Effect of Tempering Time on Carbide Evolution and Mechanical Properties of a Nb-V-Ti Micro-Alloyed Steel
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
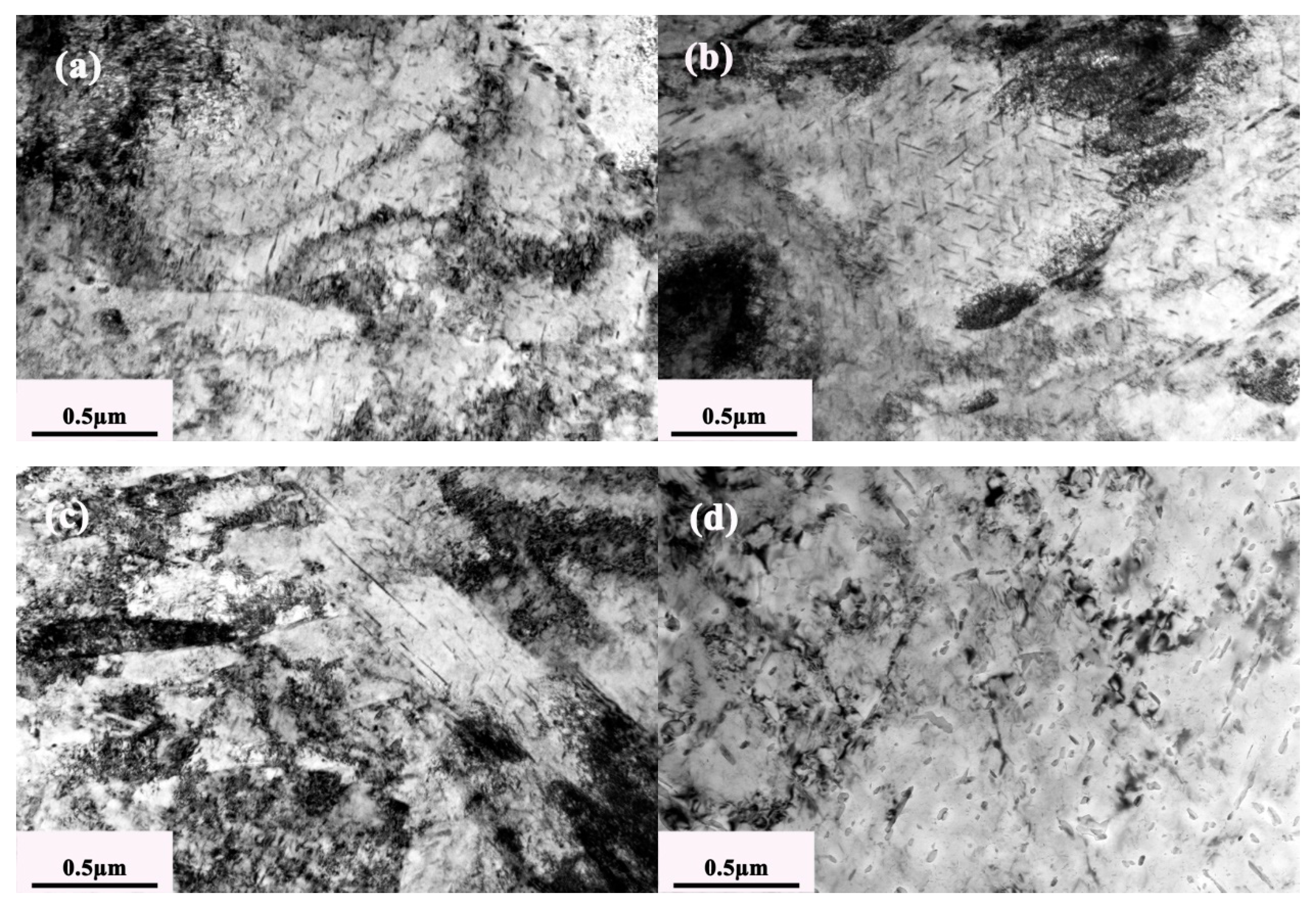
3.1. Microstructure Evolution under Different Tempering Times
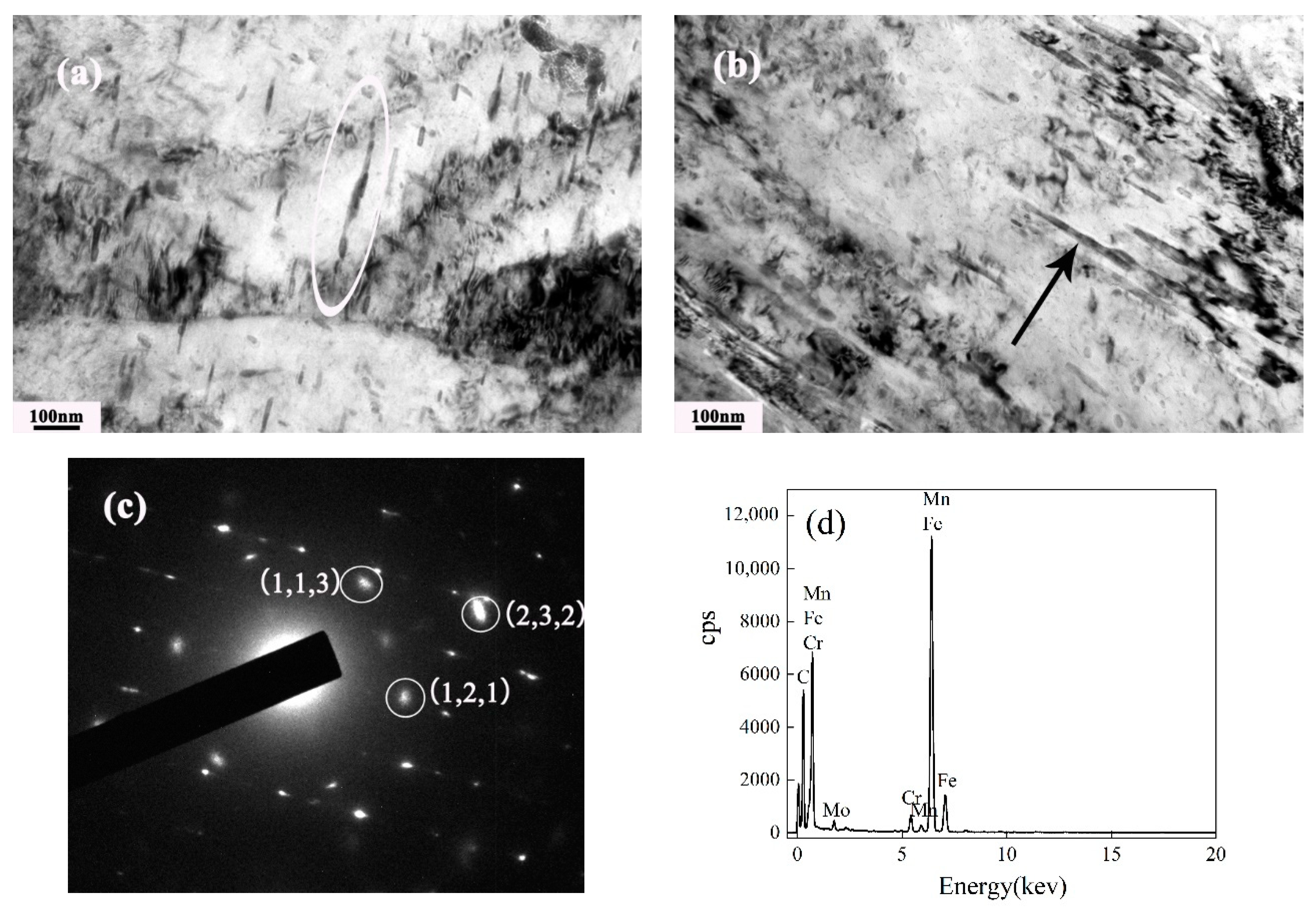
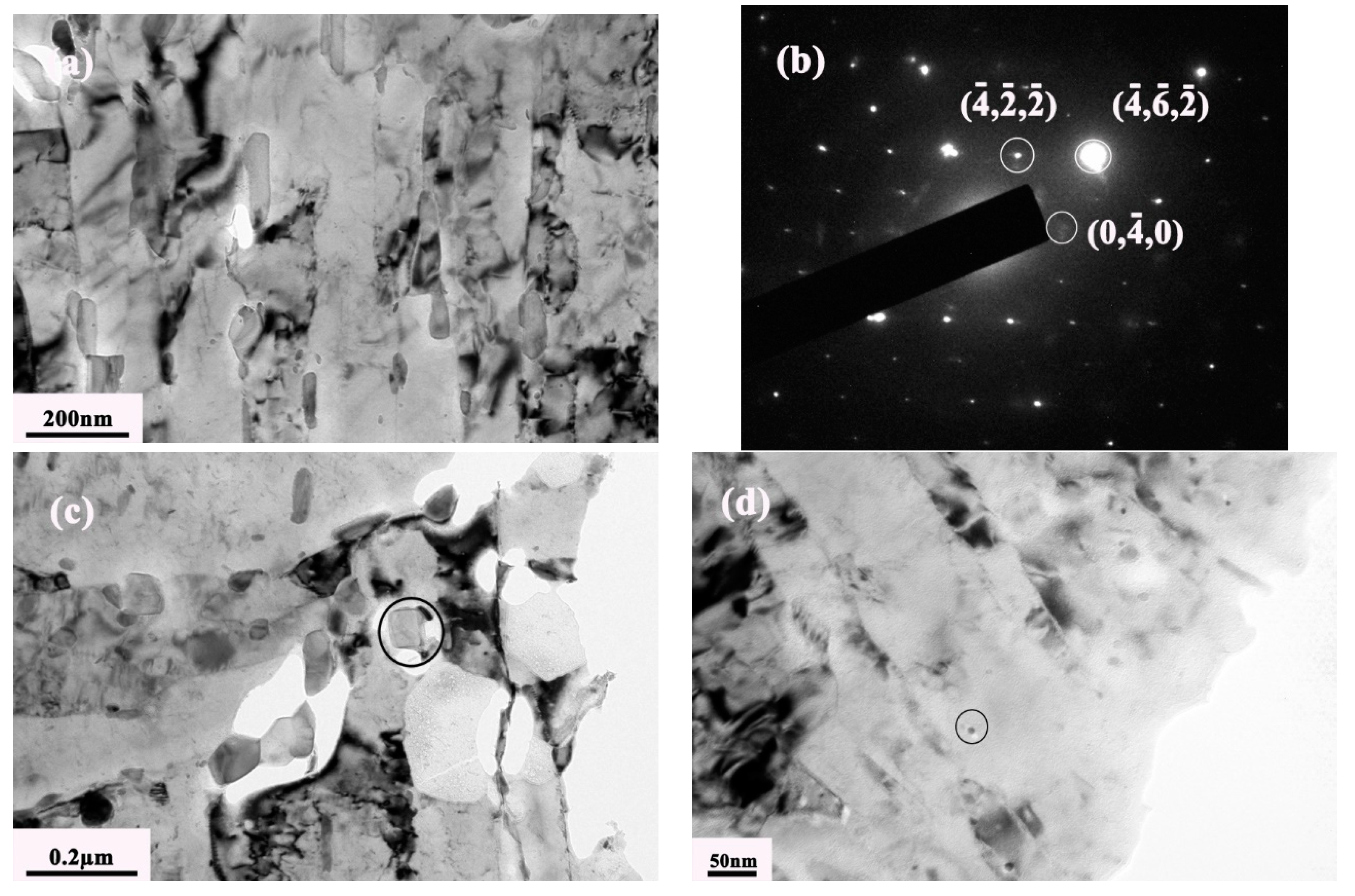
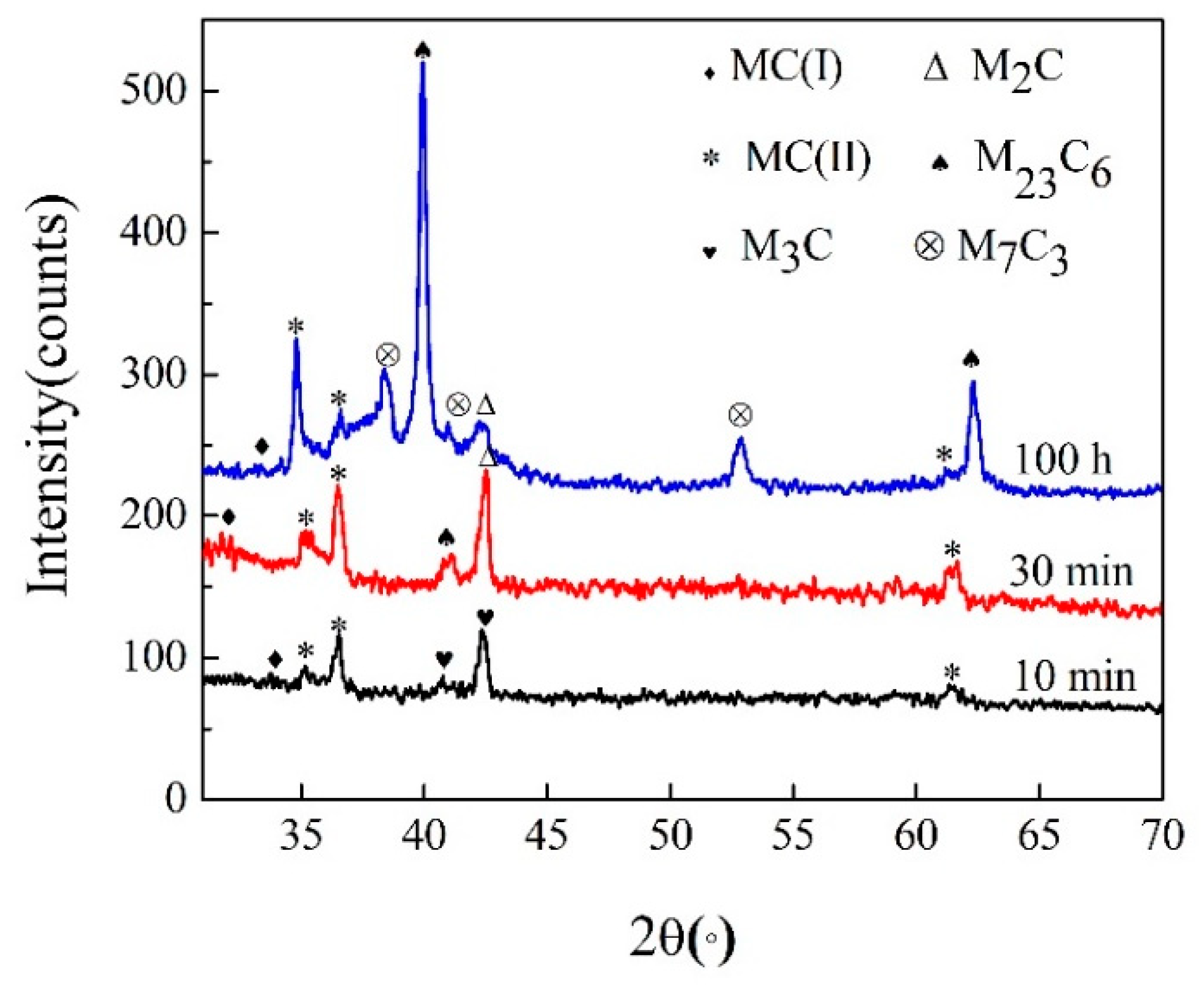
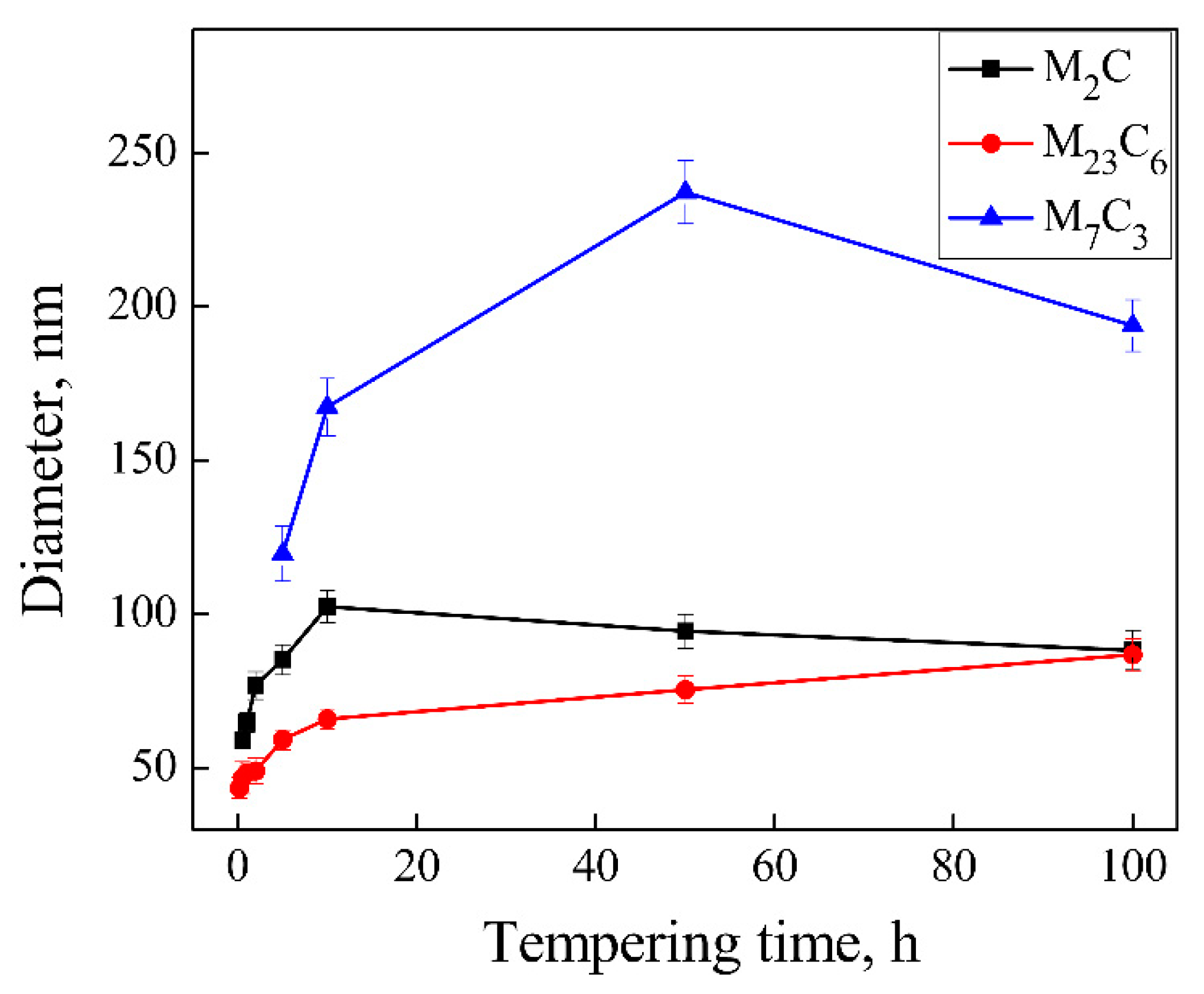
3.2. Precipitation Behavior of Carbide under Different Tempering Times
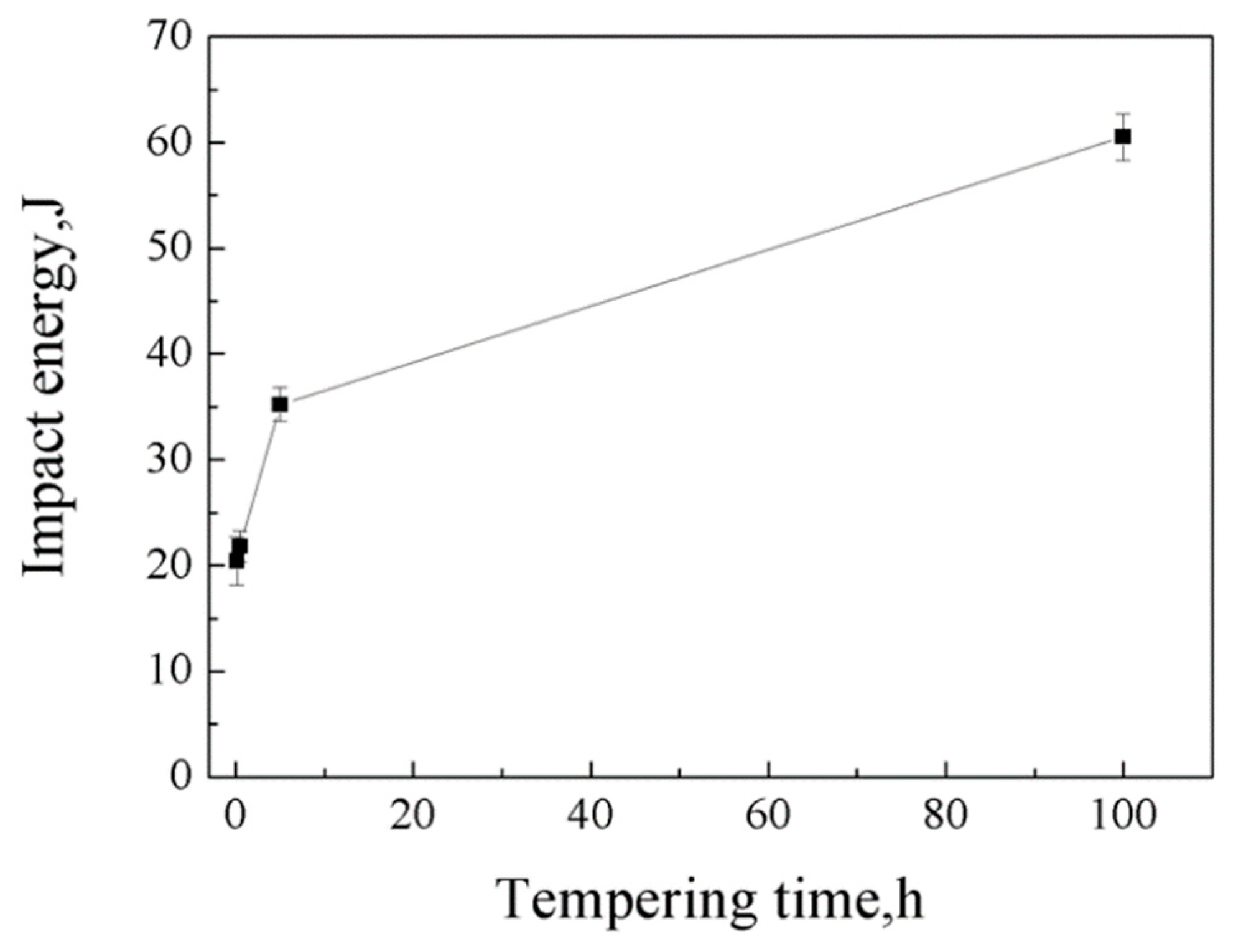
3.3. Mechanical Properties under Different Tempering Times
4. Conclusions
- The width of martensite laths increased and the retained austenite and dislocation decreased gradually with the prolongation of the tempering time.
- The evolution sequence of the carbides during tempering at 600 °C for the different times was identified as: M3C → M2C → M7C3 → M23C6. The other two kinds of MC carbides remained stable during the tempering process.
- The strength decreased and the Charpy impact toughness increased gradually with the prolongation of the tempering time. The Vickers hardness increased remarkably as the tempering time was extended to 1 h and then decreased sharply with a further increase in the tempering time up to 5 h. When the tempering time was between 5 and 100 h, the Vickers hardness values decreased gradually.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.J.; Xiao, N.M.; Li, D.X.; Zhang, J.Y.; Luo, Y.J.; Zhang, R.X. Effect of microstructure evolution on strength and impact toughness of G18CrMo2-6 heat-resistant steel during tempering. Mater. Sci. Eng. A 2014, 604, 103–110. [Google Scholar] [CrossRef]
- Sun, J.; Lian, F.L.; Sun, Y.; Wang, Y.J.; Guo, S.W.; Liu, Y.N. The Fine Grain Effect on a New Carbide Fe4C3 Formed in Pipeline Steel X80. JOM 2023, 75, 417–427. [Google Scholar] [CrossRef]
- Li, X.; Shi, L.; Liu, Y.; Gan, K.; Liu, C. Achieving a desirable combination of mechanical properties in HSLA steel through step quenching. Mater. Sci. Eng. A 2020, 772, 138683. [Google Scholar] [CrossRef]
- Mandal, A.; Barik, R.K.; Bhattacharya, A.; Chakrabarti, D.; Davis, C. The Correlation Between Bending, Tensile and Charpy Impact Properties of Ultra-high-Strength Strip Steels. Metall. Mater. Trans. A 2023, 1–24. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, C.; Yan, Z.; Li, H.; Liu, Y. Formation mechanism and control methods of acicular ferrite in HSLA steels: A review. J. Mater. Sci. Technol. 2018, 34, 737–744. [Google Scholar] [CrossRef]
- Kan, L.; Ye, Q.; Wang, Z.; Zhao, T. Improvement of strength and toughness of 1 GPa Cu-bearing HSLA steel by direct quenching. Mater. Sci. Eng. A 2022, 855, 143875. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Liao, T.; Wang, S.; Lv, B.; Guo, H.; Huang, Y.; Yong, Q.; Mao, X. Revealing the precipitation kinetics and strengthening mechanisms of a 450 MPa grade Nb-bearing HSLA steel. Mater. Sci. Eng. A 2023, 884, 145506. [Google Scholar] [CrossRef]
- Kar, S.; Srivastava, V.C.; Mandal, G.K. Low-density nano-precipitation hardened Ni-based medium entropy alloy with excellent strength-ductility synergy. J. Alloys Compd. 2023, 963, 171213. [Google Scholar] [CrossRef]
- Huang, W.; Lei, L.; Fang, G. Optimizing Heat Treatment to Improve the Microstructures and Mechanical Properties of 5CrNiMoV Steel. Metals 2023, 13, 1263. [Google Scholar] [CrossRef]
- Shi, L.; Yan, Z.; Liu, Y.; Zhang, C.; Qiao, Z.; Ning, B.; Li, H. Improved toughness and ductility in ferrite/acicular ferrite dual-phase steel through intercritical heat treatment. Mater. Sci. Eng. A 2014, 590, 7–15. [Google Scholar] [CrossRef]
- Dudko, V.; Belyakov, A.; Molodov, D.; Kaibyshev, R. Microstructure evolution and pinning of boundaries by precipitates in a 9 pct Cr heat resistant steel during creep. Metall. Mater. Trans. A 2013, 44, 162–172. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, C.; Zhu, J.; Yang, Z.; Ding, R.; Zhang, C.; Yang, Z. Austenite/ferrite interface migration and alloying elements partitioning: An overview. Acta Metall. Sin. 2017, 54, 217–227. [Google Scholar]
- Dong, J.; Zhou, X.; Liu, Y.; Li, C.; Liu, C.; Li, H. Effects of quenching-partitioning-tempering treatment on microstructure and mechanical performance of Nb-V-Ti microalloyed ultra-high strength steel. Mater. Sci. Eng. A 2017, 690, 283–293. [Google Scholar] [CrossRef]
- Roy, S.; Romualdi, N.; Yamada, K.; Poole, W.; Militzer, M.; Collins, L. The Relationship Between Microstructure and Hardness in the Heat-Affected Zone of Line Pipe Steels. JOM 2022, 74, 2395–2401. [Google Scholar] [CrossRef]
- Wu, H.; Ju, B.; Tang, D.; Hu, R.; Guo, A.; Kang, Q.; Wang, D. Effect of Nb addition on the microstructure and mechanical properties of an 1800 MPa ultrahigh strength steel. Mater. Sci. Eng. A 2015, 622, 61–66. [Google Scholar] [CrossRef]
- Mandal, A.; Bandyopadhay, T.K. Effect of tempering on microstructure and tensile properties of niobium modified martensitic 9Cr heat resistant steel. Mater. Sci. Eng. A 2015, 620, 463–470. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, H.; Shang, Z.; Xu, Z. Precipitate phases in normalized and tempered ferritic/martensitic steel P92. J. Nucl. Mater. 2015, 465, 373–382. [Google Scholar] [CrossRef]
- Moon, J.; Choi, J.; Han, S.K.; Huh, S.; Kim, S.J.; Lee, C.H.; Lee, T.H. Influence of precipitation behavior on mechanical properties and hydrogen induced cracking during tempering of hot-rolled API steel for tubing. Mater. Sci. Eng. A 2016, 652, 120–126. [Google Scholar] [CrossRef]
- Tao, X.G.; Han, L.Z.; Gu, J.F. Effect of tempering on microstructure evolution and mechanical properties of X12CrMoWVNbN10-1-1 steel. Mater. Sci. Eng. A 2014, 618, 189–204. [Google Scholar] [CrossRef]
- Stornelli, G.; Tselikova, A.; Mirabile Gattia, D.; Mortello, M.; Schmidt, R.; Sgambetterra, M.; Testani, C.; Zucca, G.; Di Schino, A. Influence of vanadium micro-alloying on the microstructure of structural high strength steels welded joints. Materials 2023, 16, 2897. [Google Scholar] [CrossRef]
- Janovec, J.; Svoboda, M.; Kroupa, A.; Výrostková, A. Thermal-induced evolution of secondary phases in Cr–Mo–V low alloy steels. J. Mater. Sci. 2006, 41, 3425–3433. [Google Scholar] [CrossRef]
- Janovec, J.; Svoboda, M.; Výrostková, A.; Kroupa, A. Time–temperature–precipitation diagrams of carbide evolution in low alloy steels. Mater. Sci. Eng. A 2005, 402, 288–293. [Google Scholar] [CrossRef]
- Jia, C.; Liu, Y.; Liu, C.; Li, C.; Li, H. Precipitates evolution during tempering of 9CrMoCoB (CB2) ferritic heat-resistant steel. Mater. Charact. 2019, 152, 12–20. [Google Scholar] [CrossRef]
- Janovec, J.; Vyrostkova, A.; Svoboda, M. Influence of tempering temperature on stability of carbide phases in 2.6 Cr–0.7 Mo–0.3 V steel with various carbon content. Metall. Mater. Trans. A 1994, 25, 267–275. [Google Scholar] [CrossRef]
- Li, C.; Duan, R.; Fu, W.; Gao, H.; Wang, D.; Di, X. Improvement of mechanical properties for low carbon ultra-high strength steel strengthened by Cu-rich multistructured precipitation via modification to bainite. Mater. Sci. Eng. A 2021, 817, 141337. [Google Scholar] [CrossRef]
- Thomson, R.C.; Bhadeshia, H.K.D.H. Atom probe and STEM studies of carbide precipitation in 2sol14Cr1Mo steel. Appl. Surf. 1993, 67, 334–341. [Google Scholar] [CrossRef]
- Jain, D.; Isheim, D.; Hunter, A.H.; Seidman, D.N. Multicomponent high-strength low-alloy steel precipitation-strengthened by sub-nanometric Cu precipitates and M2C carbides. Metall. Mater. Trans. A 2016, 47, 3860–3872. [Google Scholar] [CrossRef]
- Lee, W.B.; Hong, S.G.; Park, C.G.; Park, S.H. Carbide precipitation and high-temperature strength of hot-rolled high-strength, low-alloy steels containing Nb and Mo. Metall. Mater. Trans. A 2002, 33, 1689–1698. [Google Scholar] [CrossRef]
- Fedoseeva, A.; Dudova, N.; Glatzel, U.; Kaibyshev, R. Effect of W on tempering behaviour of a 3% Co modified P92 steel. J. Mater. Sci. 2016, 51, 9424–9439. [Google Scholar] [CrossRef]
- Chakraborty, G.; Das, C.R.; Albert, S.K.; Bhaduri, A.K.; Paul, V.T.; Panneerselvam, G.; Dasgupta, A. Study on tempering behaviour of AISI 410 stainless steel. Mater. Charact. 2015, 100, 81–87. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Liu, Y.; Li, C.; Liu, C.; Guo, Q. Carbide precipitation in Nb-V-Ti microalloyed ultra-high strength steel during tempering. Mater. Sci. Eng. A 2017, 683, 215–226. [Google Scholar] [CrossRef]
- Hou, Z.; Hedström, P.; Chen, Q.; Xu, Y.; Wu, D.; Odqvist, J. Quantitative modeling and experimental verification of carbide precipitation in a martensitic Fe–0.16 wt% C–4.0 wt% Cr alloy. Calphad 2016, 53, 39–48. [Google Scholar] [CrossRef]
- Kasana, S.S.; Pandey, O.P. Effect of electroslag remelting and homogenization on hydrogen flaking in AMS-4340 ultra-high-strength steels. Int. J. Min. Met. Mater. 2019, 26, 611–621. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, Y. Influence of carbides on the high-temperature tempered martensite embrittlement of martensitic heat-resistant steels. Mater. Sci. Eng. A 2016, 670, 256–263. [Google Scholar] [CrossRef]
- Kolluri, M.; Martin, O.; Naziris, F.; D’Agata, E.; Gillemot, F.; Brumovsky, M.; Ulbricht, A.; Autio, J.-M.; Shugailo, A.; Horvath, A. Structural MATerias research on parameters influencing the material properties of RPV steels for safe long-term operation of PWR NPPs. Nucl. Eng. Des. 2023, 406, 112236. [Google Scholar] [CrossRef]
- Tao, X.; Gu, J.; Han, L. Characterization of precipitates in X12CrMoWVNbN10-1-1 steel during heat treatment. J. Nucl. Mater. 2014, 452, 557–564. [Google Scholar] [CrossRef]
- Maddi, L.; Deshmukh, G.S.; Ballal, A.R.; Peshwe, D.R.; Paretkar, R.K.; Laha, K.; Mathew, M.D. Effect of Laves phase on the creep rupture properties of P92 steel. Mater. Sci. Eng. A 2016, 668, 215–223. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Ren, J.; Li, R.; Wang, M.; Zhang, F.; Yan, Z. Effect of microstructure on the strength of 25CrMo48V martensitic steel tempered at different temperature and time. Mater. Des. 2012, 36, 220–226. [Google Scholar] [CrossRef]
- Caballero, F.G.; Santofimia, M.J.; García-Mateo, C.; Chao, J.; De Andres, C.G. Theoretical design and advanced microstructure in super high strength steels. Mater. Des. 2009, 30, 2077–2083. [Google Scholar] [CrossRef]
- Dai, L.; Liu, Z.; Yu, L.; Liu, Y.; Shi, Z. Microstructural characterization of Mg–Al–O rich nanophase strengthened Fe–Cr alloys. Mater. Sci. Eng. A 2020, 771, 138664. [Google Scholar] [CrossRef]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzaki, K.; Furuhara, T. Stress–strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta Mater. 2015, 83, 383–396. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Bao, H.; Weng, Y.; Liu, W. Effect of tempering temperature on the toughness of 9Cr–3W–3Co martensitic heat resistant steel. Mater. Des. 2014, 54, 874–879. [Google Scholar] [CrossRef]
- Li, X.; Cai, Z.; Hu, M.; Li, K.; Hou, M.; Pan, J. Effect of NbC precipitation on toughness of X12CrMoWNbVN10-1-1 martensitic heat resistant steel for steam turbine blade. J. Mater. Res. Technol. 2021, 11, 2092–2105. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Zhan, M.; Zheng, Z.; Gao, J.; Shao, G. Electron force-induced dislocations annihilation and regeneration of a superalloy through electrical in-situ transmission electron microscopy observations. J. Mater. Sci. Technol. 2020, 36, 79–83. [Google Scholar] [CrossRef]
- Martınez-de-Guerenu, A.; Arizti, F.; Gutiérrez, I. Recovery during annealing in a cold rolled low carbon steel. Part II: Modelling the kinetics. Acta Mater. 2004, 52, 3665–3670. [Google Scholar] [CrossRef]







| Tempering Time | YS, MPa | UTS, MPa | TE, % |
|---|---|---|---|
| 10 min | 1138 ± 16 | 1231 ± 20 | 8.8 ± 0.3 |
| 30 min | 1112 ± 12 | 1193 ± 18 | 8.4 ± 0.2 |
| 5 h | 1055 ± 14 | 1132 ± 21 | 11.5 ± 0.5 |
| 100 h | 835 ± 11 | 896 ± 15 | 12.3 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Qiao, Z.; Dong, J. Effect of Tempering Time on Carbide Evolution and Mechanical Properties of a Nb-V-Ti Micro-Alloyed Steel. Metals 2023, 13, 1495. https://doi.org/10.3390/met13081495
Zhao Q, Qiao Z, Dong J. Effect of Tempering Time on Carbide Evolution and Mechanical Properties of a Nb-V-Ti Micro-Alloyed Steel. Metals. 2023; 13(8):1495. https://doi.org/10.3390/met13081495
Chicago/Turabian StyleZhao, Qian, Zhixia Qiao, and Ji Dong. 2023. "Effect of Tempering Time on Carbide Evolution and Mechanical Properties of a Nb-V-Ti Micro-Alloyed Steel" Metals 13, no. 8: 1495. https://doi.org/10.3390/met13081495






