Experiments on Oxidation and Combustion Behaviors of Cerium Metal Slice with Slow Heating under O2/Ar Atmospheric Conditions
Abstract
:1. Introduction
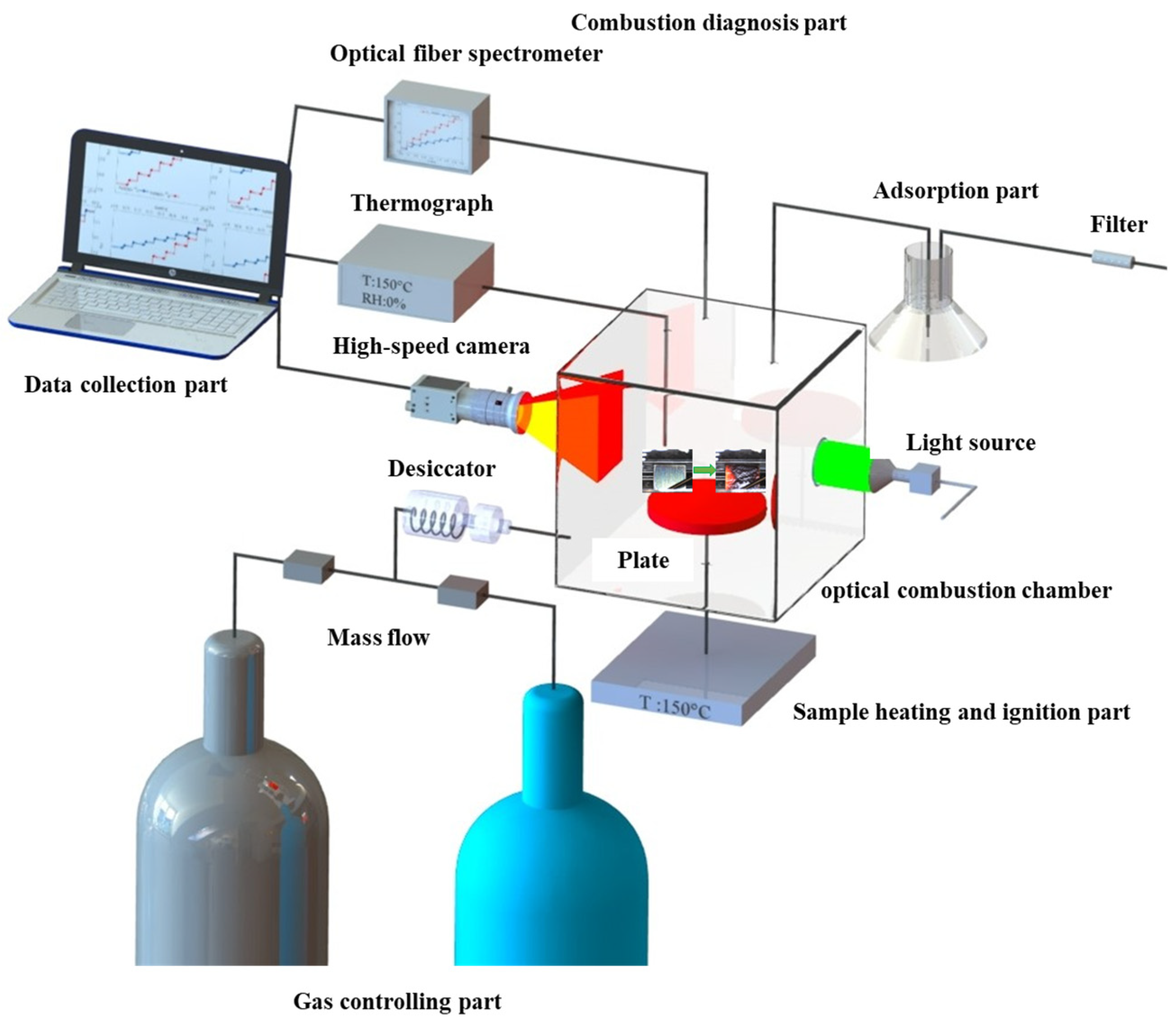
2. Materials and Methods
3. Results and Discussion
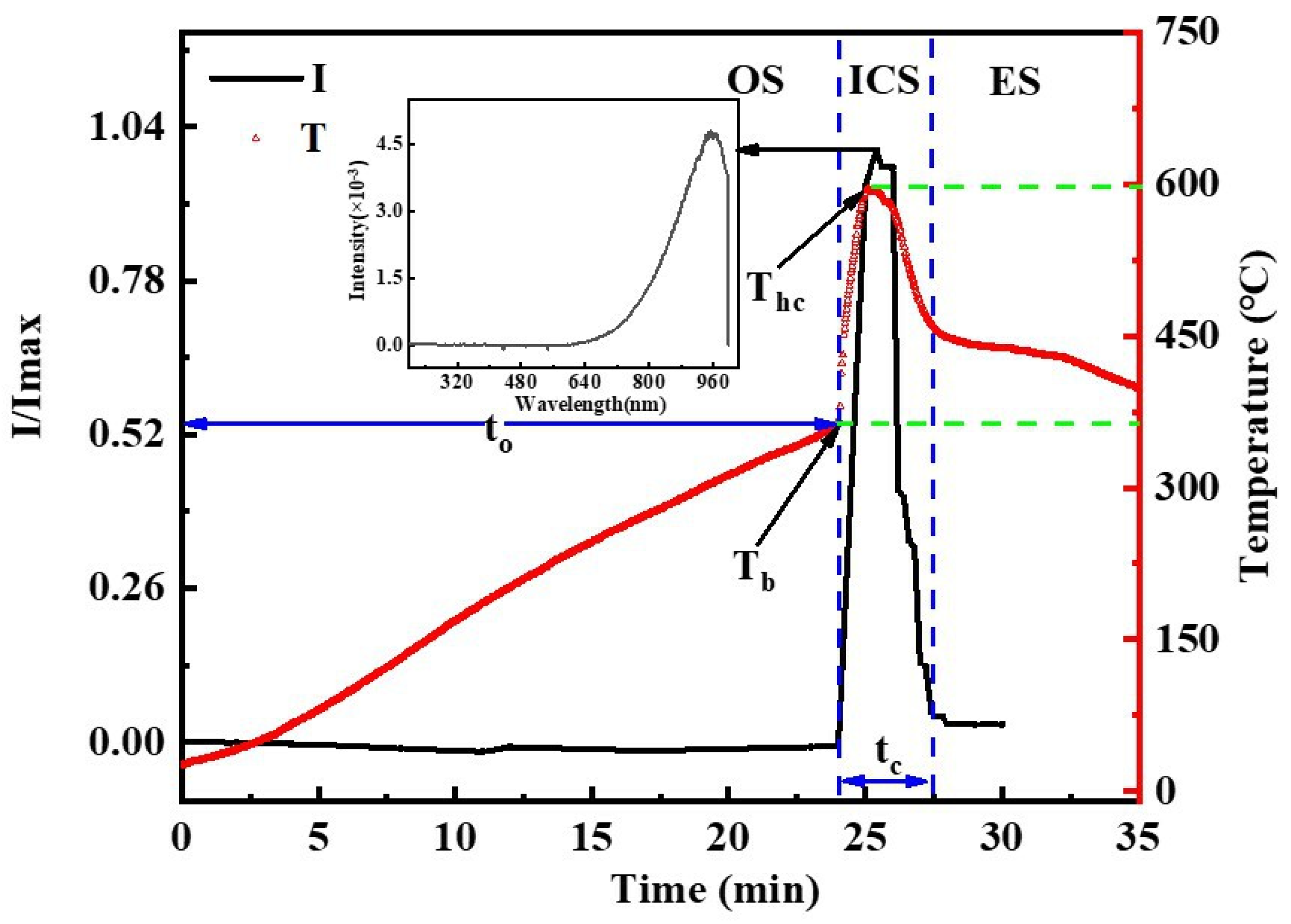
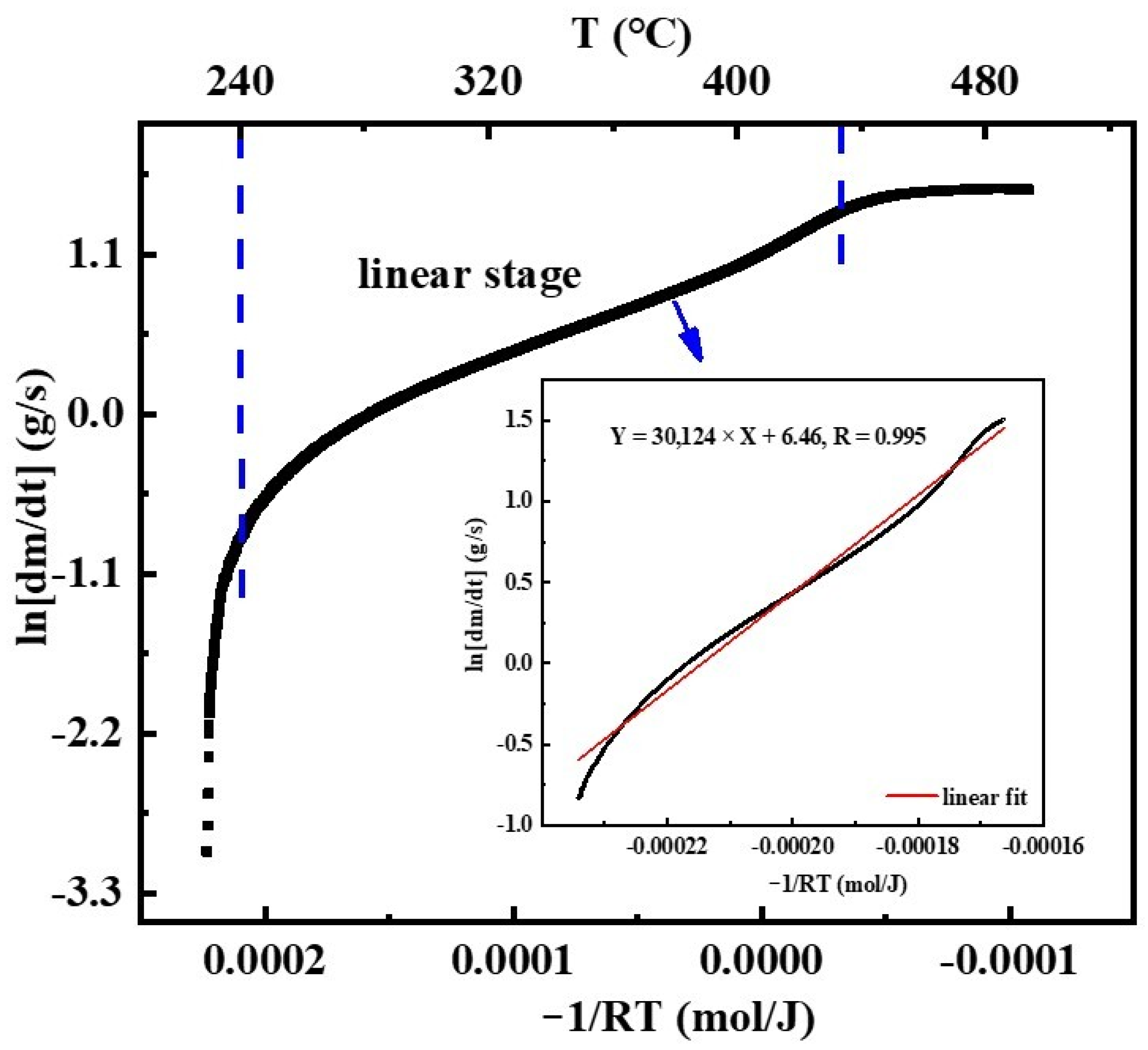
3.1. The Oxidation-to-Combustion Transition of CMS
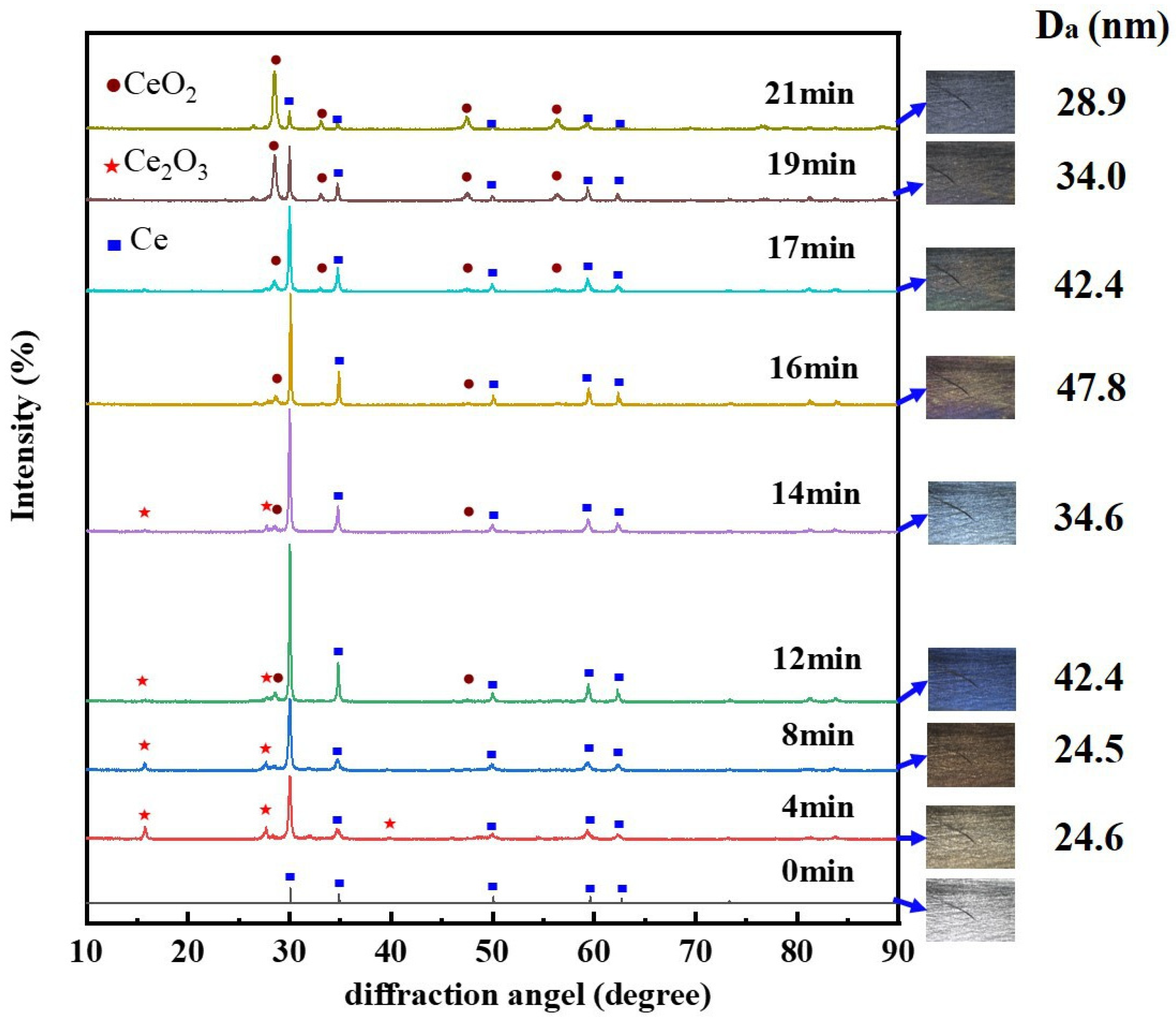
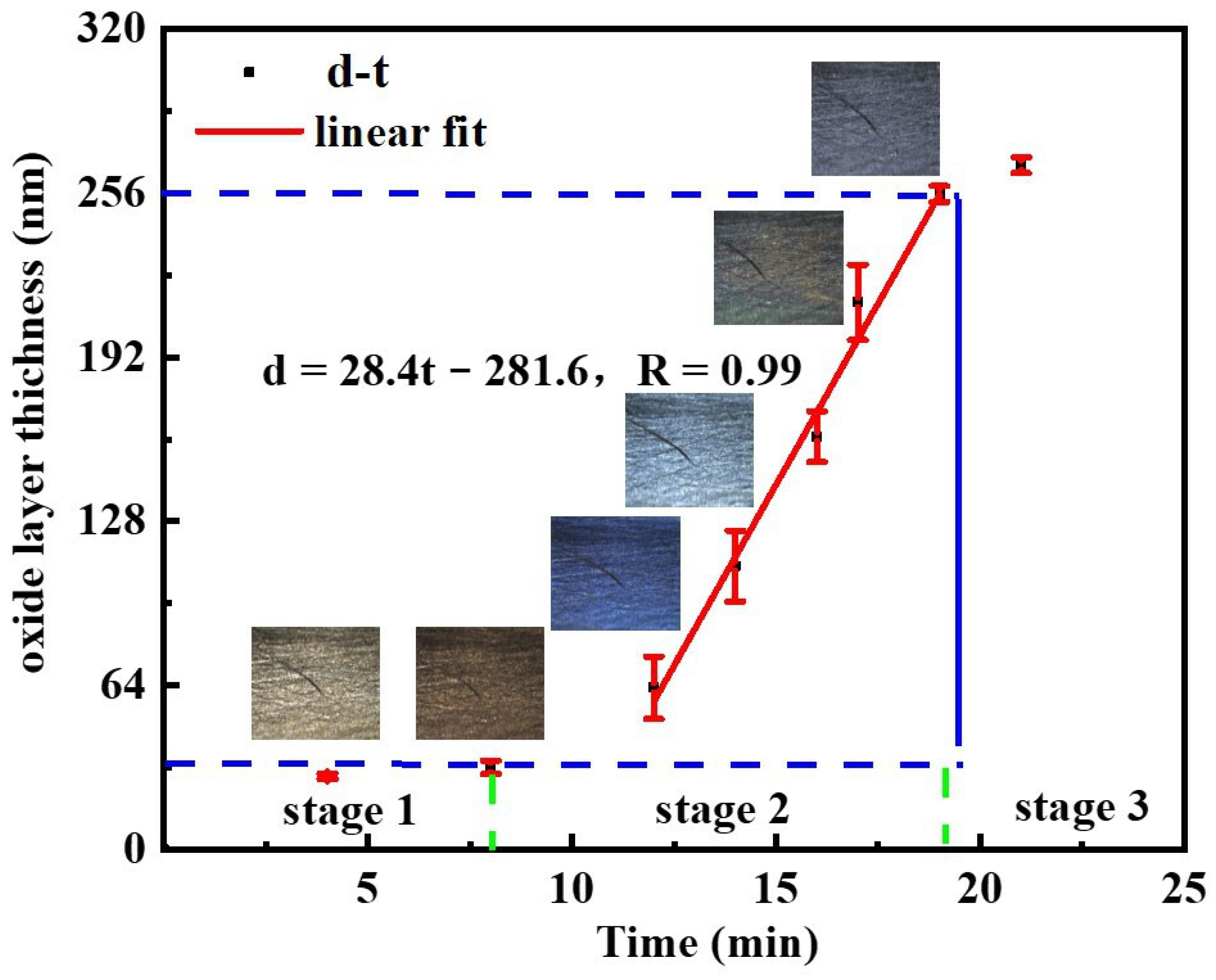
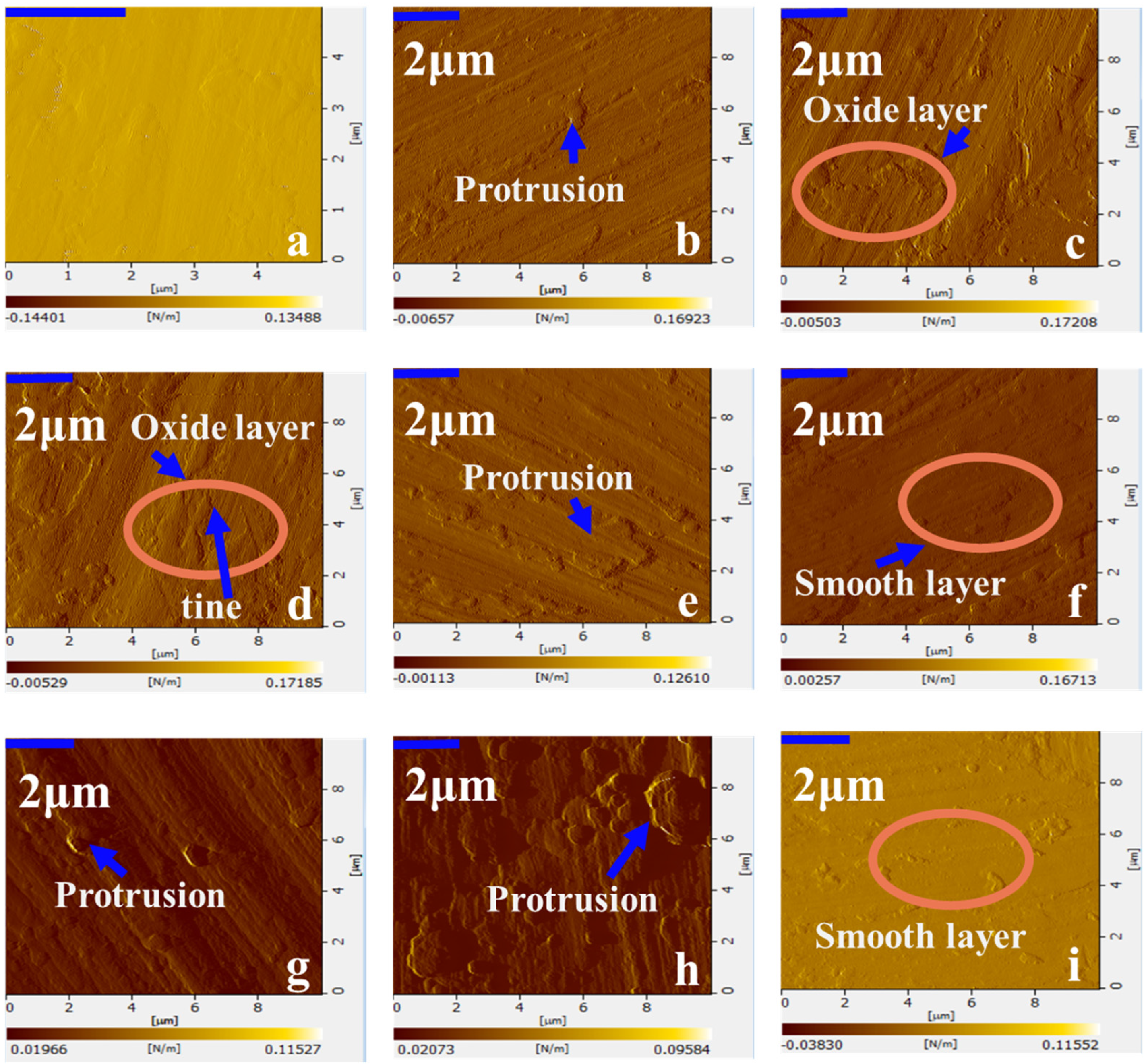
3.2. The Oxidation Reactions and Behaviors of CMS
3.3. The Combustion Behaviors of CMS
3.4. The Oxidation and Combustion Reactivity of Cerium
4. Conclusions
- According to the evolutions of surface morphology, surface temperature and emission spectrum, the oxidation-to-combustion process of CMS can be divided into the oxidation stage (OS), ignition and combustion stage (ICS) and extinction stage (ES).
- At the OS, fresh cerium is first oxidized to cerium oxide. Then, the oxides aggregate and grow to form a dense oxide layer. The overall growth rate of cerium oxide layer is generally consistent with the linear stage, yet the growth rate varies with the Ce-O reaction. The growth rate of oxide layer thickness is slow in the time of 0 min to 4 min, when cerium is oxidized to Ce2O3. Soon afterwards, both Ce2O3 and Ce are oxidized to CeO2, leading to a faster growth rate of oxide layer thickness in the time of 4 min to 12 min. After 12 min, Ce is directly oxidized to be CeO2 without the intermediate oxidization of Ce2O3, resulting in a reduction in the growth rate of oxide layer thickness.
- With cerium oxide layer growing thicker and denser, along with the accumulation of heat release from Ce-O reaction, Ce is ignited to burn once the temperature rises to the ignition point and emits luminescent radiation with a sharp rise in sample temperature. Moreover, the morphology of final cerium oxide product indicates that cerium inside is melted, and thus oxide layer cracks during ICS, resulting in a fierce heterogeneous combustion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rinaudo, M.G.; Cadús, L.E.; Morales, M.R. Mechanochemical Activation of CeO2 in Order to Boost Physicochemical Properties for Catalytic Applications. Eng. Proc. 2023, 56, 49–53. [Google Scholar]
- Ahmad, I.; Akhtar, M.S.; Manzoor, M.F.; Wajid, M.; Noman, M.; Ahmed, E.; Ahmad, M.; Khan, W.Q.; Rana, A.M. Synthesis of yttrium and cerium doped ZnO nanoparticles as highly inexpensive and stable photocatalysts for hydrogen evolution. J. Rare Earths 2021, 39, 440–445. [Google Scholar] [CrossRef]
- Kim, S.; Choi, M.; Park, J. Cerium-Doped Oxide-Based Materials for Energy and Environmental Applications. Crystals 2023, 13, 1631. [Google Scholar] [CrossRef]
- Chatzichristodoulou, C.; Blennow, P.T.; Søgaard, M.; Hendriksen, P.V.; Mogensen, M.B. Ceria and its use in solid oxide cells and oxygen membranes. Catal. Sci. Ser. 2013, 12, 623–782. [Google Scholar]
- Taye, M.B.; Ningsih, H.S.; Shih, S.-J. Antibacterial and In Vitro Bioactivity Studies of Silver-Doped, Cerium-Doped, and Silver–Cerium Co-Doped 80S Mesoporous Bioactive Glass Particles via Spray Pyrolysis. Appl. Sci. 2023, 13, 12637. [Google Scholar] [CrossRef]
- Aljarrah, M.; Alnahas, J.; Alhartomi, M. Thermodynamic Modeling and Mechanical Properties of Mg-Zn-{Y, Ce} Alloys: Review. Crystals 2021, 11, 1592. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.H.; Cao, S.J.; Peng, J.H.; Guo, P.; Long, C.; Wang, S.; Chen, C.; Wu, Y.; Huang, J.A.; et al. Effect of cerium alloying on microstructure, texture and mechanical properties of magnesium during cold-rolling process. J. Rare Earths 2023, in press. [Google Scholar] [CrossRef]
- Han, S.; Geng, R.; Lei, S.; Li, Y.; Wang, C. Effect of Cerium on Inclusion Modification in a Secondary-Hardening Steel. Materials 2023, 16, 3972. [Google Scholar] [CrossRef]
- Luginin, N.; Eroshenko, A.; Khimich, M.; Prosolov, K.; Kashin, A.; Uvarkin, P.; Tolmachev, A.; Glukhov, I.; Panfilov, A.; Sharkeev, Y. Severe Plastic Deformation of Mg–Zn–Zr–Ce Alloys: Advancing Corrosion Resistance and Mechanical Strength for Medical Applications. Metals 2023, 13, 1847. [Google Scholar] [CrossRef]
- Fan, J.F.; Yang, C.L.; Han, G.; Fang, S.; Yang, W.D.; Xu, B.S. Oxidation behavior of ignition-proof magnesium alloys with rare earth addition. J Alloy Compd. 2011, 509, 2137–2142. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, Y.X.; Liu, Y.; Xu, H.; Ying, Q.J.; Wu, Z.B. Insight into the Role of Cerium in the Enhanced Performances during Catalytic Combustion of Acetonitrile over Core–Shell-like Cu–Ce/ZSM-5 Catalysts. ACS ES&T Eng. 2022, 2, 1709–1721. [Google Scholar]
- Zheng, J.; Wang, Z.; Chen, Z.; Zuo, S.F. Mechanism of CeO2 synthesized by thermal decomposition of Ce-MOF and its performance of benzene catalytic combustion. J. Rare Earths 2021, 37, 790–796. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Z.Y.; Dong, J.; Jin, L.; Ding, W.J. Selective oxidation behavior of an ignition-proof Mg-Y-Ca-Ce alloy. J. Rare Earths 2013, 31, 1003–1008. [Google Scholar] [CrossRef]
- Kang, W.; Ozgur, D.O.; Varma, A. Solution combustion synthesis of high surface area CeO2 nanopowders for catalytic applications: Reaction mechanism and properties. ACS Appl. Nano Mater. 2018, 1, 675–685. [Google Scholar] [CrossRef]
- Trojanowski, R.; Fthenakis, V. Nanoparticle emissions from residential wood combustion: A critical literature review, characterization, and recommendations. Renew. Sust. Energ. Rev. 2019, 103, 515–528. [Google Scholar] [CrossRef]
- Gantt, B.; Hoque, S.; Willis, R.D.; Fahey, K.M.; Delgado-Saborit, J.M.; Harrison, R.M.; Erdakos, G.B.; Bhave, P.V.; Zhang, K.M.; Kovalcik, K.; et al. Near-Road modeling and measurement of cerium-containing particles generated by nanoparticle diesel fuel additive use. Environ. Sci. Technol. 2014, 48, 10607–10613. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.Y.; Shi, W.; Li, J.; Ye, C.B.; Fang, R.M.; Zhu, H.F.; Chen, S.H.; Lan, L. Effect of yttrium and manganese addition on catalytic soot combustion activity and anti-high-temperature stability of CeO2 catalyst. J. Rare Earths. 2023, in press. [Google Scholar] [CrossRef]
- Andreescu, D.; Bulbul, G.; Özel, R.E.; Hayat, A.; Sardesai, N.; Andreescu, S. Applications and implications of nanoceria reactivity: Measurement tools and environmental impact. Environ. Sci. Nano 2014, 1, 445–458. [Google Scholar] [CrossRef]
- Muhammad, R.; Ali, U. Optimized cerium addition for microstructure and mechanical properties of SAC305. Solder. Surf. Mt. Technol. 2020, 33, 197. [Google Scholar] [CrossRef]
- Hoang, A.T. Combustion behavior, performance and emission characteristics of diesel engine fueled with biodiesel containing cerium oxide nanoparticles: A review. Fuel Process. Technol. 2021, 218, 106840. [Google Scholar] [CrossRef]
- Fan, J.F.; Yang, G.C.; Cheng, S.L.; Xie, H.; Hao, W.X.; Wang, M.; Yang, G.C.; Zhou, Y.H. Surface oxidation behavior of Mg-Y-Ce alloys at high temperature. Metall. Mater. Trans. A 2005, 36, 235–239. [Google Scholar] [CrossRef]
- Lin, P.Y.; Zhou, H.; Li, W.; Li, W.P.; Sun, N.; Yang, R. Interactive effect of cerium and aluminum on the ignition point and the oxidation resistance of magnesium alloy. Corros. Sci. 2008, 50, 2669–2675. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.X.; Li, W.; Wang, L.; Zhao, Y. Effect of Ce addition on ignition point of AM50 alloy powder. Mater. Lett. 2006, 60, 3238–3240. [Google Scholar]
- Cubicciotti, D. The Reaction of Cerium with Oxygen. J. Am. Chem. Soc. 1952, 74, 1200–1201. [Google Scholar] [CrossRef]
- Wheeler, D.W. Kinetics and mechanism of the oxidation of cerium in air at ambient temperature. Corros. Sci. 2016, 111, 52–60. [Google Scholar] [CrossRef]
- Karasev, V.V.; Onishchuk, A.A.; Khromova, S.A.; Glotov, O.G.; Zarko, V.E.; Pilyugina, E.A.; Tsai, C.T. Formation of metal oxide nanoparticles in combustion of titanium and aluminum droplets. Combust. Explos. Shock Waves 2006, 42, 649–662. [Google Scholar] [CrossRef]
- Smovzh, D.V.; Kalyuzhnyĭ, N.A.; Zaikovsky, A.V.; Novopashin, S.A. Synthesis of hollow nanoparticles γ-Al2O3. J. Nanopart. Res. 2013, 2, 120–124. [Google Scholar]
- Zarko, V.; Glazunov, A. Review of experimental methods for measuring the ignition and combustion characteristics of metal nanoparticles. Nanomaterials 2020, 10, 2008. [Google Scholar] [CrossRef]
- Shih, T.S.; Wang, J.H.; Chong, K.Z. Combustion of magnesium alloys in air. Mater. Chem. Phys. 2004, 85, 302–309. [Google Scholar] [CrossRef]
- Haschke, J.M.; Allen, T.H.; Morales, L.A. Surface and corrosion chemistry of plutonium. Los Alamos Sci. 2000, 26, 252–273. [Google Scholar]
- Joseph, C.M.; John, M.H.; Jerry, L. A mechanism for plutonium pyrophoricity. J. Nucl. Mater. 1994, 210, 130–142. [Google Scholar]
- Han, D.; Zhang, J.; Huang, J.F.; Lian, Y.; He, G.Y. A review on ignition mechanisms and characteristics of magnesium alloys. J. Magnes. Alloy 2020, 8, 329–344. [Google Scholar] [CrossRef]




| Time | Zone 1 | Zone 2 | ||
|---|---|---|---|---|
| Ce Content (%) | O Content (%) | Ce Content (%) | O Content (%) | |
| 0 | 100 | 0 | 100 | 0 |
| 4 | 67.5 | 32.5 | 48.6 | 51.4 |
| 8 | 28.1 | 71.9 | 54.5 | 45.5 |
| 12 | 56.3 | 43.7 | 43.3 | 56.7 |
| 14 | 39.5 | 60.5 | 26.4 | 73.6 |
| 16 | 32.3 | 67.7 | 23.7 | 76.3 |
| 17 | 45.6 | 54.4 | 44.1 | 55.9 |
| 19 | 31.3 | 68.9 | 30.7 | 69.3 |
| 21 | 28.1 | 71.9 | 37.0 | 63.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zheng, X.; Wang, Y.; Pan, S.; Qi, Q.; Shi, Z.; Wu, Y.; Wang, X. Experiments on Oxidation and Combustion Behaviors of Cerium Metal Slice with Slow Heating under O2/Ar Atmospheric Conditions. Metals 2024, 14, 3. https://doi.org/10.3390/met14010003
Li S, Zheng X, Wang Y, Pan S, Qi Q, Shi Z, Wu Y, Wang X. Experiments on Oxidation and Combustion Behaviors of Cerium Metal Slice with Slow Heating under O2/Ar Atmospheric Conditions. Metals. 2024; 14(1):3. https://doi.org/10.3390/met14010003
Chicago/Turabian StyleLi, Shun, Xianglong Zheng, Yongpeng Wang, Sheqi Pan, Qin Qi, Zhanghong Shi, Yingchun Wu, and Xiaolin Wang. 2024. "Experiments on Oxidation and Combustion Behaviors of Cerium Metal Slice with Slow Heating under O2/Ar Atmospheric Conditions" Metals 14, no. 1: 3. https://doi.org/10.3390/met14010003
APA StyleLi, S., Zheng, X., Wang, Y., Pan, S., Qi, Q., Shi, Z., Wu, Y., & Wang, X. (2024). Experiments on Oxidation and Combustion Behaviors of Cerium Metal Slice with Slow Heating under O2/Ar Atmospheric Conditions. Metals, 14(1), 3. https://doi.org/10.3390/met14010003







