A Kinetic Study on the Preparation of Al-Mn Alloys by Aluminothermic Reduction of Mn3O4 and MnO Powders
Abstract
:1. Introduction
Aluminothermic Reduction Reactions
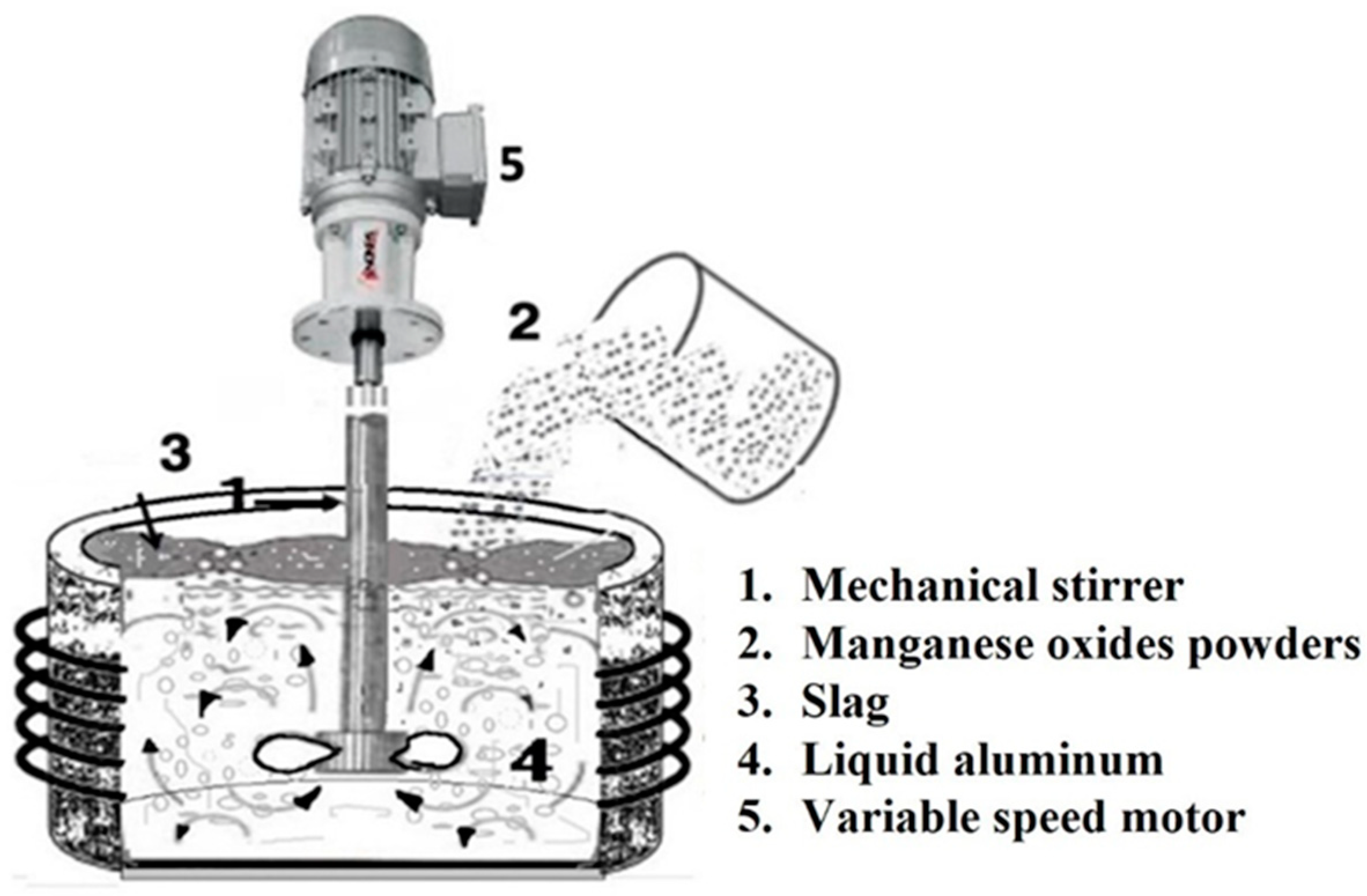
2. Materials and Methods
3. Results and Discussion
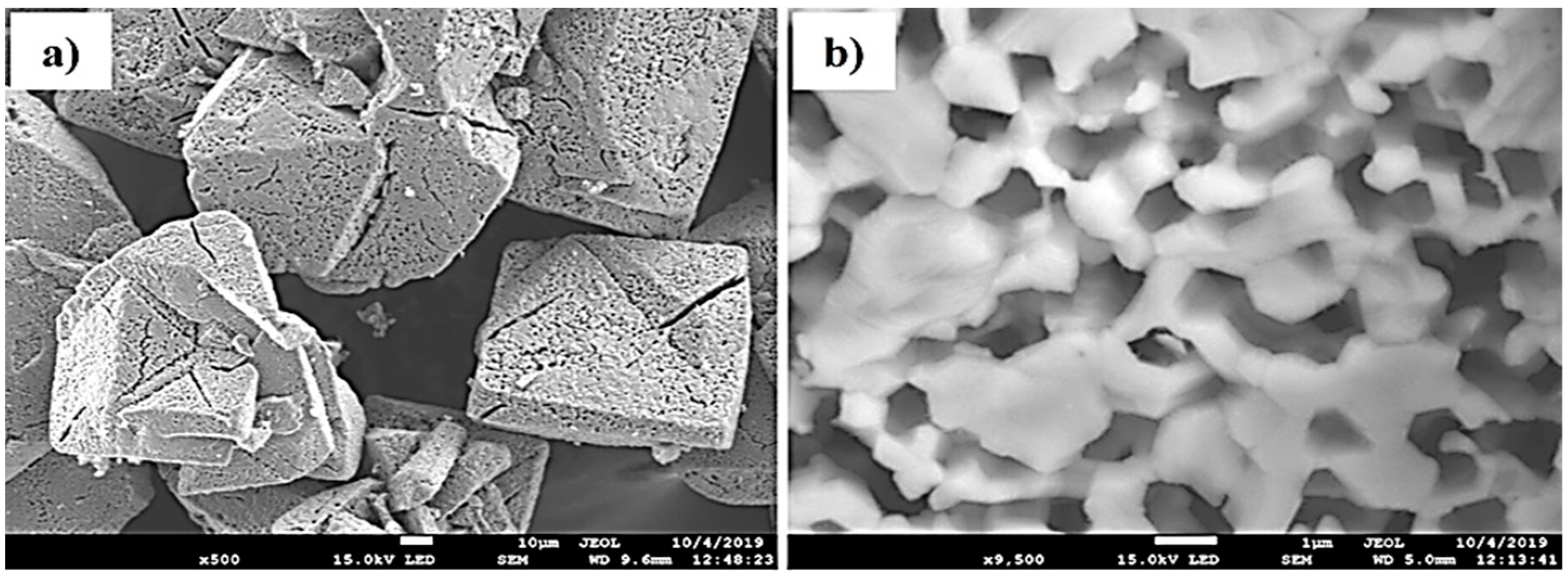
3.1. Characterization of the MnO and Mn3O4 Powders
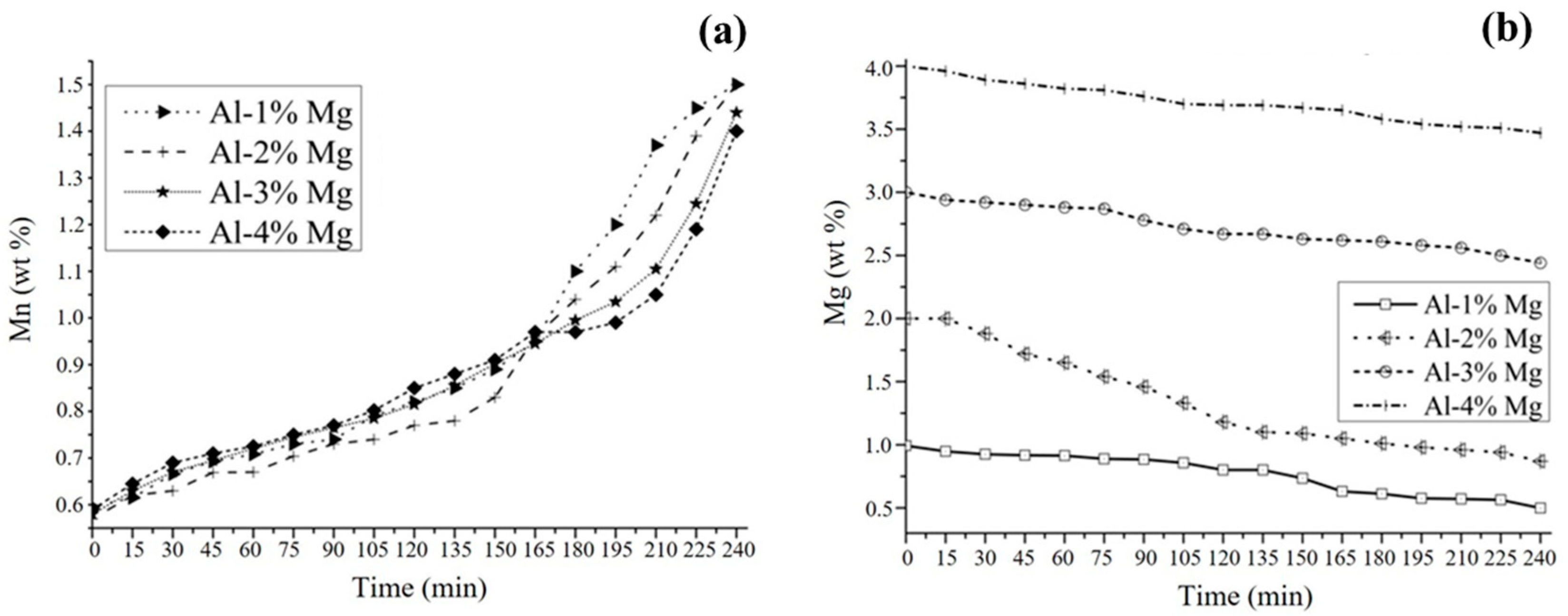
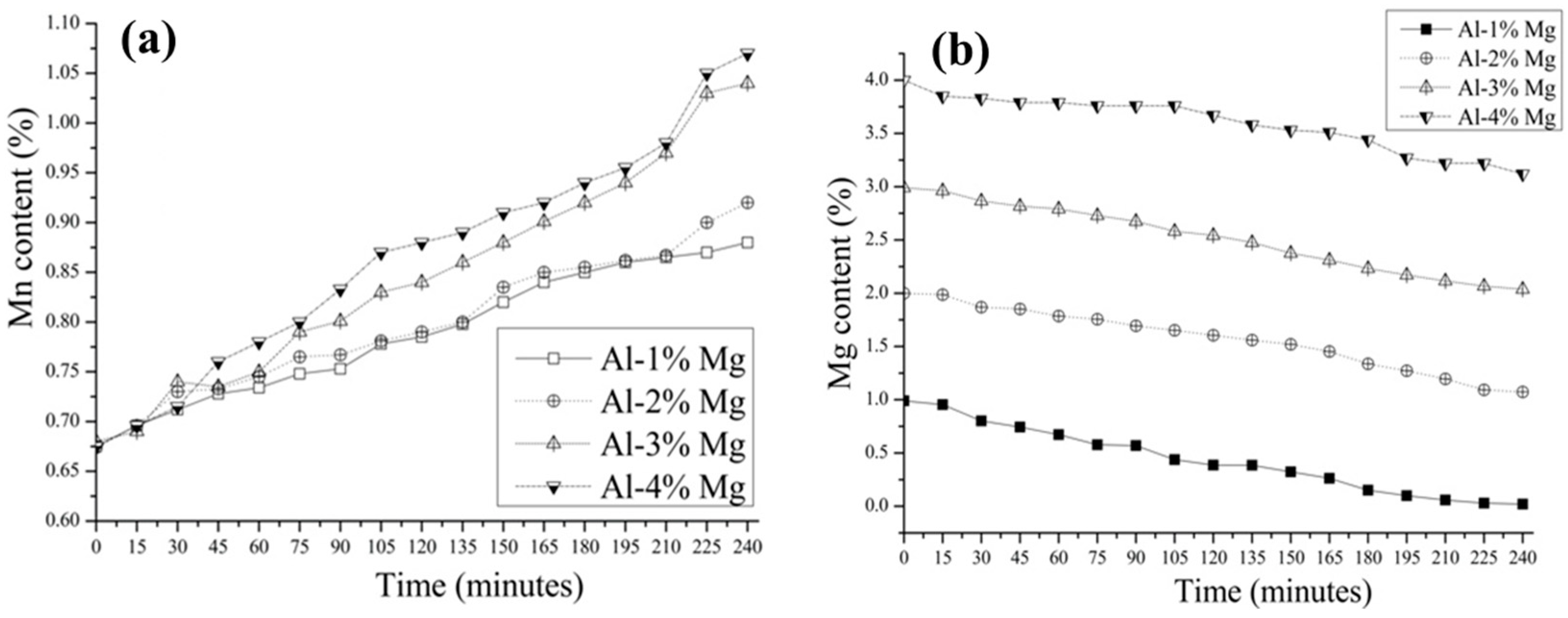
3.2. Aluminothermic Reduction
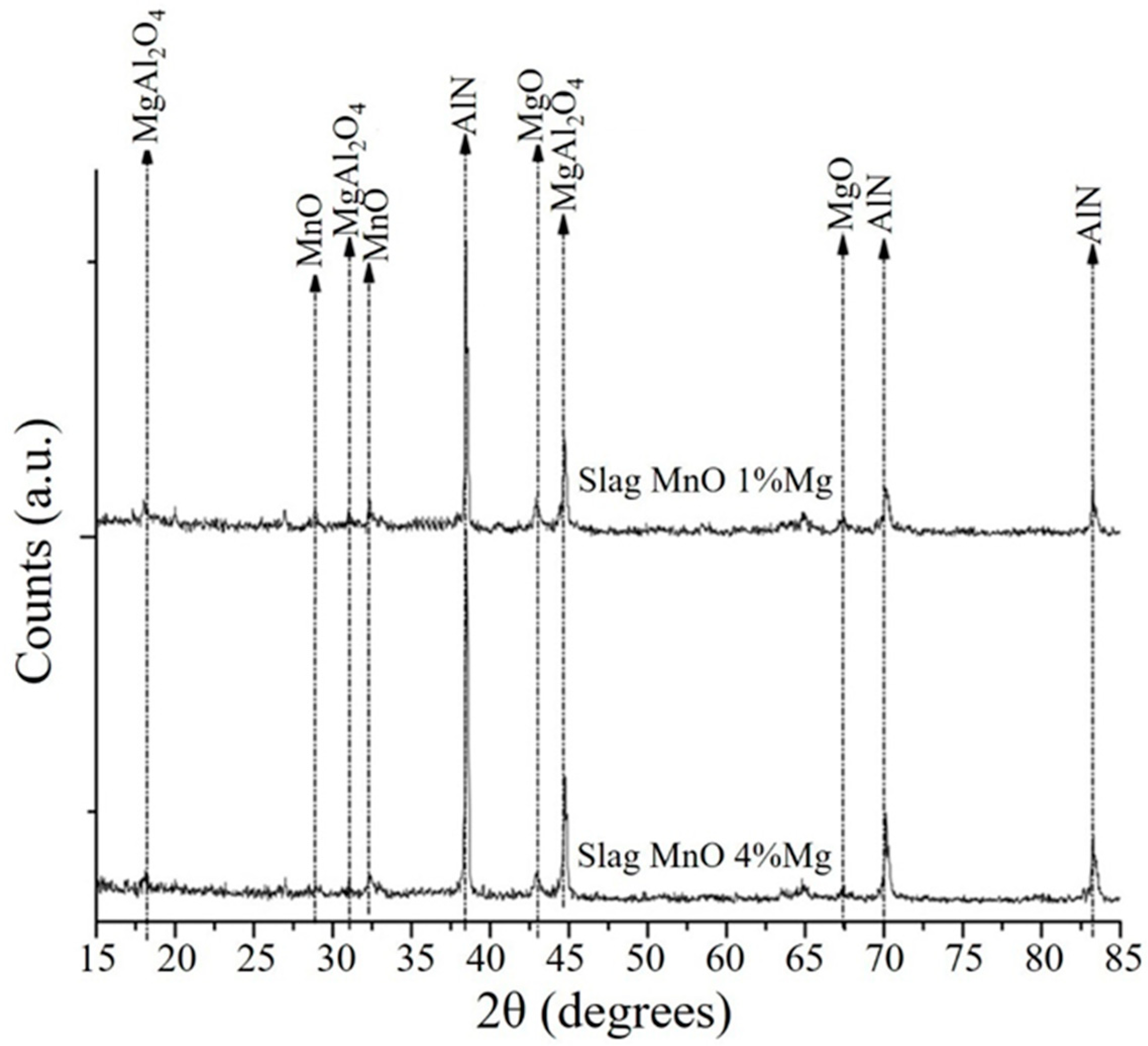
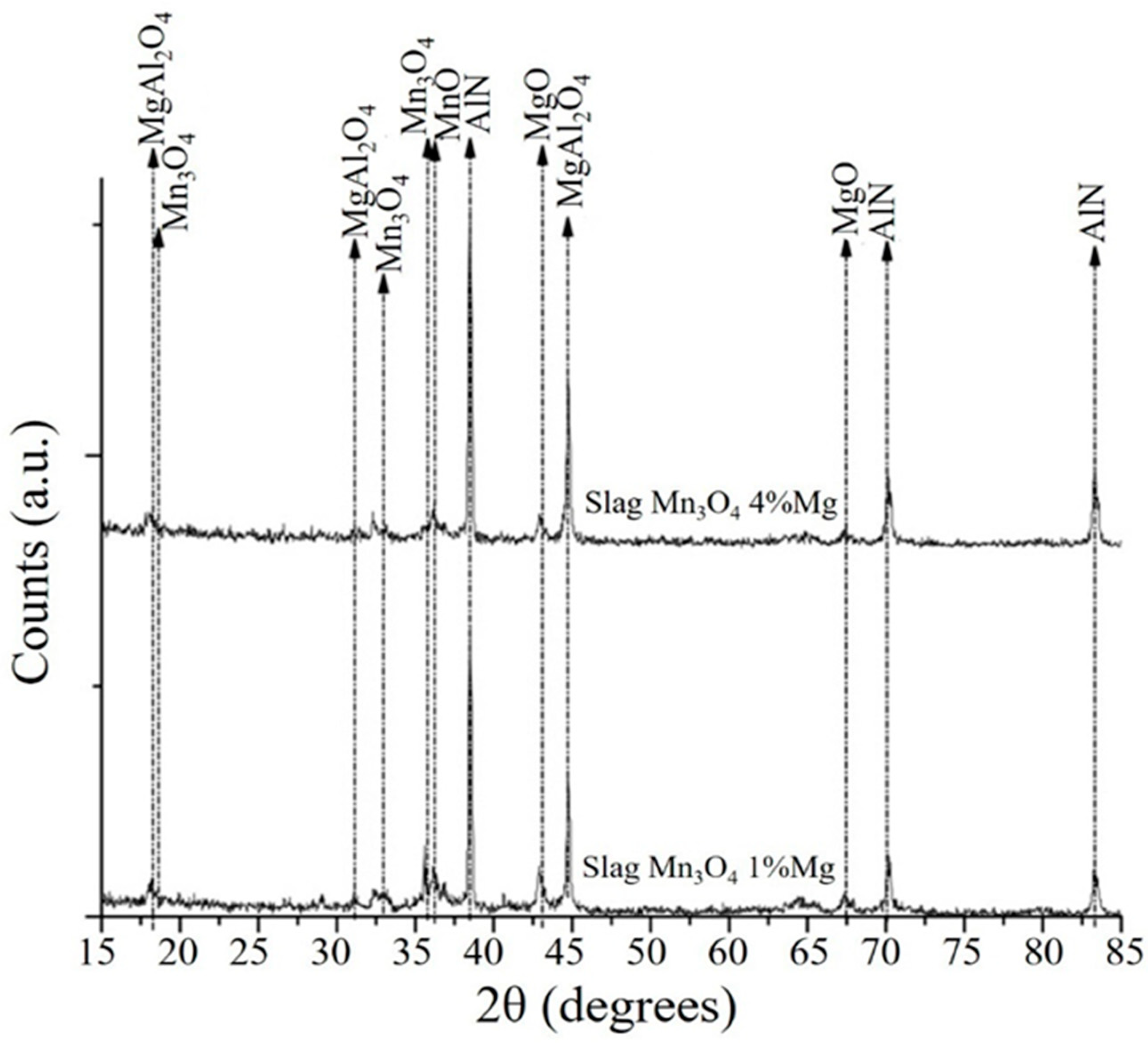
3.3. Analysis of the Slags
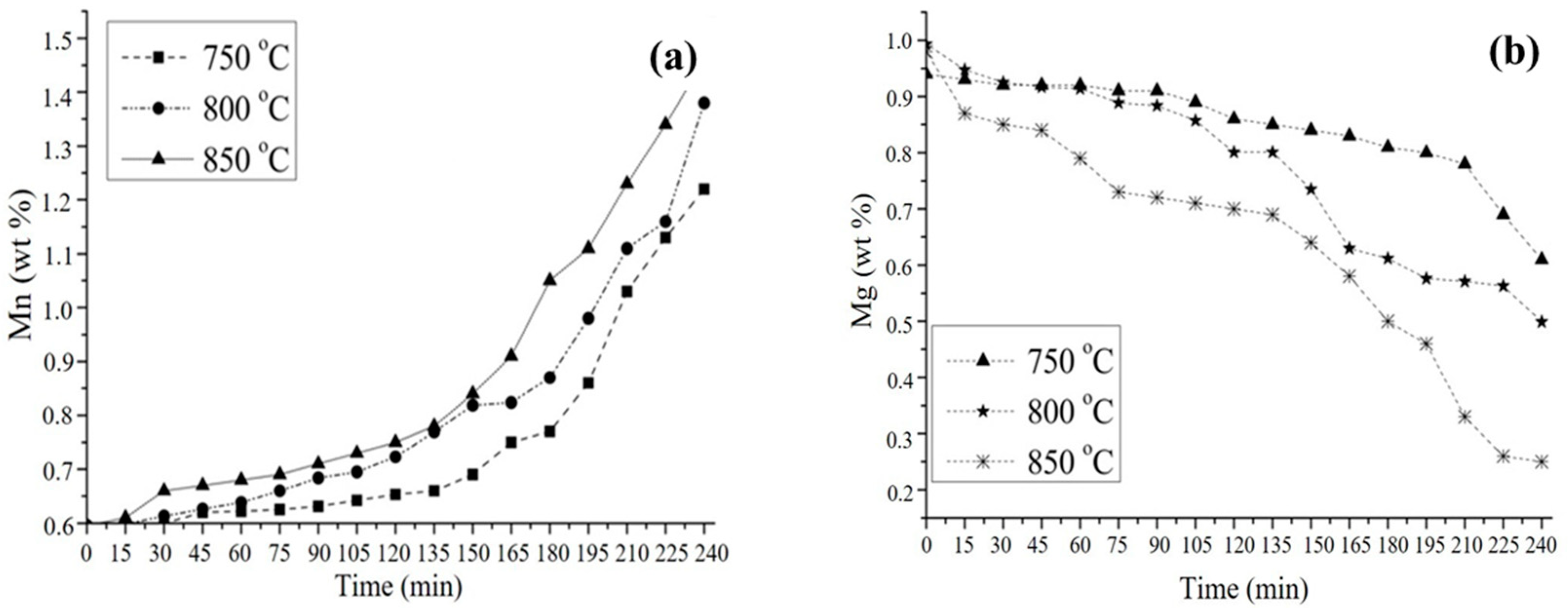
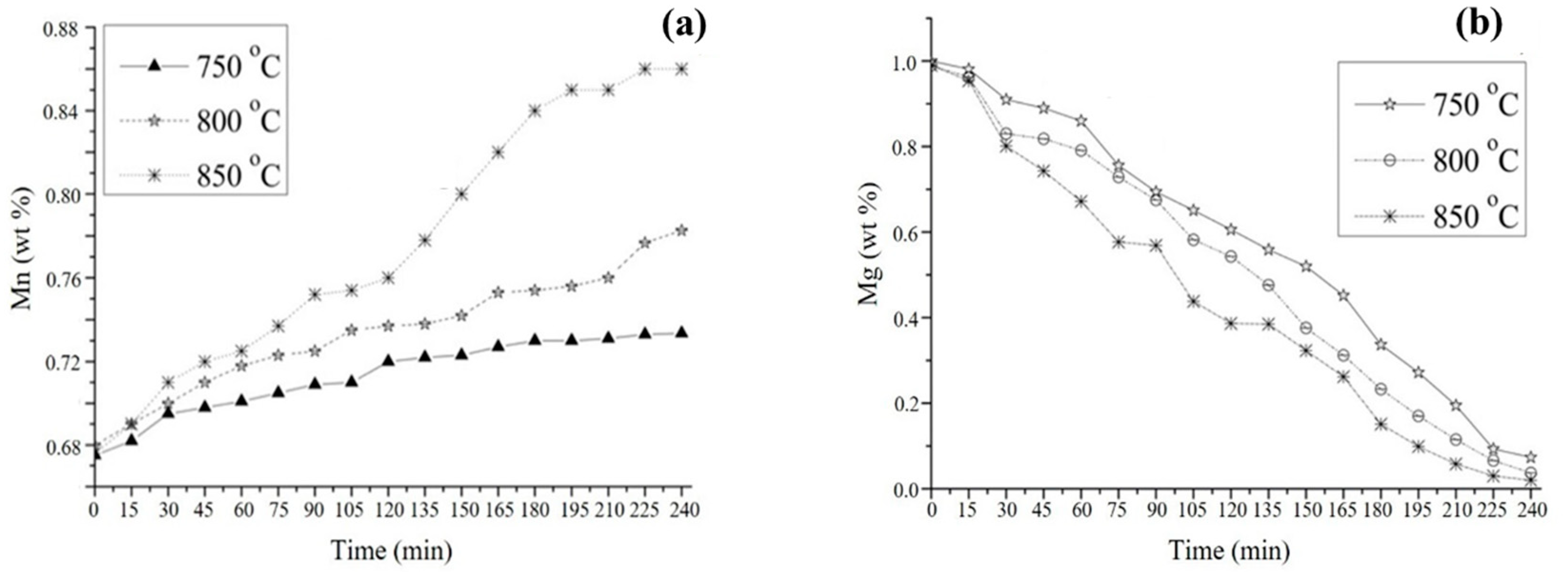
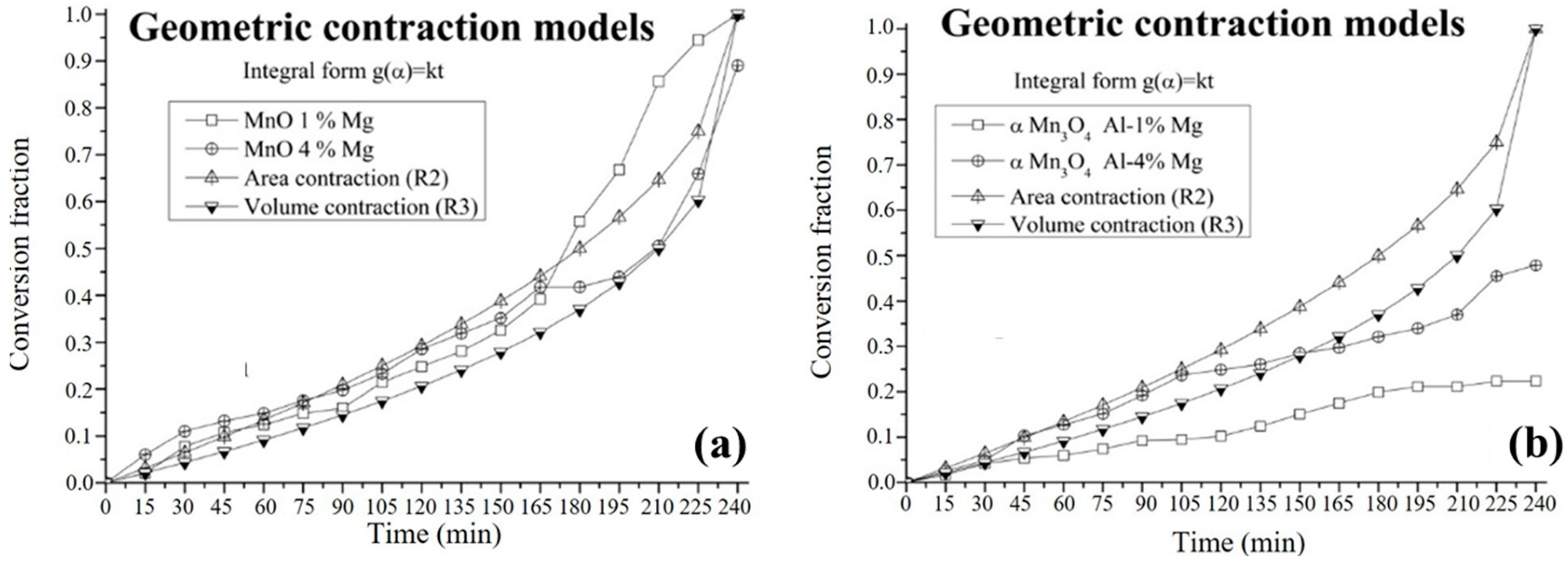
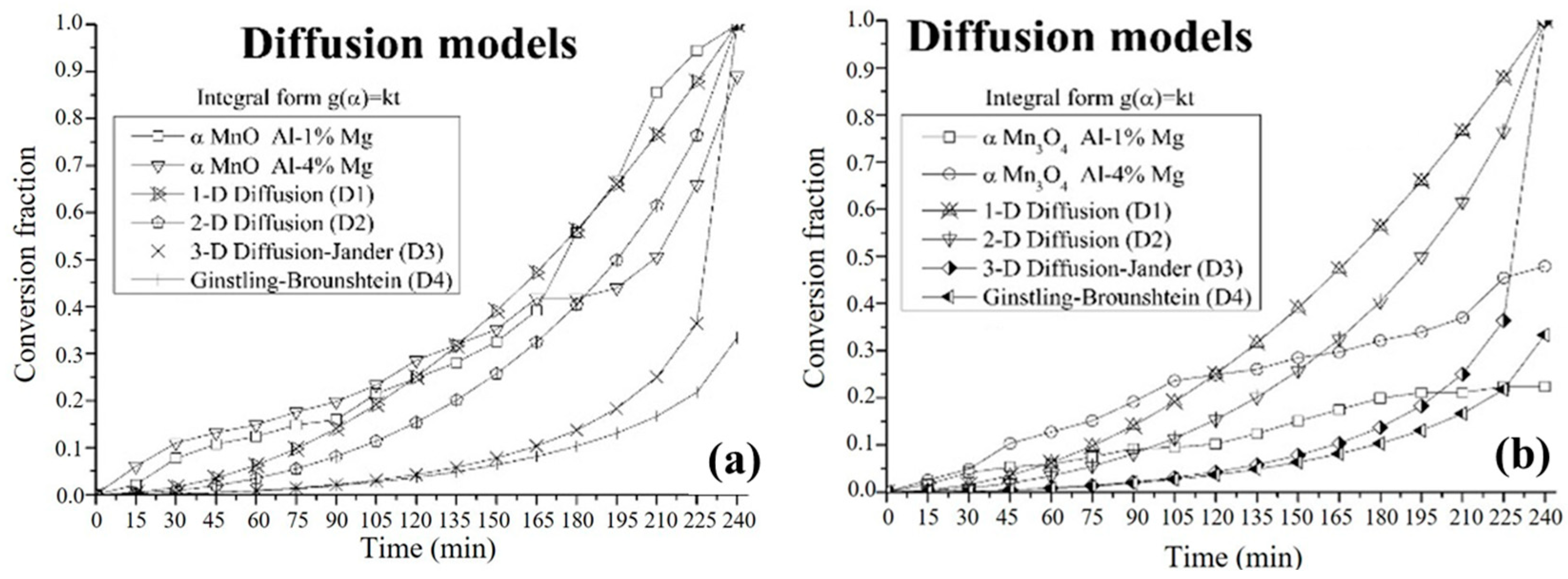
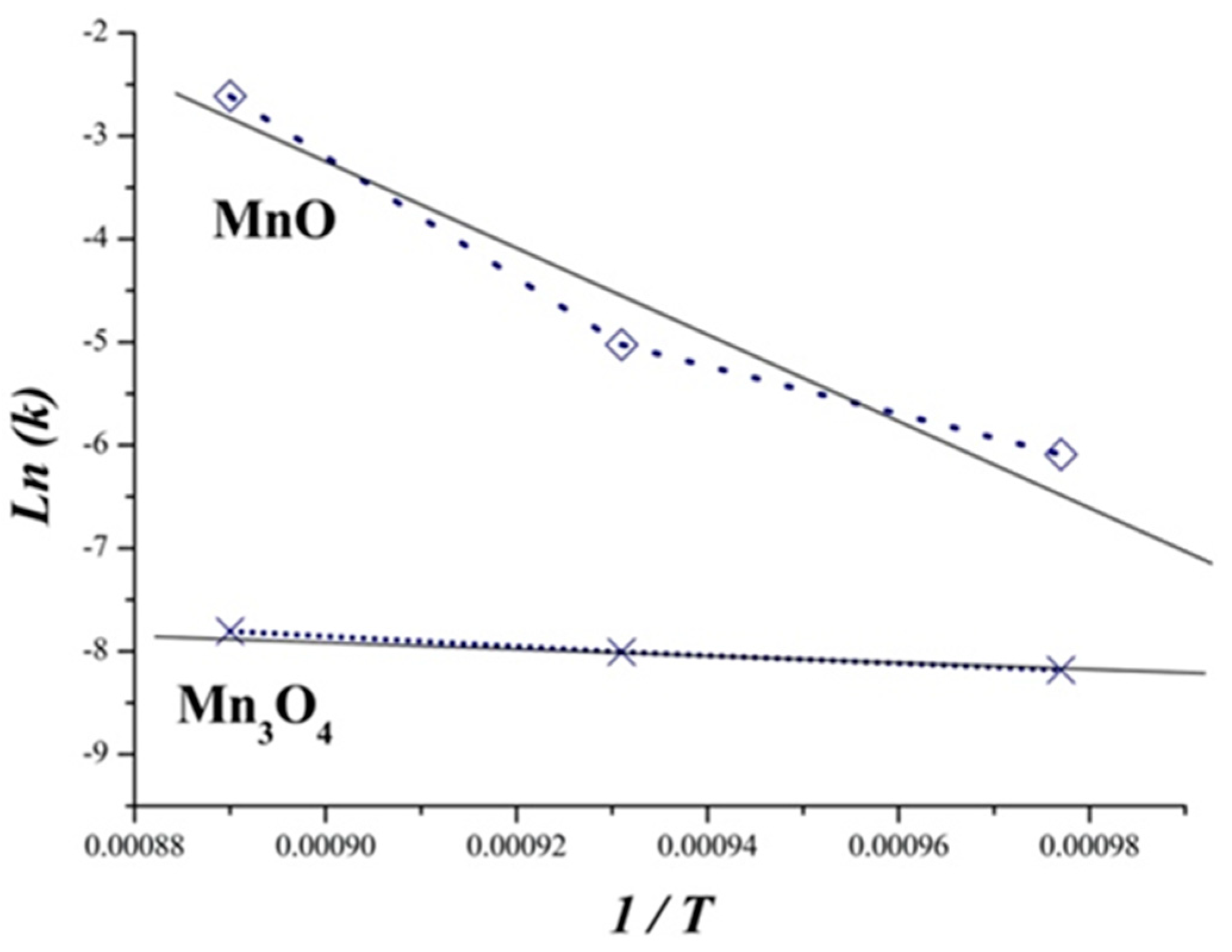
3.4. Kinetic Study
3.5. Thermodynamic Consideration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romano, D.C.; Moura, A.; Tenório, J.A. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Lindermann, W. Process for the Recovery of Raw Materials from Presorted Collected Waste, Especially Scrap Electrochemical Batteries and Accumulators. U.S. Patent US5575907A, 19 November 1996. [Google Scholar]
- Poinsignon, C.; Lucienne, J. Method for Electrolytical Processing of Used Batteries. European Patent EP0620607A11997, 6 August 1994. [Google Scholar]
- Ahmed, F. The battery recycling loop: A European perspective. J. Power Sources 1996, 59, 107–111. [Google Scholar] [CrossRef]
- Ming, Y.; En, J.; Cheng, C.; Yu, J.; Ping, G. Vitrification for reclaiming spent alkaline batteries. Waste Manag. 2009, 29, 2132–2139. [Google Scholar]
- Jordi, H. A financing system for battery recycling in Switzerland. J. Power Sources 1995, 57, 51–53. [Google Scholar] [CrossRef]
- Ferella, F.; Michelis, I.M.; Vegliò, F. Process for the recycling of alkaline and zinc–carbon spent batteries. J. Power Sources 2008, 183, 805–811. [Google Scholar] [CrossRef]
- Mukhlis, R.; Mackenzie, A.; Rhamdhani, M.A. Small scale recycling process for spent alkaline batteries: Technoeconomic analysis and potential use of solar energy. Resour. Conserv. Recycl. 2021, 166, 105367. [Google Scholar] [CrossRef]
- Klejnowska, K.; Sydow, M.; Michalski, R.; Bogacka, M. Life Cycle Impacts of Recycling of Black Mass Obtained from End-Of-Life Zn-C and Alkaline Batteries Using Waelz Kiln. Energies 2023, 16, 49. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Lach, J.; Wróbel, K.; Domańska, U. New method for recovery of nickel and cadmium from the “black mass” of spent Ni-Cd batteries by solvent extraction. J. Mol. Liq. 2022, 357, 119087. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Lach, J.; Wróbel, K.; Domańska, U. Recovery of zinc and manganese from “black mass” of waste Zn-MnO2 alkaline batteries by solvent extraction technique with ionic liquids, DESs and organophosphorous-based acids. J. Mol. Liq. 2021, 338, 116590. [Google Scholar] [CrossRef]
- Sadeghi, S.M.; Jesus, J.; Soares, H.M.V.M. A critical updated review of the hydrometallurgical routes for recycling zinc and manganese from spent zinc-based batteries. Waste Manag. 2020, 113, 342–350. [Google Scholar] [CrossRef]
- Yang, Z.; Uhrynowski, W.; Jakusz, G.; Retka, J.; Karczewska-Golec, J.; Debiec-Andrzejewska, K.; Rogulski, Z.; Drewniak, L. Biochemical treatment of leachates from hydrometallurgical recycling of spent alkaline batteries. Hidrometallurgy 2020, 191, 105223. [Google Scholar] [CrossRef]
- Petranikova, M.; Ebin, B.; Mikhailova, S.; Steenari, B.M.; Ekberg, C. Investigation of the effects of thermal treatment on the leachability of Zn and Mn from discarded alkaline and Znsingle bondC batteries. J. Clean. Prod. 2018, 170, 1195–1205. [Google Scholar] [CrossRef]
- Andak, B.; Özduğan, E.; Türdü, S.; Bulutcu, A.N. Recovery of zinc and manganese from spent zinc-carbon and alkaline battery mixtures via selective leaching and crystallization processes. J. Environ. Chem. Eng. 2019, 7, 103372. [Google Scholar] [CrossRef]
- Flores, O.; Torres, J.; Flores, A. Effect of Mg Concentration on the aluminothermic reduction of Mn2O3 articles obtained from cathodes of discharged alkaline batteries: Mathematical Modeling and Experimental Results. Metals 2019, 9, 49. [Google Scholar]
- Torres, J.; Flores, A.; Almanza, J.M. Elaboration of Al-Mn Alloys by Aluminothermic Reduction of Mn2O3. Mater. Today Proc. 2015, 2, 4963–4970. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Wang, Z.; Mo, Y.; Luo, X.; Yang, F.; Lv, M.; Lid, Z.; Liu, X. Manganese-based oxide electrocatalysts for the oxygen evolution reaction: A review. J. Mater. Chem. A 2023, 11, 5476–5494. [Google Scholar] [CrossRef]
- Ochoa, R.M.; Flores, A.; Torres, J.; Escobedo, J.C. Manufacture of Al-Zn-Mg alloys using spent alkaline Batteries and Cans. Mater. Today Proc. 2015, 9, 4971–4977. [Google Scholar] [CrossRef]
- Flores, A.; Juarez, R.; Torres, J.; Ayala, Z.; Ochoa, R.M. A kinetic study on the aluminothermic reduction of ZrO2. Mater. Today Proc. 2019, 10, 332–339. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yang, Y.; Zhang, Y.; Wang, Z. Thermodynamics, kinetics and mechanism analysis of aluminothermic reduction for preparing Al-Zr alloy. Mater. Today Commun. 2022, 31, 103714. [Google Scholar] [CrossRef]
- Jinzhong, Y.; Peng, W.Y.J.; Di, Y. Reaction Mechanism and Kinetics of Ferrotitanium Preparation by Aluminothermic Reduction of CaTiO3. Mater. Today Comun. 2022, 30, 102995. [Google Scholar] [CrossRef]
- Larionov, A.V.; Chumarev, V.M.; Udoeva, L.Y.; Mansurova, A.N.; Rylov, A.N.; Raikov, A.Y.; Aleshiin, A.P.; Trubachev, M.V. Simulation of Aluminothermic Smeltingof Al–Zr and Al–Zr–Mo–Sn Alloys. Russ. Metall. Met. 2013, volume, 564–569. [Google Scholar] [CrossRef]
- Alonso, E.; Hutter, C.; Romero, M.; Steinfeld, A.; Gonzale, J.z. Kinetics of Mn2O3–Mn3O4 and Mn3O4–MnO Redox Reactions Performed under Concentrated Thermal Radiative Flux. Energy Fuels 2013, 27, 4884–4890. [Google Scholar] [CrossRef]
- Coudurier, L.; Hopkins, D.W.; Wilkomirsk, I.y. Chapter 1—Thermodynamic Functions. In Fundamentals of Metallurgical Processes, 2nd ed.; Coudurier, L., Hopkins, D.W., Wilkomirsky, I., Eds.; Elsevier: Amsterdam, The Netherlands; Pergamon Press: Oxford, UK, 1985; pp. 1–17. [Google Scholar]
- Rosenqvist, T. Principles of Extractive Metallurgy, 2nd ed.; Tapir Academic Press: Trondheim, Norway, 2004; pp. 236–270. [Google Scholar]
- Song, M.; Yu, B.; Cui, W.; Yang, W.; Bai, Q.; Zhang, C. A self-healing room-temperature liquid eutectic GaSn anode with improved wettability for advanced Mg ion batteries. Chem. Eng. J. 2022, 435, 134903. [Google Scholar] [CrossRef]
- Yang, N.; Shen, P.; Yang, B.; Guo, R.; Jiang, Q. Significant improvement in the wettability of ZrO2 by molten Al under the application of a direct current. Mater. Des. 2016, 111, 158–163. [Google Scholar] [CrossRef]
- Teng, L.; Yang, P.S.B.; Fen, R.; Zhang, N.; Chuan, Q. Polarity effects on the wettability and interfacial chemistry at Cu–YSZ interface by applying a direct current. J. Eur. Ceram. Soc. 2018, 38, 1790–1795. [Google Scholar] [CrossRef]
- Ochoa, R.M.; Flores, A.; Torres, J.; Guía, J.; Muñiz, R. Kinetic study on the metallothermic reduction of chromite ore using magnesium scrap. Can. Metall. Q. 2016, 55, 210–220. [Google Scholar] [CrossRef]
- Ramirez, A.; Hillebrand, P.; Stellmach, D.; May, M.M. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Zhong, Y.L.; Ying, J.Y. Porous MnO/Mn3O4 nanocomposites for electrochemical energy storage. Nano Energy 2015, 13, 902–908. [Google Scholar] [CrossRef]
- Nabi, G.; Lu, W.K. The Kinetics of Hematite to Magnetite Reduction in Hydrogen-Water and Hydrogen-Water-Nitrogen Mixtures. Ind. Eng. Chem. Fundamen. 1974, 13, 311–316. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-state kinetic models: Basics and mathematical fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Chakraborty, A. Kinetics of the reduction of hematite to magnetite near its Curie transition. J. Magn. Magn. Mater. 1999, 204, 57–60. [Google Scholar] [CrossRef]
- Kudyba, A.; Akhtar, S.; Johanse, I.; Safarian, J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials 2021, 14, 356. [Google Scholar] [CrossRef]
- Kudyba, A.; Safarian, J. Manganese and Aluminium Recovery from Ferromanganese Slag and Al White Dross by a High Temperature Smelting-Reduction Process. Materials 2022, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Valorization of Aluminum Dross with Copper via High Temperature Melting to Produce Al-Cu Alloys. Materials 2021, 14, 4117. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, P.E. Aluminium salt slag characterization and utilization—A review. J. Hazard. Mater. 2012, 217–218, 1–10. [Google Scholar] [CrossRef]



| Si | Fe | Cu | Mn | Mg | Ni | Zn | Ti | Co | Sr | V | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.26 | 0.73 | 0.18 | 0.6 | 0.9 | 0.012 | 0.12 | 0.01 | 0.004 | 0.001 | 0.01 | 97.2 |
| Parameters | Oxide | Temperature (°C) | Stirring Speed (RPM) | Mg (wt.%) |
|---|---|---|---|---|
| Type and range | MnO, Mn3O4 | 750, 800, 850 | 200, 250, 300 | 1, 2, 3, 4 |
| Powder | Ea (kJ/mol) | R2 |
|---|---|---|
| MnO (Al-1%Mg) | 329.56 | 0.93 |
| Mn3O4 (Al-4%Mg) | 35.88 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas Avilés, J.A.; Flores Valdés, A.; Torres Torres, J.; Ochoa Palacios, R.M.; Flores Saldívar, A.A. A Kinetic Study on the Preparation of Al-Mn Alloys by Aluminothermic Reduction of Mn3O4 and MnO Powders. Metals 2023, 13, 1556. https://doi.org/10.3390/met13091556
Salas Avilés JA, Flores Valdés A, Torres Torres J, Ochoa Palacios RM, Flores Saldívar AA. A Kinetic Study on the Preparation of Al-Mn Alloys by Aluminothermic Reduction of Mn3O4 and MnO Powders. Metals. 2023; 13(9):1556. https://doi.org/10.3390/met13091556
Chicago/Turabian StyleSalas Avilés, Juan Alberto, Alfredo Flores Valdés, Jesús Torres Torres, Rocío Maricela Ochoa Palacios, and Alfredo Alan Flores Saldívar. 2023. "A Kinetic Study on the Preparation of Al-Mn Alloys by Aluminothermic Reduction of Mn3O4 and MnO Powders" Metals 13, no. 9: 1556. https://doi.org/10.3390/met13091556






