Lattice Dynamics, Transport and Thermoelectric Properties of Bi-Sb Alloys Obtained by Mechanical Alloying and Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
3. Results
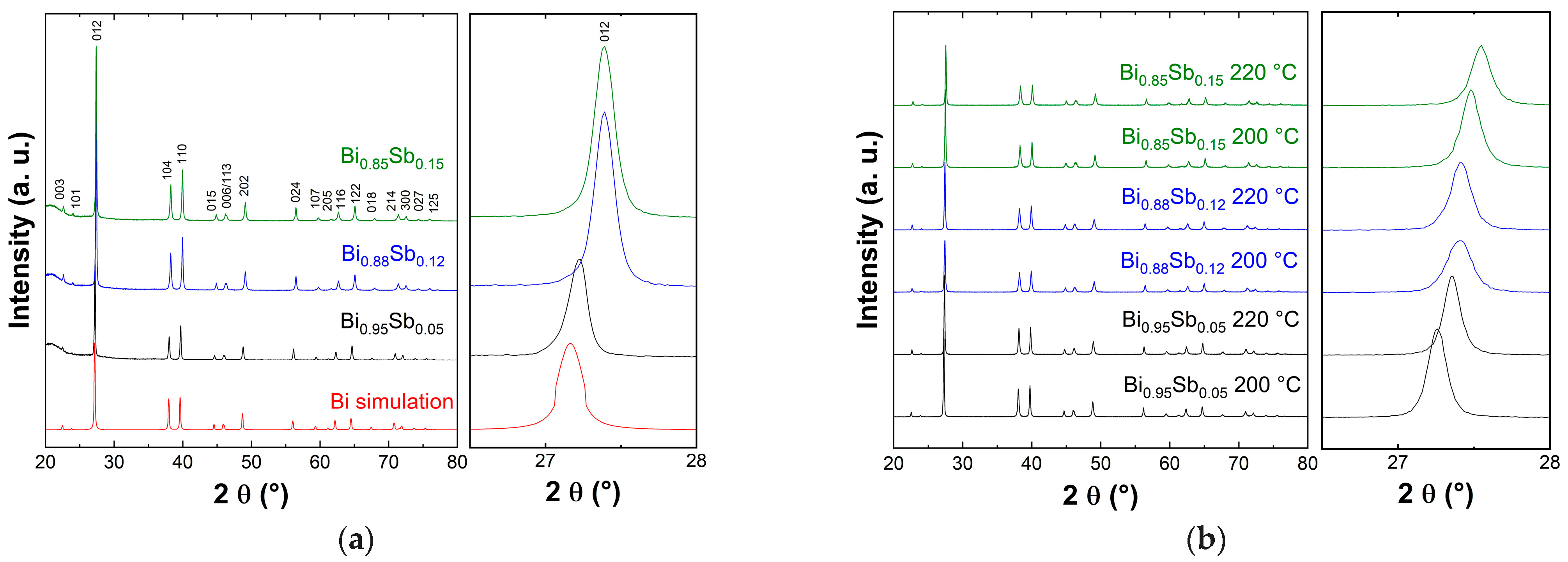
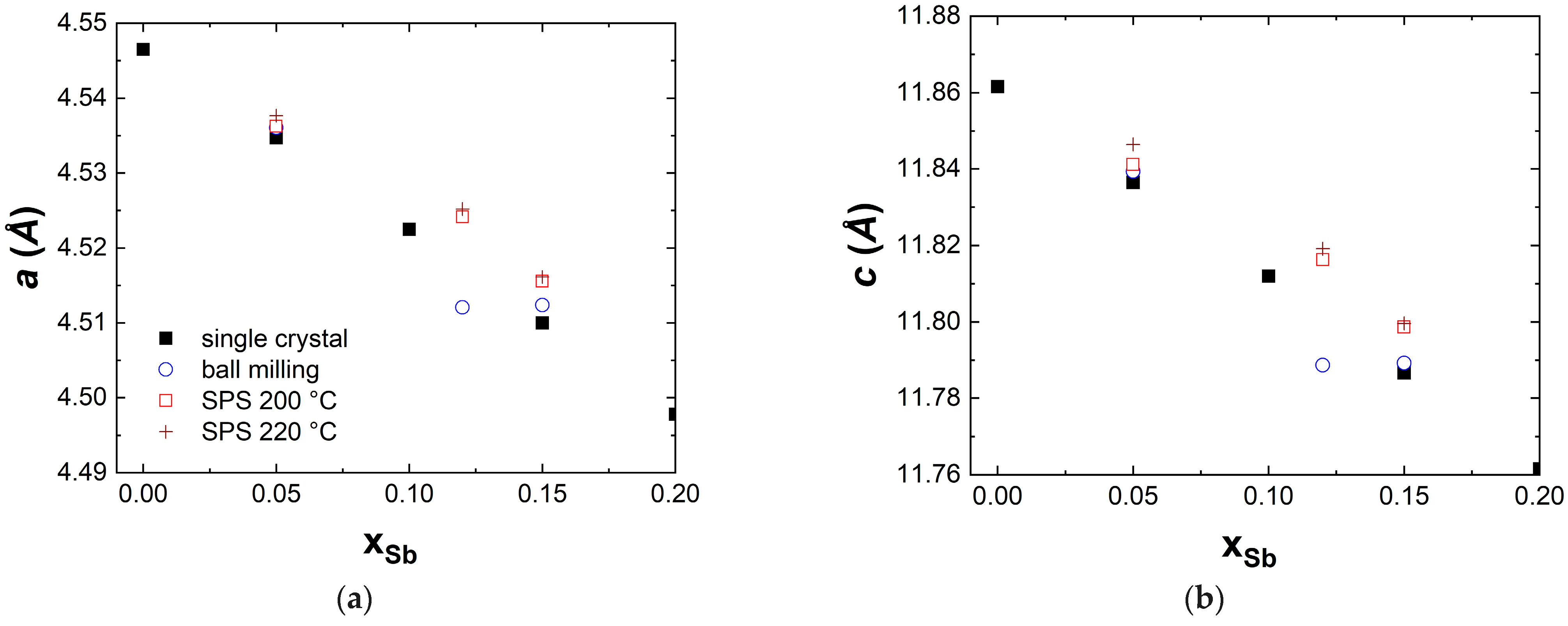
3.1. Structural Characterization
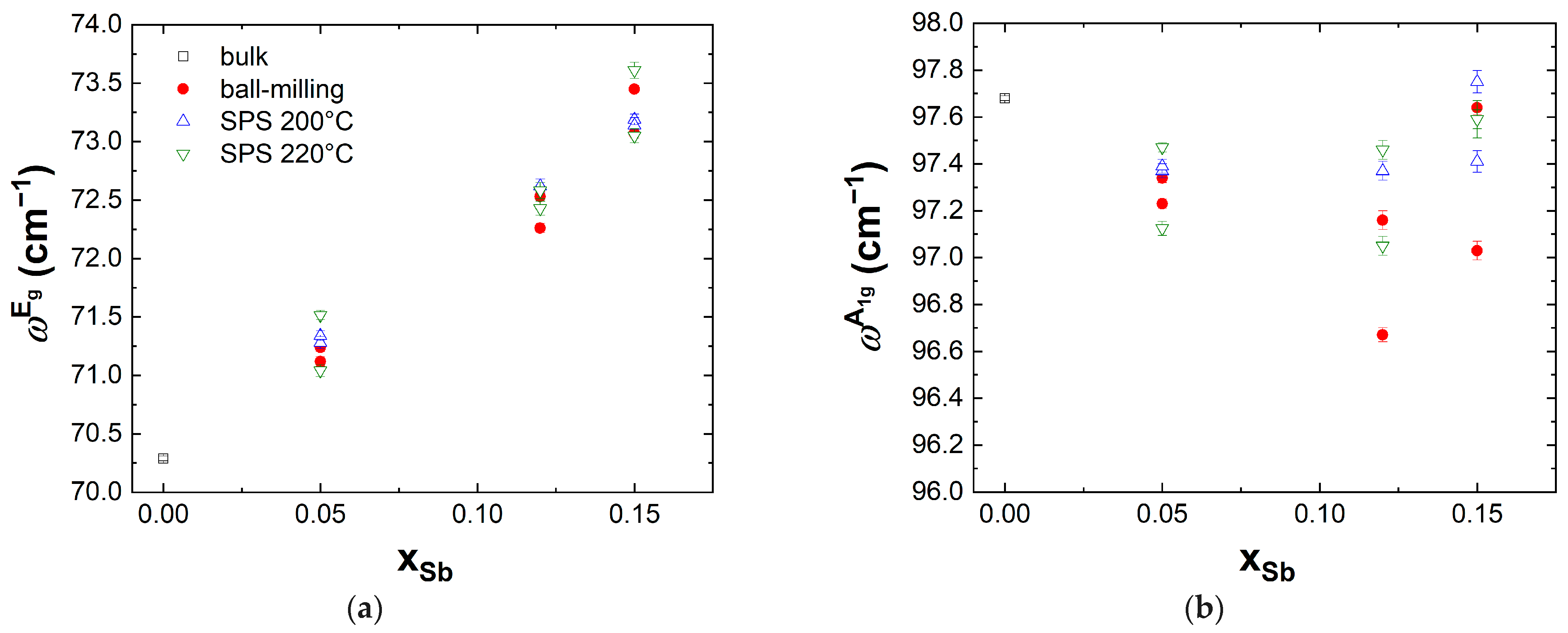
3.2. Raman Scattering
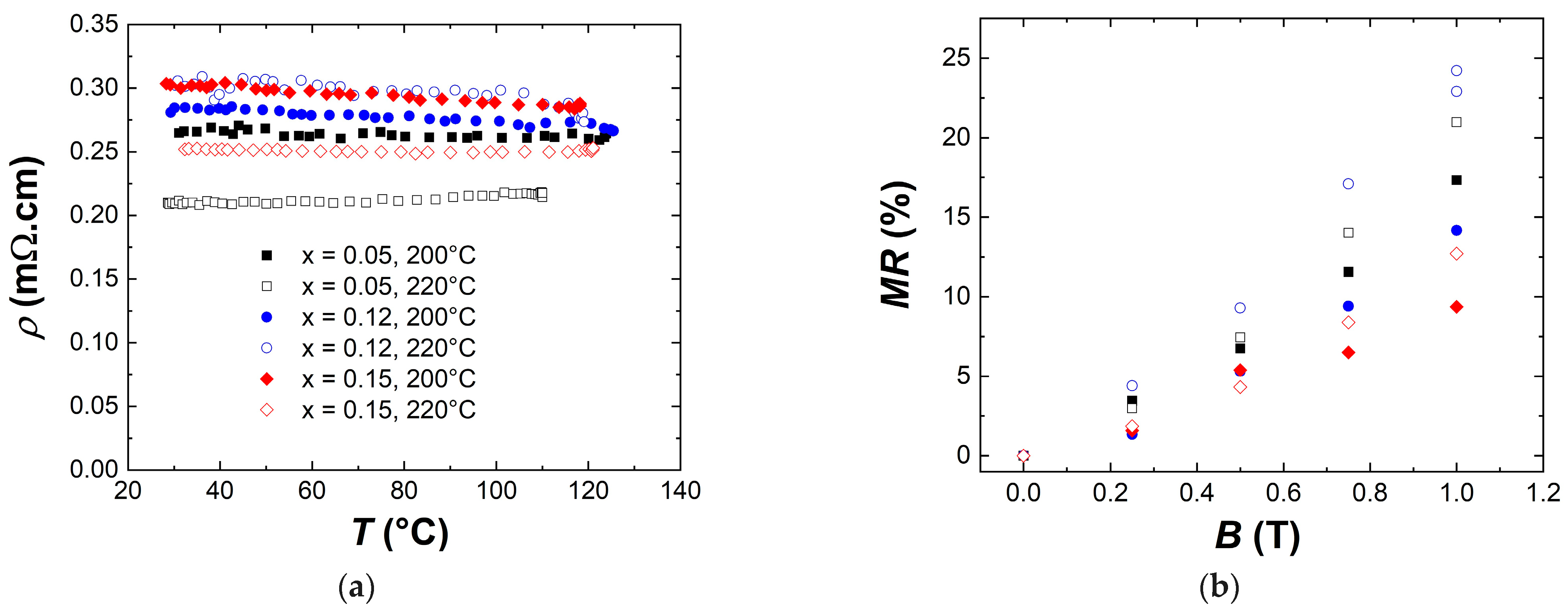
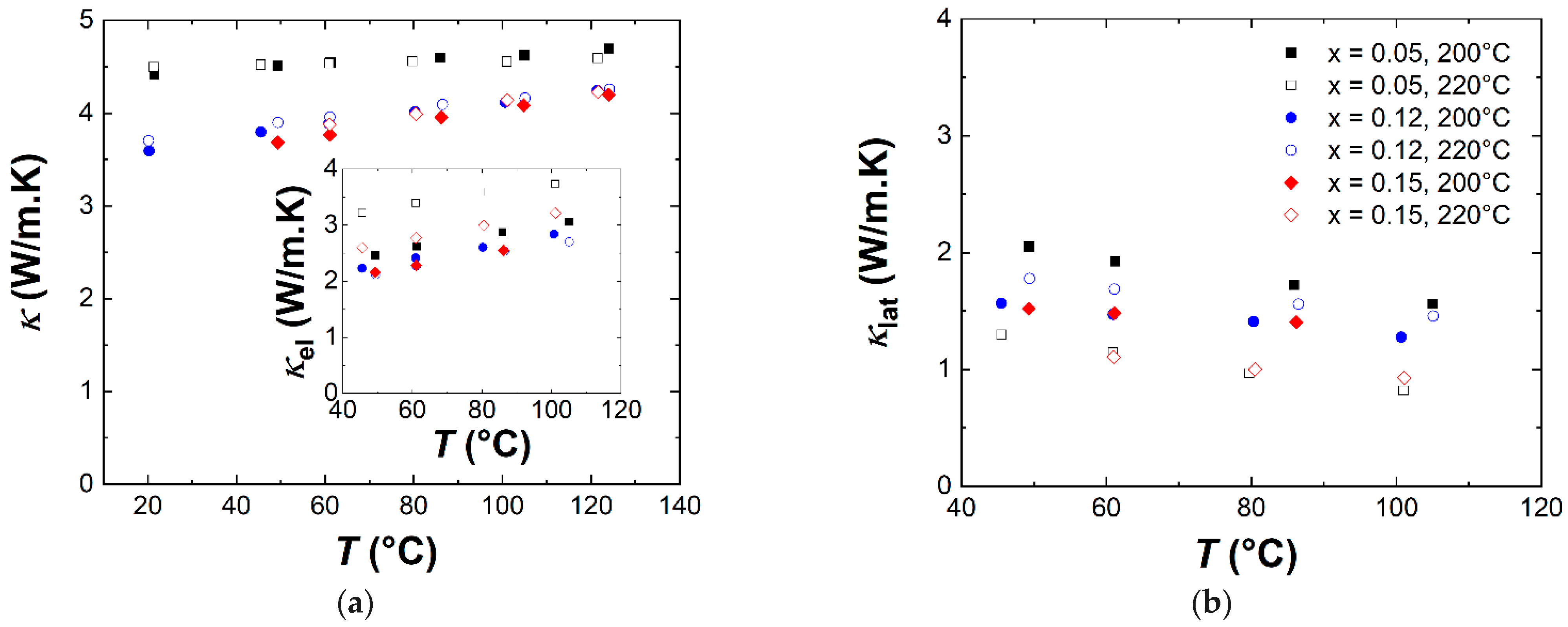
3.3. Transport and Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, J.; Chen, G.; Ren, Z. Thermoelectric cooling materials. Nat. Mater. 2021, 20, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Li, R.; Li, Y.; Bai, S. Strategies to improve the thermoelectric figure of Merit in thermoelectric functional materials. Front. Chem. 2022, 10, 865281. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, B.; Scherrer, H.; Caillat, T. An overview of recent developments of BiSb alloys. Semicond. Semimet. 2001, 69, 101–137. [Google Scholar]
- Jin, H.; Jaworski, C.M.; Heremans, J.P. Enhancement in the figure of merit of p-type Bi100−xSbx alloys through multiple valence-band doping. Appl. Phys. Lett. 2012, 101, 053904. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, M.; Huang, R.; Huang, C.; Song, C.; Zhou, Y.; Li, L. Thermoelectric performance of p-type (Bi85Sb15)1−xSnx materials prepared by a pressureless sintering technique. J. Electron. Mater. 2012, 41, 1725–1729. [Google Scholar] [CrossRef]
- Yim, W.M.; Amith, A. Bi-Sb alloys for magneto-thermoelectric and thermomagnetic cooling. Solid State Electron. 1972, 15, 1141–1165. [Google Scholar] [CrossRef]
- Fu, L.; Kane, C.L. Topological insulator with inversion symmetry. Phys. Rev. B 2007, 76, 045302. [Google Scholar] [CrossRef]
- Teo, J.C.Y.; Fu, L.; Kane, C.L. Surface states and topological invariants in three-dimensional topological insulators: Application to Bi1−xSbx. Phys. Rev. B 2008, 78, 045426. [Google Scholar] [CrossRef]
- Hsieh, D.; Xia, Y.; Wray, L.; Qian, D.; Hor, Y.S.; Cava, R.J.; Hasan, M.Z. A topological Dirac insulator in a quantum spin Hall phase. Nature 2008, 452, 970–975. [Google Scholar] [CrossRef]
- Hsieh, D.; Qian, D.; Wray, L.; Xia, Y.; Pal, A.; Dil, J.H.; Osterwalder, J.; Meier, F.; Bihlmeyer, G.; Kane, C.L.; et al. Observation of unconventional quantum spin textures in topological insulators. Science 2008, 323, 919–922. [Google Scholar] [CrossRef]
- Nakamura, F.; Kousa, Y.; Taskin, A.A.; Takeichi, Y.; Nishide, A.; Kakizaki, A.; D’Angelo, M.; Lefevre, P.; Bertran, F.; Talb-Ibrahimi, A.; et al. Topological transition in Bi1−xSbx studied as function of Sb doping. Phys. Rev. B 2011, 84, 235308. [Google Scholar] [CrossRef]
- Guo, H.; Sugawara, K.; Takayama, A.; Souma, S.; Sato, T.; Satoh, N.; Ohnishi, A.; Kitaura, M.; Sasaki, M.; Xue, Q.-K.; et al. Evolution of surface states in Bi1−xSbx alloys across the topological phase transition. Phys. Rev. B 2011, 83, 201104. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, K.-S.; Wang, J.-F.; Sasaki, M.; Satoh, N.; Ohnishi, A.; Kitaura, M.; Yang, M.; Li, L. Dirac, versus Weyl fermions in topological insulators: Adler-Bell-Jackiw anomaly in transport phenomena. Phys. Rev. Lett. 2013, 111, 246603. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, Y.; Sasaki, M.; Jeong, Y.H.; Weickert, F.; Betts, J.B.; Kim, H.-J.; Kim, K.-S.; Kim, J. Violation of Ohm’s law in a Weyl metal. Nat. Mater. 2015, 16, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Kang, N.H.D.; Yao, K.; Hai, P.N. Epitaxial growth and characterization of Bi1−xSbx spin Hall thin films on GaAs(111)A substrates. Appl. Phys. Lett. 2017, 110, 062401. [Google Scholar] [CrossRef]
- Park, H.; Rho, S.; Kim, J.; Kim, D.; Kang, C.; Cho, M.-H. Topological surface-dominated spintronic THz emission in topologically nontrivial Bi1−xSbx films. Adv. Sci. 2022, 9, 2200948. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Dresselhaus, M.S. Phase diagram of Bi1−xSbx thin films with different growth orientations. Phys. Rev. B 2012, 86, 075436. [Google Scholar] [CrossRef]
- Tang, S.; Dresselhaus, M.S. Electronic properties of nano-structured bismuth-antimony materials. J. Mater. Chem. C 2014, 2, 4710–4726. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef]
- Martin-Lopez, R.; Lenoir, B.; Devaux, X.; Dauscher, A.; Scherrer, H. Mechanical alloying of BiSb semiconducting alloys. Mater. Sci. Eng. A 1998, 248, 147–152. [Google Scholar] [CrossRef]
- Luo, T.; Wang, S.; Li, H.; Tang, X. Low temperature thermoelectric properties of melt spun Bi85Sb15 alloys. Intermetallics 2013, 32, 96–102. [Google Scholar] [CrossRef]
- Gao, S.; Gaskins, J.; Hu, X.; Tomko, K.; Hopkins, P.; Poon, S.J. Enhanced figure of merit in bismuth-antimony fine-grained alloys at cryogenic temperatures. Sci. Rep. 2019, 9, 14892. [Google Scholar] [CrossRef] [PubMed]
- Devaux, X.; Brochin, F.; Dauscher, A.; Lenoir, B.; Martin-Lopez, R.; Scherrer, H.; Scherrer, S. Production of ultrafine powders of Bi-Sb solid solution. Nanostructured Mater. 1997, 8, 137–147. [Google Scholar] [CrossRef]
- Kaspar, K.; Fritsch, K.; Habicht, K.; Willenberg, H.; Hillebrecht, H. Solvent-based synthesis of nano- Bi0.85Sb0.15 for low-temperature thermoelectric applications. J. Electron. Mater. 2017, 46, 92–100. [Google Scholar] [CrossRef]
- Zhou, B.; Zhu, J.-J. A chemical solution route to rapid synthesis of homogenous Bi100−xSbx alloys nanoparticle at room temperature. J. Nanosci. Nanotech. 2007, 7, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Sumithra, S.; Misra, D.K.; Wei, C.; Gabrisch, H.; Poudeu, P.F.P.; Stokes, K.L. Solvothermal synthesis and analysis of Bi1−xSbx nanoparticles. Mater. Sci. Eng. B 2011, 176, 246–251. [Google Scholar] [CrossRef]
- Shi, J.J.; Wang, Y.-J.; Ma, Y.; Shen, Q.-M.; Zhu, J.-J. Sonoelectrochemical synthesis and assembly of bismuth-antimony alloy: From nanocrystals to nanoflakes. Ultrason. Sonochem. 2012, 19, 1039–1043. [Google Scholar] [CrossRef]
- Devaux, X.; Brochin, F.; Martin-Lopez, R.; Scherrer, H. Study of the microstructure influence on the transport properties of Bi86.5Sb13.5 polycrystalline alloy. J. Phys. Chem. Solids 2002, 53, 119–125. [Google Scholar] [CrossRef]
- Miyajima, M.; Takagi, K.; Okamura, H.; Lee, G.G.; Noda, Y.; Watanabe, R. Thermoelectric properties of Bi-Sb composite prepared by mechanical alloying method. In Proceedings of the 15th International Conference on Thermoelectrics, Clemson, PA, USA, 26–29 March 1996; pp. 18–21. [Google Scholar]
- Martin-Lopez, R.; Dauscher, A.; Scherrer, H.; Hejtmanek, J.; Kenzari, H.; Lenoir, B. Thermoelectric properties of mechanically alloyed Bi-Sb alloys. Appl. Phys. A 1999, 68, 597–602. [Google Scholar] [CrossRef]
- Rodriguez, J.E.; Cadavid, D. Seebeck coefficient of Bi-Sb samples grown by mechanical alloying. In Proceedings of the 24th International Conference on Thermoelectrics, Clemson, OA, USA, 18–23 June 2005; pp. 399–401. [Google Scholar]
- Liu, H.J.; Li, L.F.; Shi, D. High-pressure preparation and thermoelectric properties of Bi0.85Sb0.15. J. Electron. Mater. 2006, 35, L7–L10. [Google Scholar] [CrossRef]
- Huang, R.; Song, C.; Cai, F.; Li, L. Thermoelectric performance of ternary Bi-Sb-Ag alloys prepared by mechanical alloying and subsequent pressureless sintering. J. Phys. Chem. Solids 2007, 68, 1400–1404. [Google Scholar]
- Song, C.; Huang, R.; Zhou, M.; Gong, L.; Li, L. Low-temperature thermoelectric properties of Bi85Sb15-xNbx alloys prepared by high-press sintering. J. Phys. Chem. Solids 2010, 71, 999–1003. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Z.; Chu, X.; Li, L. Effect of nano-ZrW2O8 on the thermoelectric properties of Bi85Sb15/ZrW2O8. J. Electron. Mater. 2012, 41, 1263–1266. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Y.M.; Zhou, M.; Song, C.M.; Huang, R.J.; Zhou, Y.; Li, L.F. Improved thermoelectric properties of p-type bismuth antimony-based alloys prepared by spark plasma sintering. J. Electron. Mater. 2014, 43, 1295–1301. [Google Scholar] [CrossRef]
- Dutta, S.; Shubbha, V.; Ramesh, T.G.; D’Sa, F. Thermal and electronic properties of Bi1−xSbx alloys. J. Alloys Compds 2009, 467, 305–309. [Google Scholar] [CrossRef]
- Landschreiber, B.; Günes, E.; Homm, G.; Will, C.; Tomes, P.; Rohner, C.; Sesselmann, A.; Klar, P.J.; Paschen, S.; Müller, E.; et al. Structural and thermoelectric properties of Bi1−xSbx nanoalloys prepared by mechanical alloying. J. Electron. Mater. 2013, 42, 2356–2361. [Google Scholar] [CrossRef]
- Günes, E.; Gundlach, F.; Elm, M.T.; Klar, P.J.; Schlecht, S.; Wickleder, M.S.; Müller, E. Nanostructured composites of Bi1−xSbx nanoparticles and carbon nanotubes and the characterization of their thermoelectric properties. ACS Appl. Mater. Interfaces 2017, 9, 44756–44765. [Google Scholar] [CrossRef]
- Günes, E.; Landschreiber, B.; Homm, G.; Wiegand, C.; Tomes, P.; Will, C.; Elm, M.T.; Paschen, S.; Klar, P.J.; Schlecht, S.; et al. Structure and thermoelectric properties of nanostructured Bi1−xSbx alloys synthesized by mechanical alloying. J. Electron. Mater. 2018, 47, 6007–6015. [Google Scholar] [CrossRef]
- Combe, E.; Funahashi, R.; Takeuchi, T.; Barbier, T.; Yubuta, K. Thermal deformation effects in thermoelectric properties for Bi0.82Sb0.18 alloys. J. Alloys Compd. 2017, 692, 563–568. [Google Scholar] [CrossRef]
- Flores-Conde, A.; Diaz-Torres, E.; Morales-Leal, F.; Tritt, T.M.; Matsumoto, Y.; Ortega-Lopez, M. Spark plasma sintered Bi0.90Sb0.10 and Bi0.86Sb0.14 alloys and their electrical and thermal transport properties. Mater. Sci. Semicond. Process. 2020, 120, 105280. [Google Scholar] [CrossRef]
- El-Asfoury, M.S.; Abdou, S.M.; Nassef, A. Boosting thermoelectric-mechanical properties of BiSb-based material by SiC nacocomposite. JOM 2021, 73, 2808–2818. [Google Scholar] [CrossRef]
- El-Asfoury, M.S.; Abdou, S.M.; Nassef, A. Unraveling the thermoelectric performances of bismuth antimony/graphene composite synthesized by spark plasma extrusion. J. Alloys Compd. 2021, 887, 161399. [Google Scholar] [CrossRef]
- El-Asfoury, M.S.; Nasr, M.N.A.; Nakamura, K.; Abdel-Moneim, A. Thermoelectric power factor performance of Bi85Sb15/graphene composite. Jpn J. Appl. Phys. 2016, 55, 045802. [Google Scholar] [CrossRef]
- El-Asfoury, M.S.; Nasr, M.N.A.; Nakamura, K. Abdel-Moneim, Structural and thermoelectric properties of Bi85Sb15 prepared by non-equal channel angular extrusion. J. Electron. Mater. 2018, 47, 242–250. [Google Scholar] [CrossRef]
- Will, C.H.; Elm, M.T.; Klar, P.J.; Landschreiber, B.; Günes, E.; Schlecht, S. Effect of nanostructuring on the band structure and the galvanomagnetic properties of Bi1−xSbx alloys. J. Appl. Phys. 2013, 114, 193707. [Google Scholar] [CrossRef]
- Lee, Y.H.; Koyanagi, T. Thermoelectric properties of n Bi-Sb sintered alloys prepared by spark plasma sintering method. In Proceedings of the 20th International Conference on Thermoelectrics, Beijing, China, 8–11 June 2001; pp. 278–281. [Google Scholar]
- Rodriguez-Carvajal, J. FULLPROF: A program for Rietveld refinement and pattern matching analysis. In Proceedings of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 19–28 July 1990; p. 127. [Google Scholar]
- Rogocheva, E.I.; Doshenko, A.N.; Nashchekina, O.N. Temperature and concentration dependences of specific heat of Bi1−xSbx solid solutions. Funct. Mater. 2018, 25, 720–728. [Google Scholar] [CrossRef]
- Dismukes, J.P.; Paff, R.J.; Smith, R.T.; Ulmer, R. Lattice parameter and density in bismuth-antimony alloys. J. Chem. Eng. Data 1968, 13, 317–320. [Google Scholar] [CrossRef]
- Malik, K.; Das, D.; Mondal, D.; Chattopadhyay, D.; Deb, A.K.; Bandyopadhyay, S.; Banerjee, A. Sb concentration dependent structural and resistive properties of polycrystalline Bi-Sb alloys. J. Appl. Phys. 2012, 112, 083706. [Google Scholar] [CrossRef]
- Zhao, Y.; Clément, S.; Haines, J.; Viennois, R. Lattice dynamics study of elemental bismuth under high pressure. J. Phys. Chem. C 2020, 124, 26659–26669. [Google Scholar] [CrossRef]
- Zitter, R.N.; Watson, P.C. Raman and x-ray spectra of single-crystals Bi-Sb alloys. Phys. Rev. B 1974, 10, 607–611. [Google Scholar] [CrossRef]
- Lannin, J.S. First-order Raman scattering in Bi1−xSbx alloys. Phys. Rev. B 1979, 19, 2390–2393. [Google Scholar] [CrossRef]
- Olijnyk, H.; Nakano, S.; Takemura, K. First- and second order Raman scattering in Sb and Bi at high pressure. Phys. Status Solidi (B) 2007, 244, 3572–3582. [Google Scholar] [CrossRef]
- Sultana, R.; Gurjar, G.; Gathori, B.; Patnaik, S.; Awana, V.P.S. Flux free crystal growth and detailed physical property characterization of Bi1−xSbx (x = 0.05, 0.1 and 0.15) topological insulator. Mater. Res. Express 2019, 6, 106102. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, Y.; Zhao, Y.; Chen, J.; Chang, B.; Huang, H.; Xu, T.; Sun, L.; Chen, Y.; Sha, J.; et al. Large-area 2D bismuth antimonide with enhanced thermoelectric properties via multiscale electron-phonon decoupling. Mater. Horiz. 2023, 10, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Awana, V.P.S. Surface states induced weak anti-localization effect in Bi0.85Sb0.15 topological single crystal. Mater. Res. Express 2022, 9, 056301. [Google Scholar] [CrossRef]
- Lee, W.J.; Salawu, Y.A.; Kim, H.-J.; Jang, C.W.; Kim, S.; Ratcliff, T.; Elliman, R.G.; Yue, Z.; Wang, X.; Lee, S.-E.; et al. Possible permanent Dirac- to Weyl-semimetal phase transition by ion implantation. NPG Asia Mater. 2022, 14, 31. [Google Scholar] [CrossRef]
- Höhne, J.; Wenning, U.; Schulz, H.; Hüfner, S. Temperature dependence of the k = 0 optical phonons of Bi and Sb. Z. Physik B 1977, 27, 297–302. [Google Scholar] [CrossRef]
- Lu, D.; Luo, S.; Liu, S.; Yao, H.; Ren, X.; Zhou, W.; Tang, D.; Qi, X.; Zhong, J. Anomalous temperature-dependent Raman scattering of vapour deposited two-dimensional Bi thin films. J. Phys. Chem. C 2018, 122, 24459–24466. [Google Scholar] [CrossRef]
- Lenoir, B.; Cassart, M.; Michenaud, J.-P.; Scherrer, H.; Scherrer, S. Transport of Bi-rich Bi-Sb alloys. J. Phys. Chem. Solids 1996, 57, 89–99. [Google Scholar] [CrossRef]
- Teramoto, T.; Komine, T.; Yamamoto, S.; Kuraishi, M.; Sugita, R.; Hasegawa, Y.; Nakamura, H. Influence of the band structure of BiSb alloy on the magneto-Seebeck coefficient. J. Appl. Phys. 2008, 104, 053714. [Google Scholar] [CrossRef]
- Cho, S.; DiVenere, A.; Wong, K.G.; Ketterson, J.B.; Meyer, J.R. Transport properties of Bi1−xSbx alloy thin film grown on CdTe (111)B. Phys. Rev. B 1999, 59, 10691–10696. [Google Scholar] [CrossRef]
- Morelli, D.T.; Partin, D.L.; Heremans, J. Galvanomagnetic properties of single-crystal bismuth-antimony thin films. Semicond. Sci. Technol. 1990, 5, S257–S259. [Google Scholar] [CrossRef]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]





| Nominal x | Sintering T | x from EDXS | RH (C/cm−3) | n (1018 cm−3) | µ (V/m.s) |
|---|---|---|---|---|---|
| 0.05 | 200 °C | 0.050(5) | −0.802 | 7.8 | 3024 |
| 0.05 | 220 °C | 0.054(4) | −0.797 | 7.84 | 3796 |
| 0.12 | 200 °C | 0.106(3) | −0.761 | 8.21 | 2709 |
| 0.12 | 220 °C | 0.109(5) | −0.916 | 6.82 | 3035 |
| 0.15 | 200 °C | 0.142(3) | −0.703 | 8.89 | 2316 |
| 0.15 | 220 °C | 0.144(2) | −0.964 | 6.48 | 3827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viennois, R.; Alvarez, L.; Coulomb, L.; El Mastour, Y.; Fabbiani, M.; Villeroy, B.; Bérardan, D.; Moll, A.; Beaudhuin, M. Lattice Dynamics, Transport and Thermoelectric Properties of Bi-Sb Alloys Obtained by Mechanical Alloying and Spark Plasma Sintering. Metals 2024, 14, 40. https://doi.org/10.3390/met14010040
Viennois R, Alvarez L, Coulomb L, El Mastour Y, Fabbiani M, Villeroy B, Bérardan D, Moll A, Beaudhuin M. Lattice Dynamics, Transport and Thermoelectric Properties of Bi-Sb Alloys Obtained by Mechanical Alloying and Spark Plasma Sintering. Metals. 2024; 14(1):40. https://doi.org/10.3390/met14010040
Chicago/Turabian StyleViennois, Romain, Laurent Alvarez, Loïc Coulomb, Yassine El Mastour, Marco Fabbiani, Benjamin Villeroy, David Bérardan, Adrien Moll, and Mickaël Beaudhuin. 2024. "Lattice Dynamics, Transport and Thermoelectric Properties of Bi-Sb Alloys Obtained by Mechanical Alloying and Spark Plasma Sintering" Metals 14, no. 1: 40. https://doi.org/10.3390/met14010040





