The Interfacial Reaction between Amorphous Ni-W-P Coating and Sn-58Bi Solder
Abstract
:1. Introduction
Ni2+ + 2H2O = NiO + 2H+
P + 4H2O = PO43− + 8H+ + 5e−
2PO43− + 3Ni2+ = Ni3(PO4)2
W + 4H2O = WO3 + 6H+ + 6e−
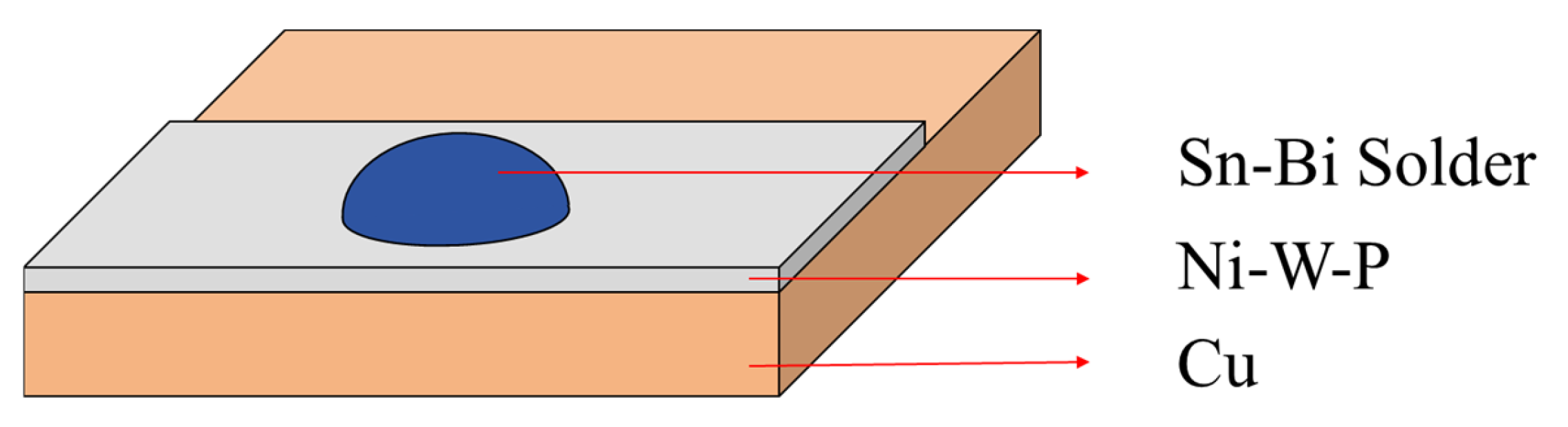
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Main Salt Concentration on Coating Composition
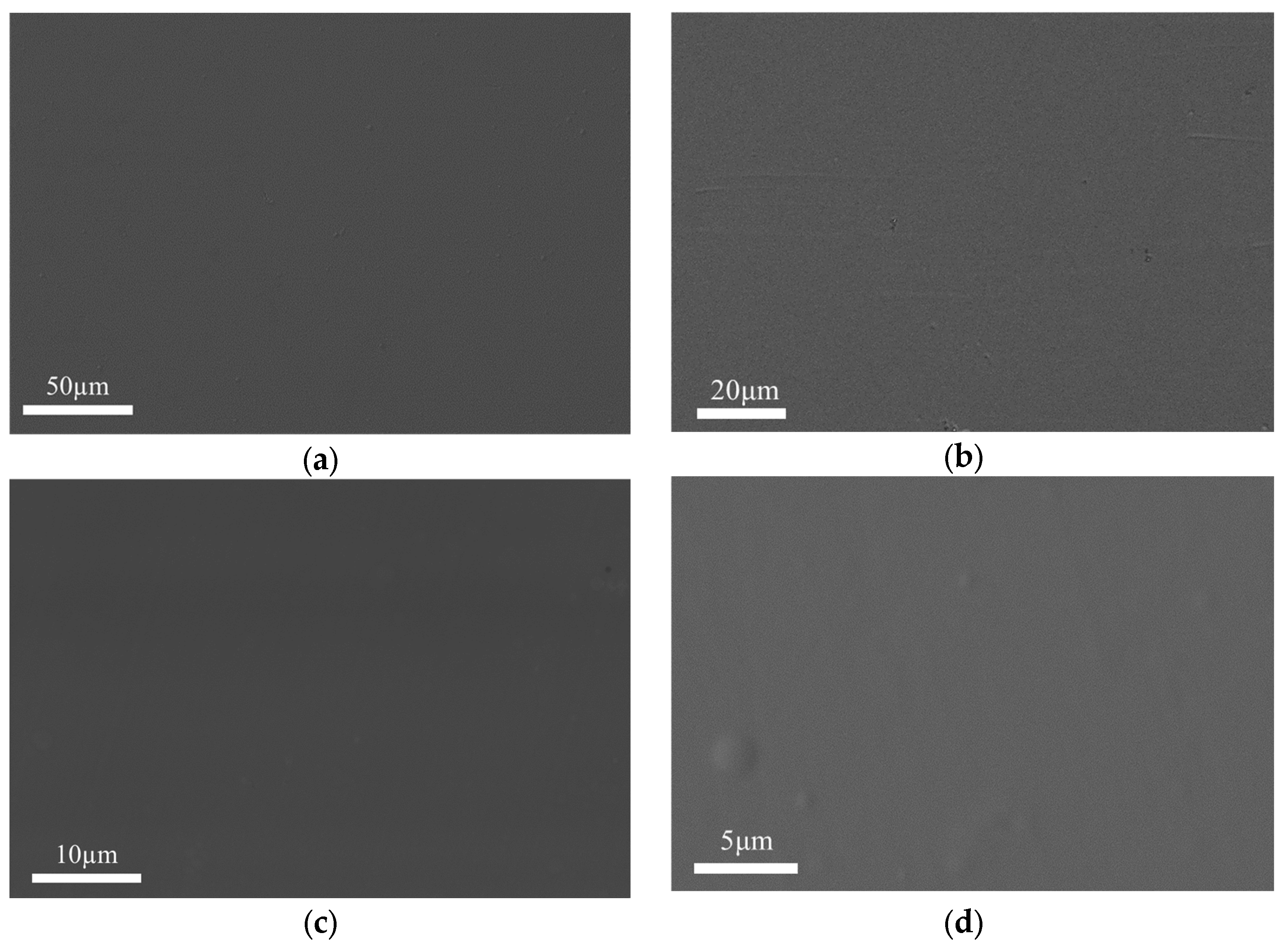
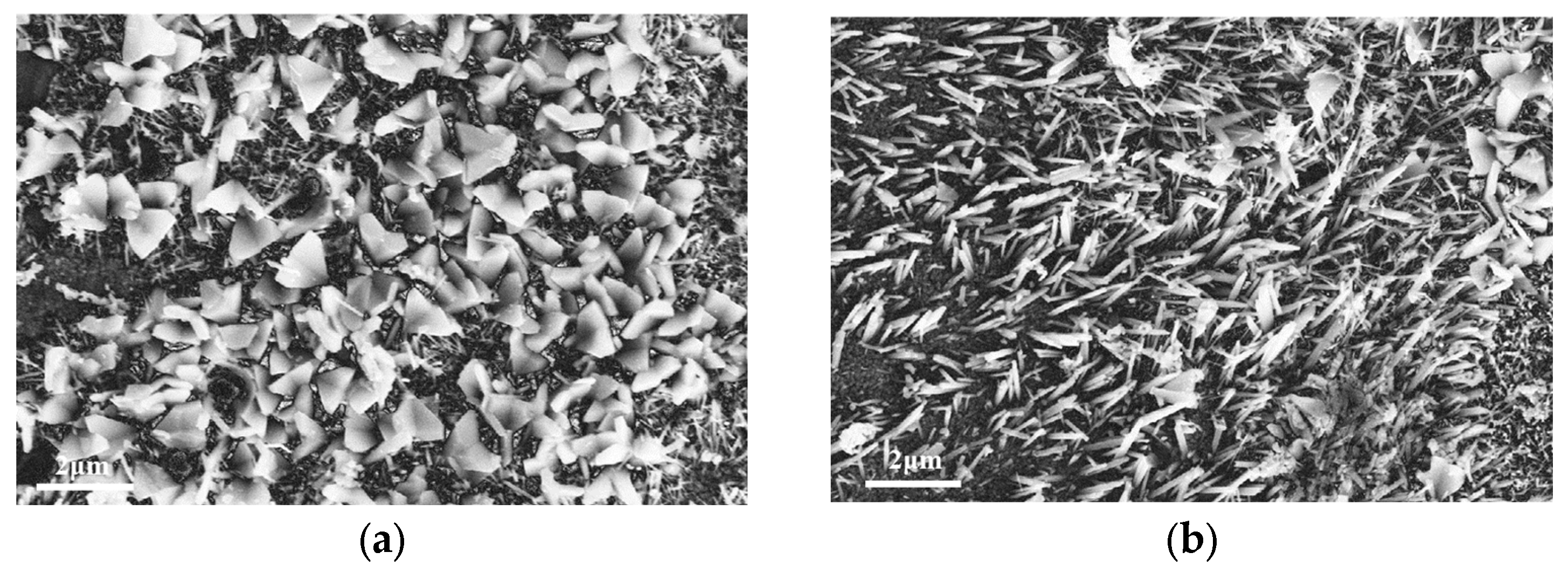
3.2. Surface Topography and Crystal Structure of the Coating
3.3. Electrical Conductivity and Wettability
3.4. Interface Reactions
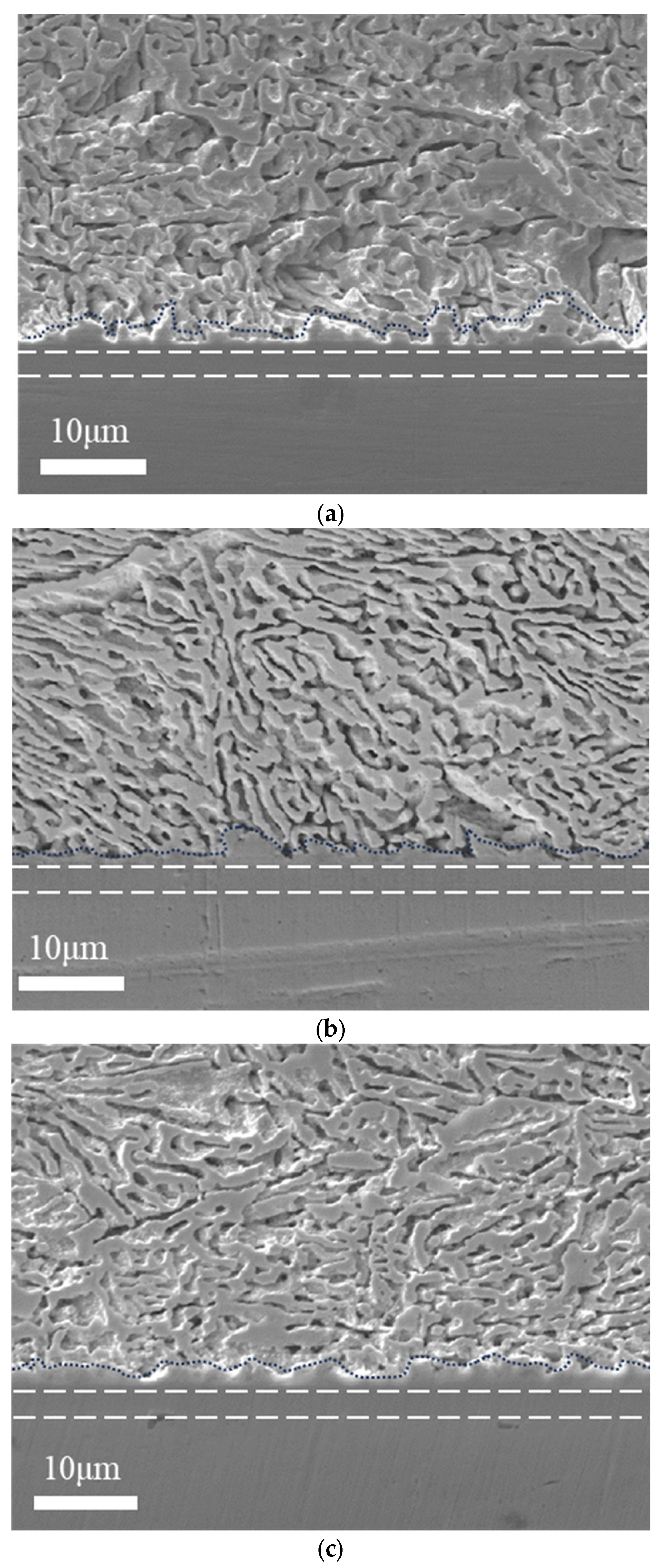
3.4.1. IMC Layer Growth Morphology and Composition
3.4.2. IMC Layer Thickness
3.4.3. Interface Reaction Rate
3.4.4. Isothermal Aging Treatment
4. Conclusions
- (1)
- By adjusting the concentration of additives and the electroplating process parameters, a coating with excellent surface quality can be obtained, and under these conditions, the thickness of the coating after 20 min of pulse electroplating is approximately 3 to 4 μm.
- (2)
- During the co-deposition process of electroplating, tungsten (W) and phosphorus (P) exhibit a competitive relationship, and the content of W and P does not have a linear correlation with the ratio of the main salt concentration in the plating solution.
- (3)
- The plating is amorphous, the addition of W to the Ni-P plating improves the electrical conductivity of the plating, and its square resistance decreases from 0.616 mΩ/sq to 0.534 mΩ/sq.
- (4)
- The growth rate of the IMC layer at the interface is very fast when the welding time is from 60 s to 180 s. However, the growth rate of the IMC layer is greatly reduced after increasing the welding time, which shows the excellent barrier effect of the Ni-W-P coating.
- (5)
- For Ni-W-P coatings with the same welding time, the higher the W content, the slower the growth rate of the IMCs layer at the interface.
- (6)
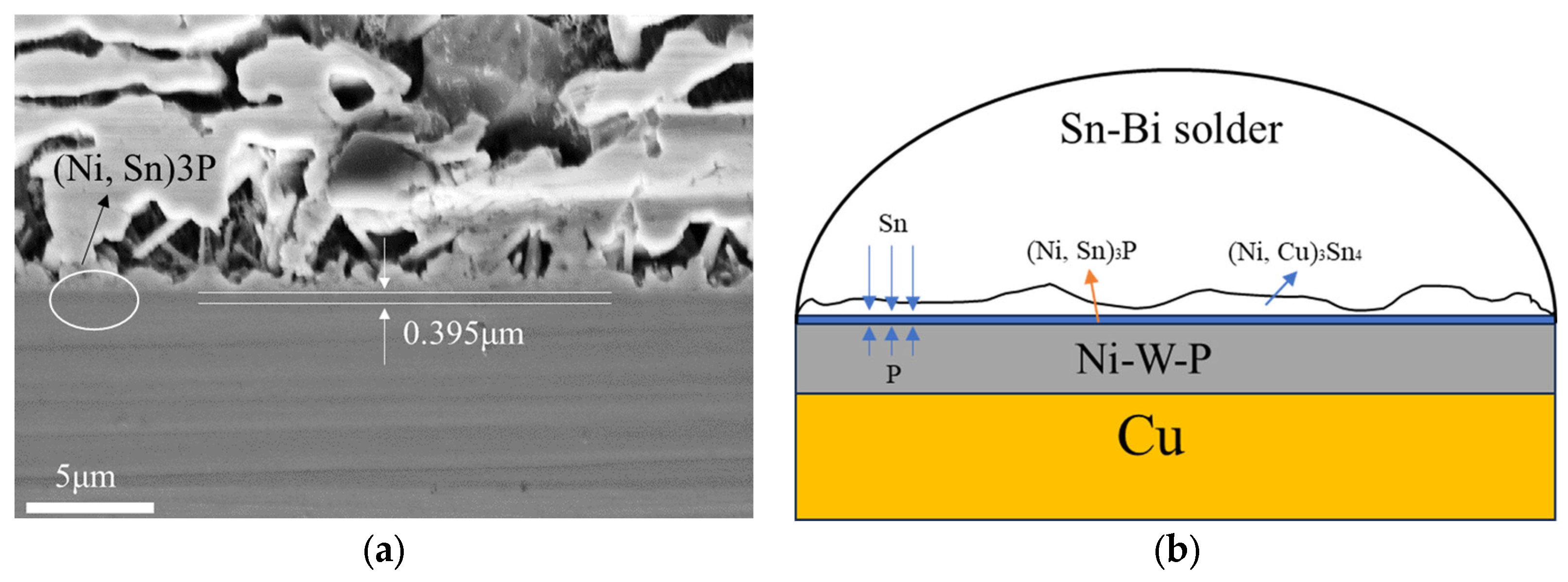
- The Ni-W-P coating effectively prevents the diffusion of copper (Cu) atoms, and after isothermal aging at 100 °C for 240 h, the Ni-W-P coating is transformed into only 0.395 μm, which is less than one-tenth of the total thickness of the coating, and it has good thermal stability.
- (7)
- The Ni-W-P coating will gradually transform into (Ni, Sn)3P, which may eventually lead to its failure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abtew, M.; Selvaduray, G. Lead-free Solders in Microelectronics. Mater. Sci. Eng. R Rep. 2000, 27, 95–141. [Google Scholar] [CrossRef]
- Islam, R.A.; Chan, Y.C.; Jillek, W.; Islam, S. Comparative study of wetting behavior and mechanical properties (microhardness) of Sn–Zn and Sn–Pb solders. Microelectron. J. 2006, 37, 705–713. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Feng, H.; Ke, W. Effect of Ag3Sn intermetallic compounds on corrosion of Sn-3.0Ag-0.5Cu solder under high-temperature and high-humidity condition. Corros. Sci. 2012, 63, 20–28. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.C.; Han, Y.J.; Wan, J.B.; Yang, K. Microstructures of eutectic Sn–Ag–Zn solder solidified with different cooling rates. J. Alloys Compd. 2008, 464, 301–305. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.T.; Ansari, M.; Hanim, M.A.; Oyekanmi, A.A.; Ojo-Kupoluyi, O.J.; Atiqah, A. Recent advances in Sn-based lead-free solder interconnects for microelectronics packaging: Materials and technologies. J. Mater. Res. Technol. 2023, 25, 4231–4263. [Google Scholar] [CrossRef]
- Lau, J.H. Recent advances and trends in advanced packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 228–252. [Google Scholar] [CrossRef]
- Li, Y.; Fu, G.; Wan, B.; Wu, Z.; Yan, X.; Zhang, W. A study on the effects of electrical and thermal stresses on void formation and migration lifetime of Sn3.0Ag0.5Cu solder joints. Solder. Surf. Mt. Technol. 2022, 34, 162–173. [Google Scholar] [CrossRef]
- Li, Y.; Fu, G.; Wan, B.; Yan, X.; Zhang, W.; Li, W. Phase-field modeling of lead-free solder joint void growth under thermal-electrical coupled stress. J. Electron. Mater. 2022, 51, 259–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Wang, J.; Han, Y.; Yu, Z.; Wang, Q.; Zhang, P.; Yang, S. Effects of solder thickness on interface behavior and nanoindentation characteristics in Cu/Sn/Cu microbumps. Weld. World 2022, 66, 973–983. [Google Scholar] [CrossRef]
- McCluskey, P.; Munamarty, R.; Pecht, M. Popcorning in PBGA Packages During IR Reflow Soldering. Microelectron. Int. 1997, 14, 20–23. [Google Scholar] [CrossRef]
- Singh, A.; Durairaj, R. Study on Hardness and Shear Strength with Microstructure Properties of Sn52Bi/Cu + 1% Al2O3 Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 834, 012075. [Google Scholar] [CrossRef]
- Hua, F.; Mei, Z.; Glazer, J. In Eutectic Sn–Bi as an Alternative to Pb-Free Solders. In Proceedings of the 48th Electronic Components and Technology Conference, Seattle, WA, USA, 25–28 May 1998. [Google Scholar]
- Mei, Z.; Morris, J.W. Characterization of eutectic Sn–Bi solder joints. J. Electron. Mater 1992, 21, 599–607. [Google Scholar] [CrossRef]
- Liu, P.L.; Shang, J.K. Interfacial embrittlement by bismuth segregation copper/tin–bismuth Pb-free solder interconnect. J. Mater. Res. 2001, 16, 1651–1659. [Google Scholar] [CrossRef]
- Zou, H.F.; Zhang, Q.K.; Zhang, Z.F. Transition of Bi embrittlement of SnBi/Cu joint couples with reflow temperature. J. Mater. Res. 2010, 26, 449–454. [Google Scholar] [CrossRef]
- Mo, C.C.; Tran, D.P.; Juang, J.Y.; Chen, C. Effect of Intermetallic Compound Bridging on the Cracking Resistance of Sn2.3Ag Microbumps with Different UBM Structures under Thermal Cyclin. Metals 2021, 11, 1065. [Google Scholar] [CrossRef]
- Okamoto, H. Ni-Zn (nickel-zinc). J. Phase Equilibria 2003, 24, 280–281. [Google Scholar] [CrossRef]
- Alam, M.O.; Chan, Y.C.; Tu, K.N. Correlation between chemical reaction and brittle fracture found in electroless Ni(P)/immersion gold–solder interconnection. J. Appl. Phys. 2003, 94, 4108. [Google Scholar] [CrossRef]
- Zeng, K.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free SnAgCu solder and Ni(P) surface finish on printed circuit boards. IEEE Trans. Electron. Packag. Manuf. 2002, 25, 162–167. [Google Scholar] [CrossRef]
- Chin, Y.T.; Lam, P.K.; Yow, H.K.; Tou, T.Y. Transmission electron microscopy studies of interfacial reactions and void formation in lead-free solders with minor elements. J. Mater. 2010, 25, 1304–1311. [Google Scholar] [CrossRef]
- Yang, Y.; Balaraju, J.N.; Chong, S.C.; Xu, H.; Liu, C.; Silberschmidt, V.V.; Chen, Z. Significantly retarded interfacial reaction between an electroless Ni-W-P metallization and lead-free Sn-3.5Ag solder. J. Alloys Compd. 2013, 565, 11–16. [Google Scholar] [CrossRef]
- Bae, S.H.; Nguyen, Y.N.; Son, I. Ni-W-P layer-induced strength improvement of solder joint in Bi2Te3 thermoelectric module. Sci. Technol. Weld 2023, 28, 399–406. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys; Academic Press Inc.: New York, NY, USA, 1963; pp. 160–165. [Google Scholar]
- Lin, C.S.; Lee, C.Y.; Chen, F.J.; Li, W.C. Structural Evolution and Internal Stress of Nickel-Phosphorus Electrodeposits. Electrochem. Soc. 2005, 152, C370–C375. [Google Scholar] [CrossRef]
- Lai, G.-Q.; Liu, H. Electrodeposition of functionally graded Ni-W/Er2O3 rare earth nanoparticle composite film. Int. J. Miner. Metall. Mater. 2020, 27, 818–829. [Google Scholar] [CrossRef]
- Alam, M.O.; Chan, Y.C.; Hung, K.C. Preparation and wear resistance of electrodeposited Ni–W/diamond composite coatings. Microelectron. Reliab. 2002, 4, 1065. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, P.; Voyles, P.M.; Liu, X. Effect of tungsten alloying on short-to-medium-range-order evolution and crystallization behavior of near-eutectic amorphous Ni–P. Acta Mater. 2017, 122, 400–441. [Google Scholar] [CrossRef]
- Farrell, T.; Greig, D. The electrical resistivity of nickel and its alloys. J. Phys. C Solid State Phys. 1968, 1, 1359. [Google Scholar] [CrossRef]
- Petroff, P.; Sheng, T.T.; Sinha, A.K.; Rozgonyi, G.A.; Alexander, F.B. Microstructure, growth, resistivity, and stresses in thin tungsten films deposited by rf sputtering. J. Appl. Phys. 1973, 44, 2545–2554. [Google Scholar] [CrossRef]




| Element | Compositions (g/L) | Information on Chemical Reagents |
|---|---|---|
| NiSO4·6H2O | 40 | DAMAO, Tianjin, China |
| NaH2PO2·H2O | 10–30 | DAMAO, Tianjin, China |
| Na2WO4·2H2O | 33 | DAMAO, Tianjin, China |
| C6H5Na3O7·2H2O | 100 | DAMAO, Tianjin, China |
| H3BO3 | 20 | Aladdin, Shanghai, China |
| C7H4NaO3S7·2H2O | 6 | Aladdin, Shanghai, China |
| CH3(CH2)11OSO3Na | 0.3 | Aladdin, Shanghai, China |
| Element | W:P = 1:2 | W:P = 1:1 | W:P = 2:1 |
|---|---|---|---|
| Ni | 73.31% | 76.71% | 74.50% |
| P | 26.37% | 21.29% | 23.13% |
| W | 0.32% | 2.00% | 2.37% |
| Plated Layer | Ni-P | Ni-W-P |
|---|---|---|
| Sheet Resistance mΩ/sq | 0.610 | 0.535 |
| 0.620 | 0.532 | |
| 0.617 | 0.534 | |
| Average | 0.616 | 0.534 |
| Soldering Time | Ni-P | Ni-W-P |
|---|---|---|
| 1 s | 46.31° | 43.60° |
| 60 s | 43.41° | 42.43° |
| 300 s | 44.75° | 40.84° |
| Composition | W:P = 1:2 | W:P = 1:1 | W:P = 2:1 |
|---|---|---|---|
| Plating thickness | 4.03 μm | 3.89 μm | 3.08 μm |
| Time | W:P = 1:2 | W:P = 1:1 | W:P = 2:1 |
|---|---|---|---|
| 60 s | 0.924 μm | 0.929 μm | 1.068 μm |
| 180 s | 1.321 μm | 1.203 μm | 1.145 μm |
| 300 s | 1.371 μm | 1.374 μm | 1.267 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Su, X.; Zhang, Z.; Ma, H.; Yao, J.; Xia, H.; Zhao, Y. The Interfacial Reaction between Amorphous Ni-W-P Coating and Sn-58Bi Solder. Metals 2024, 14, 1107. https://doi.org/10.3390/met14101107
Li C, Su X, Zhang Z, Ma H, Yao J, Xia H, Zhao Y. The Interfacial Reaction between Amorphous Ni-W-P Coating and Sn-58Bi Solder. Metals. 2024; 14(10):1107. https://doi.org/10.3390/met14101107
Chicago/Turabian StyleLi, Chenyu, Xiaolin Su, Zhongxu Zhang, Haitao Ma, Jinye Yao, Haohao Xia, and Yuanbang Zhao. 2024. "The Interfacial Reaction between Amorphous Ni-W-P Coating and Sn-58Bi Solder" Metals 14, no. 10: 1107. https://doi.org/10.3390/met14101107





