BMPD-Assisted Enhancement of Corrosion Resistance of Carbon Steel: Experimental and First-Principle DFTB Insights
Abstract
:1. Introduction
2. Experimental
2.1. Corrosive Electrolyte and Materials
2.2. Weight Loss Method
2.3. Electrochemical Measurements
2.4. Surface Morphology
2.5. Density Functional Theory Calculations
3. Results and Discussion
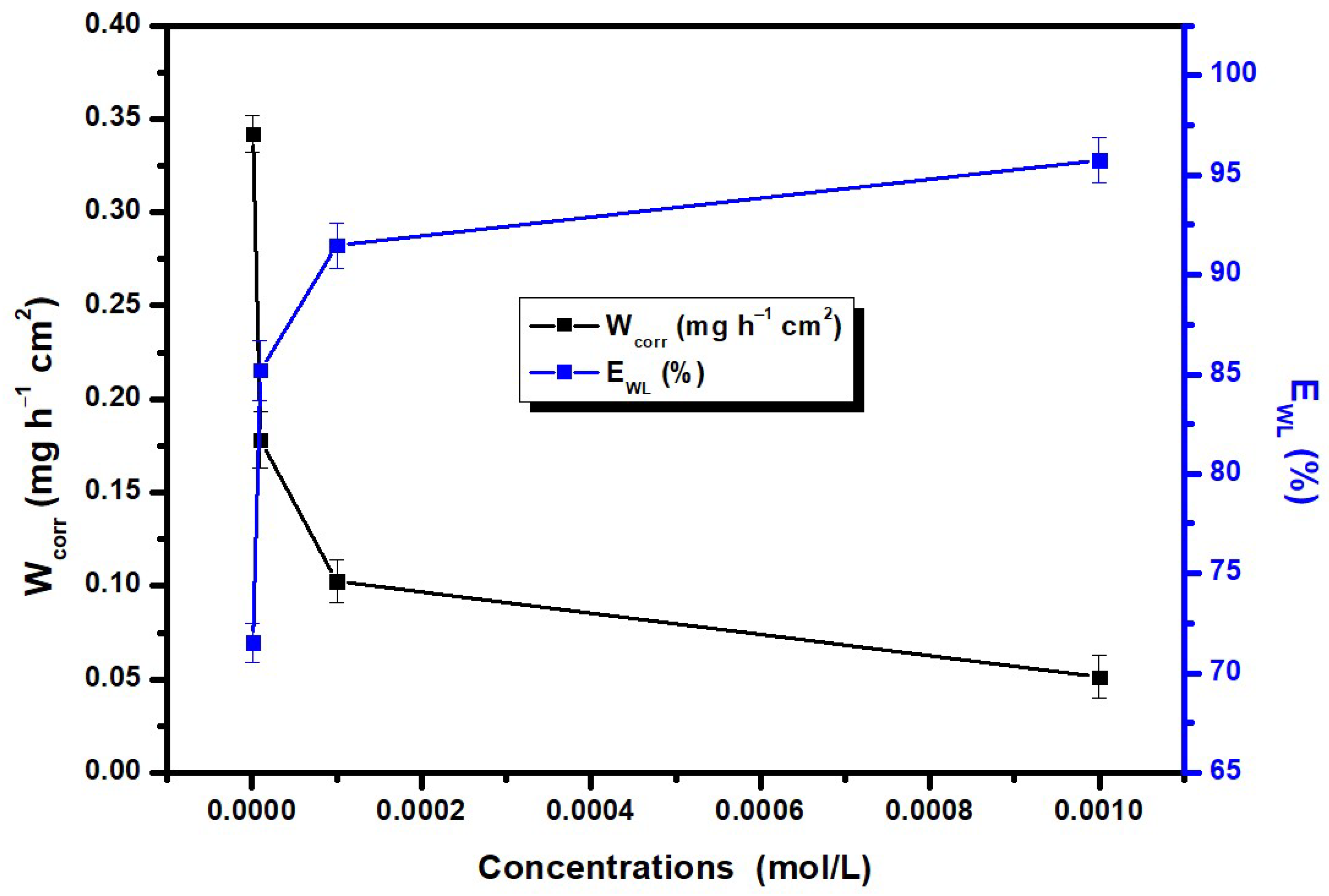
3.1. Gravimetric Experiments
3.2. Electrochemical Behavior by BMPD Inhibitor
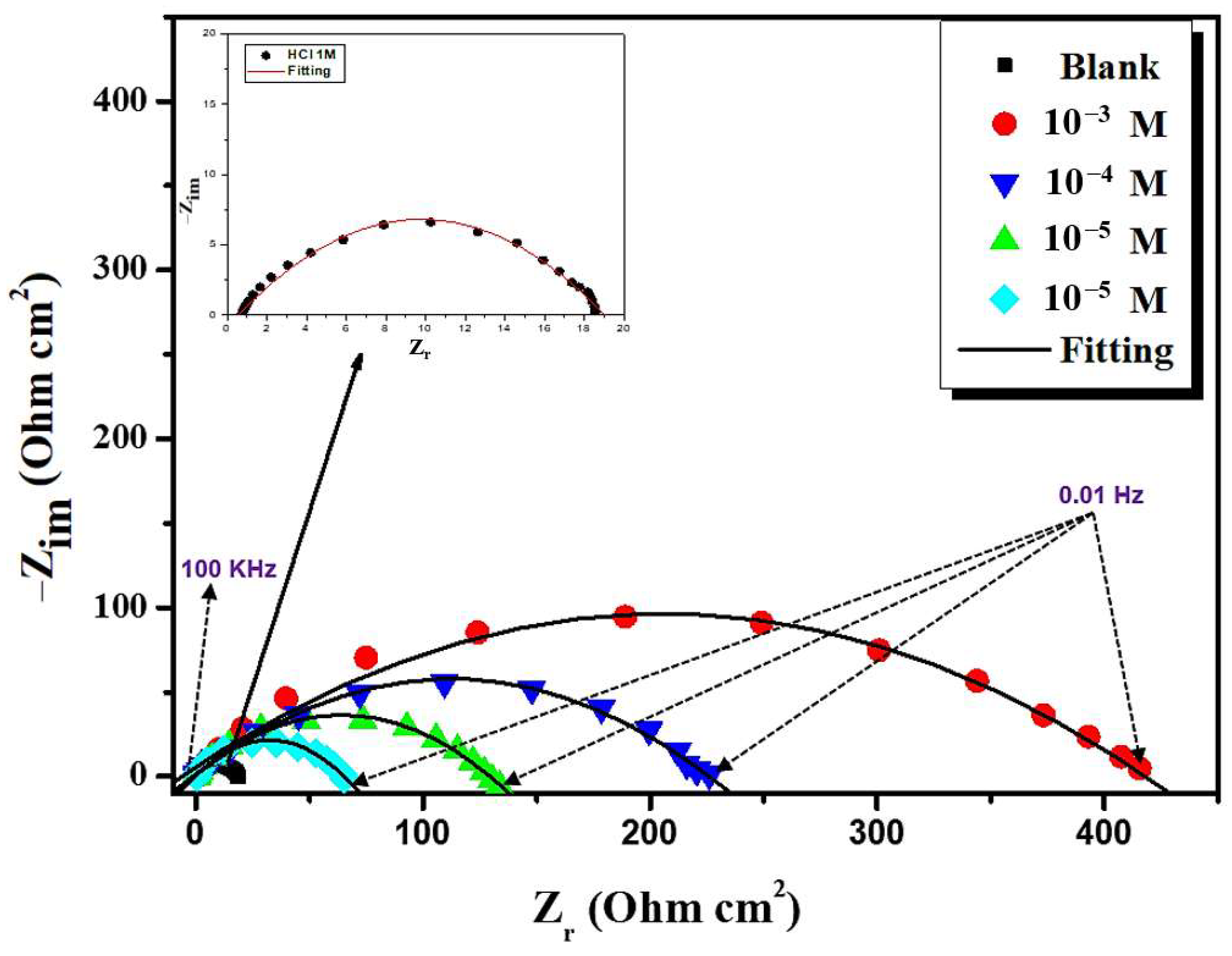
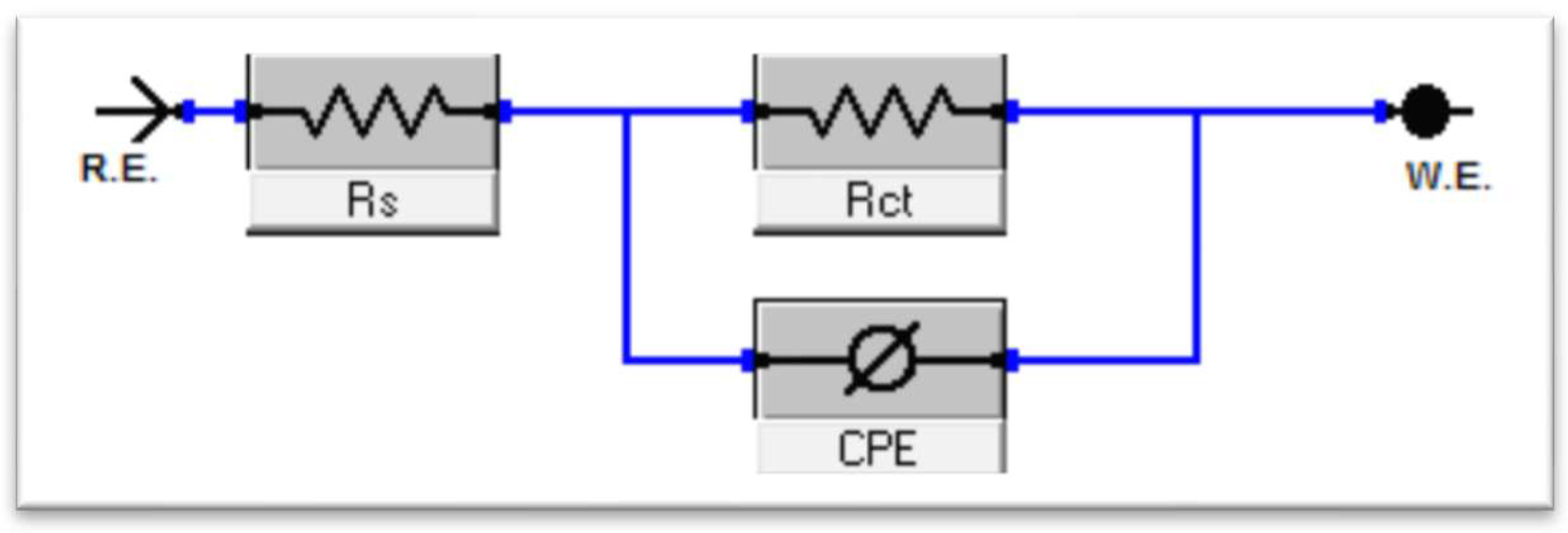
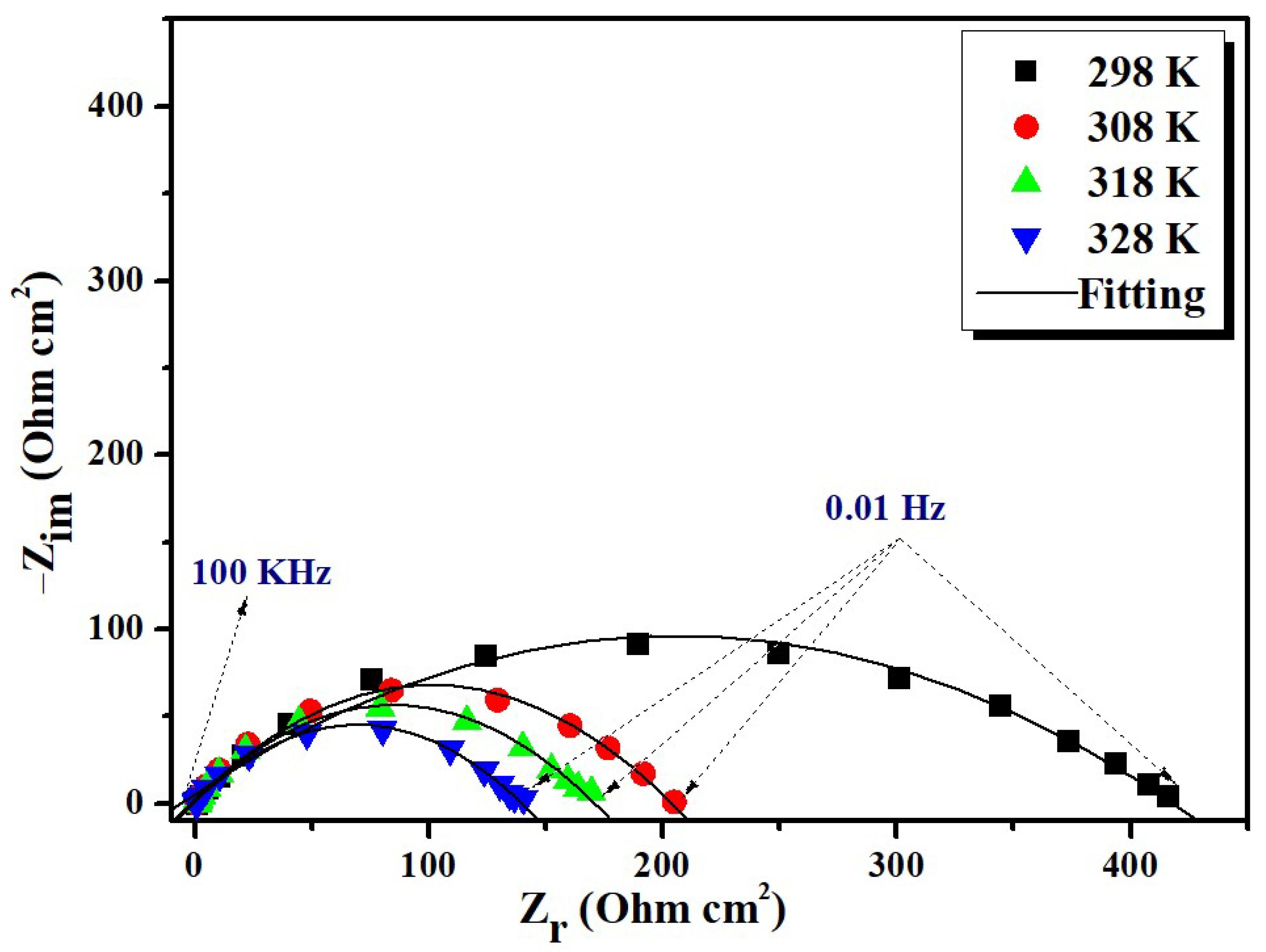
3.2.1. EIS Behavior
3.2.2. Polarization Behavior
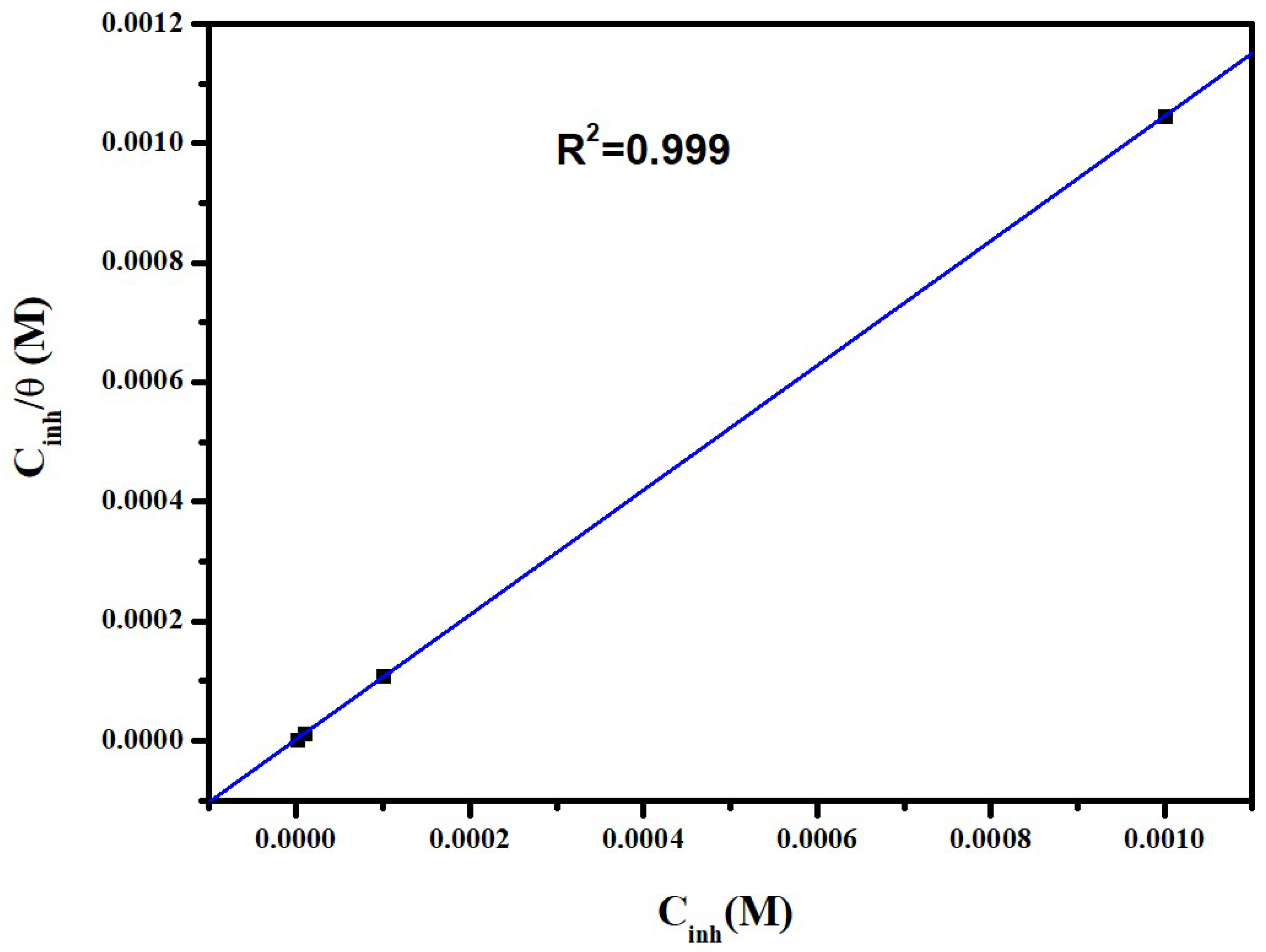
3.3. Adsorption Isotherm
3.4. Influence of the Temperature on Inhibition Performances
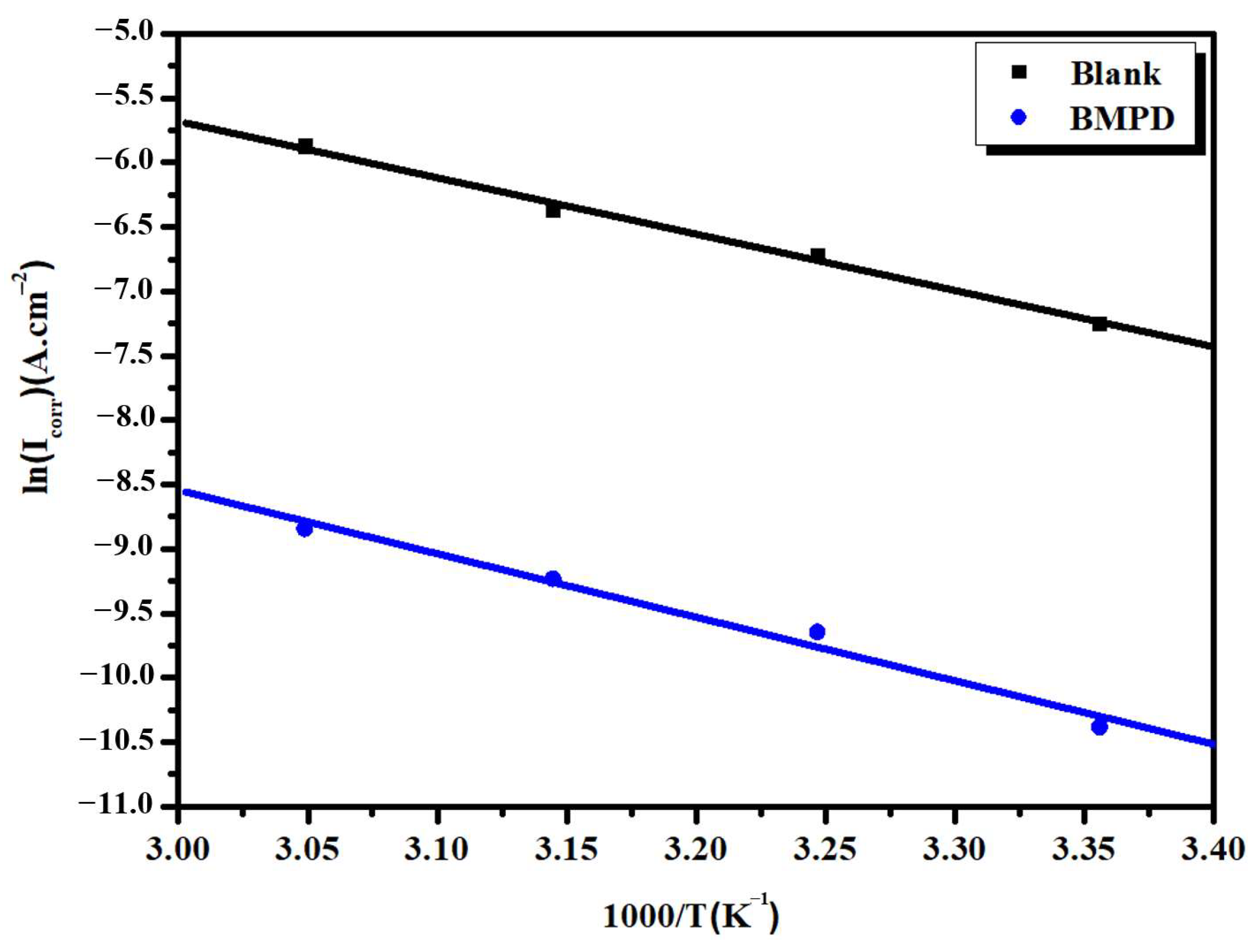
3.5. Kinetic-Thermodynamic Corrosion Parameters
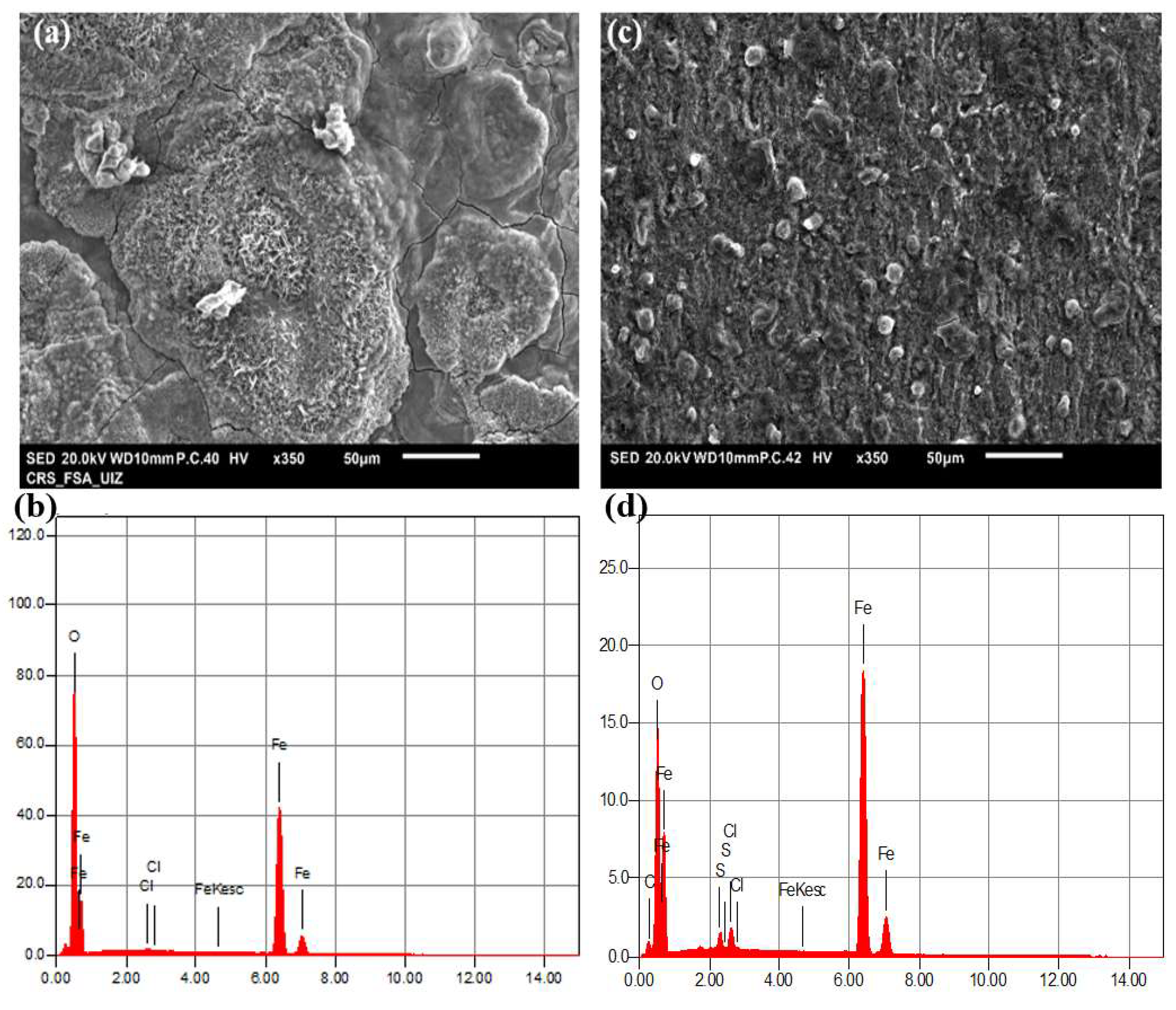
3.6. Morphological Observation of Protective Organic Layer
3.7. Predicting Reactive Sites and Possible Adsorption Behavior Based on DFT/DFTB Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, I.M.; Malathy, R.; Kim, S.H.; Kalaiselvi, K.; Prabakaran, M.; Gopiraman, M. Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J. Adhes. Sci. Technol. 2020, 34, 1483–1506. [Google Scholar] [CrossRef]
- Karthik, G.; Sundaravadivelu, M. Investigations of the inhibition of copper corrosion in nitric acid solutions by levetiracetam drug. Egypt. J. Pet. 2015, 25, 481–493. [Google Scholar] [CrossRef]
- Mert, B.; Mert, M.; Kardaş, G.; Yazıcı, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]
- Popoola, L.T. Progress on pharmaceutical drugs, plant extracts and ionic liquids as corrosion inhibitors. Heliyon 2019, 5, e01143. [Google Scholar] [CrossRef] [PubMed]
- Jeeva, M.; Prabhu, G.V.; Boobalan, M.S.; Rajesh, C.M. Interactions and Inhibition Effect of Urea-Derived Mannich Bases on a Mild Steel Surface in HCl. J. Phys. Chem. C 2015, 119, 22025–22043. [Google Scholar] [CrossRef]
- Subrahmanya, B.K.; Lgaz, H.; Salghi, R.; Chaouiki, R.; Shubhalaxmi; Jodeh, S. Pyrazoline derivatives as possible corrosion inhibitors for mild steel in acidic media: A combined experimental and theoretical approach. Cogent Eng. 2018, 5, 1441585. [Google Scholar]
- Zinad, D.S.; Hanoon, M.; Salim, R.D.; Ibrahim, S.I.; Al-Amiery, A.A.; Takriff, M.S.; Kadhum, A.A.H. A new synthesized coumarin-derived Schiff base as a corrosion inhibitor of mild steel surface in HCl medium: Gravimetric and DFT studies. Int. J. Corros. Scale Inhib. 2020, 9, 228–243. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Al-Baghdadi, S.B.; Hashim, F.G.; Salam, A.Q.; Abed, T.K.; Gaaz, T.S.; Al-Amiery, A.A.; Kadhum, A.A.H.; Reda, K.S.; Ahmed, W.K. Synthesis and corrosion inhibition application of NATN on mild steel surface in acid media complemented with DFT studies. Results Phys. 2018, 8, 1178–1184. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhim, A.; Al-Adili, A.; Tawfiq, Z.H. Limits and developments in ecofriendly corrosion inhibitors of mild steel: A critical review. Part 1: Coumarins. Int. J. Corros. Scale Inhib. 2021, 10, 1355–1384. [Google Scholar] [CrossRef]
- Deyab, M.A.; Osman, M.M.; Elkholya, A.E.; Heakal, F.E.-T. Green approach towards corrosion inhibition of carbon steel in produced oilfield water using lemongrass extract. RSC Adv. 2017, 7, 45241–45251. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, I.H.; Masroor, S.; Chung, I.M. Green corrosion inhibition of mild steel by hydrazone derivatives in 1.0 M HCl. Coatings 2020, 10, 640. [Google Scholar] [CrossRef]
- Luo, Z.-G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.-F.; Liao, B.-K.; Guo, X.-P. Modified Nano-Lignin as a Novel Biomass-Derived Corrosion Inhibitor for Enhanced Corrosion Resistance of Carbon Steel. Corros. Sci. 2023, 227, 111705. [Google Scholar] [CrossRef]
- Vagapov, R.K.; Kantyukov, R.R.; Zapevalov, D.N. Investigation of the corrosiveness of moisture condensation conditions at gas production facilities in the presence of CO2. Int. J. Corros. Scale Inhib. 2021, 10, 994–1010. [Google Scholar]
- Rahimi, A.; Farhadian, A.; Berisha, A.; Shaabani, A.; Varfolomeev, M.A.; Mehmeti, V.; Zhong, X.; Yousefzadeh, S.; Djimasbe, R. Novel Sucrose Derivative as a Thermally Stable Inhibitor for Mild Steel Corrosion in 15% HCl Medium: An Experimental and Computational Study. Chem. Eng. J. 2022, 446, 136938. [Google Scholar] [CrossRef]
- Junaedi, S.; Kadhum, A.A.H.; Al-Amiery, A.; Mohamad, A.B.; Takriff, M.S. Synthesis and characterization of novel corrosion inhibitor derived from oleic acid: 2-Amino-5-Oleyl 1,3,4-Thiadiazol (AOT). Int. J. Electrochem. Sci. 2012, 7, 3543–3554. [Google Scholar] [CrossRef]
- Benali, O.; Zebida, M.; Maschke, U. Synthesis and inhibition corrosion effect of two thiazole derivatives for in 1 M HCl. J. Indian Chem. Soc. 2021, 98, 100113. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, W.; Wang, M.; Yu, C.; Ma, Y.; Bian, J.; Yang, X.; Zhang, D. Anthocyanin as sustainable and non-toxic corrosion inhibitor for mild steel in HCl media: Electrochemical, surface morphology and theoretical investigations. J. Mol. Liq. 2021, 344, 117721. [Google Scholar] [CrossRef]
- Zarrok, H.; Zarrouk, A.; Hammouti, B.; Salghi, R.; Jama, C.; Bentiss, F. Corrosion control of carbon steel in phosphoric acid by purpald–Weight loss, electrochemical and XPS studies. Corros. Sci. 2012, 64, 243. [Google Scholar] [CrossRef]
- Hmamou, D.B.; Zarrouk, R.S.; Zarrok, H.; Hammouti, B.; Al-Deyab, S.S.; Bouachrine, M.; Chakir, A.; Zougagh, M. The inhibited effect of phenolphthalein towards the corrosion of C38 steel in hydrochloric acid. Int. J. Electrochem. Sci. 2012, 7, 5716. [Google Scholar] [CrossRef]
- Hazmatulhaq, F.; Suhartono, T.; Sheng, Y.; Kamil, M.P.; Chaouiki, A.; Ko, Y.G. Amelioration of 2D hybrid architecture for protective surface using thiourea-based inhibitor: Electrochemical and computational perspectives. Sustain. Mater. Technol. 2023, 38, e00757. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Ko, Y.G. Nature-inspired architecture combining organic–inorganic frameworks: Unique structure and active sites toward a stable anti-corrosion coating. Appl. Mater. Today 2023, 32, 101852. [Google Scholar] [CrossRef]
- Chaouiki, A.; Al Zoubi, W.; Ko, Y.G. Advanced prediction of organic–metal interactions through DFT study and electrochemical displacement approach. J. Magnes. Alloys. 2023, 11, 301–316. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Arinkoola, A.O.; Eletta, O.A.; Agbede, O.O.; Osho, Y.A.; Morakinyo, A.F.; Hamed, J.O. Green corrosion inhibition and adsorption characteristics of Luffa cylindrical leaf extract on mild steel in hydrochloric acid environment. Heliyon 2020, 6, e03205–e03216. [Google Scholar] [CrossRef] [PubMed]
- Bentiss, F.; Lagrenee, M. Heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid medium—Correlation between electronic structure and inhibition efficiency. J. Mater. Environ. Sci. 2011, 2, 13–17. [Google Scholar]
- Shamnamol, G.K.; Sreelakshmi, K.P.; Ajith, G.; Jacob, J.M. Effective utilization of drugs as green corrosion inhibitor: A review. AIP Conf. Proc. 2020, 2225, 070006. [Google Scholar] [CrossRef]
- Schmitzhaus, T.E.; OrtegaVega, M.R.; Schroeder, R.; Mattedi, S.; de Fraga Malfatti, C. An amino-based protic ionic liquid as a corrosion inhibitor of mild steel in aqueous chloride solutions. Mater. Corros. 2020, 71, 1175–1193. [Google Scholar] [CrossRef]
- Issaadi, S.; Douadi, T.; Zouaoui, A.; Chafaa, S.; Khan, M.A.; Bouet, G. Novel thiophene symmetrical Schif base compounds as corrosion inhibitor for mild steel in acidic media. Corros. Sci. 2011, 53, 1484–1488. [Google Scholar] [CrossRef]
- Obayes, H.R.; Al-Amiery, A.A.; Alwan, G.H.; Abdullah, T.A.; Kadhum, A.A.H.; Mohamad, A.B. Sulphonamides as corrosion inhibitor: Experimental and DFT studies. J. Mol. Struct. 2017, 1138, 27–34. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Suhartono, T.; Hazmatulhaq, F.; Ko, Y.G. Interface engineering of LDH-based material as efficient anti-corrosive system via synergetic performance of host, interlayers, and morphological features of nature-mimic architectures. Chem. Eng. J. 2023, 462, 142239. [Google Scholar] [CrossRef]
- Jamil, D.M.; Al-Okbi, A.K.; Al-Baghdadi, S.B.; Al-Amiery, A.A.; Kadhim, A.; Gaaz, T.S.; Kadhum, A.A.H.; Mohamad, A.B. Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem. Cent. J. 2018, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, H.J.; Luaibi, H.M.; Dakhil, R.M.; Kadhum, A.A.H.; Al-Amiery, A.A.; Gaaz, T.S. Development of new corrosion inhibitor tested on mild steel supported by electrochemical study. Results Phys. 2018, 8, 1260–1267. [Google Scholar] [CrossRef]
- Salman, T.A.; Zinad, D.S.; Jaber, S.H.; Shayaa, M.; Mahal, A.; Takriff, M.S.; Al-Amiery, A.A. Effect of 1,3,4-thiadiazole scafold on the corrosion inhibition of mild steel in acid medium: An experimental and computational study. J. Bio Tribo-Corros. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Khillah extract as inhibitor for acid corrosion of SX 316 steel. Appl. Surf. Sci. 2006, 252, 8521–8525. [Google Scholar] [CrossRef]
- Scully, J.; Baboian, R. Standard Practice for Laboratory Immersion Corrosion Testing of Metals; ASTM: Phila, PA, USA, 1995. [Google Scholar]
- Kadhim, A.; Al-Okbi, A.K.; Jamil, D.M.; Qussay, A.; Al-Amiery, A.A.; Gaas, T.S.; Kadhum, A.A.H.; Mohamad, A.B.; Nassir, M.H. Experimental and theoretical studies of benzoxazines corrosion inhibitors. Results Phys. 2017, 7, 4013–4019. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Shaker, L.M. Corrosion inhibition of mild steel using novel pyridine derivative in 1 M hydrochloric acid. Koroze Ochr. Mater. 2020, 64, 59–64. [Google Scholar] [CrossRef]
- Nahlé, A.; Salim, R.; El Hajjaji, F.; Aouad, M.; Messali, M.; Ech-Chihbi, E.; Hammouti, B.; Taleb, M. Novel triazole derivatives as ecological corrosion inhibitors for mild steel in 1.0 M HCl: Experimental & theoretical approach. RSC Adv. 2021, 11, 4147–4162. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, D.; Huang, Y.; Huang, L.; Wang, P.; Qian, H.; Li, X.; Terryn, H.A.; Mol, J.M. Dual-action self-healing protective coatings with photothermal responsive corrosion inhibitor nanocontainers. Chem. Eng. J. 2021, 404, 127118. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, N.; Huang, X.; Shang, W.; Wu, L. Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: Experimental and theoretical studies. RSC Adv. 2016, 6, 22250–22268. [Google Scholar] [CrossRef]
- Chen, W.; Hong, S.; Xiang, B.; Luo, H.; Li, M.; Li, N. Corrosion inhibition of copper in hydrochloric acid by coverage with trithiocyanuric acid self-assembled monolayers. Corros. Eng. Sci. Technol. 2013, 48, 98–107. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1 M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Ren, C.; Ju, P.; Huang, Y.; Zhang, F.; Zhao, F.; Zhang, Z.; Zhang, D. Detection of corrosion inhibitor adsorption via a surface-enhanced Raman spectroscopy (SERS) silver nanorods tape sensor. Sens. Actuators B Chem. 2020, 321, 128617. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma Lingwei Ren, C.; Zhang, D.; Ma, L.; Sun, M. Qualitative and quantitative detection of corrosion inhibitors using surface-enhanced Raman scattering coupled with multivariate analysis. Appl. Surf. Sci. 2021, 568, 150967. [Google Scholar] [CrossRef]
- E. A. Flores; et al. Sodium phthalamates as corrosion inhibitors for carbon steel in aqueous hydrochloric acid solution. Corros. Sci. 2011, 53, 3899–3913. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Zarrok, H.; Al-Deyab, S.S.; Warad, I. Thermodynamic study of metal corrosion and inhibitor adsorption processes in copper/N-1-naphthylethylenediamine dihydrochloride monomethanolate/nitric acid system: Part 2. Res. Chem. Intermed. 2012, 38, 1655–1668. [Google Scholar] [CrossRef]
- Ouici, H.; Tourabi, M.; Benali, O.; Selles, C.; Jama, C.; Zarrouk, A.; Bentiss, F. Adsorption and Corrosion Inhibition Properties of 5-amino 1,3,4-Thiadiazole-2-Thiol on the Mild Steel in Hydrochloric Acid Medium: Thermodynamic, Surface and Electrochemical Studies. J. Electroanal. Chem. 2017, 803, 125–134. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Verma, C.; Quraishi, M.A. Experimental and Quantum Chemical Analysis of 2-amino-3-((4-((S)-2-Amino-2- Carboxyethyl)-1h-Imidazol-2-Yl)thio) Propionic Acid as New and green Corrosion Inhibitor for Mild Steel in 1 M Hydrochloric Acid Solution. J. Mol. Liq. 2017, 225, 848–855. [Google Scholar] [CrossRef]
- Anusuya, N.; Saranya, J.; Sounthari, P.; Zarrouk, A.; Chitra, S. Corrosion Inhibition and Adsorption Behaviour of Some Bis-Pyrimidine Derivatives on Mild Steel in Acidic Medium. J. Mol. Liq. 2017, 225, 406–417. [Google Scholar] [CrossRef]
- Fadhil, A.A.; Khadom, A.A.; Liu, H.; Fu, C.; Wang, J.; Fadhil, N.A.; Mahood, H.B. Corrosion inhibitor of steel in acidic solution: Gravimetrical, electrochemical, surface morphology and theoretical simulation. J. Mol. Liq. 2019, 276, 503–518. [Google Scholar] [CrossRef]
- Fouda, A.S.; Abdel-Latif, E.; Helal, H.M.; El-Hossiany, A. Synthesis and Characterization of Some Novel Thiazole Derivatives and Their Applications as Corrosion Inhibitors for Zinc in 1 M Hydrochloric Acid Solution. Russ. J. Electrochem. 2021, 57, 159–171. [Google Scholar] [CrossRef]
- Bentiss, F.; Bouanis, M.; Mernari, B.; Traisnel, M.; Vezin, H.; Lagrenée, M. Understanding the adsorption of 4H-1,2,4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl. Surf. Sci. 2007, 253, 3696–3704. [Google Scholar] [CrossRef]
- Sliem, M.H.; El Basiony, N.M.; Zaki, E.G.; Sharaf, M.A.; Abdullah, A.M. Corrosion Inhibition of Mild Steel in Sulfuric Acid by a Newly Synthesized Schiff Base: An Electrochemical, DFT, and Monte Carlo Simulation Study. Electroanalysis 2020, 32, 3145–3158. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Tang, H.; Yan, X. Experimental and Theoretical Studies of Cinnamyl Alcohol as a Novel Corrosion Inhibitor for Copper Foils in Rolling Oil. Mater. Corros. 2020, 72, 534–542. [Google Scholar] [CrossRef]
- El Faydy, M.; Lakhrissi, B.; Jama, C.; Zarrouk, A.; Olasunkanmi, L.O.; Ebenso, E.E.; Bentiss, F. Electrochemical, surface and computational studies on the inhibition performance of some newly synthesized 8-hydroxyquinoline derivatives containing benzimidazole moiety against the corrosion of carbon steel in phosphoric acid. J. Mater. Res. Technol. 2019, 9, 727–748. [Google Scholar] [CrossRef]
- Ahchouch, H.; Chaouiki, A.; Talhajt, S.A.; Bammou, L.; Belkhaouda, M.; Salghi, R.; Ko, Y.G. Inter-and Intra-Molecular Synergism in Designing MgO-MCC Composite-Based Coating: An Efficient Inhibitor for Excellent Anticorrosion Performance. Process Saf. Environ. Prot. 2023, 177, 1461–1476. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. 2-Mercaptopyrimidine as an effective inhibitor for the corrosion of cold rolled steel in HNO3 solution. Corros. Sci. 2017, 118, 202–216. [Google Scholar] [CrossRef]
- Nahle, A.; El-Hajjaji, F.; Ghazoui, A.; Benchat, N.-E.; Taleb, M.; Saddik, R.; Elaatiaoui, A.; Koudad, M.; Hammouti, B. Effect of Substituted Methyl Group by Phenyl Group in Pyridazine Ring on the Corrosion Inhibition of Mild Steel in 1.0 M HCl. Anti-Corros. Methods Mater. 2018, 65, 87–96. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Rahimi, A.; Mendgaziev, R.I.; Semenov, A.P.; Stoporev, A.S.; Vinogradova, S.S.; Karwt, R.; Kelland, M.A. Gas Hydrate and Corrosion Inhibition Performance of the Newly Synthesized Polyurethanes: Potential Dual Function Inhibitors. Energy Fuels 2021, 35, 6113–6124. [Google Scholar] [CrossRef]
- Benabdellah, M.; Benkaddour, M.; Hammouti, B.; Bendahhou, M.; Aouniti, A. Inhibition of Steel Corrosion in 2M H3PO4 by Artemisia Oil. Appl. Surf. Sci. 2006, 252, 6212–6217. [Google Scholar] [CrossRef]
- Dahmani, M.; Et-Touhami, A.; Al-Deyab, S.S.; Hammouti, B.; Bouyanzer, A. Corrosion Inhibition of C38 Steel in 1 M HCl: A Comparative Study of Black Pepper Extract and Its Isolated Piperine. Int. J. Electrochem. Sci. 2010, 5, 1060–1069. [Google Scholar] [CrossRef]
- Khadraoui, A.; Khelifa, A.; Hadjmeliani, M.; Mehdaoui, R.; Hachama, K.; Tidu, A.; Azari, Z.; Obot, I.B.; Zarrouk, A. Extraction, Characterization and Anti-corrosion Activity of Mentha Pulegium Oil: Weight Loss, Electrochemical, Thermodynamic and Surface Studies. J. Mol. Liq. 2016, 216, 724–731. [Google Scholar] [CrossRef]
- Sliem, M.H.; Afifi, M.; Bahgat Radwan, A.; Fayyad, E.M.; Shibl, M.F.; Heakal, F.E.-T.; Abdullah, A.M. AEO7 Surfactant as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl Solution. Sci. Rep. 2019, 9, 2319. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.; Bilgic, S.; Yılmaz, H. The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl. Surf. Sci. 2002, 195, 1–7. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chauhan, D.; Ahamad, I.; Quraishi, M. Electrochemical behavior of steel/acid interface: Adsorption and inhibition effect of oligomeric aniline. RSC Adv. 2013, 3, 632–646. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Three pyrazine derivatives as corrosion inhibitors for steel in 1.0 M H2SO4 solution. Corros. Sci. 2011, 53, 3241–3247. [Google Scholar] [CrossRef]
- Gomma, G.K.; Wahdan, M.H. Schiff Bases as Corrosion Inhibitors for Aluminium in Hydrochloric Acid Solution. Mater. Chem. Phys. 1995, 39, 209–213. [Google Scholar] [CrossRef]
- Ateya, B.; El-Anadouli, B.; El-Nizamy, F. The effect of thiourea on the corrosion kinetics of mild steel in H2SO4. Corros. Sci. 1984, 24, 497–507. [Google Scholar] [CrossRef]
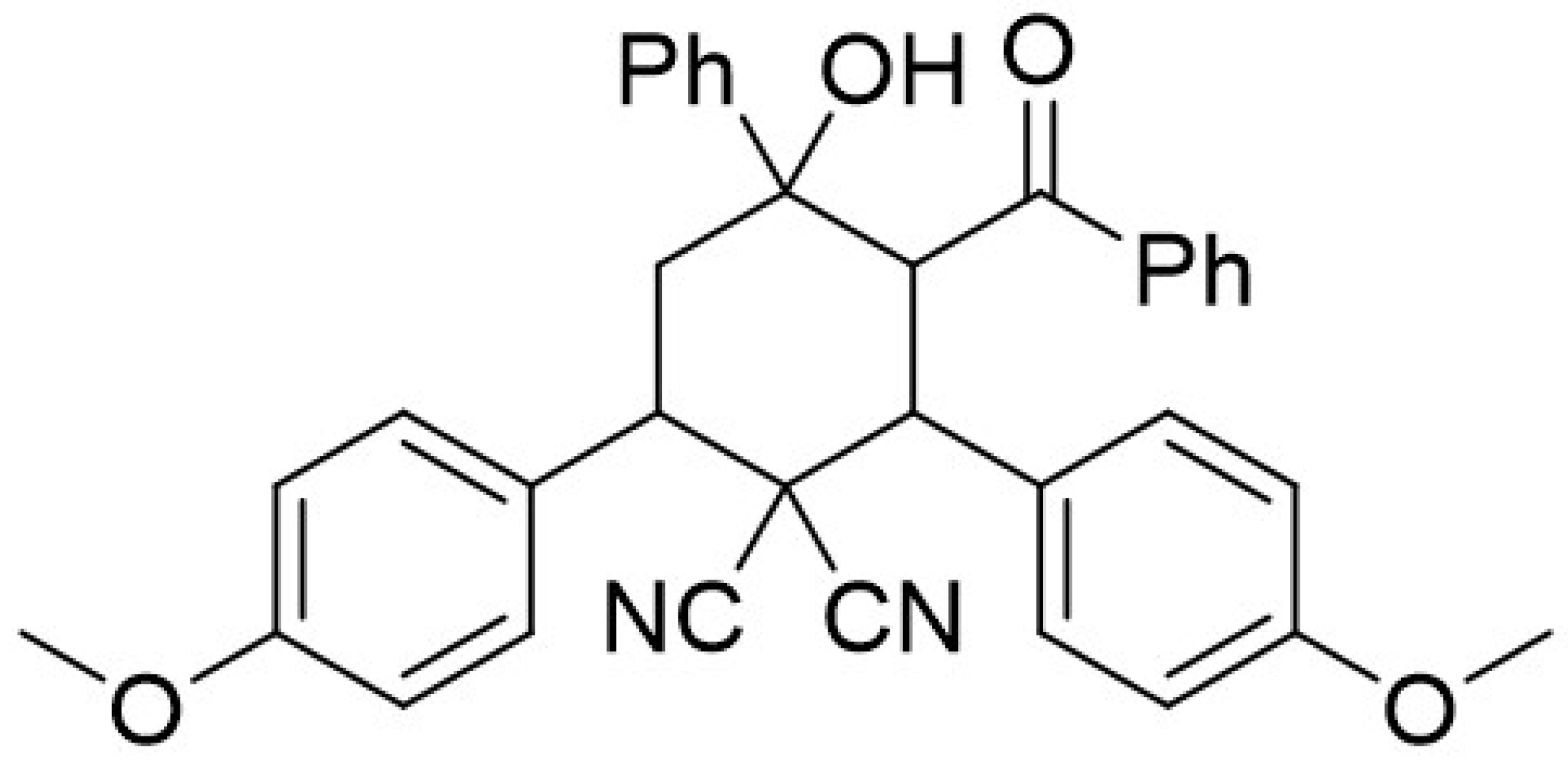





| Inhibitor | C (mol/L) | Rct (Ω·cm2) | n | Q × 10 ̶ 4 (sn·Ω−1·cm−2) | Cdl (μF·cm−2) | ERct (%) |
|---|---|---|---|---|---|---|
| Blank | - | 18 | 0.79 | 2.09 | 47 | - |
| BMPD | 10 ̶ 3 | 415 | 0.60 | 1.23 | 16 | 92.92 |
| 10 ̶ 4 | 215 | 0.61 | 1.55 | 17 | 89.30 | |
| 10 ̶ 5 | 127 | 0.68 | 1.78 | 29 | 85.82 | |
| 10 ̶ 6 | 65 | 0.75 | 1.85 | 42 | 81.53 |
| Inhibitor | C (mol/L) | −Ecorr (mV/SCE) | −βc (mV·dec ̶ 1) | βa (mV·dec−1) | Icorr (μA·cm−2) | EI (%) |
|---|---|---|---|---|---|---|
| Blank | - | 463 | 168 | 129 | 636 | - |
| BMPD | 10−3 | 505 | 149 | 102 | 37 | 94.18 |
| 10−4 | 519 | 144 | 109 | 68 | 89.30 | |
| 10−5 | 520 | 142 | 106 | 112 | 82.38 | |
| 10−6 | 472 | 150 | 103 | 184 | 71.06 |
| Inhibitor | Slope | Kads (L·mol−1) | R2 | (kJ/mol) |
|---|---|---|---|---|
| BMPD | 1.04 | 49.441 | 0.999 | −42.41 |
| Inhibitor | Temperature (K) | Rct (Ω·cm2) | n | Q × 10 ̶ 4 (sn·Ω ̶ 1 cm ̶ 2) | Cdl (μF·cm ̶ 2) | ERct (%) |
|---|---|---|---|---|---|---|
| Blank | 298 | 18 | 0.79 | 2.09 | 47 | - |
| 308 | 11 | 0.77 | 2.69 | 7 | - | |
| 318 | 8 | 0.80 | 2.37 | 5 | - | |
| 328 | 5 | 0.81 | 2.17 | 4 | - | |
| BMPD | 298 | 415 | 0.60 | 1.23 | 16 | 95.66 |
| 308 | 205 | 0.70 | 1.45 | 32 | 94.63 | |
| 318 | 140 | 0.72 | 1.63 | 34 | 94.28 | |
| 328 | 98 | 0.74 | 1.38 | 38 | 94.89 |
| Inhibitor | Ea (kJ/mol) | ∆H* (kJ/mol) | ∆S* (J/mol·K) | Ea−ΔH* |
|---|---|---|---|---|
| Blank | 36.38 | 33.79 | −191.53 | 2.60 |
| BMPD | 41.02 | 38.42 | −201.43 | 2.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Id El Mouden, O.; Al-Moubaraki, A.H.; Chafiq, M.; Bakhouch, M.; Batah, A.; Bammou, L.; Belkhaouda, M.; Chaouiki, A.; Ko, Y.G. BMPD-Assisted Enhancement of Corrosion Resistance of Carbon Steel: Experimental and First-Principle DFTB Insights. Metals 2024, 14, 69. https://doi.org/10.3390/met14010069
Id El Mouden O, Al-Moubaraki AH, Chafiq M, Bakhouch M, Batah A, Bammou L, Belkhaouda M, Chaouiki A, Ko YG. BMPD-Assisted Enhancement of Corrosion Resistance of Carbon Steel: Experimental and First-Principle DFTB Insights. Metals. 2024; 14(1):69. https://doi.org/10.3390/met14010069
Chicago/Turabian StyleId El Mouden, Omar, Aisha H. Al-Moubaraki, Maryam Chafiq, Mohamed Bakhouch, Ahmed Batah, Lahcen Bammou, M’hammed Belkhaouda, Abdelkarim Chaouiki, and Young Gun Ko. 2024. "BMPD-Assisted Enhancement of Corrosion Resistance of Carbon Steel: Experimental and First-Principle DFTB Insights" Metals 14, no. 1: 69. https://doi.org/10.3390/met14010069
APA StyleId El Mouden, O., Al-Moubaraki, A. H., Chafiq, M., Bakhouch, M., Batah, A., Bammou, L., Belkhaouda, M., Chaouiki, A., & Ko, Y. G. (2024). BMPD-Assisted Enhancement of Corrosion Resistance of Carbon Steel: Experimental and First-Principle DFTB Insights. Metals, 14(1), 69. https://doi.org/10.3390/met14010069









