Deciphering Microstructures and Phases of Gas-Atomised Novel Al-Fe-Si-Cr-Ni Alloys
Abstract
:1. Introduction
2. Materials and Methods
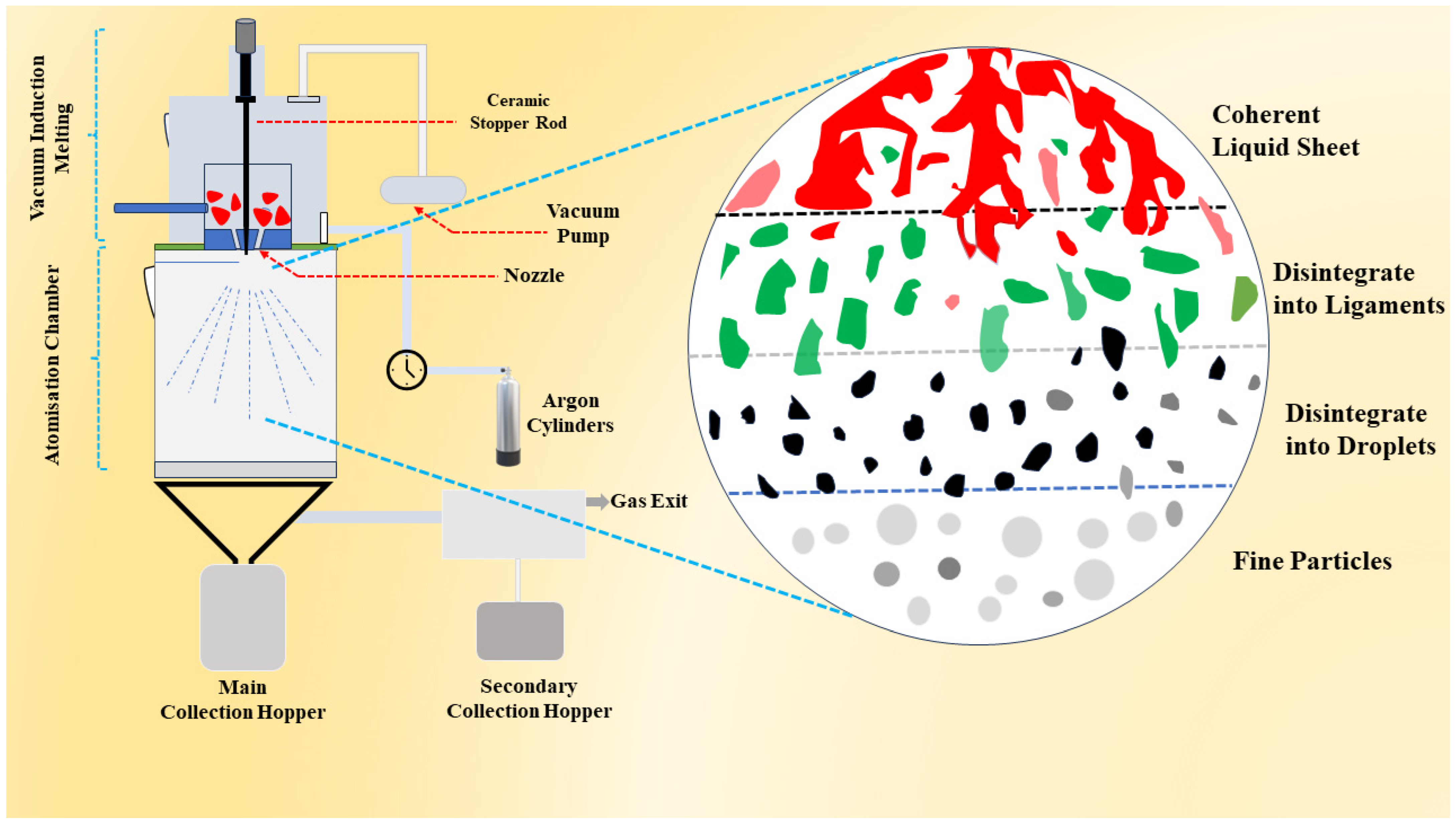
2.1. Powder Production
2.2. Powder Characterisations
3. Results
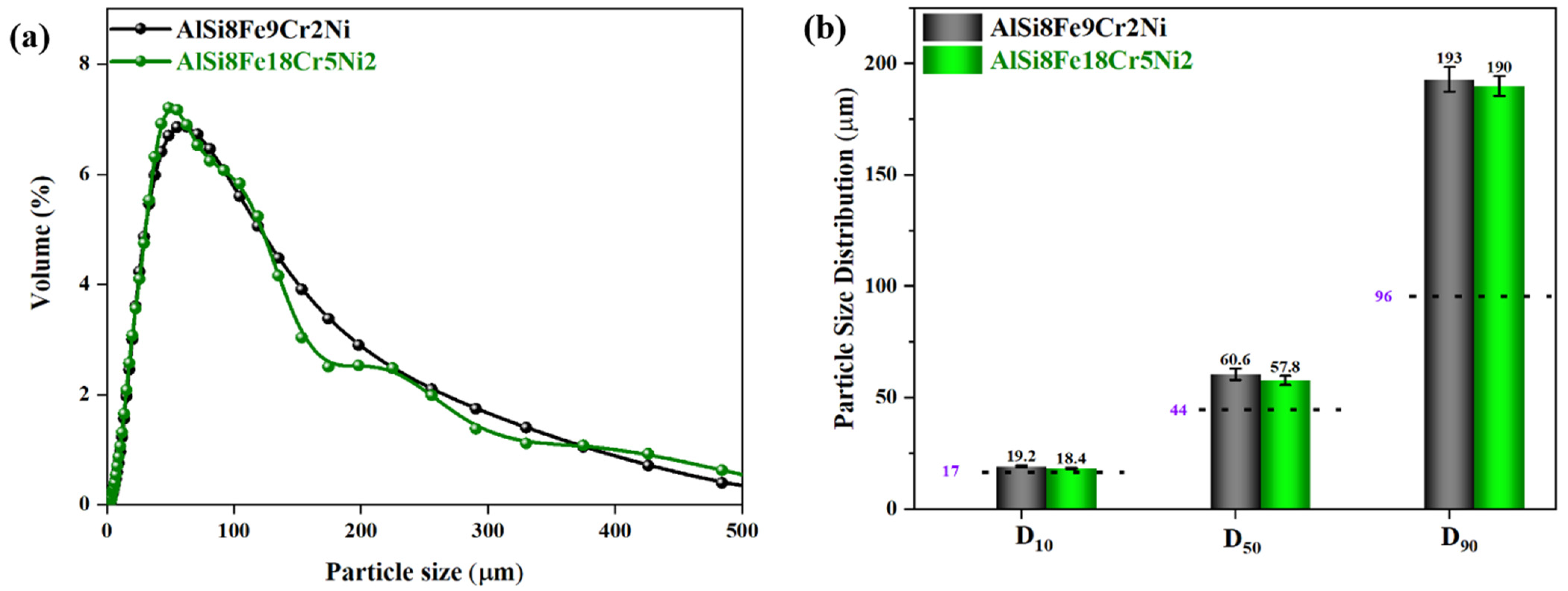
3.1. Particle Size Distribution
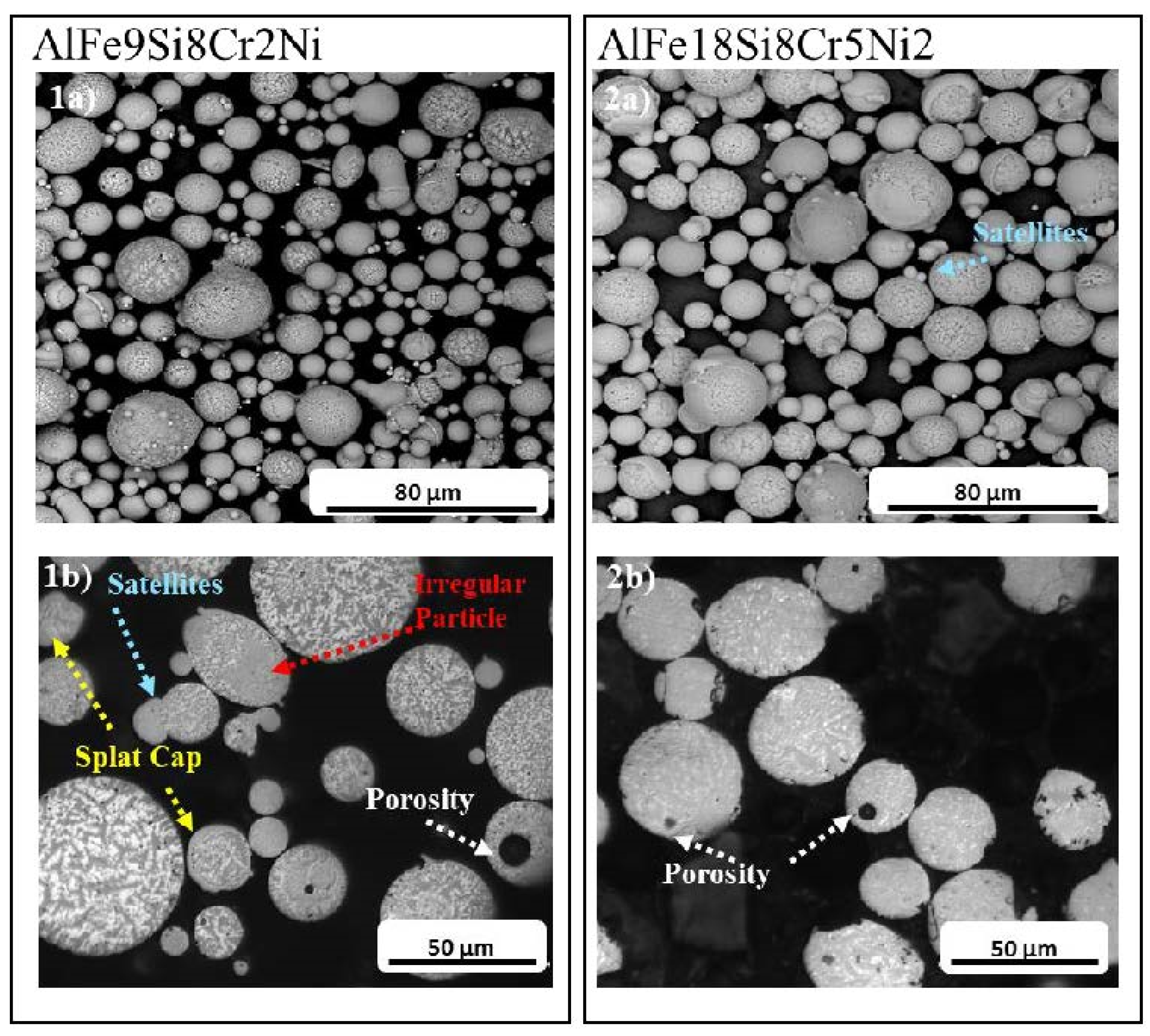
3.2. Morphological Characterisation
3.3. Effect of Cooling Rate on Microstructural Evolution of AlFe9Si8Cr2Ni and AlFe18Si8Cr5Ni2 Powders
3.4. Compositional Characterisation
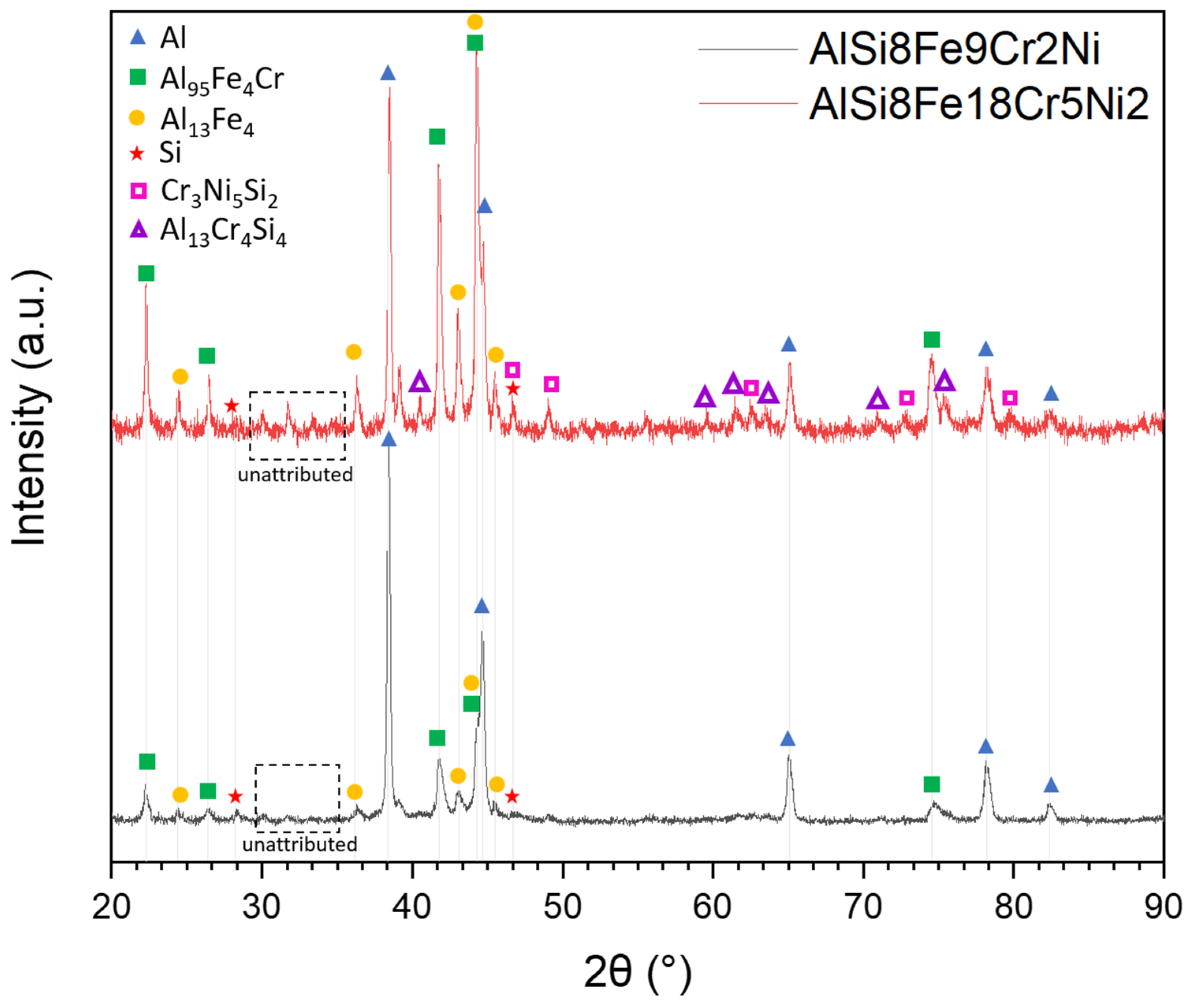
3.4.1. Phase Identification through XRD
3.4.2. Compositional Analysis by EDS
3.4.3. Thermal Analysis by DSC
4. Conclusions
- IGAed compositions exhibited asymmetrical PSD curves, with D50 and D90 percentiles higher than AlSi10Mg, attributed to the presence of Fe, Cr, and Ni altering the molten stream viscosity during gas atomisation.
- AlFe9Si8Cr2Ni and AlFe18Si8Cr5Ni2 particle morphologies were predominantly spherical, with just a limited presence of defects.
- Al-Fe-Si-Cr-Ni powders observed with the BSE detector showed a dendritic pattern with three distinct phases. In AlFe9Si8Cr2Ni, fine particles displayed cellular growth, transitioning to dendritic morphology as particle size increased. In contrast, the AlFe18Si8Cr5Ni2 composition exhibited more elongated and consistent dendritic solidification regardless of particle size.
- In XRD analysis, AlFe9Si8Cr2Ni exhibited intense peaks of fcc-Al, with additional peaks mainly attributed to intermetallic phases Al95Fe4Cr and Al13Fe4. In AlFe18Si8Cr5Ni2, the same but more pronounced peaks were detected with the addition of peaks related to the presence of the Cr3Ni5Si2 and Al13Cr4Si4 phases.
- EDS mapping of AlFe9Si8Cr2Ni particles showed dendritic structures enriched in Fe and Cr, confirming the Al95Fe4Cr phase detected with XRD, and interdendritic regions with Al-Si alternations resembling the lamellar structure of AlSi10Mg. In AlFe18Si8Cr5Ni2, dendritic structures appeared to be enriched in Fe and Cr, Al-Si alternations were not detected, and the more uniform Cr distribution justifies the appearance of Cr-containing phases identified by XRD.
- DSC analyses confirmed the higher amount of eutectic Al-Si in the AlFe9Si8Cr2Ni system and revealed a melting temperature of the two compositions well above that of AlSi10Mg.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cáceres, C.H.; Selling, B.I. Casting Defects and the Tensile Properties of an Al-Si-Mg Alloy. Mater. Sci. Eng. A 1996, 220, 109–116. [Google Scholar] [CrossRef]
- Javidani, M.; Larouche, D. Application of Cast Al-Si Alloys in Internal Combustion Engine Components. Int. Mater. Rev. 2014, 59, 132–158. [Google Scholar] [CrossRef]
- Mbuya, T.O.; Odera, B.O.; Ng’ang’a, S.P. Influence of Iron on Castability and Properties of Aluminium Silicon Alloys: Literature Review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Callegari, B.; Lima, T.N.; Coelho, R.S. The Influence of Alloying Elements on the Microstructure and Properties of Al-Si-Based Casting Alloys: A Review. Metals 2023, 13, 1174. [Google Scholar] [CrossRef]
- Seifeddine, S.; Johansson, S.; Svensson, I.L. The Influence of Cooling Rate and Manganese Content on the β-Al5FeSi Phase Formation and Mechanical Properties of Al-Si-Based Alloys. Mater. Sci. Eng. A 2008, 490, 385–390. [Google Scholar] [CrossRef]
- Lan, X.; Li, K.; Wang, J.; Yang, M.; Lu, Q.; Du, Y. Developing Al–Fe–Si Alloys with High Thermal Stability through Tuning Fe, Si Contents and Cooling Rates. Intermetallics 2022, 144, 107505. [Google Scholar] [CrossRef]
- Petr, J. The Iron Correctors in Al-Si Alloys. Int. J. Eng. 2011, 9, 401–405. [Google Scholar]
- Aranda, V.A.; Figueroa, I.A.; González, G.; García-Hinojosa, J.A.; Alfonso, I. Study of the Microstructure and Mechanical Properties of Al-Si-Fe with Additions of Chromium by Suction Casting. J. Alloys Compd. 2021, 853, 157155. [Google Scholar] [CrossRef]
- Školáková, A.; Novák, P.; Vojtěch, D.; Kubatík, T.F. Microstructure and Mechanical Properties of Al-Si-Fe-X Alloys. Mater. Des. 2016, 107, 491–502. [Google Scholar] [CrossRef]
- Petrík, J. The Application of Ni for Improvement of Al-Si-Fe Alloys. Mater. Eng. 2009, 16, 29–32. [Google Scholar]
- Xavier, M.G.C.; Freitas, B.J.M.; Koga, G.Y.; Spinelli, J.E. Effects of Ni and Co on the Corrosion Resistance of Al-Si-Cu-Zn-Fe Alloys in NaCl Solution. Metals 2022, 12, 645. [Google Scholar] [CrossRef]
- Lavernia, E.J.; Srivatsan, T.S. The Rapid Solidification Processing of Materials: Science, Principles, Technology, Advances, and Applications. J. Mater. Sci. 2010, 45, 287–325. [Google Scholar] [CrossRef]
- Dunkley, J.J. Metal Powder Atomisation Methods for Modern Manufacturing. Johns. Matthey Technol. Rev. 2019, 63, 226–232. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, S.Y.; Tan, P.; Wang, J.; Xiang, C.S.; Tang, H.P. A Comparative Study of Ti-6Al-4V Powders for Additive Manufacturing by Gas Atomization, Plasma Rotating Electrode Process and Plasma Atomization. Powder Technol. 2018, 333, 38–46. [Google Scholar] [CrossRef]
- Jones, H. On the Prediction of Lattice Parameter vs. Concentration for Solid Solutions Extended by Rapid Quenching from the Melt. Scr. Metall. 1983, 17, 97–100. [Google Scholar] [CrossRef]
- Geoffrey, V.; Raynor, V.G.R. Phase Equilibria in Iron Ternary Alloys: A Critical Assessment of the Experimental Literature; Institute of Metals: London, UK; Institute of Metals North American Publications Center: Brookfield, VT, USA, 1988. [Google Scholar]
- Ünal, A. Effect of Processing Variables on Particle Size in Gas Atomization of Rapidly Solidified Aluminium Powders. Mater. Sci. Technol. 1987, 3, 1029–1039. [Google Scholar] [CrossRef]
- Prasad, A.; Mosbah, S.; Henein, H.; Gandin, C.A. A Solidification Model for Atomization. ISIJ Int. 2009, 49, 992–999. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Hasebe, Y.; Bian, H.; Yamanaka, K.; Aoyagi, K.; Hagisawa, T.; Chiba, A. Controlling Factors Determining Flowability of Powders for Additive Manufacturing: A Combined Experimental and Simulation Study. Powder Technol. 2021, 393, 482–493. [Google Scholar] [CrossRef]
- Fischmeister, H.F.; Ozerskii, A.D.; Olsson, L. Solidification Structure of Gas-Atomized High-Speed Steel Powders. Powder Metall. 1982, 25, 1–9. [Google Scholar] [CrossRef]
- Xiong, L.; Chuang, A.C.; Thomas, J.; Prost, T.; White, E.; Anderson, I.; Singh, D. Defect and Satellite Characteristics of Additive Manufacturing Metal Powders. Adv. Powder Technol. 2022, 33, 103486. [Google Scholar] [CrossRef]
- Kumar, G.S.; Sateshwar, M.; Sharma, A.R.; Palit, M.; Sarkar, R.; Ghosal, P.; Rao, G.A. Particle Size Dependent Microstructure Evolution of Inert Gas Atomized Nickel Base Superalloy Powders. J. Alloys Compd. 2022, 909, 164772. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, M.; Song, C.; Han, Q.; Zhai, Q. Solidification Structure of C2.08Cr25.43Si1.19Mn0.43Fe70.87 Powders Fabricated by High Pressure Gas Atomization. Mater. Charact. 2010, 61, 116–122. [Google Scholar] [CrossRef]
- Fang, P.; Xu, Y.; Li, X.; Chen, Y. Influence of Atomizing Gas and Cooling Rate on Solidification Characterization of Nickel-Based Superalloy Powders. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2018, 47, 423–430. [Google Scholar] [CrossRef]
- Bassini, E.; Galech, U.; Soria, T.; Aristizabal, M.; Iturriza, I.; Biamino, S.; Ugues, D. Effect of the Particle Size Distribution on Physical Properties, Composition, and Quality of Gas Atomized Astroloy Powders for HIP Application. J. Alloys Compd. 2022, 890, 161631. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Li, R.; Zhou, N.; Zheng, K.; Fu, Y.; Zhang, W. Study of the Evolution Mechanisms of Fe-Rich Phases in Al-Si-Fe Alloys with Mn Modification Using Synchrotron X-ray Imaging. J. Alloys Compd. 2022, 915, 165378. [Google Scholar] [CrossRef]
- Marola, S.; Manfredi, D.; Fiore, G.; Poletti, M.G.; Lombardi, M.; Fino, P.; Battezzati, L. A comparison of Selective Laser Melting with bulk rapid solidification of AlSi10Mg alloy. J. Alloys Compd. 2018, 742, 271–279. [Google Scholar] [CrossRef]




| Elements | Cr | Ni | C | Mn | P | S | Si | N | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Composition (wt.%) | 18.0 | 8.0 | 0.02 | 1.50 | 0.03 | 0.03 | 0.41 | 0.08 | Bal |
| Elements | Si | Mg | Fe | Mn | Zn | Al |
|---|---|---|---|---|---|---|
| Composition (wt.%) | 9.0–11.0 | 0.2–0.45 | 0.55 | 0.45 | 0.10 | Bal |
| Alloy | Fe | Si | Cr | Ni | Al |
|---|---|---|---|---|---|
| AlFe9Si8Cr2Ni (wt.%) | 9.14 ± 0.30 | 8.60 ± 0.38 | 1.82 ± 0.10 | 0.77 ± 0.07 | Bal |
| AlFe18Si8Cr5Ni2 (wt.%) | 18.73 ± 0.45 | 7.42 ± 0.20 | 5.82 ± 0.28 | 2.12 ± 0.02 | Bal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, B.; Martucci, A.; Virgillito, E.; Gobber, F.; Bondioli, F.; Manfredi, D.; Lombardi, M.; Fino, P. Deciphering Microstructures and Phases of Gas-Atomised Novel Al-Fe-Si-Cr-Ni Alloys. Metals 2024, 14, 17. https://doi.org/10.3390/met14010017
Bhatt B, Martucci A, Virgillito E, Gobber F, Bondioli F, Manfredi D, Lombardi M, Fino P. Deciphering Microstructures and Phases of Gas-Atomised Novel Al-Fe-Si-Cr-Ni Alloys. Metals. 2024; 14(1):17. https://doi.org/10.3390/met14010017
Chicago/Turabian StyleBhatt, Bhaskaranand, Alessandra Martucci, Enrico Virgillito, Federico Gobber, Federica Bondioli, Diego Manfredi, Mariangela Lombardi, and Paolo Fino. 2024. "Deciphering Microstructures and Phases of Gas-Atomised Novel Al-Fe-Si-Cr-Ni Alloys" Metals 14, no. 1: 17. https://doi.org/10.3390/met14010017
APA StyleBhatt, B., Martucci, A., Virgillito, E., Gobber, F., Bondioli, F., Manfredi, D., Lombardi, M., & Fino, P. (2024). Deciphering Microstructures and Phases of Gas-Atomised Novel Al-Fe-Si-Cr-Ni Alloys. Metals, 14(1), 17. https://doi.org/10.3390/met14010017











