Effect of Dissolved Oxygen Content on Tribo-Corrosion Behavior of Monel 400 Alloy in Seawater
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Machine
2.2. Test Process
2.3. The Calculation of Each Component in the Process of Tribo-Corrosion
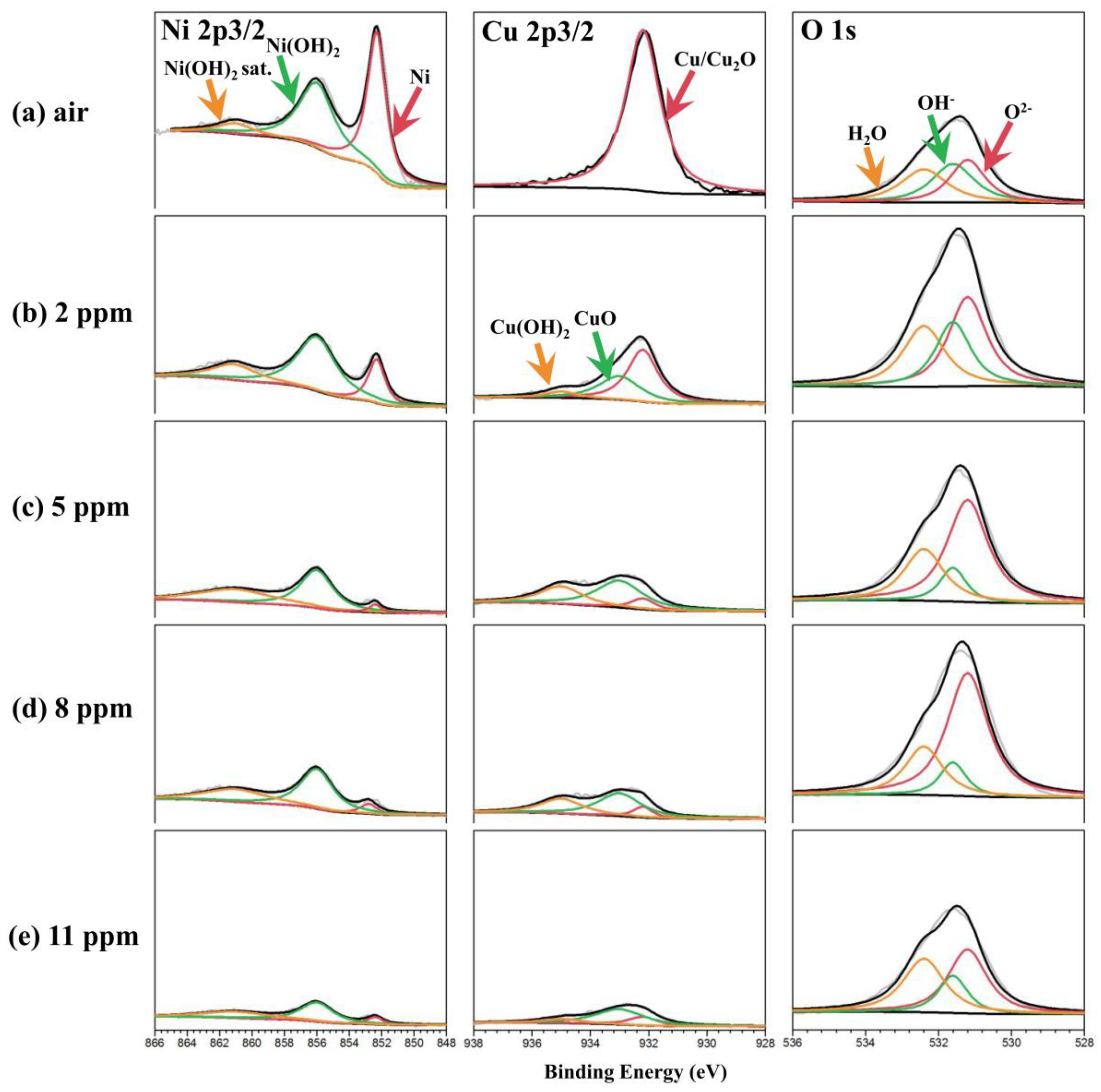
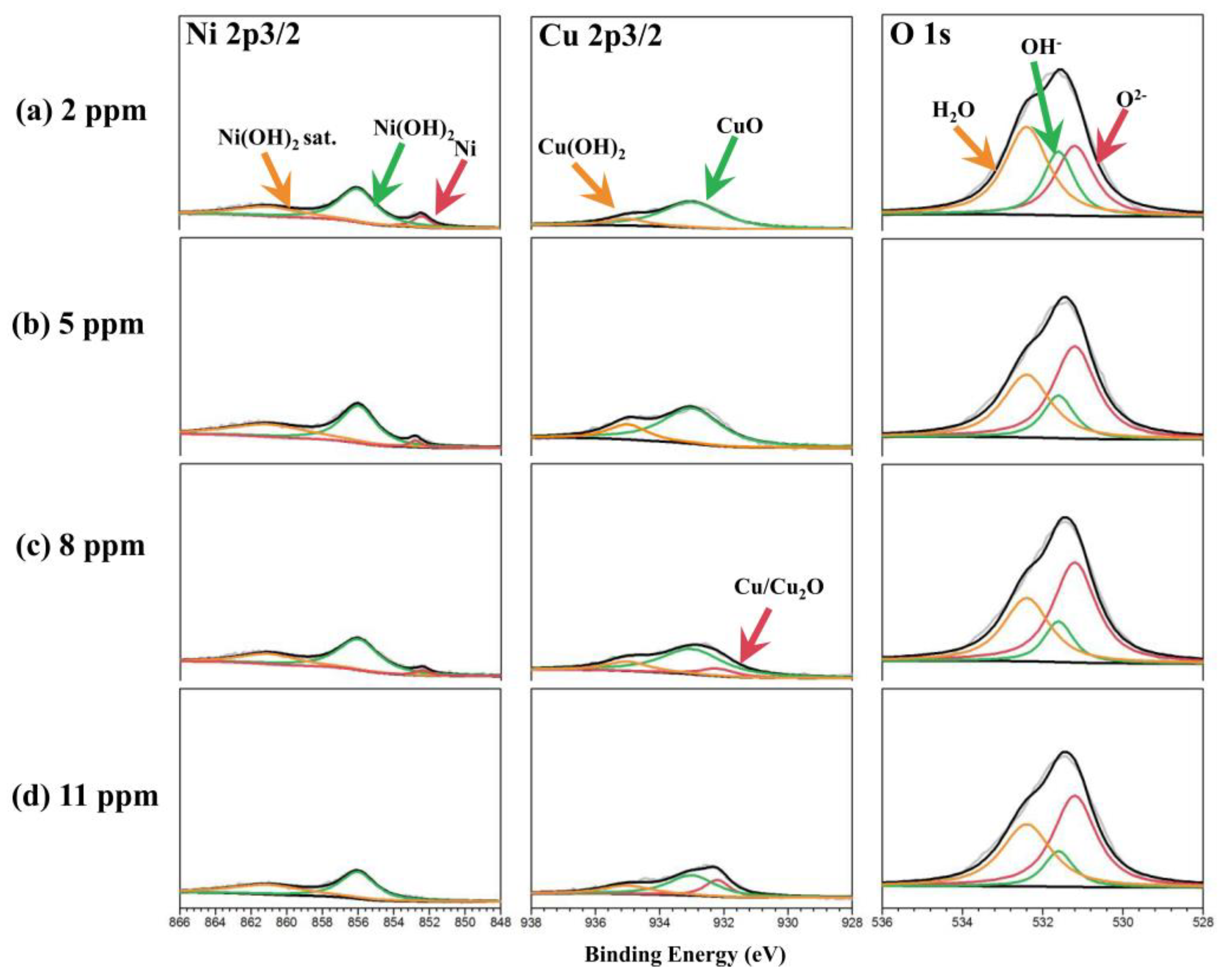
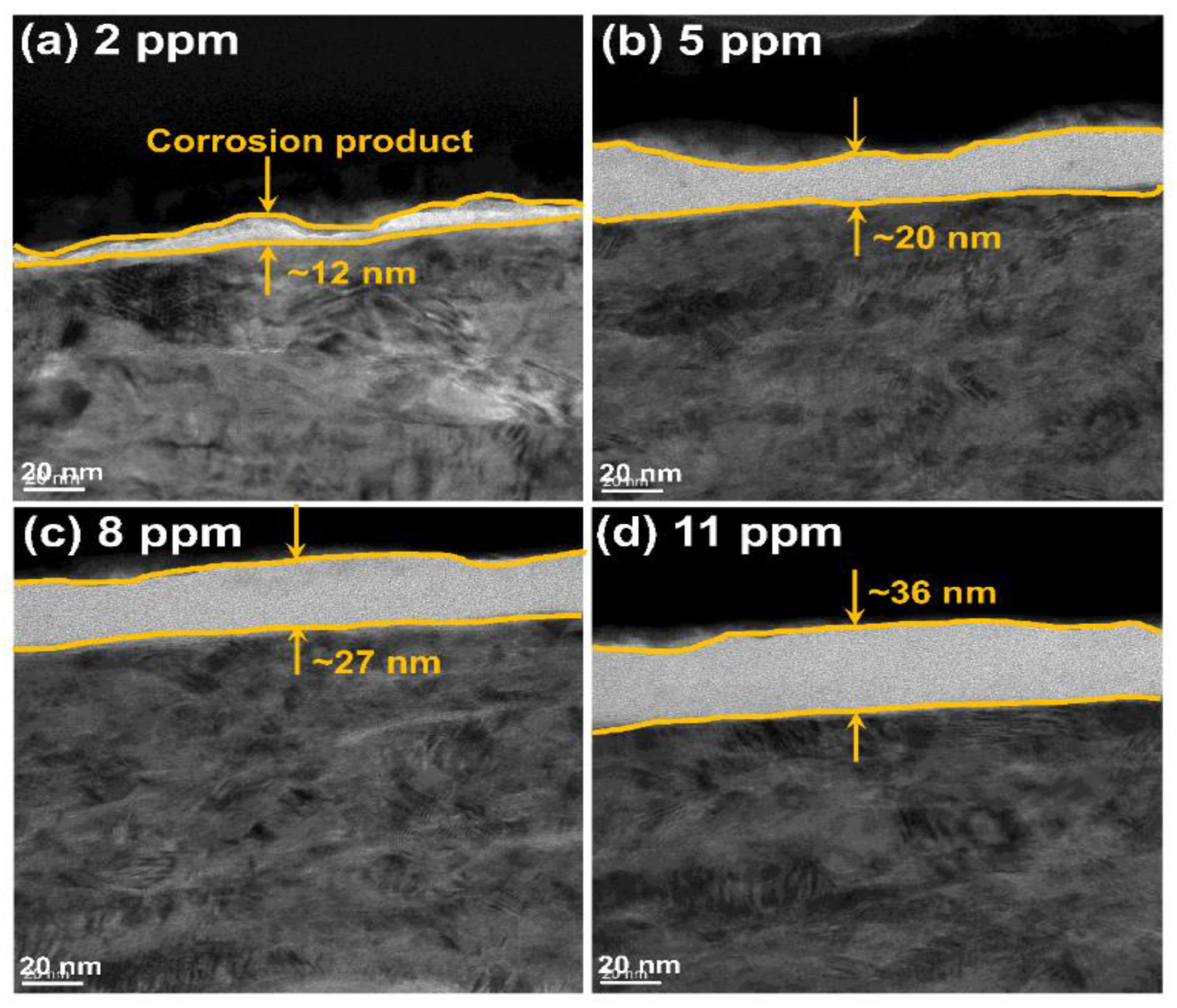
2.4. Characterization
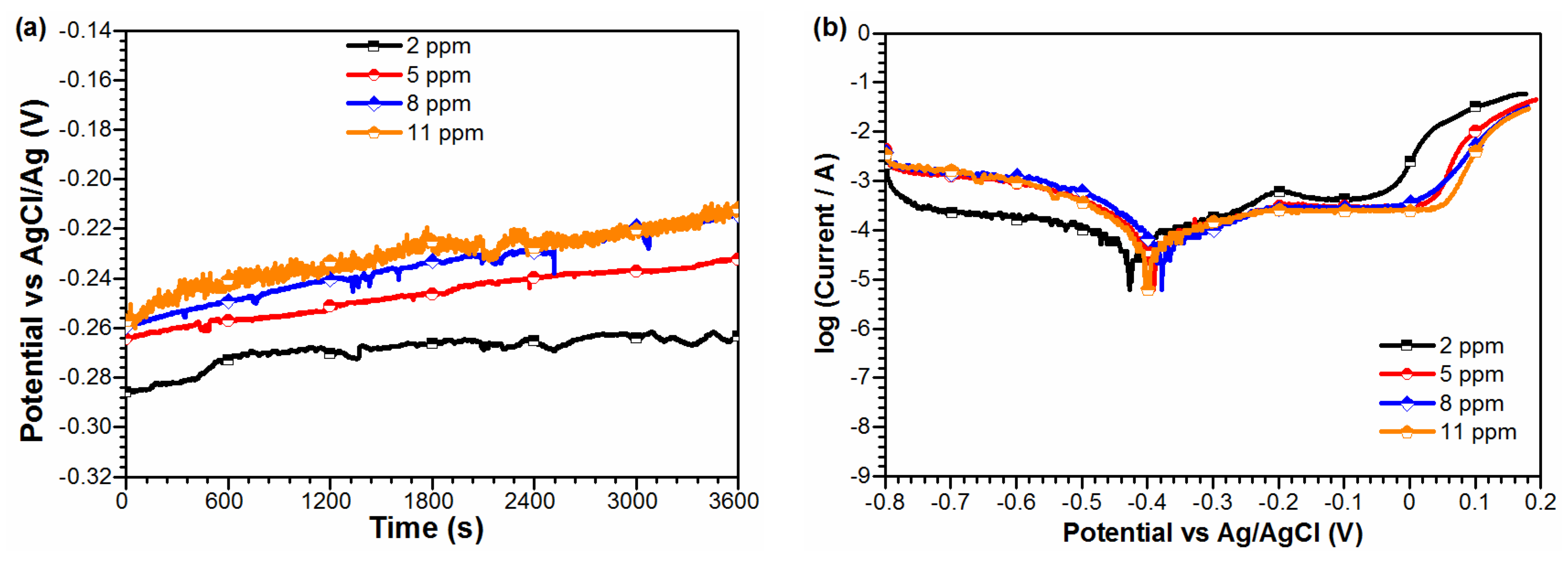
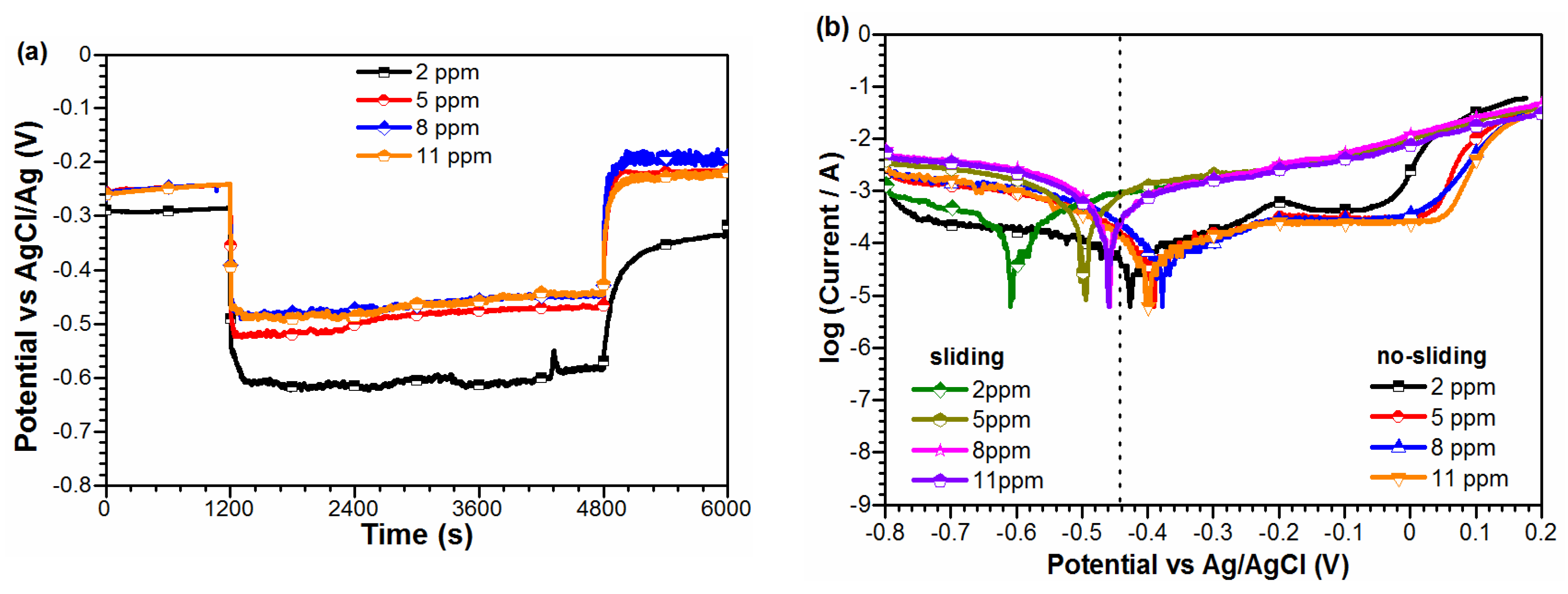
3. Result
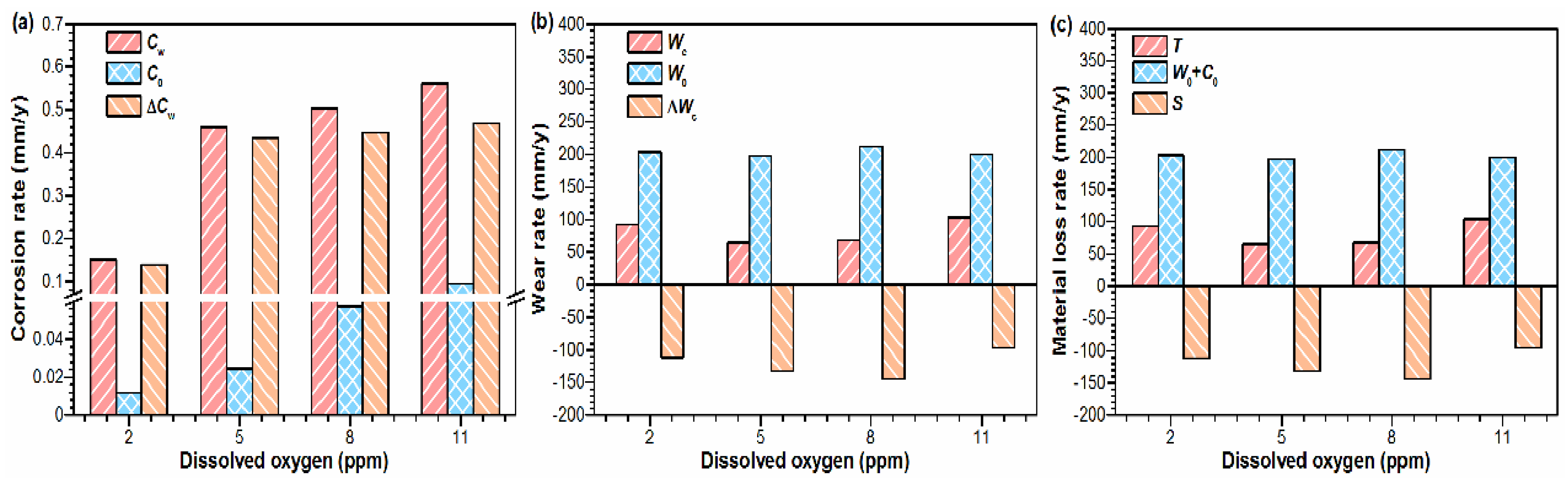
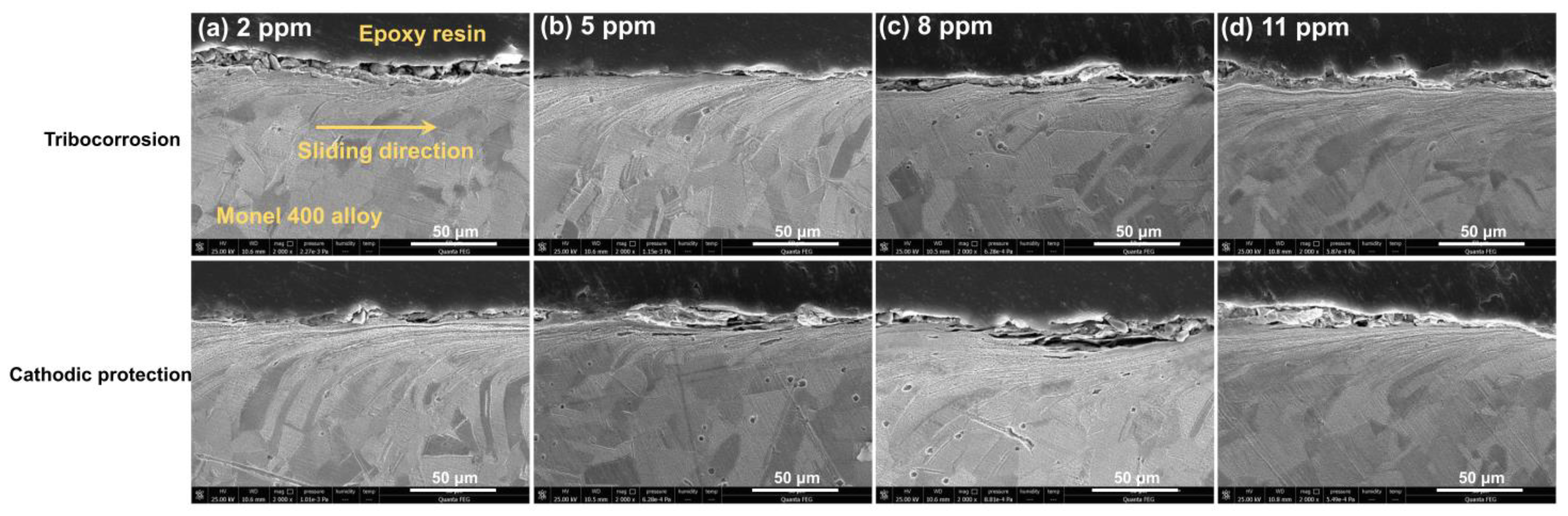
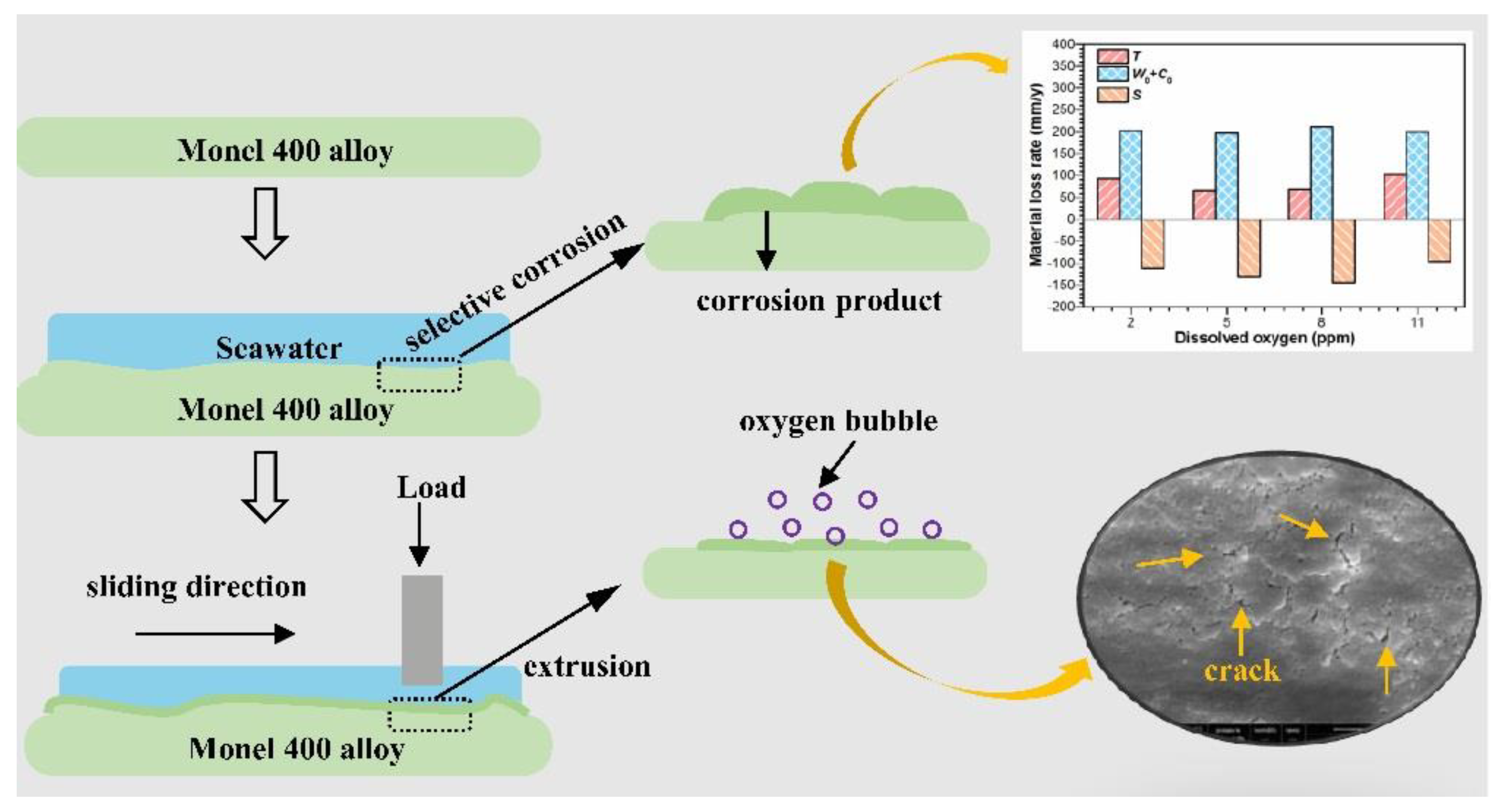
4. Discussion
5. Conclusions
- (1)
- Under the same DO content, the material loss rates (T) after tribo-corrosion tests are significantly lower than those after mechanical wear tests, which is attributed to the corrosion products with excellent lubrication properties generated on the damaged surface of the alloy under tribo-corrosion conditions.
- (2)
- Under tribo-corrosion conditions, with the increase in DO content, although the shrouded area and thickness of the corrosion products on the surface of Monel 400 alloy increases, the wear rate of the alloy decreases first and then increases because the oxygen bubbles generated under DO content of 11 ppm (supersaturated DO) attack the corrosion product layer on the damaged surface of the alloy, causing the corrosion product layer to break and eventually fall from the damaged surface, resulting in material loss.
- (3)
- Because DO controls the corrosion kinetics of the alloy, it indirectly affects the wear of the alloy under tribo-corrosion conditions. If the corrosion is inhibited (cathodic protection test), the DO content has little effect on the wear of the alloy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, M.; Li, Y.; Li, Y.; Pang, S.; Zhang, J. A fast way of single-beacon localization for AUVs. Appl. Ocean Res. 2022, 119, 103037. [Google Scholar] [CrossRef]
- Vedachalam, N.; Ravindran, M.; Atmanand, M.A. Technology developments for the strategic Indian blue economy. Mar. Georesour. Geotechnol. 2019, 37, 828–844. [Google Scholar] [CrossRef]
- Jouffray, J.-B.; Blasiak, R.; Norstrom, A.V.; Osterblom, H.; Nystrom, M. The Blue Acceleration: The Trajectory of Human Expansion into the Ocean. One Earth 2020, 2, 43–54. [Google Scholar] [CrossRef]
- Zinke, J.; Nilsson, E.D.; Zieger, P.; Salter, M.E. The Effect of Seawater Salinity and Seawater Temperature on Sea Salt Aerosol Production. J. Geophys. Res. Atmos. 2022, 127, e2021JD036005. [Google Scholar] [CrossRef]
- Toloei, A.; Atashin, S.; Pakshir, M. Corrosion rate of carbon steel under synergistic effect of seawater parameters including pH, temperature, and salinity in turbulent condition. Corros. Rev. 2013, 31, 135–144. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Y.; Zhao, Y.; Zhang, W.; Yao, J.; Wei, M.; Zhou, K.; Ma, X. Quantitative Understanding of the Environmental Effect on B10 Copper Alloy Corrosion in Seawater. Metals 2021, 11, 1080. [Google Scholar] [CrossRef]
- Wang, J.; Wen, W.; Cheng, J.; Dai, L.; Li, S.; Zhang, X.; Yang, Y.; Li, H.; Hou, X.; Wu, B.; et al. Tribocorrosion behavior of high-entropy alloys FeCrNiCoM (M = Al, Mo) in artificial seawater. Corros. Sci. 2023, 218, 111165. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Chen, B.; Yan, F. Tribocorrosion Behaviors of Inconel 625 Alloy Sliding against 316 Steel in Seawater. Tribol. Trans. 2011, 54, 514–522. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Wang, Y.; Huang, Z.; Zhou, Q.; Wang, H. Tribocorrosion Behavior of CoCrNi Medium Entropy Alloy in Simulated Seawater. Metals 2022, 12, 401. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Zeng, Z.; Gao, Y. Tribocorrosion behaviour of F690 and 316L steel in artificial seawater. Lubr. Sci. 2018, 30, 365–375. [Google Scholar] [CrossRef]
- Ma, F.; Gao, Y.; Zeng, Z.; Liu, E. Improving the Tribocorrosion Resistance of Monel 400 Alloy by Aluminizing Surface Modification. J. Mater. Eng. Perform. 2018, 27, 3439–3448. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yan, F. Load-dependent tribocorrosion behaviour of nickel-aluminium bronze in artificial seawater. Corros. Sci. 2018, 131, 252–263. [Google Scholar] [CrossRef]
- Ren, P.; Meng, H.; Xia, Q.; Zhu, Z.; He, M. Influence of seawater depth and electrode potential on the tribocorrosion of Ti6Al4V alloy under the simulated deep-sea environment by in-situ electrochemical technique. Corros. Sci. 2021, 180, 109185. [Google Scholar] [CrossRef]
- Pejakovic, V.; Totolin, V.; Ripoll, M.R. Tribocorrosion behaviour of Ti6A14V in artificial seawater at low contact pressures. Tribol. Int. 2018, 119, 55–65. [Google Scholar] [CrossRef]
- Han, G.; Jiang, P.; Wang, J.; Yan, F. Effects of NaCl concentration on wear-corrosion behavior of SAF 2507 super duplex stainless steel. Rsc Adv. 2016, 6, 111261–111268. [Google Scholar] [CrossRef]
- Paul, S. Estimation of Corrosion Rate of Mild Steel in Sea Water and Application of Genetic Algorithms to Find Minimum Corrosion Rate. Can. Metall. Q. 2010, 49, 99–106. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, P.; Liu, S.; Fan, L.; Guo, W.; Hou, J. Study on the classification of seawater corrosivity of typical sea areas in China. Corros. Rev. 2020, 38, 323–330. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tsai, A.P.; Sumita, M.; Hanawa, T. Effects of surface finishing and dissolved oxygen on the polarization behavior of Zr65Al7.5Ni10Cu17.5 amorphous alloy in phosphate buffered solution. Corros. Sci. 2000, 42, 2167–2185. [Google Scholar] [CrossRef]
- Su, H.; Liang, Y.; Wang, Y.; Wang, B.; Tong, H.; Yuan, Y.; Wei, S. Effect of Dissolved Oxygen on Pitting Corrosion Behavior of Low-Alloy Steel under Hydrostatic Pressure. Int. J. Electrochem. Sci. 2019, 14, 4812–4827. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, L.; Cui, Z.; Sun, M. Effect of temperature and dissolved oxygen on the passivation behavior of Ti-6Al-3Nb-2Zr-1Mo alloy in artificial seawater. J. Mater. Res. Technol. 2022, 17, 374–391. [Google Scholar] [CrossRef]
- Rezakhani, D. The effects of temperature, dissolved oxygen and the velocity of seawater, on the corrosion behavior of condenser alloys. Anti-Corros. Methods Mater. 2011, 58, 90–94. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, B.; Zhang, Z.; Xiao, K.; Wu, J.; Yao, Q.; Ma, G.; Sun, G. Passivation behavior of 316L stainless steel in artificial seawater: Effects of pH and dissolved oxygen. Anti-Corros. Methods Mater. 2021, 68, 122–129. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, S.; Dou, Y.; Han, S.; Wang, L.; Man, C.; Wang, X.; Chen, S.; Cheng, Y.F.; Li, X. Passivation behavior and surface chemistry of 2507 super duplex stainless steel in artificial seawater: Influence of dissolved oxygen and pH. Corros. Sci. 2019, 150, 218–234. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, Y.; Yang, J.; Ren, M.; Liu, X. Experimental Analysis of the Effect of Wear Factors on Guide Vane of Hydraulic Turbine. Machines 2022, 10, 264. [Google Scholar] [CrossRef]
- Xin, L.; Han, Y.; Ling, L.; Lu, Y.; Shoji, T. Effects of dissolved oxygen on partial slip fretting corrosion of Alloy 690TT in high temperature pure water. J. Nucl. Mater. 2020, 542, 152483. [Google Scholar] [CrossRef]
- Goto, H. Influence of mechanical and chemical factors on transition between severe and mild wear in saline solution. J. Tribol. Trans. Asme 1997, 119, 619–625. [Google Scholar] [CrossRef]
- ASTM D1141-98; Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2021.
- Zhu, Y.; Wang, J.; Liu, H.; Ren, P.; Yan, F. The tribocorrosion behavior of Monel 400 alloy in seawater at different temperatures. Tribol. Int. 2023, 189, 108975. [Google Scholar] [CrossRef]
- ASTM G119-09; Standard Guide for Determining Synergism Between Wear and Corrosion. ASTM International: West Conshohocken, PA, USA, 2009.
- Pham, T.T.; Nguyen, T.D.; Nguyen, A.S.; Nguyen, T.T.; Gonon, M.; Belfiore, A.; Paint, Y.; To, T.X.H.; Olivier, M.-G. Role of Al and Mg alloying elements on corrosion behavior of zinc alloy-coated steel substrates in 0.1 M NaCl solution. Mater. Corros.-Werkst. Und Korros. 2023, 74, 903–919. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Udoh, K. Effects of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure titanium, and titanium alloys. Dent. Mater. J. 2002, 21, 83–92. [Google Scholar] [CrossRef]
- Zheng, J. Seawater splitting for high-efficiency hydrogen evolution by alloyed PtNix electrocatalysts. Appl. Surf. Sci. 2017, 413, 360–365. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O. Surface characterization and corrosion behavior of 70/30 Cu-Ni alloy in pristine and sulfide-containing simulated seawater. Corros. Sci. 2007, 49, 1276–1304. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. The role of the Auger parameter in XPS studies of nickel metal, halides and oxides. Phys. Chem. Chem. Phys. 2012, 14, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-H.; Kim, S.H.; Chu, J.H.; Kim, S.Y.; Kim, J.H.; Kwon, S.-Y. Enhancement of seawater corrosion resistance in copper using acetone-derived graphene coating. Nanoscale 2014, 6, 4379–4386. [Google Scholar] [CrossRef]
- Rao, B.V.A.; Kumar, K.C.; Hebalkar, N.Y. X-ray photoelectron spectroscopy depth-profiling analysis of surface films formed on Cu-Ni (90/10) alloy in seawater in the absence and presence of 1,2,3-benzotriazole. Thin Solid Film 2014, 556, 337–344. [Google Scholar]
- Ma, A.L.; Jiang, S.L.; Zheng, Y.G.; Ke, W. Corrosion product film formed on the 90/10 copper-nickel tube in natural seawater: Composition/structure and formation mechanism. Corros. Sci. 2015, 91, 245–261. [Google Scholar] [CrossRef]
- Zhang, J.X.; Liang, B.N.; Guo, X.R. Tribocorrosion Behaviors of NiTi/AlNi2Ti Intermetallic Alloy in NaCl Solution. Russ. J. Phys. Chem. A 2022, 96, 2894–2899. [Google Scholar] [CrossRef]
- Mantyranta, A.; Heino, V.; Isotandon, E.; Salminen, T.; Huttunen-Saarivirta, E. Tribocorrosion behaviour of two low-alloy steel grades in simulated waste solution. Tribol. Int. 2019, 138, 250–262. [Google Scholar] [CrossRef]
- Mraied, H.; Cai, W. The effects of Mn concentration on the tribocorrosion resistance of Al-Mn alloys. Wear 2017, 380–381, 191–202. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Liu, H.; Yan, F. The Tribo-Corrosion Behavior of Monel 400 Alloy in Marine Environment at Varied Rotational Velocities. Metals 2022, 12, 1503. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Wang, J.; Yan, F. Antagonistic effect of electrochemical corrosion on the mechanical wear of Monel 400 alloy in seawater. Corros. Sci. 2022, 198, 110120. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Zhang, D.; Wang, J.; Yan, F. Effect of polarization potentials on tribocorrosion behavior of Monel 400 alloy in seawater environment. Tribol. Int. 2022, 168, 107445. [Google Scholar] [CrossRef]



| Components | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | SrCl2 | H3BO3 | NaF |
| Contents (g/L) | 24.53 | 5.20 | 4.09 | 1.16 | 0.695 | 0.201 | 0.101 | 0.025 | 0.027 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, J.; Liu, H.; Ren, P.; Yan, F. Effect of Dissolved Oxygen Content on Tribo-Corrosion Behavior of Monel 400 Alloy in Seawater. Metals 2024, 14, 6. https://doi.org/10.3390/met14010006
Zhu Y, Wang J, Liu H, Ren P, Yan F. Effect of Dissolved Oxygen Content on Tribo-Corrosion Behavior of Monel 400 Alloy in Seawater. Metals. 2024; 14(1):6. https://doi.org/10.3390/met14010006
Chicago/Turabian StyleZhu, Yuhua, Jianzhang Wang, Hao Liu, Pengwei Ren, and Fengyuan Yan. 2024. "Effect of Dissolved Oxygen Content on Tribo-Corrosion Behavior of Monel 400 Alloy in Seawater" Metals 14, no. 1: 6. https://doi.org/10.3390/met14010006




