Laser Powder Bed Fusion Fabrication of a Novel Carbide-Free Bainitic Steel: The Possibilities and a Comparative Study with the Conventional Alloy
Abstract
:1. Introduction
2. Experimental Procedures
2.1. L-PBF Fabrication and Parameter Optimization
2.2. Porosity, Melt Pool, and Microstructural Analysis
2.3. Hardness Measurements
2.4. Heat Treatment and Comparison with Conventionally Fabricated Material
3. Results
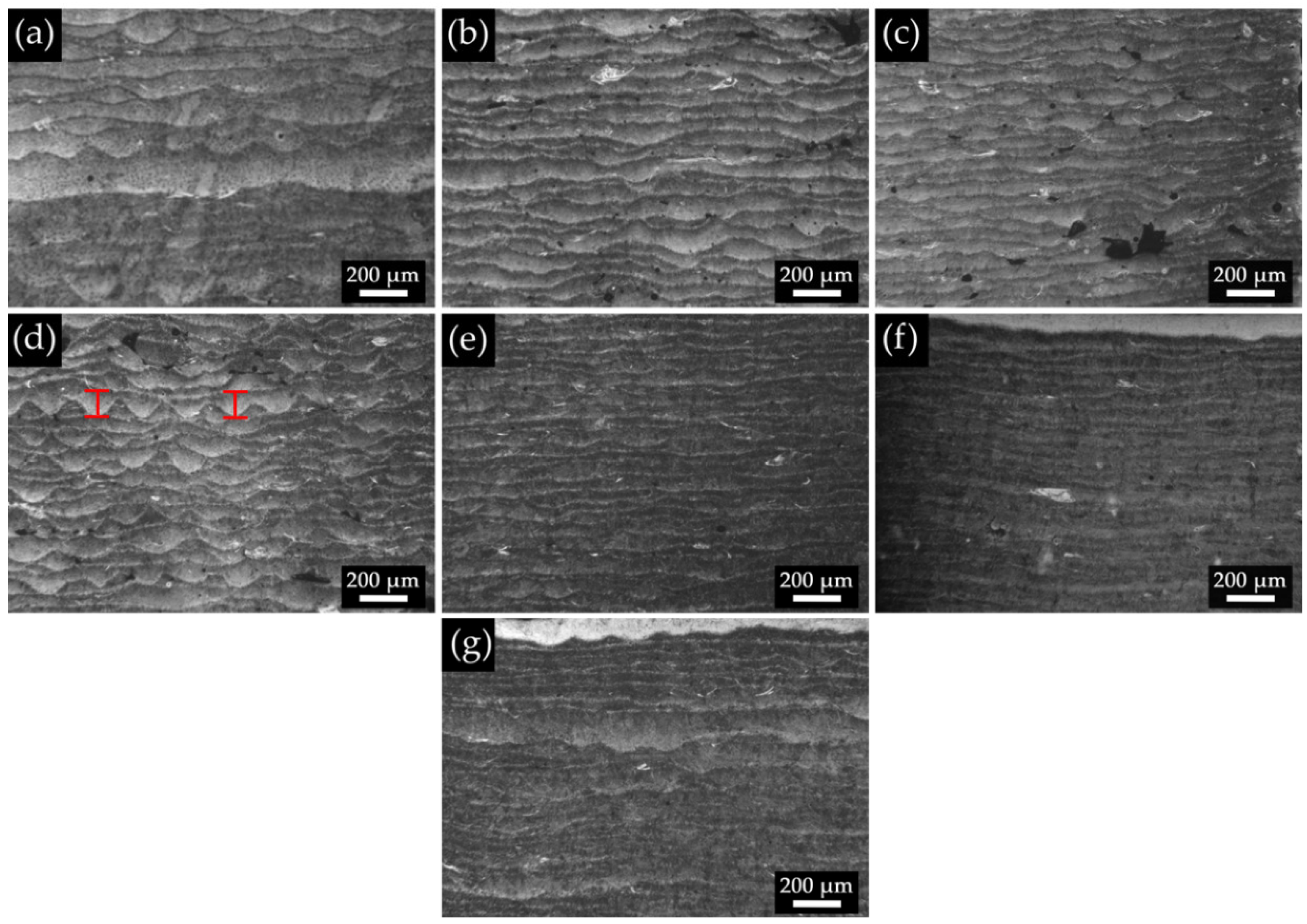
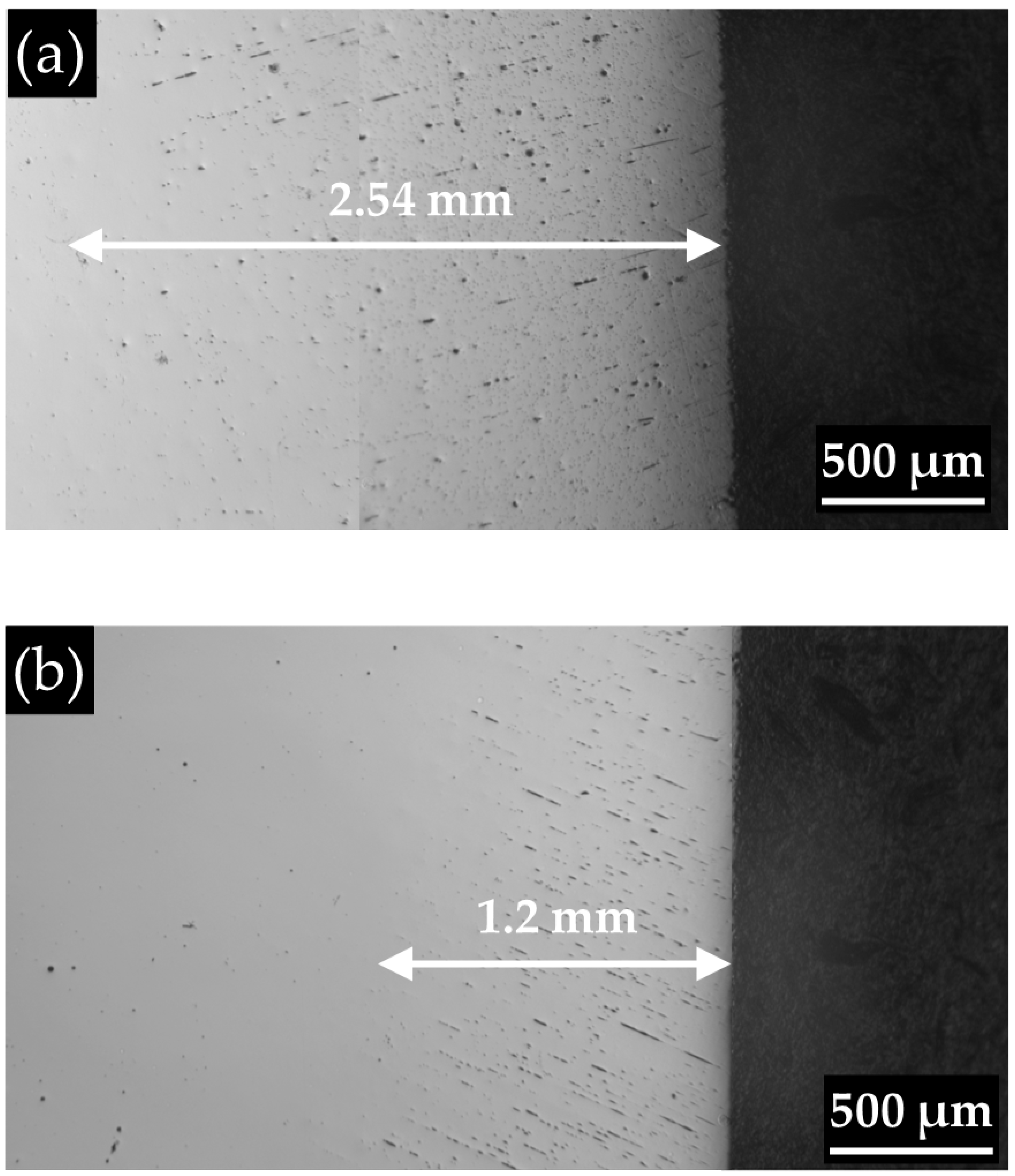
3.1. General Characterization
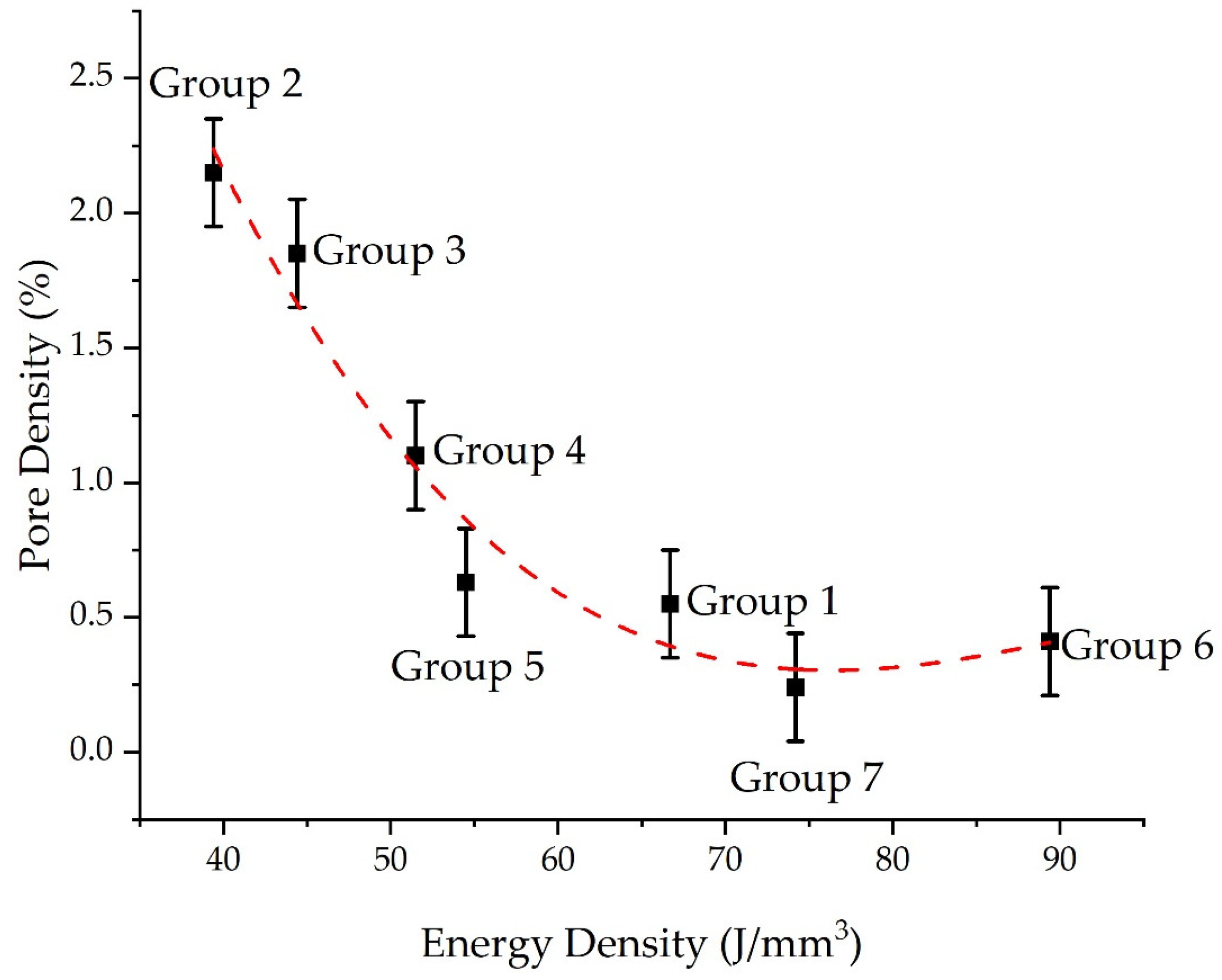
3.2. Pore Density
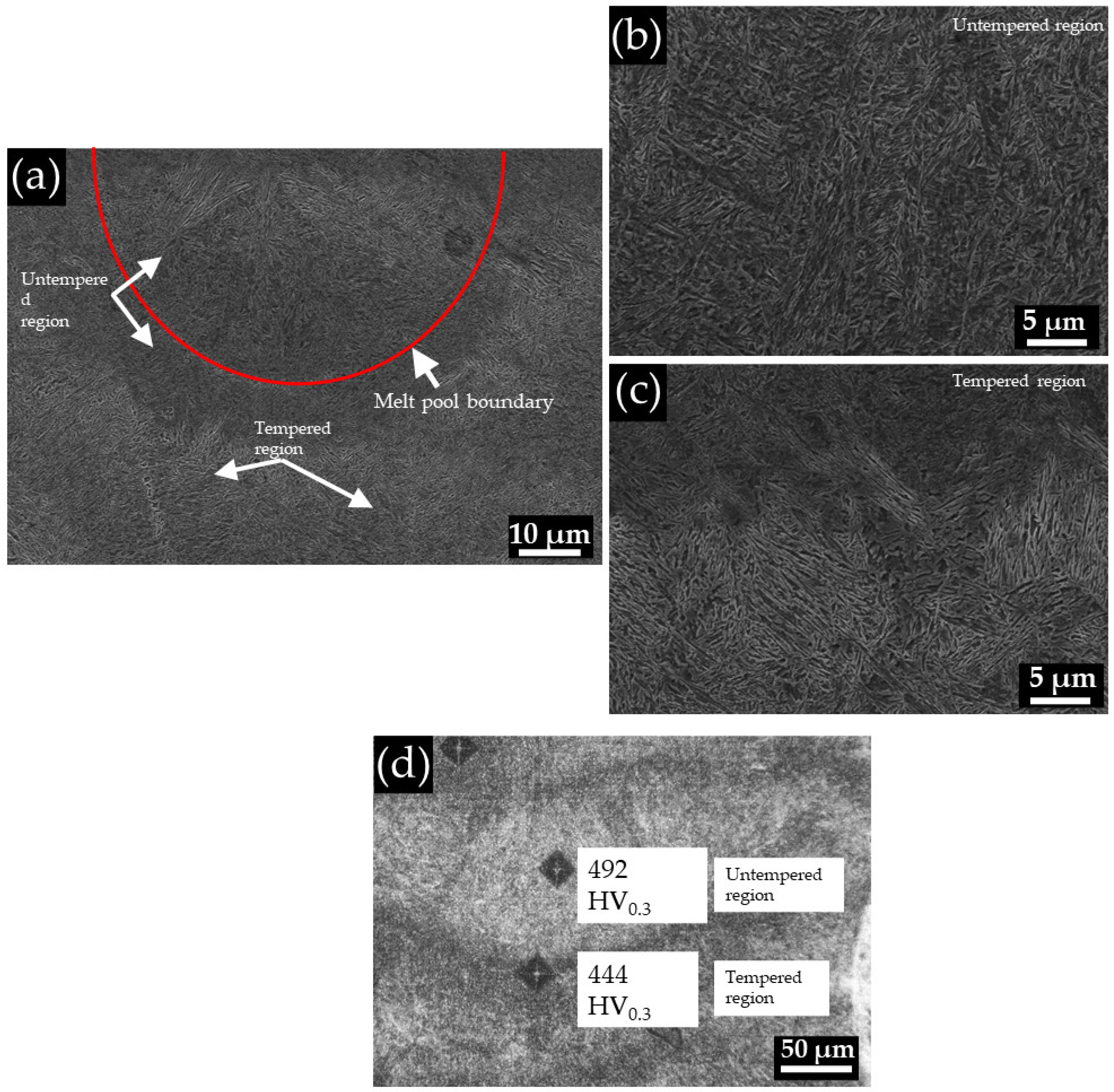
3.3. Microstructural Analysis
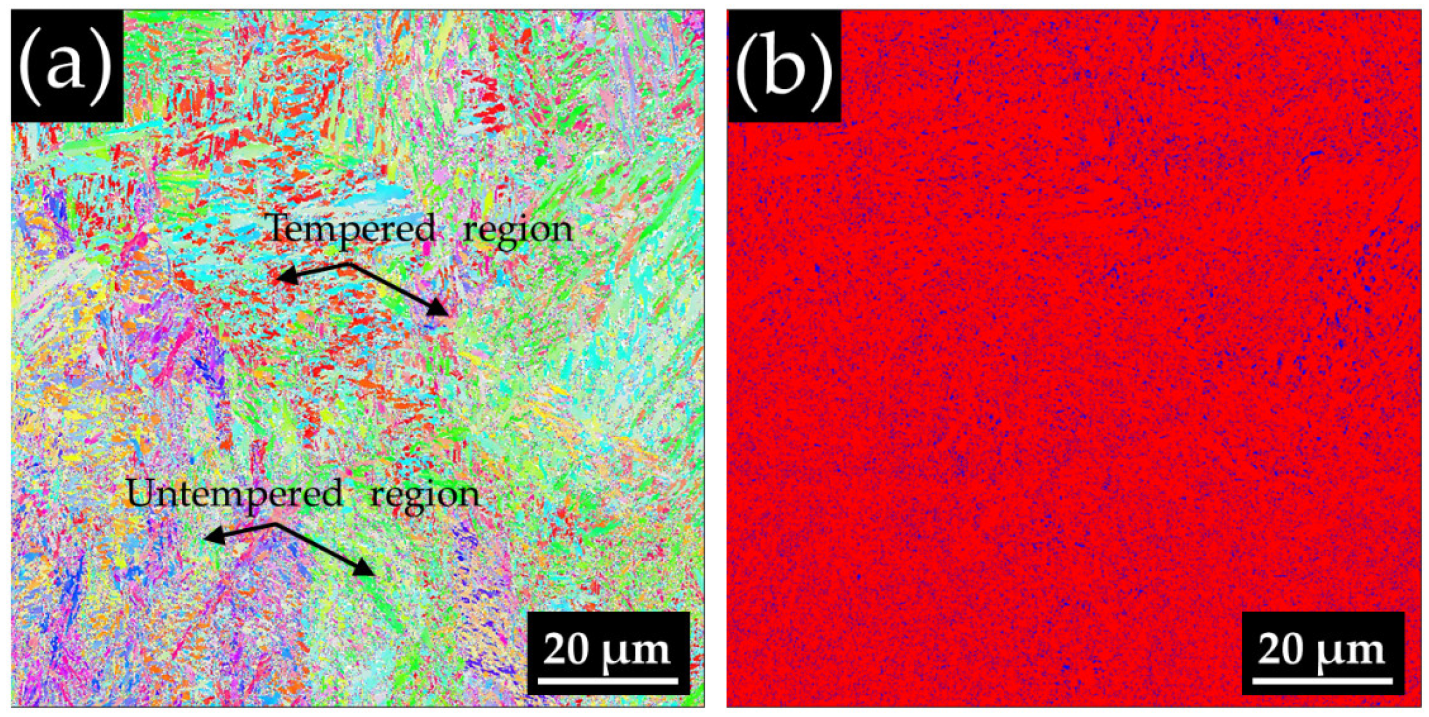
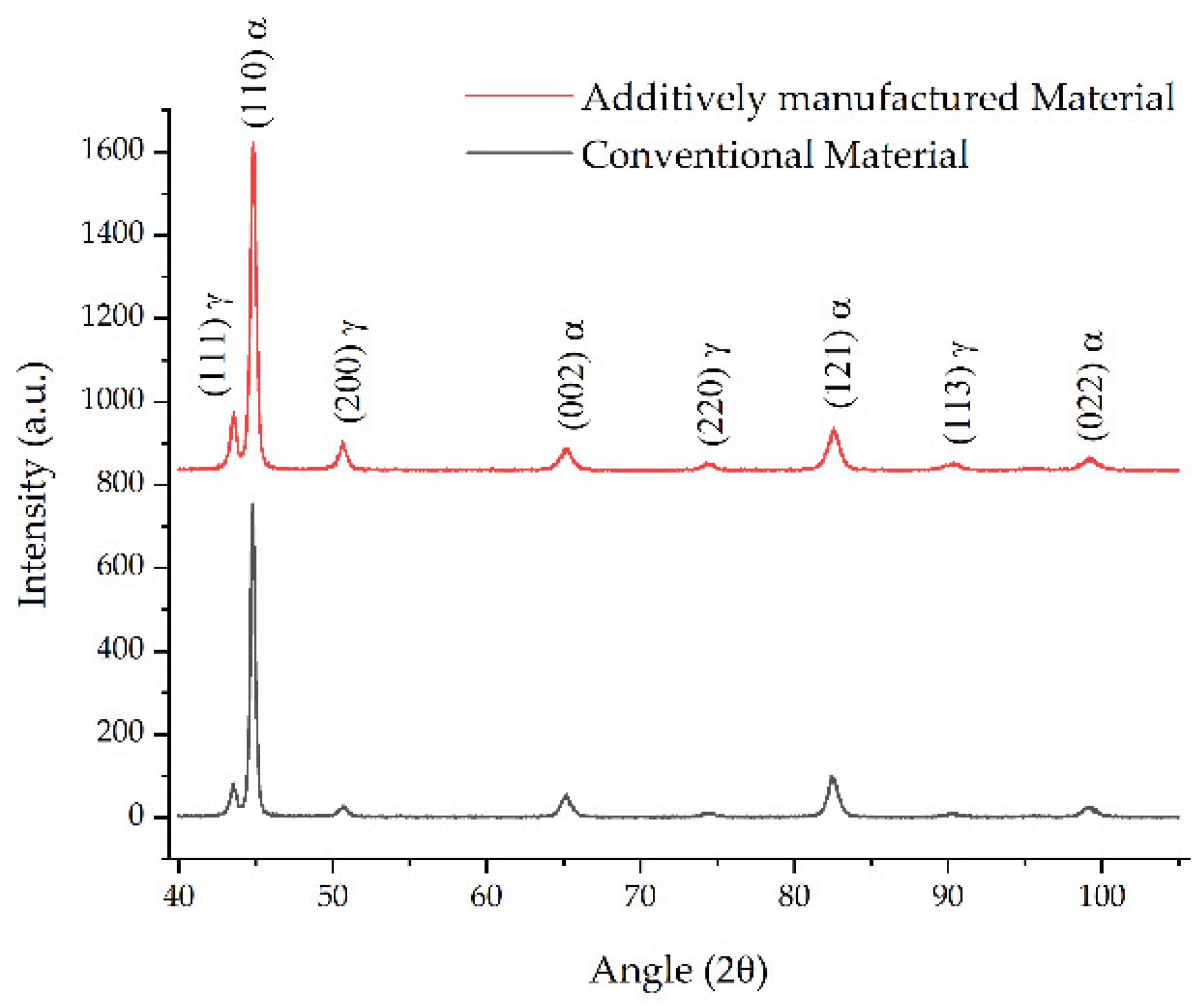
3.4. Comparative Study after Heat Treatment between Conventional Material and L-PBF-Fabricated Sample
4. Discussion
4.1. General Aspects and Challenges
4.2. As-Built Microstructure
4.3. Response of the L-PBF-Fabricated and the Conventional Alloys to Austempering Treatments
5. Conclusions
- The pore density decreases as the energy density input decreases;
- L-PBF-fabricated samples exhibited a carbide-free bainitic microstructure with fine bainitic ferrite plates in comparison with the conventionally fabricated material due to the finer prior austenite grains generated as a consequence of the L-PBF process;
- L-PBF-fabricated samples showed a faster transformation kinetic of the bainitic transformation due to acceleration provided by the finer prior austenite grain size.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sing, S.L.; Yeong, W.Y. Laser Powder Bed Fusion for Metal Additive Manufacturing: Perspectives on Recent Developments. Virtual Phys. Prototyp. 2020, 15, 359–370. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, L.; Pi, Z.; Wang, Z.; Yan, C.; Shi, Y. Robot-Assisted Laser Additive Manufacturing for High-Strength/Low-Porosity Continuous Fiber-Reinforced Thermoplastic Composites. Compos. Sci. Technol. 2024, 247, 110397. [Google Scholar] [CrossRef]
- Merz, B.; Nilsson, R.; Garske, C.; Hilgenberg, K. Camera-Based High Precision Position Detection for Hybrid Additive Manufacturing with Laser Powder Bed Fusion. Int. J. Adv. Manuf. Technol. 2023, 125, 2409–2424. [Google Scholar] [CrossRef]
- Gisario, A.; Kazarian, M.; Martina, F.; Mehrpouya, M. Metal Additive Manufacturing in the Commercial Aviation Industry: A Review. J. Manuf. Syst. 2019, 53, 124–149. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive Manufacturing of Metallic Components—Process, Structure and Properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Khademzadeh, S.; Carmignato, S.; Parvin, N.; Zanini, F.; Bariani, P.F. Micro Porosity Analysis in Additive Manufactured NiTi Parts Using Micro Computed Tomography and Electron Microscopy. Mater. Des. 2016, 90, 745–752. [Google Scholar] [CrossRef]
- Khademzadeh, S.; Gennari, C.; Zanovello, A.; Franceschi, M.; Campagnolo, A.; Brunelli, K. Development of Micro-Laser Powder Bed Fusion for Precision Additive Manufacturing of Inconel 718. Materials 2022, 15, 5231. [Google Scholar] [CrossRef]
- King, W.E.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A.; Rubenchik, A.M. Laser Powder Bed Fusion Additive Manufacturing of Metals; Physics, Computational, and Materials Challenges. Appl. Phys. Rev. 2015, 2, 041304. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Franceschi, M.; Revilla, R.I.; Khademzadeh, S.; De Graeve, I.; Dabalà, M. Revealing the Stress Corrosion Cracking Mechanism and Superior Electrochemical Behaviour of Alloy 718 Prepared by Laser Powder Bed Fusion Assessed by Microcapillary Method. Corros. Sci. 2022, 208, 110642. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Franceschi, M.; Rebesan, P.; Dabalà, M. Correlation of Lack of Fusion Pores with Stress Corrosion Cracking Susceptibility of L-PBF 316L: Effect of Surface Residual Stresses. Materials 2022, 15, 7151. [Google Scholar] [CrossRef]
- Bian, L.; Shamsaei, N.; Usher, J.M. Laser-Based Additive Manufacturing of Metal Parts: Modeling, Optimization, and Control of Mechanical Properties; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498739986. [Google Scholar]
- Sun, J.; Yang, Y.; Wang, D. Parametric Optimization of Selective Laser Melting for Forming Ti6Al4V Samples by Taguchi Method. Opt. Laser Technol. 2013, 49, 118–124. [Google Scholar] [CrossRef]
- Song, B.; Zhao, X.; Li, S.; Han, C.; Wei, Q.; Wen, S.; Liu, J.; Shi, Y. Differences in Microstructure and Properties between Selective Laser Melting and Traditional Manufacturing for Fabrication of Metal Parts: A Review. Front. Mech. Eng. 2015, 10, 111–125. [Google Scholar] [CrossRef]
- Lopez Taborda, L.L.; Maury, H.; Pacheco, J. Design for Additive Manufacturing: A Comprehensive Review of the Tendencies and Limitations of Methodologies. Rapid Prototyp. J. 2021, 27, 918–966. [Google Scholar] [CrossRef]
- Thomas, D.S.; Gilbert, S.W. Costs and Cost Effectiveness of Additive Manufacturing: A Literature Review and Discussion; NIST: Gaithersburg, MD, USA, 2014; pp. 1–96. [Google Scholar]
- Bhadeshia, H.K.D.H. Bainite in Steels: Theory and Practice; Maney Publishing: London, UK, 2006; Volume 19, ISBN 9781909662742. [Google Scholar]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Very Strong Low Temperature Bainite. Mater. Sci. Technol. 2002, 18, 279–284. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Bhadeshia, H.K.D.H. Nucleation Theory for High-Carbon Bainite. Mater. Sci. Eng. A 2004, 378, 289–292. [Google Scholar] [CrossRef]
- Morales-Rivas, L.; Yen, H.W.; Huang, B.M.; Kuntz, M.; Caballero, F.G.; Yang, J.R.; Garcia-Mateo, C. Tensile Response of Two Nanoscale Bainite Composite-Like Structures. Jom 2015, 67, 2223–2235. [Google Scholar] [CrossRef]
- Morales-Rivas, L.; Garcia-Mateo, C.; Sourmail, T.; Kuntz, M.; Rementeria, R.; Caballero, F.G. Ductility of Nanostructured Bainite. Metals 2016, 6, 302. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Sourmail, T.; Kuntz, M.; Cornide, J.; Smanio, V.; Elvira, R. Tensile Behaviour of a Nanocrystalline Bainitic Steel Containing 3 wt% Silicon. Mater. Sci. Eng. A 2012, 549, 185–192. [Google Scholar] [CrossRef]
- Avishan, B.; Yazdani, S.; Caballero, F.G.; Wang, T.S.; Garcia-Mateo, C. Characterisation of Microstructure and Mechanical Properties in Two Different Nanostructured Bainitic Steels. Mater. Sci. Technol. 2015, 31, 1508–1520. [Google Scholar] [CrossRef]
- Seede, R.; Shoukr, D.; Zhang, B.; Whitt, A.; Gibbons, S.; Flater, P.; Elwany, A.; Arroyave, R.; Karaman, I. An Ultra-High Strength Martensitic Steel Fabricated Using Selective Laser Melting Additive Manufacturing: Densification, Microstructure, and Mechanical Properties. Acta Mater. 2020, 186, 199–214. [Google Scholar] [CrossRef]
- Franceschi, M.; Miotti Bettanini, A.; Pezzato, L.; Dabalà, M.; Jacques, P.J. Effect of Multi-Step Austempering Treatment on the Microstructure and Mechanical Properties of a High Silicon. Metals 2021, 11, 2055. [Google Scholar] [CrossRef]
- Franceschi, M.; Pezzato, L.; Settimi, A.G.A.G.; Gennari, C.; Pigato, M.; Polyakova, M.; Konstantinov, D.; Brunelli, K.; Dabalà, M.; Dabal, M. Effect of Different Austempering Heat Treatments on Corrosion Properties of High Silicon Steel. Materials 2021, 14, 288. [Google Scholar] [CrossRef]
- Franceschi, M.; Pezzato, L.; Gennari, C.; Fabrizi, A.; Polyakova, M.; Konstantinov, D.; Brunelli, K.; Dabalà, M.; Dabal, M. Effect of Intercritical Annealing and Austempering on the Microstructure and Mechanical Properties of a High Silicon Manganese Steel. Metals 2020, 10, 448. [Google Scholar] [CrossRef]
- Franceschi, M.; Soffritti, C.; Fortini, A.; Pezzato, L.; Garagnani, G.L.; Dabalà, M. Evaluation of Wear Resistance of a Novel Carbide-Free Bainitic Steel. Tribol. Int. 2023, 178, 108071. [Google Scholar] [CrossRef]
- Franceschi, M.; Bertolini, R.; Fabrizi, A.; Dabalà, M.; Pezzato, L. Effect of Ausforming Temperature on Bainite Morphology in a 3.2% Si Carbide-Free Bainitic Steel. Mater. Sci. Eng. A 2023, 864, 144553. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, Z. Development of and Perspective on High-Performance Nanostructured Bainitic Bearing Steel. Engineering 2019, 5, 319–328. [Google Scholar] [CrossRef]
- Putatunda, S.K. Fracture Toughness of a High Carbon and High Silicon Steel. Mater. Sci. Eng. A 2001, 297, 31–43. [Google Scholar] [CrossRef]
- Królicka, A.; Żak, A.M.; Caballero, F.G. Enhancing Technological Prospect of Nanostructured Bainitic Steels by the Control of Thermal Stability of Austenite. Mater. Des. 2021, 211, 110143. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Hesse, O.; Friedrich, T.; Kunert, M.; Brykov, M.N.; Shimizu, K.; Zurnadzhy, V.I.; Šuchmann, P. Two-Body Abrasion Resistance of High-Carbon High-Silicon Steel: Metastable Austenite vs Nanostructured Bainite. Wear 2019, 418–419, 24–35. [Google Scholar] [CrossRef]
- Eres-Castellanos, A.; Morales-Rivas, L.; Latz, A.; Caballero, F.G.; Garcia-Mateo, C. Effect of Ausforming on the Anisotropy of Low Temperature Bainitic Transformation. Mater. Charact. 2018, 145, 371–380. [Google Scholar] [CrossRef]
- Eres-Castellanos, A.; Caballero, F.G.; Garcia-Mateo, C. Stress or Strain Induced Martensitic and Bainitic Transformations during Ausforming Processes. Acta Mater. 2020, 189, 60–72. [Google Scholar] [CrossRef]
- Eres-Castellanos, A.; Hidalgo, J.; Morales-Rivas, L.; Caballero, F.G.; Garcia-Mateo, C. The Role of Plastic Strains on Variant Selection in Ausformed Bainitic Microstructures Studied by Finite Elements and Crystal Plasticity Simulations. J. Mater. Res. Technol. 2021, 13, 1416–1430. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Eres-Castellanos, A.; Somani, M.; Porter, D.; Latz, A.; Lieven, B.; Caballero, F.G. Developing Nanostructured Bainite by Means of Ausforming. In Proceedings of the THERMEC 2018, Paris, France, 9–13 July 2018. [Google Scholar]
- Lan, H.; Du, L.; Zhou, N.; Liu, X. Effect of Austempering Route on Microstructural Characterization of Nanobainitic Steel. Acta Metall. Sin. Engl. Lett. 2014, 27, 19–26. [Google Scholar] [CrossRef]
- Kempen, K.; Yasa, E.; Thijs, L.; Kruth, J.P.; Van Humbeeck, J. Microstructure and Mechanical Properties of Selective Laser Melted 18Ni-300 Steel. Phys. Procedia 2011, 12, 255–263. [Google Scholar] [CrossRef]
- Campanelli, S.L.; Contuzzi, N.; Ludovico, A.D. Manufacturing of 18 Ni Marage 300 Steel Samples by Selective Laser Melting. Adv. Mater. Res. 2010, 83–86, 850–857. [Google Scholar] [CrossRef]
- Santana, A.; Eres-Castellanos, A.; Jimenez, J.A.; Rementeria, R.; Capdevila, C.; Caballero, F.G. Effect of Layer Thickness and Laser Emission Mode on the Microstructure of an Additive Manufactured Maraging Steel. J. Mater. Res. Technol. 2023, 25, 6898–6912. [Google Scholar] [CrossRef]
- Karlapudy, S.P.; Nancharaiah, T.; Subba Rao, V.V. Influence of Post-Heat Treatment on Microstructure, Texture, and Mechanical Properties of 18Ni-300 Maraging Steel Fabricated by Using LPBF Technique. Prog. Addit. Manuf. 2023, 1–16. [Google Scholar] [CrossRef]
- Casalino, G.; Campanelli, S.L.; Contuzzi, N.; Ludovico, A.D. Experimental Investigation and Statistical Optimisation of the Selective Laser Melting Process of a Maraging Steel. Opt. Laser Technol. 2015, 65, 151–158. [Google Scholar] [CrossRef]
- Lemke, J.N.; Simonelli, M.; Garibaldi, M.; Ashcroft, I.; Hague, R.; Vedani, M.; Wildman, R.; Tuck, C. Calorimetric Study and Microstructure Analysis of the Order-Disorder Phase Transformation in Silicon Steel Built by SLM. J. Alloys Compd. 2017, 722, 293–301. [Google Scholar] [CrossRef]
- Garibaldi, M.; Ashcroft, I.; Simonelli, M.; Hague, R. Metallurgy of High-Silicon Steel Parts Produced Using Selective Laser Melting. Acta Mater. 2016, 110, 207–216. [Google Scholar] [CrossRef]
- Wu, B.; Pan, Z.; Ding, D.; Cuiuri, D.; Li, H.; Xu, J.; Norrish, J. A Review of the Wire Arc Additive Manufacturing of Metals: Properties, Defects and Quality Improvement. J. Manuf. Process. 2018, 35, 127–139. [Google Scholar] [CrossRef]
- Ron, T.; Levy, G.K.; Dolev, O.; Leon, A.; Shirizly, A.; Aghion, E. Environmental Behavior of Low Carbon Steel Produced by a Wire Arc Additive Manufacturing Process. Metals 2019, 9, 888. [Google Scholar] [CrossRef]
- Nagasai, B.P.; Malarvizhi, S.; Balasubramanian, V. Mechanical Properties of Wire Arc Additive Manufactured Carbon Steel Cylindrical Component Made by Gas Metal Arc Welding Process. J. Mech. Behav. Mater. 2021, 30, 188–198. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Yao, C.; Zhang, K.; Lu, F.; Feng, K.; Huang, J.; Wang, M.; Wu, Y. Microstructure Evolution of Fe-Based Nanostructured Bainite Coating by Laser Cladding. Mater. Des. 2014, 63, 100–108. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Lu, F.; Zhang, K.; Li, Z.; Hosseini, S.R.E.; Wang, M. Effects of Isothermal Heat Treatment on Nanostructured Bainite Morphology and Microstructures in Laser Cladded Coatings. Appl. Surf. Sci. 2015, 357, 309–316. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Fang, J.X.; Ma, G.Z.; Tian, H.L.; Zhang, D.B.; Cao, Y. Microstructure and Properties of an As-Deposited and Post Treated High Strength Carbide-Free Bainite Steel Fabricated via Laser Powder Deposition. Mater. Sci. Eng. A 2021, 824, 141791. [Google Scholar] [CrossRef]
- Martina, F.; Ding, J.; Williams, S.; Caballero, A.; Pardal, G.; Quintino, L. Tandem Metal Inert Gas Process for High Productivity Wire Arc Additive Manufacturing in Stainless Steel. Addit. Manuf. 2019, 25, 545–550. [Google Scholar] [CrossRef]
- Wanwan, J.; Chaoqun, Z.; Shuoya, J.; Yingtao, T.; Daniel, W.; Wen, L. Wire Arc Additive Manufacturing of Stainless Steels: A Review. Appl. Sci. 2020, 10, 1563. [Google Scholar]
- Su, A.; Aldalur, E.; Veiga, F.; Artaza, T. Wire Arc Additive Manufacturing of an Aeronautic Fitting with Different Metal Alloys: From the Design to the Part. J. Manuf. Process. 2021, 64, 188–197. [Google Scholar]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels and Properties; Butterworths-Heinemann: Oxford, UK, 2017; ISBN 9780081002704. [Google Scholar]
- Mueller, I.; Rementeria, R.; Caballero, F.G.; Kuntz, M.; Sourmail, T.; Kerscher, E. A Constitutive Relationship between Fatigue Limit and Microstructure in Nanostructured Bainitic Steels. Materials 2016, 9, 831. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Sourmail, T.; Smanio, V.; De Andres, C.G. Industrialised Nanocrystalline Bainitic Steels. Design Approach. Int. J. Mater. Res. 2014, 105, 725–734. [Google Scholar] [CrossRef]
- Fonstein, N. Advanced High Strength Sheet Steels: Physical Metallurgy, Design, Processing, and Properties; Springer: Cham, Switzerland, 2015; ISBN 9783319191652. [Google Scholar]
- Fonstein, N.; Jun, H.J.; Huang, G.; Sriram, S.; Yan, B. Effect of Bainite on Mechanical Properties of Multiphase Ferrite-Bainite-Martensite Steels. Mater. Sci. Technol. 2011, 1, 634–641. [Google Scholar]
- Saunders, N.; Guo, Z.; Li, X.; Miodownik, A.P.; Schillé, J.P. Using JMatPro to Model Materials Properties and Behavior. Jom 2003, 55, 60–65. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Revilla, R.I.; Franceschi, M.; Fabrizi, A.; Khademzadeh, S.; Khodabakhshi, M.; De Graeve, I.; Dabalà, M. Unveiling the Impact of Laser Power Variations on Microstructure, Corrosion, and Stress-Assisted Surface Crack Initiation in Laser Powder Bed Fusion-Processed Ni-Fe-Cr Alloy 718. Electrochim. Acta 2024, 476, 143723. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Corporation, O. Origin(Pro), Version 2023b; OriginLab Corporation: Northampton, MA, USA, 2022. [Google Scholar]
- Voort, V. Metallography Principles and Practice; McGrawHill: New York, NY, USA, 1999; ISBN 9780871706720. [Google Scholar]
- Lutterotti, L. Maud: A Rietveld Analysis Program Designed for the Internet and Experiment Integration. Acta Crystallogr. Sect. A Found. Crystallogr. 2000, 56, s54. [Google Scholar] [CrossRef]
- Cheng, L.; Bottger, A.; Keijser, D.T.H.; Mittemeijer, E.J. Lattice Parameters of Iron-Carbon and Iron-Nitrogen Martensites and Austenites. Scr. Metall. Mater. 1990, 24, 509–514. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Jimenez, J.A.; Lopez-Ezquerra, B.; Rementeria, R.; Morales-Rivas, L.; Kuntz, M.; Caballero, F.G. Analyzing the Scale of the Bainitic Ferrite Plates by XRD, SEM and TEM. Mater. Charact. 2016, 122, 83–89. [Google Scholar] [CrossRef]
- Kotzem, D.; Arold, T.; Niendorf, T.; Walther, F. Influence of Specimen Position on the Build Platform on the Mechanical Properties of As-Built Direct Aged Electron Beam Melted Inconel 718 Alloy. Mater. Sci. Eng. A 2020, 772, 138785. [Google Scholar] [CrossRef]
- Chua, K.H.G.; Choong, Y.Y.C.; Wong, C.H. Investigation of the Effects on the Print Location during Selective Laser Melting Process. Proc. Int. Conf. Prog. Addit. Manuf. 2018, 2018, 613–618. [Google Scholar] [CrossRef]
- Dilip, J.J.S.; Ram, G.D.J.; Starr, T.L.; Stucker, B. Selective Laser Melting of HY100 Steel: Process Parameters, Microstructure and Mechanical Properties. Addit. Manuf. 2017, 13, 49–60. [Google Scholar] [CrossRef]
- San-Martin, D.; Kuntz, M.; Caballero, F.G.; Garcia-Mateo, C. A New Systematic Approach Based on Dilatometric Analysis to Track Bainite Transformation Kinetics and the Influence of the Prior Austenite Grain Size. Metals 2021, 11, 324. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, G.; Mao, X.; Zhao, G.; Bao, S. Method to Evaluate the Kinetics of Bainite Transformation in Low-Temperature Nanobainitic Steel Using Thermal Dilatation Curve Analysis. Metals 2017, 7, 330. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C. Carbon Supersaturation of Ferrite in a Nanocrystalline Bainitic Steel. Acta Mater. 2010, 58, 2338–2343. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Deckers, J.; Yasa, E.; Wauthlé, R. Assessing and Comparing Influencing Factors of Residual Stresses in Selective Laser Melting Using a Novel Analysis Method. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2012, 226, 980–991. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser Additive Manufacturing of Metallic Components: Materials, Processes and Mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Mercelis, P.; Kruth, J.P. Residual Stresses in Selective Laser Sintering and Selective Laser Melting. Rapid Prototyp. J. 2006, 12, 254–265. [Google Scholar] [CrossRef]
- Harrison, N.J.; Todd, I.; Mumtaz, K. Reduction of Micro-Cracking in Nickel Superalloys Processed by Selective Laser Melting: A Fundamental Alloy Design Approach. Acta Mater. 2015, 94, 59–68. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Mehta, A.; Park, S.; Huynh, T.; Song, S.; Bai, Y.; Cho, K.; McWilliams, B.; Sohn, Y. Composition-Dependent Solidification Cracking of Aluminum-Silicon Alloys during Laser Powder Bed Fusion. Acta Mater. 2021, 208, 116698. [Google Scholar] [CrossRef]
- Deb, P.; Challenger, K.D.; Therrien, A.E. Structure-Property Correlation of Submerged-Arc and Gas-Metal-Arc Weldments in HY-100 Steel. Metall. Trans. A 1991, 18, 987–999. [Google Scholar] [CrossRef]
- Zhao, X.; Song, B.; Zhang, Y.; Zhu, X.; Wei, Q.; Shi, Y. Decarburization of Stainless Steel during Selective Laser Melting and Its Influence on Young’s Modulus, Hardness and Tensile Strength. Mater. Sci. Eng. A 2015, 647, 58–61. [Google Scholar] [CrossRef]
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in Additive Manufacturing: A Review of Their Microstructure and Properties. Mater. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Li, Z.X.; Li, C.S.; Ren, J.Y.; Li, B.Z.; Zhang, J.; Ma, Y.Q. Effect of Cold Deformation on the Microstructure and Impact Toughness during the Austenitizing Process of 1.0C–1.5Cr Bearing Steel. Mater. Sci. Eng. A 2016, 674, 262–269. [Google Scholar] [CrossRef]
- Hu, F.; Hodgson, P.D.; Wu, K.M. Acceleration of the Super Bainite Transformation through a Coarse Austenite Grain Size. Mater. Lett. 2014, 122, 240–243. [Google Scholar] [CrossRef]
- Chakraborty, J.; Chattopadhyay, P.P.; Bhattacharjee, D.; Manna, I. Microstructural Refinement of Bainite and Martensite for Enhanced Strength and Toughness in High-Carbon Low-Alloy Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 2871–2879. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G. Understanding the Mechanical Properties of Nanostructured Bainite; Wiley: Hoboken, NJ, USA, 2015; Volume 1, ISBN 9783527674947. [Google Scholar]
- Langford, G.; Cohen, M. Strain Hardening of Iron by Severe Plastic Deformation. ASM Trans. Quart. 1969, 62, 623–638. [Google Scholar]
- Langford, G. A Study of the Deformation of Patented Steel Wire. Metall. Mater. Trans. B 1970, 1, 465–477. [Google Scholar] [CrossRef]
- Langford, G.; Cohen, M. Calculation of Cell-Size Strengthening of Wire-Drawn Iron. Metall. Mater. Trans. 1970, 1, 1478–1480. [Google Scholar] [CrossRef]
- Zhao, L.; Qian, L.; Meng, J.; Zhou, Q.; Zhang, F. Below-Ms Austempering to Obtain Refined Bainitic Structure and Enhanced Mechanical Properties in Low-C High-Si/Al Steels. Scr. Mater. 2016, 112, 96–100. [Google Scholar] [CrossRef]
- Hou, X.Y.; Xu, Y.B.; Zhao, Y.F.; Wu, D. Microstructure and Mechanical Properties of Hot Rolled Low Silicon TRIP Steel Containing Phosphorus and Vanadium. J. Iron Steel Res. Int. 2011, 18, 40–45. [Google Scholar] [CrossRef]
- Caballero, F.G.; Chao, J.; Cornide, J.; García-Mateo, C.; Santofimia, M.J.; Capdevila, C. Toughness Deterioration in Advanced High Strength Bainitic Steels. Mater. Sci. Eng. A 2009, 525, 87–95. [Google Scholar] [CrossRef]
- Keehan, E.; Karlsson, L.; Bhadeshia, H.K.D.H.; Thuvander, M. Electron Backscattering Diffraction Study of Coalesced Bainite in High Strength Steel Weld Metals. Mater. Sci. Technol. 2008, 24, 1183–1188. [Google Scholar] [CrossRef]
- Keehan, E.; Karlsson, L.; Andren, H.O.; Bhadeshia, H.K.D.H. Understanding Mechanical Properties of Novel High Strength Steel Weld Metals through High-Resolution Microstructural Investigation, Proceedings of the International Conference on Trends in Welding Research, Pine Mountain, GA, USA, 16–20 May 2005; ASM International: Atlanta, GA, USA, 2005; pp. 16–20. [Google Scholar]
- Bhadeshia, H.K.D.H.; Keehan, E.; Karlsson, L.; Andrén, H.O. Coalesced Bainite. Trans. Indian Inst. Met. 2006, 59, 689–694. [Google Scholar]
- Pak, J.; Suh, D.W.; Bhadeshia, H.K.D.H. Promoting the Coalescence of Bainite Platelets. Scr. Mater. 2012, 66, 951–953. [Google Scholar] [CrossRef]
- Pak, J.H.; Bhadeshia, H.K.D.H.; Karlsson, L.; Keehan, E. Coalesced Bainite by Isothermal Transformation of Reheated Weld Metal. Sci. Technol. Weld. Join. 2008, 13, 593–597. [Google Scholar] [CrossRef]
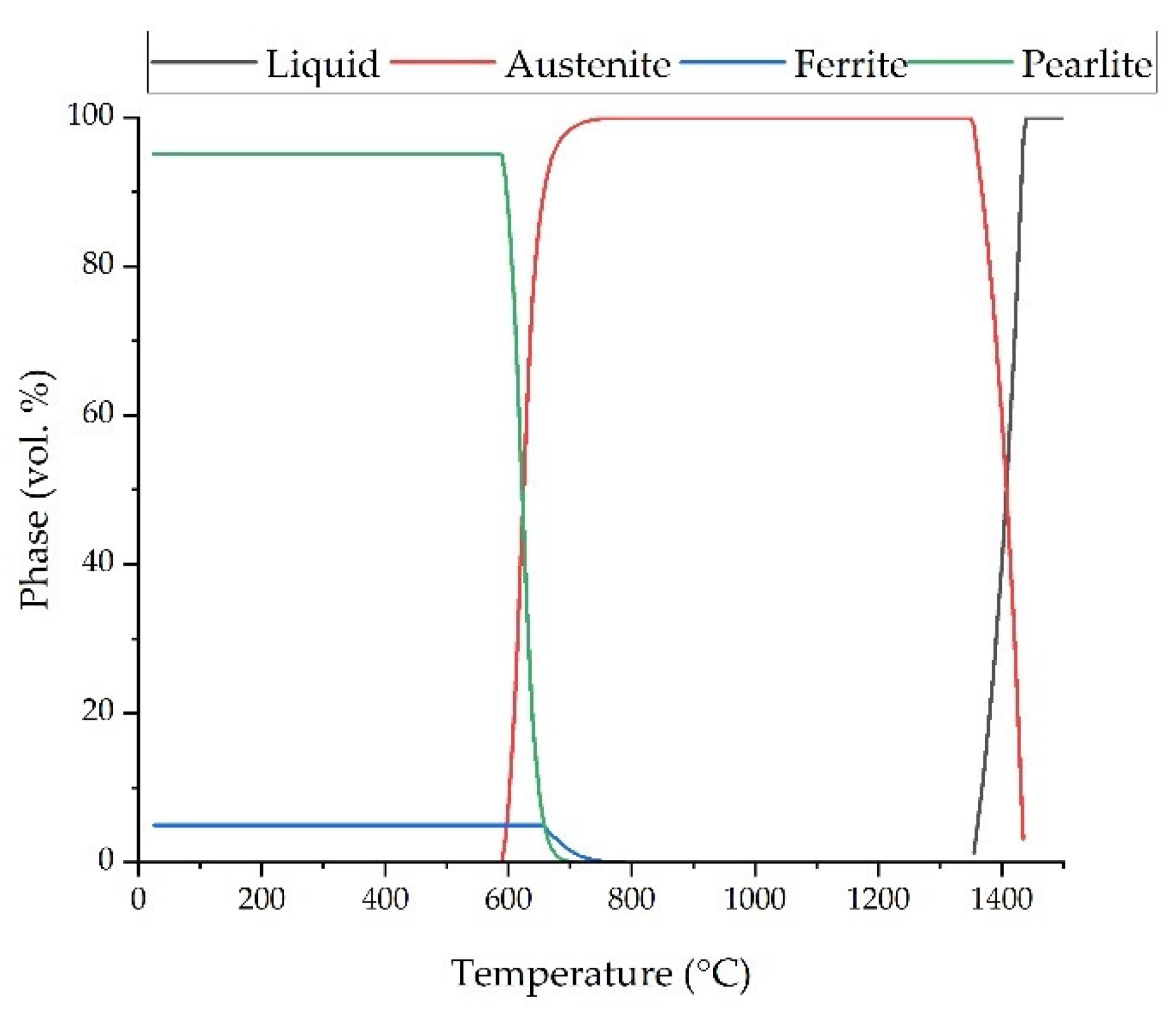
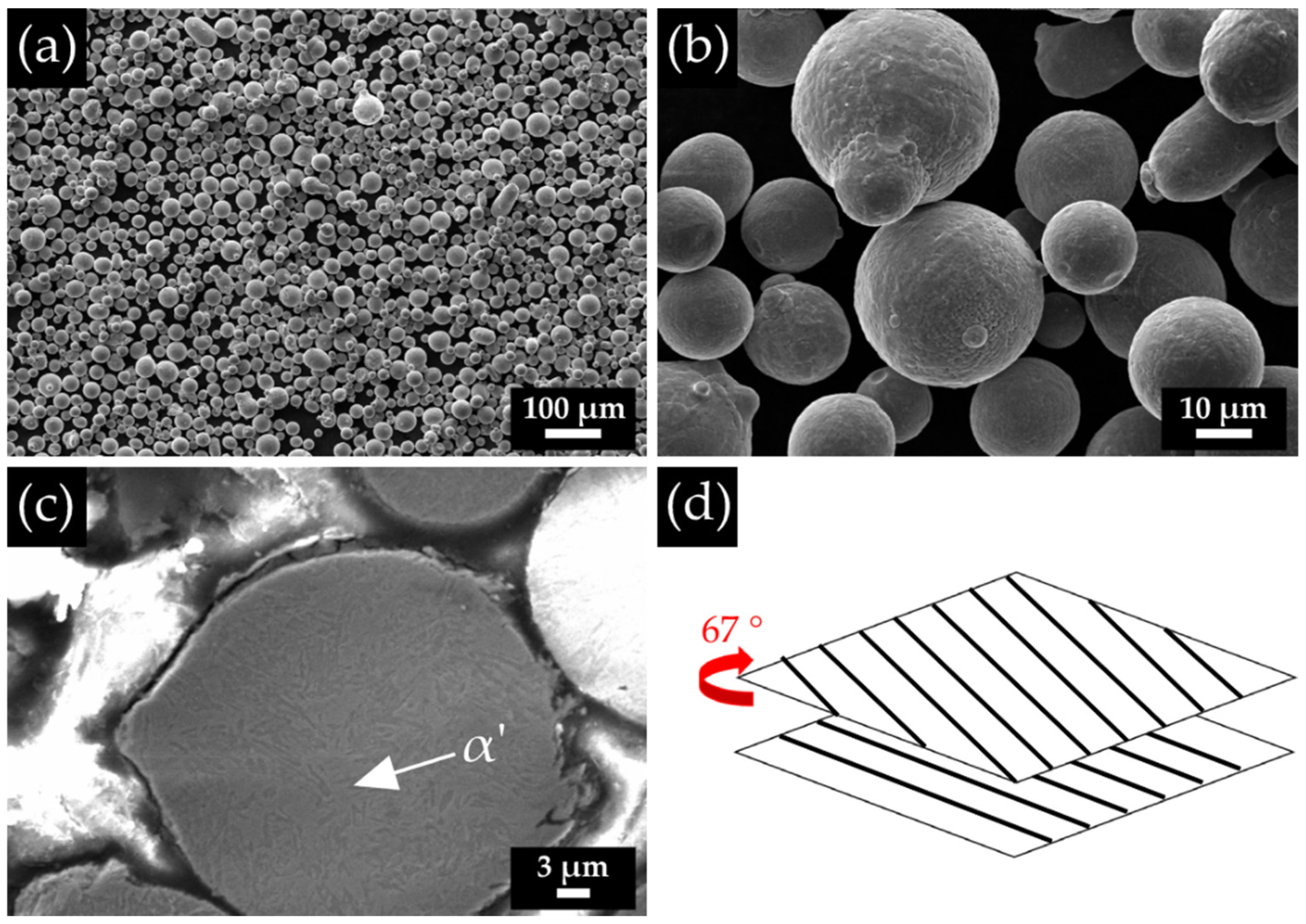
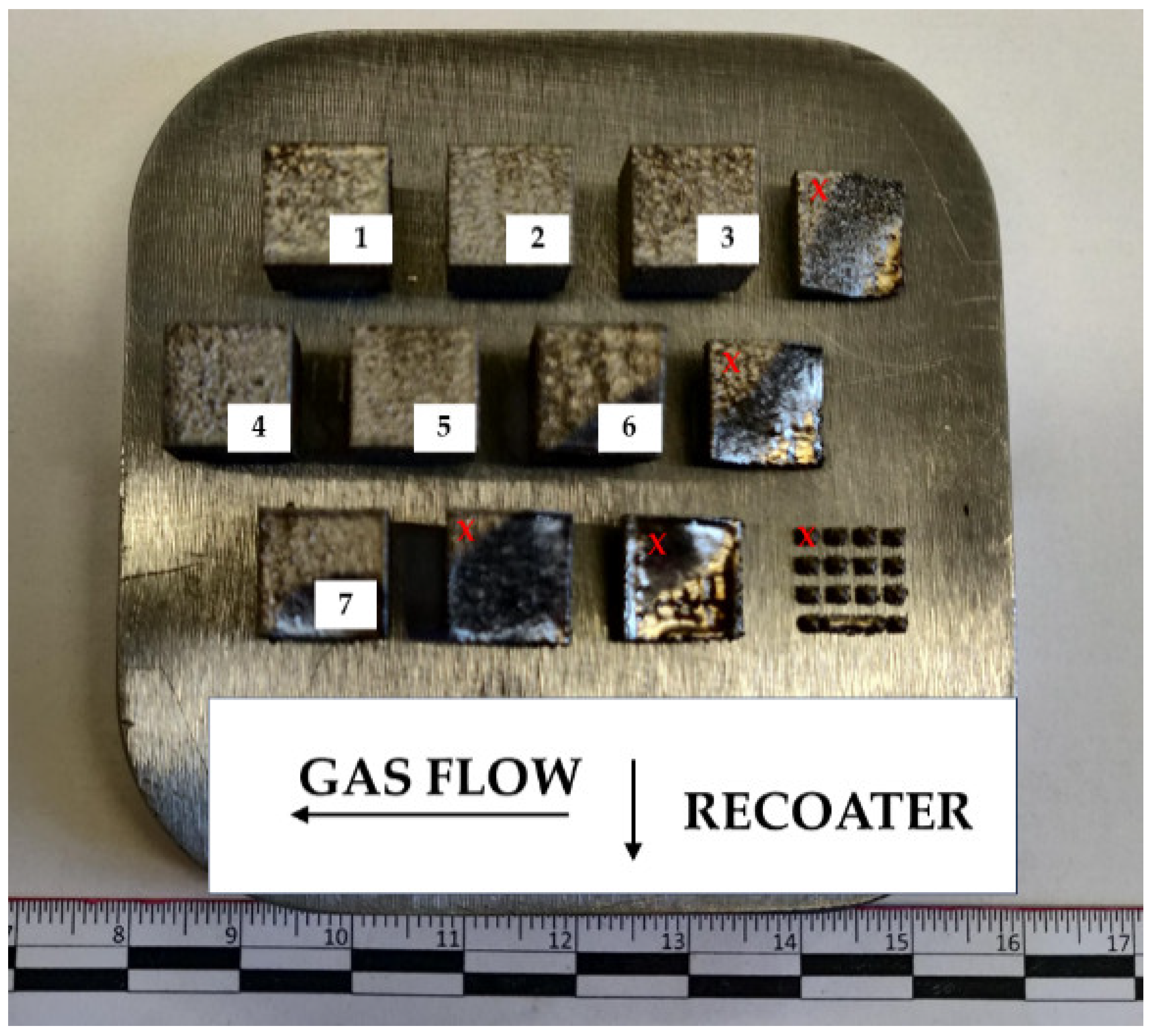
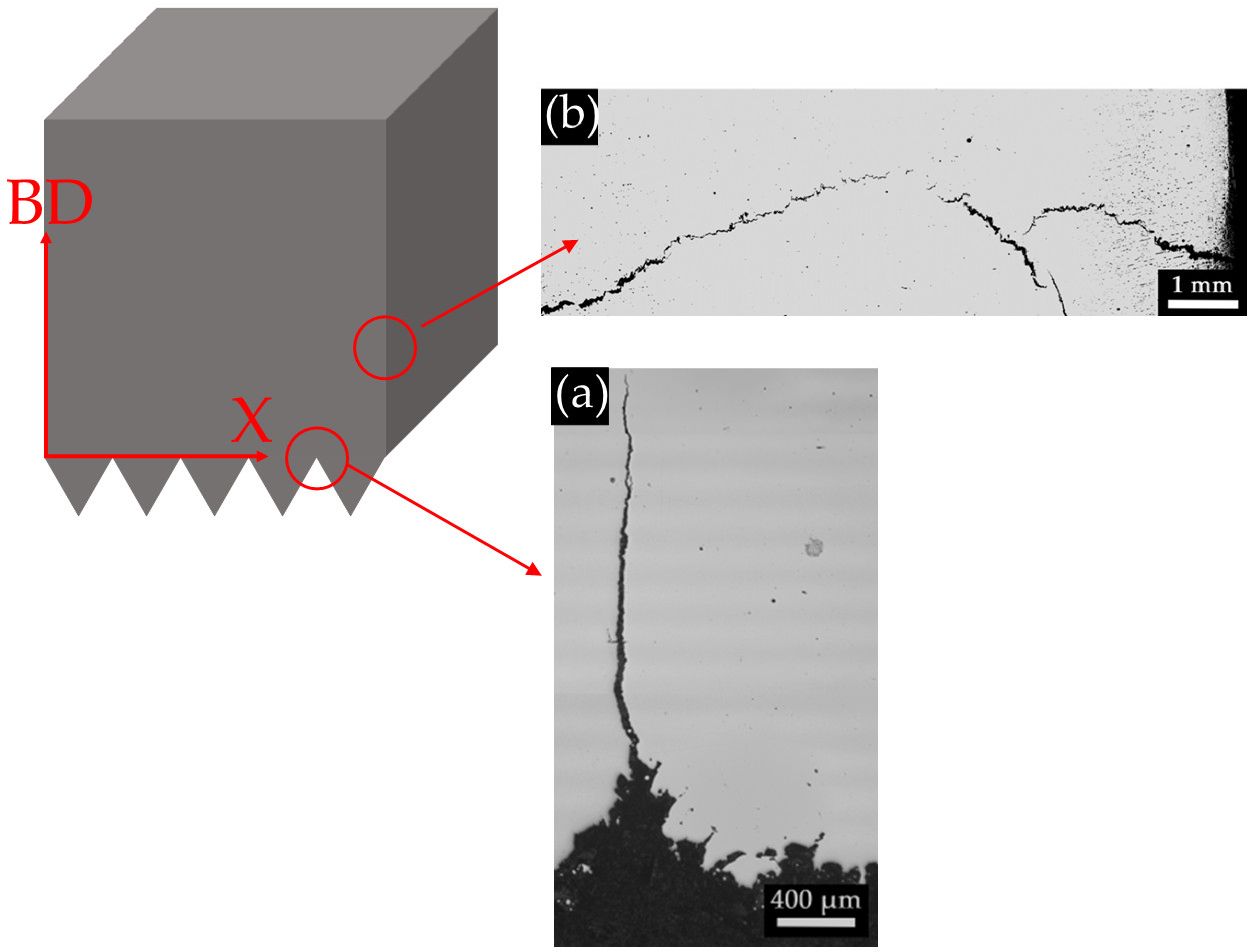







| Fe | C | Si | Mn | O | Al | Ms (Adapted from Ref. [27]) | Melting Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Bal. | 0.35 | 3.17 | 2.81 | 0.02 | 0.1 | 245 °C | 1440 |
| Parameter Group | LP (W) | SS (m/s) | HS (μm) | LT (μm) | Energy Density (J/mm3) |
|---|---|---|---|---|---|
| 1 | 220 | 0.5 | 110 | 60 | 66.7 |
| 2 | 195 | 0.75 | 110 | 60 | 39.4 |
| 3 | 220 | 0.75 | 110 | 60 | 44.4 |
| 4 | 170 | 0.5 | 110 | 60 | 51.4 |
| 5 | 270 | 0.75 | 110 | 60 | 54.5 |
| 6 | 295 | 0.5 | 110 | 60 | 89.4 |
| 7 | 245 | 0.5 | 110 | 60 | 74.2 |
| 8 | 170 | 0.75 | 110 | 60 | 22.2 |
| 9 | 245 | 0.75 | 110 | 60 | 49.5 |
| 10 | 295 | 0.75 | 110 | 60 | 59.6 |
| 11 | 195 | 0.5 | 110 | 60 | 59.1 |
| 12 | 270 | 0.5 | 110 | 60 | 81.8 |
| Group | Mean [HV0.3] |
|---|---|
| 1 | 539 ± 37 |
| 2 | 501 ± 6 |
| 3 | 485 ± 18 |
| 4 | 503 ± 12 |
| 5 | 485 ± 12 |
| 6 | 465 ± 11 |
| 7 | 478 ± 1 |
| Bulk * | 670 ± 4 |
| Group | Mean (μm2) | SD (μm2) | Median (μm2) | Min (μm2) | Max (μm2) | Q1–Q3 (μm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | C | S | C | S | C | S | C | S | C | S | C | |
| 1 | 148.76 | 65.81 | 1435.87 | 448.55 | 13.49 | 14.98 | 0.18 | 0.04 | 137,421.07 | 16,543.51 | 41.38 | 40.33 |
| 2 | 555.63 | 160.96 | 3831.04 | 1162.18 | 23.03 | 12.58 | 0.18 | 0.04 | 207,901.31 | 29,988.02 | 90.46 | 45.75 |
| 3 | 409.92 | 151.45 | 3022.70 | 1246.70 | 26.63 | 15.61 | 0.18 | 0.04 | 189,714.97 | 48,625.89 | 111.32 | 43.85 |
| 4 | 662.02 | 94.76 | 5833.39 | 532.71 | 24.73 | 7.80 | 0.18 | 0.04 | 412,577.29 | 19,110.32 | 107.57 | 30.77 |
| 5 | 286.92 | 34.44 | 2410.34 | 167.48 | 18.42 | 5.77 | 0.18 | 0.04 | 225,042.27 | 7328.49 | 73.50 | 19.47 |
| 6 | 985.80 | 29.85 | 7418.40 | 105.27 | 40.524 | 5.72 | 0.18 | 0.04 | 285,760.28 | 3291.84 | 222.51 | 18.59 |
| 7 | 682.25 | 29.78 | 6216.99 | 105.17 | 32.419 | 6.44 | 0.18 | 0.04 | 303,005.76 | 3632.25 | 97.07 | 19.12 |
| Sample | Vγ (%) | C γ (wt.%) | Vαb (%) | Cαb (wt.%) |
|---|---|---|---|---|
| Conventionally fabricated material | 25 ± 3 | 1.22 ± 0.04 | 75 ± 3 | 0.17 ± 0.04 |
| L-PBF-fabricated material | 21 ± 3 | 1.06 ± 0.04 | 79 ± 3 | 0.16 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschi, M.; Yazdanpanah, A.; Leone, D.; Pezzato, L.; Dabalà, M. Laser Powder Bed Fusion Fabrication of a Novel Carbide-Free Bainitic Steel: The Possibilities and a Comparative Study with the Conventional Alloy. Metals 2024, 14, 113. https://doi.org/10.3390/met14010113
Franceschi M, Yazdanpanah A, Leone D, Pezzato L, Dabalà M. Laser Powder Bed Fusion Fabrication of a Novel Carbide-Free Bainitic Steel: The Possibilities and a Comparative Study with the Conventional Alloy. Metals. 2024; 14(1):113. https://doi.org/10.3390/met14010113
Chicago/Turabian StyleFranceschi, Mattia, Arshad Yazdanpanah, Davide Leone, Luca Pezzato, and Manuele Dabalà. 2024. "Laser Powder Bed Fusion Fabrication of a Novel Carbide-Free Bainitic Steel: The Possibilities and a Comparative Study with the Conventional Alloy" Metals 14, no. 1: 113. https://doi.org/10.3390/met14010113





