Doped TiO2 Coating. Surf. Coat. Technol. 2021, 423, 127618. [Google Scholar] [CrossRef]
- Dorofeeva, M.S.; Dorofeeva, T.I.; Gritsenko, B.P.; Sergeev, V.P. Thermo-Barrier Nanostructure Microplasma Coatings of ZrO2. J. Phys. Conf. Ser. 2018, 1115, 032067. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xiao, G.-y.; Jiao, Y.; Zhao, X.-c.; Lu, Y.-p. Facile Preparation of Hopeite Coating on Stainless Steel by Chemical Conversion Method. Surf. Coat. Technol. 2014, 240, 361–364. [Google Scholar] [CrossRef]
- Sudagar, A.J.; Subasri, R. Fabrication and Characterization of Silver/Nickel Sulphide Solar Absorber Coatings on Stainless Steel by Chemical Bath Deposition. Mater. Chem. Phys. 2015, 163, 478–484. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Chen, Z.-y.; Li, X.-t.; Ji, K.-p.; Luo, H.-f.; Wan, G.-p.; Yang, S.-f. Influence of Thermal Diffusion Treatment on Microstructure Evolution and Corrosion Behavior of Hot-Dipped Al Coated Fe-Cr-Si-B Cast Steel. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2023, 33, 1522–1539. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, M.; Wang, J.; Li, F.; Zhu, S.; Wang, F. Enamel Coating for Protection of the 316 Stainless Steel against Tribo-Corrosion in Molten Zinc Alloy at 460 °C. J. Mater. Sci. Technol. 2021, 65, 126–136. [Google Scholar] [CrossRef]
- Luo, K.Y.; Jing, X.; Sheng, J.; Sun, G.F.; Yan, Z.; Lu, J.Z. Characterization and Analyses on Micro-Hardness, Residual Stress and Microstructure in Laser Cladding Coating of 316L Stainless Steel Subjected to Massive LSP Treatment. J. Alloys Compd. 2016, 673, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Luo, C.; Jin, J.; Zhang, Y.; Sun, Y.; Fu, L. Tribocorrosion Performance of 316L Stainless Steel Enhanced by Laser Clad 2-Layer Coating Using Fe-Based Amorphous Powder. J. Mater. Res. Technol. 2022, 17, 612–621. [Google Scholar] [CrossRef]
- Saidi, D.; Zaid, B.; Souami, N.; Saoula, N.; Siad, M.; Si Ahmed, A.; Biberian, J.P. AES Depth Profiles in Mo-Coated 304L Stainless Steel Achieved by RF-Magnetron Sputtering and Influence of Mo on the Corrosion in 3.5% NaCl Solution. J. Alloys Compd. 2015, 645, 45–50. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, J.; Hu, M.; Li, X. Investigation of High Potential Corrosion Protection with Titanium Carbonitride Coating on 316L Stainless Steel Bipolar Plates. Corros. Sci. 2021, 191, 109757. [Google Scholar] [CrossRef]
- Dorofeeva, T.; Gubaidulina, T.; Sergeev, V.; Fedorischeva, M. Si-Al-N-O Multi-Layer Coatings with Increased Corrosion Resistance Deposited on Stainless Steel by Magnetron Sputtering. Metals 2022, 12, 254. [Google Scholar] [CrossRef]
- Gubaidulina, T.A.; Dorofeeva, T.I.; Sergeev, V.P.; Kalashnikov, M.P.; Voronov, A.V. The Structure and Composition of Al-Si-N/Ni/Al-Si-N Three-Layer Coatings Deposited by Magnetron Sputtering. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 6–10 September 2021; American Institute of Physics Inc.: College Park, MD, USA, 2022; Volume 2509. [Google Scholar]
- Paschalidou, E.M.; Shu, R.; Boyd, R.; Papaderakis, A.A.; Bakhit, B.; le Febvrier, A.; Greczynski, G.; Eklund, P.; Nyholm, L. The Effect of the Nb Concentration on the Corrosion Resistance of Nitrogen-Containing Multicomponent TiZrTaNb-Based Films in Acidic Environments. J. Alloys Compd. 2022, 927, 167005. [Google Scholar] [CrossRef]
- Manivannan, R.; Sundararaj, S.; Dheenasagar, R.; Giridharan, K.; Sivaraman, P.R.; Udhayarani, V. Influence of Al2O3, SiC and B4C Covalent Multilayer PVD Coating on Surface Properties of HSS Rod. Mater. Today Proc. 2020, 39, 700–707. [Google Scholar] [CrossRef]
- Dorofeeva, T.I.; Gubaidulina, T.A.; Sergeev, V.P.; Kalashnikov, M.P.; Voronov, A.V. Change in the Structure and Corrosion Resistance of a Nickel-Chrome Coating on Stainless Steel During Implantation of High Energy Al+ and B+ Ions. Russ. Phys. J. 2020, 63, 1186–1194. [Google Scholar] [CrossRef]
- Cheon, K.H.; Park, C.; Kang, M.H.; Park, S.; Kim, J.; Jeong, S.H.; Kim, H.E.; Jung, H.D.; Jang, T.S. A Combination Strategy of Functionalized Polymer Coating with Ta Ion Implantation for Multifunctional and Biodegradable Vascular Stents. J. Magnes. Alloys 2021, 9, 2194–2206. [Google Scholar] [CrossRef]
- Wei, X.; Li, Z.; Liu, P.; Li, S.; Peng, X.; Deng, R.; Zhao, Q. Improvement in Corrosion Resistance and Biocompatibility of AZ31 Magnesium Alloy by NH2+ Ions. J. Alloys Compd. 2020, 824, 153832. [Google Scholar] [CrossRef] [Green Version]
- Dorofeeva, M.S.; Gubaidulina, T.A.; Sergeev, V.P.; Dorofeeva, T.I. Inffluence of Ion Beam Implantation on the Corrosion Properties of Stainless Steel. In Proceedings of the 2020 7th International Congress on Energy Fluxes and Radiation Effects (EFRE) 2020, Tomsk, Russia, 14–26 September 2020; pp. 766–768. [Google Scholar] [CrossRef]
- Majhi, R.; Rajbhar, M.K.; Das, P.; Elliman, R.G.; Chatterjee, S. Low energy ion beam-induced joining of TiO2 nanoparticles. J. Alloys Compd. 2022, 924, 166440. [Google Scholar] [CrossRef]
- Eisen, F.H.; Welch, B. Ion Implantation; Elsevier: Amsterdam, The Netherlands, 1971; pp. 1–4. [Google Scholar] [CrossRef]
- Pavlov, P.V.; Zorin, E.I.; Tetelbaum, D.I.; Khokhlov, A.F. Nitrogen as Dopant in Silicon and Germanium. Phys. Status Solidi 1976, 35, 11–36. [Google Scholar] [CrossRef]
- Pavlov, P.V.; Zorin, E.I.; Tetelbaum, D.I.; Lesnikov, V.P.; Ryzhkov, G.M.; Pavlov, A.V. Phase Transformations at Bombardment of Al and Fe Polycrystalline Films with B+, C+, N+, P+, and As+ Ions. Phys. Status Solidi 1973, 19, 373–378. [Google Scholar] [CrossRef]
- Langouche, G. Ion Implantation. Hyperfine Interact. 1992, 68, 95–106. [Google Scholar] [CrossRef]
- Savaloni, H.; Habibi, M. Influence of Ni Deposition and Subsequent N + Ion Implantation at Different Substrate Temperatures on Nano-Structure and Corrosion Behaviour of Type 316 and 304 Stainless Steels. Appl. Surf. Sci. 2011, 258, 103–112. [Google Scholar] [CrossRef]
- Pedraza, F.; Grosseau-Poussard, J.L.; Dinhut, J.F. Low Energy-High Flux Nitrogen Implantation of an Oxide-Dispersion- Strengthened FeAl Intermetallic Alloy. Thin Solid Films 2004, 467, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zheng, L.; Li, Q.; Zhang, F.; Chen, X.; Zhang, H. Corrosion Properties of Carbon Ions Implanted Chromium Coating Prepared on CSS-42L Aerospace Bearing Steel. Surf. Coat. Technol. 2018, 349, 392–399. [Google Scholar] [CrossRef]
- Seo, E.J.; Cho, L.; Kim, J.K.; Mola, J.; Zhao, L.; Lee, S.; De Cooman, B.C. Focused Ion Beam-Induced Displacive Phase Transformation from Austenite to Martensite during Fabrication of Quenched and Partitioned Steel Micro-Pillar. J. Alloys Compd. 2020, 812, 152061. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhang, W.; Ning, Z.; Wang, H.; Liao, J.; Yang, Y.; Liu, N.; Yang, J. Microstructural Evolution and Hardening Effect of 12Cr2W2Mn Ferritic/Martensitic Steel under Au-Ions Irradiation. J. Alloys Compd. 2020, 835, 155360. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Z.; An, X.; Xiao, S.; Cui, S.; Lin, H.; Fu, R.K.Y.; Tian, X.; Wei, R.; Chu, P.K.; et al. Excellent Adhered Thick Diamond-like Carbon Coatings by Optimizing Hetero-Interfaces with Sequential Highly Energetic Cr and C Ion Treatment; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 735, ISBN 8675526032. [Google Scholar]
- Sergeev, O.V.; Kalashnikov, M.P.; Fedorishcheva, M.V.; Sergeev, V.P.; Panin, V.E. Modification of Ti-Al-N Coatings by High Energy (Cr+ + B+) Ion Bombardment. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 19–23 September 2016; Volume 1783. [Google Scholar] [CrossRef]
- Genel, K.; Ozbek, I.; Bindal, C. Kinetics of Boriding of AISI W1 Steel. Mater. Sci. Eng. A 2003, 347, 311–314. [Google Scholar] [CrossRef]
- Campos, I.; Palomar-Pardavé, M.; Amador, A.; VillaVelázquez, C.; Hadad, J. Corrosion Behavior of Boride Layers Evaluated by the EIS Technique. Appl. Surf. Sci. 2007, 253, 9061–9066. [Google Scholar] [CrossRef]
- García-León, R.A.; Martínez-Trinidad, J.; Campos-Silva, I.; Figueroa-López, U.; Guevara-Morales, A. Development of Tribological Maps on Borided AISI 316L Stainless Steel under Ball-on-Flat Wet Sliding Conditions. Tribol. Int. 2021, 163, 107161. [Google Scholar] [CrossRef]
- Klepper, C.C.; Hazelton, R.C.; Yadlowsky, E.J.; Carlson, E.P.; Keitz, M.D.; Williams, J.M.; Zuhr, R.A.; Poker, D.B. Amorphous Boron Coatings Produced with Vacuum Arc Deposition Technology. J. Vac. Sci. Technol. A Vac. Surf. Film. 2002, 20, 725–732. [Google Scholar] [CrossRef]
- Panda, J.N.; Wong, B.C.; Medvedovski, E.; Egberts, P. Enhancement of Tribo-Corrosion Performance of Carbon Steel through Boronizing and BN-Based Coatings. Tribol. Int. 2021, 153, 106666. [Google Scholar] [CrossRef]
- Rodríguez-Castro, G.; Campos-Silva, I.; Chávez-Gutiérrez, E.; Martínez-Trinidad, J.; Hernández-Sánchez, E.; Torres-Hernández, A. Mechanical Properties of FeB and Fe2B Layers Estimated by Berkovich Nanoindentation on Tool Borided Steel. Surf. Coat. Technol. 2013, 215, 291–299. [Google Scholar] [CrossRef]
- Dellasega, D.; Russo, V.; Pezzoli, A.; Conti, C.; Lecis, N.; Besozzi, E.; Beghi, M.; Bottani, C.E.; Passoni, M. Boron Films Produced by High Energy Pulsed Laser Deposition. Mater. Des. 2017, 134, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Pelleg, J.; Judelewicz, M. Diffusion in the Bα-Fe System and Compound Formation between Electron Gun Deposited Boron Thin Films and Steel Substrate. Thin Solid Films 1992, 215, 35–41. [Google Scholar] [CrossRef]
- Upadhyay, N.; Pujar, M.G.; Das, C.R.; Krishna, N.G.; Mallika, C.; Kamachi Mudali, U. Pitting Corrosion Behaviour of Boron Added Modified 9Cr–1Mo Steel: Combined Effects of Alkali and Chloride Ions. Trans. Indian Inst. Met. 2015, 68, 129–141. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Danilionok, M.M.; Uglov, V.V.; Erdybaeva, N.K.; Kirik, G.V.; Dub, S.N.; Rusakov, V.S.; Shypylenko, A.P.; Zukovski, P.V.; Tuleushev, Y.Z. Nanocomposite Protective Coatings Based on Ti-N-Cr/Ni-Cr-B-Si-Fe, Their Structure and Properties. Vacuum 2009, 83, 235–239. [Google Scholar] [CrossRef]
- Henriksen, O.; Johnson, E.; Johansen, A.; Sarholt-Kristensen, L.; Wood, J.V. The effect of boron implantation on the corrosion behaviour, microhardness and contact resistance of copper and silver surfaces. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1987, 19–20, 253–258. [Google Scholar] [CrossRef]
- Sourani, F.; Enayati, M.H.; Ashrafizadeh, F.; Sayyedan, F.S.; Chu, P.K. Enhancing Surface Properties of (Fe,Cr)Al–Al2O3 Nanocomposite by Oxygen Ion Implantation. J. Alloys Compd. 2021, 853, 156892. [Google Scholar] [CrossRef]
- Bystrov, S.G.; Reshetnikov, S.M.; Borisova, E.M.; Klimova, I.N.; Kolotov, A.A.; Zhikharev, A.V.; Bayankin, V.Y. Effect of Implantation of Argon and Oxygen Ions on the Physicochemical Properties and Corrosion and Electrochemical Behavior of 14Cr17Ni2 Chromium–Nickel Steel. Inorg. Mater. Appl. Res. 2022, 13, 666–673. [Google Scholar] [CrossRef]
- Nieh, C.W.; Xiong, F.; Ahn, C.C.; Zhou, Z.; Jamieson, D.N.; Vreeland, T.; Fultz, B.; Tombrello, T.A. Formation of Buried Oxide in Mev Oxygen Implanted Silicon. MRS Proc. 1987, 107, 73–78. [Google Scholar] [CrossRef]
- Vorobiev, V.L.; Bakieva, O.R.; Gilmutdinov, F.Z.; Kolotov, A.A.; Averkiev, I.K.; Borisova, E.M.; Reshetnikov, S.M. Effect of Oxygen Ion Implantation on Physicochemical Structure and Corrosion-Electrochemical Behavior of Copper–Manganese Alloy. Inorg. Mater. Appl. Res. 2021, 12, 581–587. [Google Scholar] [CrossRef]
- Ren, J.; Yu, L.; Liu, Y.; Ma, Z.; Liu, C.; Li, H.; Wu, J. Corrosion Behavior of an Al Added High-Cr ODS Steel in Supercritical Water at 600 °C. Appl. Surf. Sci. 2019, 480, 969–978. [Google Scholar] [CrossRef]
- Tang, M.; Wang, J.; Feng, Z.; Li, G.; Yan, Z.; Zhang, R. Corrosion Resistance of AlN and Fe3Al Reinforced Fe-Based Plasma Cladding Layer in 3.5 wt% NaCl Solution. Ceram. Int. 2019, 45, 16918–16926. [Google Scholar] [CrossRef]
- Hamdy, E.; Liu, F.; Geers, C. Superior Protection by α-Al2O3/α-LiAlO2 Double Oxide Scales against Alkali Carbonate Corrosion. Corros. Sci. 2023, 218, 111217. [Google Scholar] [CrossRef]
- Gavrilov, N.V.; Kamenetskikh, A.S.; Tretnikov, P.V.; Chukin, A.V. Ion Assisted Deposition of α-Al2O3 Coatings by Anodic Evaporation in the Arc Discharge. Surf. Coat. Technol. 2018, 337, 453–460. [Google Scholar] [CrossRef]
- Cordes, S.E. Thermal Stability of γ-Alumina PVD Coatings and Analysis of Their Performance in Machining of Austenitic Stainless Steels. CIRP J. Manuf. Sci. Technol. 2012, 5, 20–25. [Google Scholar] [CrossRef]
- Xin, Y.C.; Chu, P.K. Plasma Immersion Ion Implantation (PIII) of Light Alloys. In Surface Engineering of Light Alloys: Aluminium, Magnesium and Titanium Alloys; Woodhead Publishing: Sawston, UK, 2010; pp. 362–397. [Google Scholar] [CrossRef]
- Sreenivas Rao, K.V.; Tejaswini, G.C.; Girisha, K.G. Corrosion Behavior of Plasma Sprayed Cr2O3–Al2O3–ZrO2 Multilayer Coatings on Mild Steel. Mater. Today Proc. 2018, 5, 24068–24074. [Google Scholar] [CrossRef]
- Sreenu, A.V. Aluminium-Boron Nitride Nano Composite Coating by Friction Surfacing on Low Carbon Steel Substrate—A Feasibility Study. Mater. Today Proc. 2018, 5, 26829–26835. [Google Scholar] [CrossRef]
- Zepon, G.; Nascimento, A.R.C.; Kasama, A.H.; Nogueira, R.P.; Kiminami, C.S.; Botta, W.J.; Bolfarini, C. Design of Wear Resistant Boron-Modified Supermartensitic Stainless Steel by Spray Forming Process. Mater. Des. 2015, 83, 214–223. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Zhong, Q.; Zhou, Q.; Zhang, L. Preparation of Fe2B Boride Coating on Low-Carbon Steel Surfaces and Its Evaluation of Hardness and Corrosion Resistance. Surf. Coat. Technol. 2011, 206, 473–478. [Google Scholar] [CrossRef]
- Kuterbekov, K.A.; Nurkenov, S.A.; Kislitsin, S.B.; Kuketayev, T.A.; Tussupbekova, A.K. Investigation of Structure and Properties of Barrier Layers in Metals (Fe, Cu) at Low Temperatures. Russ. Phys. J. 2016, 59, 978–983. [Google Scholar] [CrossRef]
- Gardin, E.; Zanna, S.; Seyeux, A.; Allion-Maurer, A.; Marcus, P. Comparative Study of the Surface Oxide Films on Lean Duplex and Corresponding Single Phase Stainless Steels by XPS and ToF-SIMS. Corros. Sci. 2018, 143, 403–413. [Google Scholar] [CrossRef]
- Voyshnis, S.; Seyeux, A.; Zanna, S.; Martin-Cabanas, B.; Couvant, T.; Marcus, P. Oxide Layer Growth on Nickel-Base Alloy Surfaces in High Temperature Water and in O2 Studied by ToF-SIMS with Isotopic Tracers. Corros. Sci. 2018, 145, 212–219. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, H.; Tang, J.; Guo, J.; Pi, J.; Ye, K. Corrosion Properties of Phase Reversion Induced Nano/Ultrafine Grained AISI 304 Metastable Austenite Stainless Steel. Mater. Res. Bull. 2018, 107, 421–429. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, Y.; Yang, J. Denoising Star Map Data via Sparse Representation and Dictionary Learning. Optik 2015, 126, 1133–1137. [Google Scholar] [CrossRef]
- Wesolowski, D.J.; Ziemniak, S.E.; Anovitz, L.M.; Machesky, M.L.; Bénézeth, P.; Palmer, D.A. Solubility and Surface Adsorption Characteristics of Metal Oxides. In Aqueous Systems at Elevated Temperatures and Pressures: Physical Chemistry in Water, Steam and Hydrothermal Solutions; Academic Press: Cambridge, MA, USA, 2004; pp. 493–595. [Google Scholar] [CrossRef]
- Peter, R.; Saric, I.; Piltaver, I.K.; Badovinac, I.J.; Petravic, M. Oxide Formation on Chromium Metal Surfaces by Low-Energy Oxygen Implantation at Room Temperature. Thin Solid Films 2017, 636, 225–231. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Bai, J.; Dong, N.; Liu, Y.; Zhang, C.; Han, P. Effect of B Addition on the Microstructure and Corrosion Resistance of S31254 Super Austenitic Stainless Steels after Solid Solution Treatment. Mater. Lett. 2019, 252, 60–63. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Sokolova, T.S.; Dymshits, A.M.; Litasov, K.D. Thermodynamic properties of rock-forming oxides, α-Al2O3, Cr2O3, α-Fe2O3, and Fe3O4 at high temperatures and pressures. Geodyn. Tectonophys. 2016, 7, 459–476. [Google Scholar] [CrossRef] [Green Version]
- Avdienko, K.I.; Avdienko, A.A.; Kovalenko, I.A. Effect of the Elemental Composition of an Ion Beam on the Phase Formation and Surface Strengthening of Structural Materials. Phys. Met. Metallogr. 2001, 92, 623–627. [Google Scholar]



| Mode Parameters | Stage I | Stage II |
|---|---|---|
| Installation | Dionis | Diana |
| Implanted ions | O+ | Al+B+ |
| Gas | O2 | N2 |
| Target | - | Al-B |
| Residual pressure, A | 1 × 10−8 | 1 × 10−8 |
| Gas pressure, A | 1 × 10−6 | 1 × 10−7 |
| Emission current, mA | 100 | - |
| Beam current, mA | 15 | 300 |
| Accelerating voltage, kV | 10 | 40 ÷ 80 |
| Implantation dose, ion/cm2 | 1 × 1018 | 4 × 1017 |
| Specimen | E, mV | lgicor, A/cm2 | icor × 10−6, A/cm2 | Icor, A |
|---|---|---|---|---|
| Initial | 52 ± 3 | −5.9 ± 0.2 | 1.26 | 1.26 × 10−6 |
| O/Al-B ion-implanted | 151 ± 6 | −6.1 ± 0.2 | 0.71 | 0.71 × 10−6 |
| Depth, nm | Fe3+ Peak (%) | Fe2+ Peak (%) | Metallic Fe Peak (%) | Fe2+/Fe3+ Ratio |
|---|---|---|---|---|
| 0 nm | 15.06 | 73.964 | 10.97 | 4.9/1 |
| 150 nm | 23.84 | 41.76 | 1.8/1 | |
| 250 nm | 14.76 | 18.25 | 48.93 | 1.3/1 |
| Depth, nm | Cr3+ Peak (%) | Cr6+ Peak (%) | Metallic Cr Peak (%) | Cr3+/Cr6+Ratio |
|---|---|---|---|---|
| 0 nm | 91.25094 | 6.6155 | 2.133565 | 13.8/1 |
| 150 nm | 60.66318 | 2.807458 | 36.52937 | 21.6/1 |
| 250 nm | 37.82 | 0 | 62.17 |
| State | Me-O Peak (%) | Al-O Peak (%) | O-OH Peak (%) |
|---|---|---|---|
| Before corrosion | |||
| 0 nm | 44.8089 | 28.02857 | 27.16253 |
| 150 nm | 37.88082 | 27.2717 | 34.84748 |
| After corrosion | |||
| 0 nm | 51.92621 | 30.36293 | 17.71086 |
| 150 nm | 44.06879 | 34.0043 | 21.92691 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorofeeva, T.I.; Fedorischeva, M.V.; Gubaidulina, T.A.; Sergeev, O.V.; Sungatulin, A.R.; Sergeev, V.P. Investigation of Corrosion Properties and Composition of the Surface Formed on AISI 321 Stainless Steel by Ion Implantation. Metals 2023, 13, 1468. https://doi.org/10.3390/met13081468
Dorofeeva TI, Fedorischeva MV, Gubaidulina TA, Sergeev OV, Sungatulin AR, Sergeev VP. Investigation of Corrosion Properties and Composition of the Surface Formed on AISI 321 Stainless Steel by Ion Implantation. Metals. 2023; 13(8):1468. https://doi.org/10.3390/met13081468
Chicago/Turabian StyleDorofeeva, Tamara I., Marina V. Fedorischeva, Tatiana A. Gubaidulina, Oleg V. Sergeev, Alfred R. Sungatulin, and Viktor P. Sergeev. 2023. "Investigation of Corrosion Properties and Composition of the Surface Formed on AISI 321 Stainless Steel by Ion Implantation" Metals 13, no. 8: 1468. https://doi.org/10.3390/met13081468
APA StyleDorofeeva, T. I., Fedorischeva, M. V., Gubaidulina, T. A., Sergeev, O. V., Sungatulin, A. R., & Sergeev, V. P. (2023). Investigation of Corrosion Properties and Composition of the Surface Formed on AISI 321 Stainless Steel by Ion Implantation. Metals, 13(8), 1468. https://doi.org/10.3390/met13081468









