Effect of Heating Rate on Hydride Reorientation Behavior of Zirconium Alloy Tubes under Non-Stress Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Hydrogenation Experiment
2.3. Microstructure Characterizations
3. Results
3.1. Influencing Factors of Hydride Orientation Change
3.2. Effect of Heating Rate on Microstructure of Zirconium Alloy Matrix
3.3. Effect of Heating Rate on Microstructure of Hydride
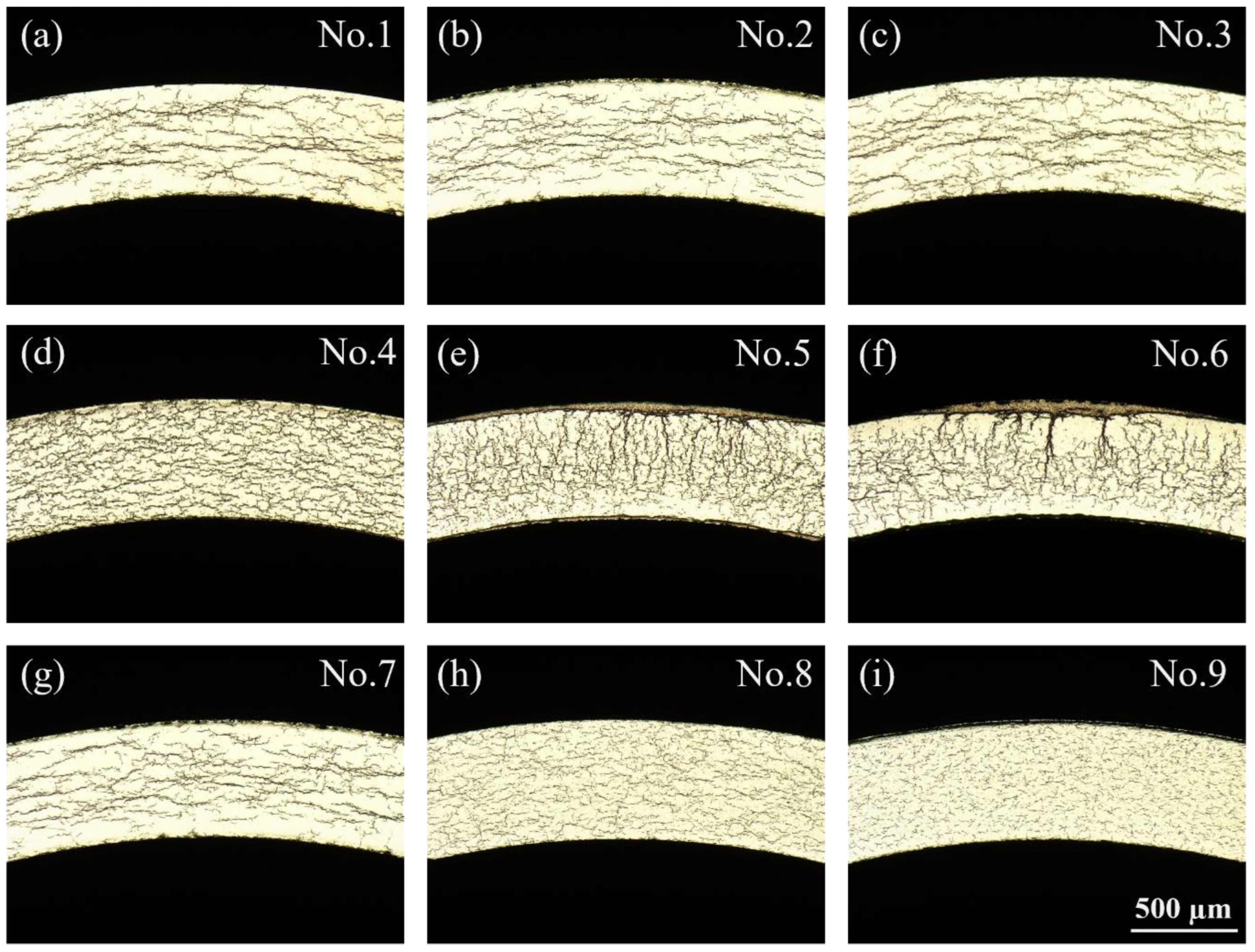
3.3.1. Effect of Heating Rate on Hydride Distribution
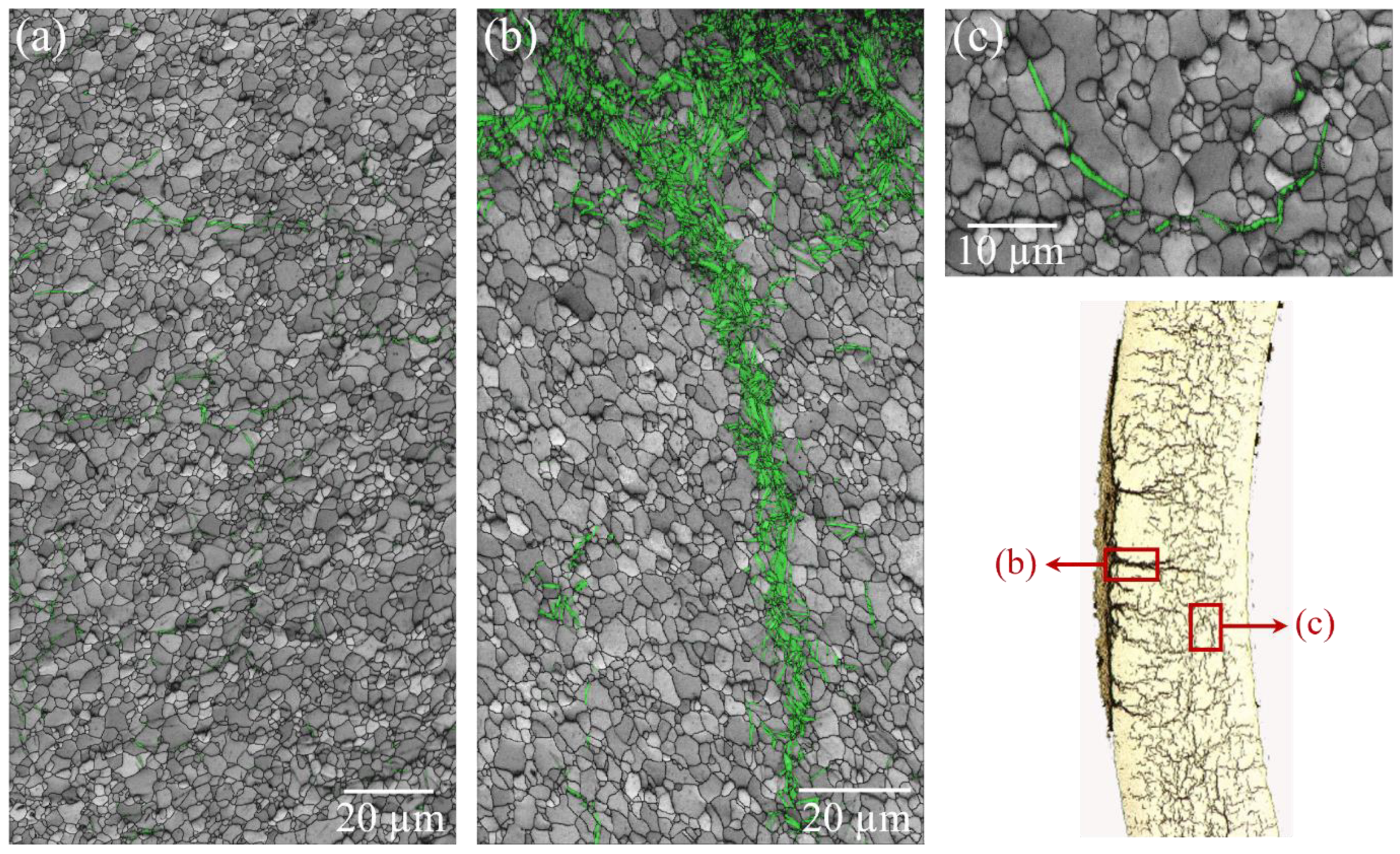
3.3.2. Effect of Heating Rate on Hydride Orientation
4. Discussion
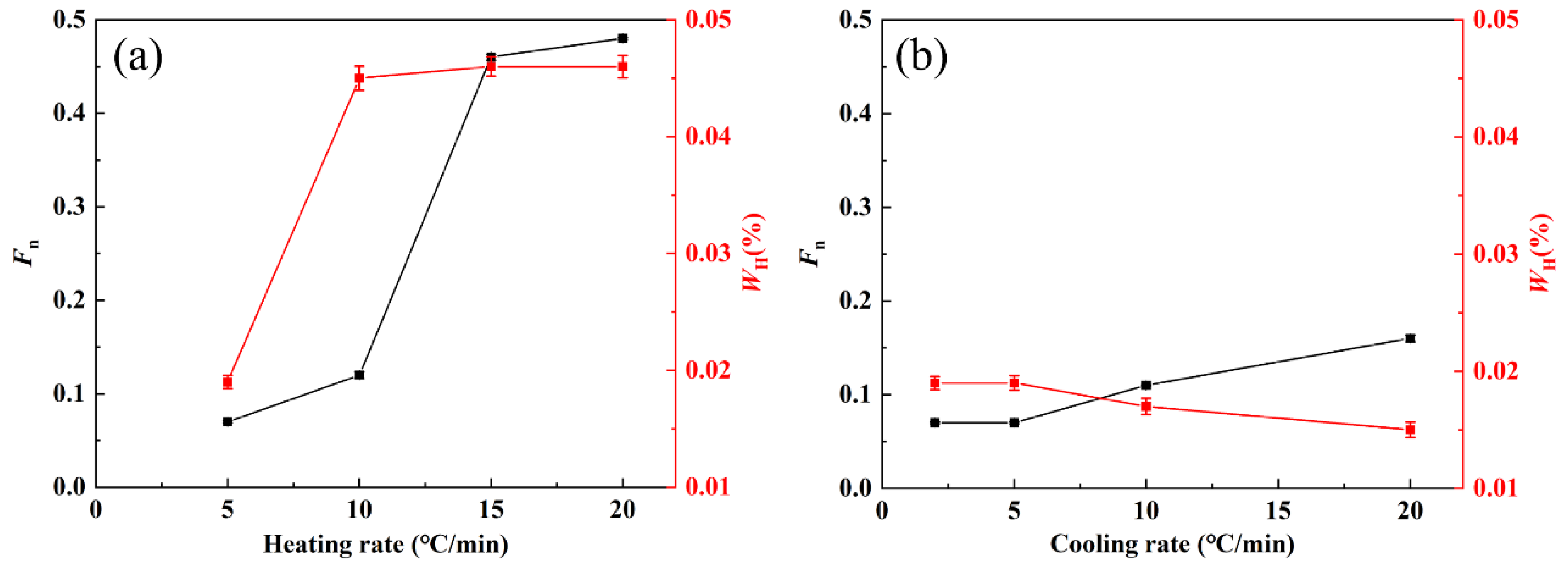
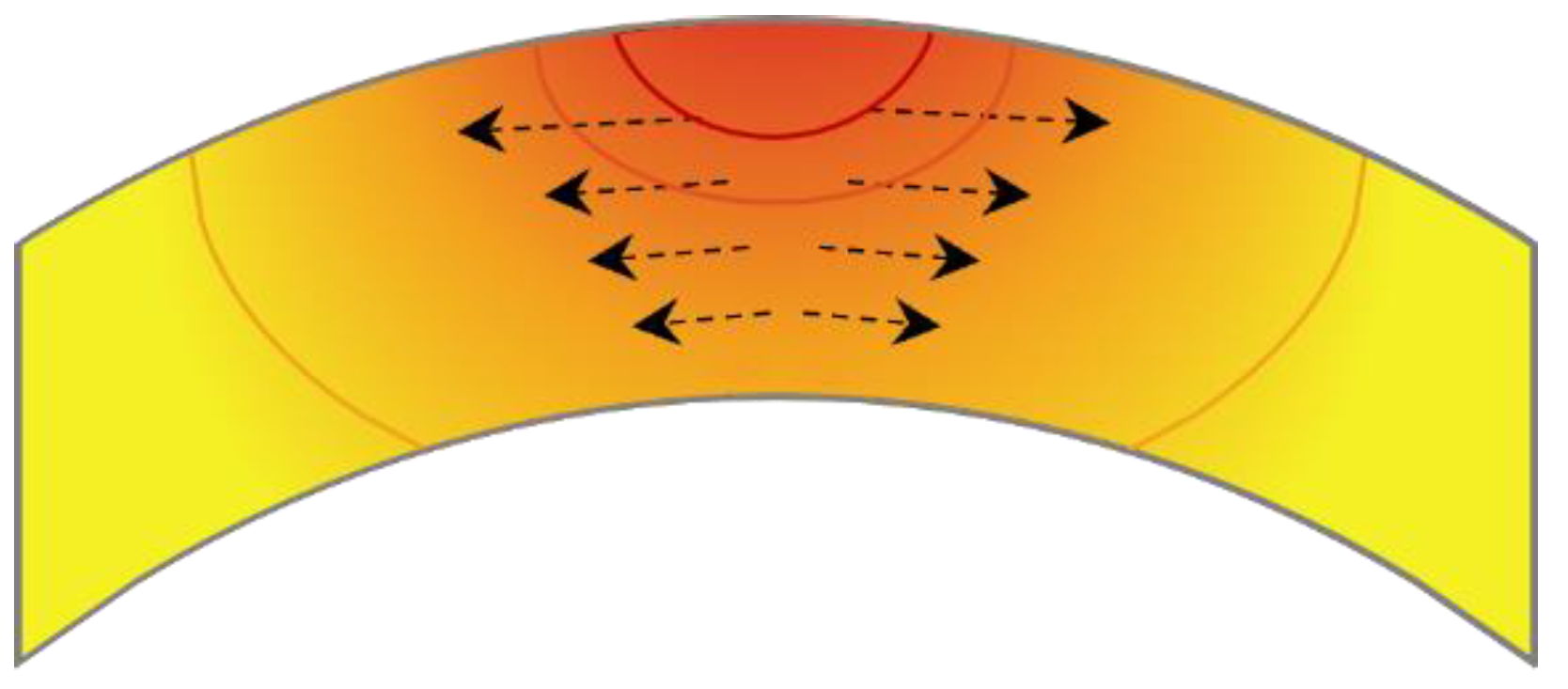
4.1. Effect of Heating Rate on Internal Stress Distribution of Zirconium Alloy Tube
4.2. Effect of Heating Rate on Hydrogen Content
4.3. Effect of Stress Distribution on Hydride Reorientation
5. Conclusions
- (1)
- With an increase in heating rate, the size and proportion of radial hydrides within the zirconium alloy envelope increase, leading to the formation of macroscopic hydrides resembling “sun spots”. Conversely, as the cooling rate increases, uniformly dispersed hydrides are formed.
- (2)
- The high heating rate induces the cladding tube to form an uneven stress gradient along the heat transfer direction, resulting in hydrogen diffusion to the high-stress region and precipitation perpendicular to the tensile stress direction.
- (3)
- The hydride and substrate exhibit the orientation relationship {0001}α∥{111}δ, <11–20>α∥<110>δ. As the heating rate increases, the orientation relationship is disrupted, making it easier for cracks to form.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kautz, E.; Gwalani, B.; Yu, Z.; Varga, T.; Geelhood, K.; Devaraj, A.; Senor, D. Investigating Zirconium Alloy Corrosion with Advanced Experimental Techniques: A Review. J. Nucl. Mater. 2023, 585, 154586. [Google Scholar] [CrossRef]
- Than, Y.R.; Grimes, R.W.; Bell, B.D.C.; Wenman, M.R. Understanding the Role of Fe, Cr and Ni in Zircaloy-2 with Special Focus on the Role of Ni on Hydrogen Pickup. J. Nucl. Mater. 2020, 530, 151956. [Google Scholar] [CrossRef]
- Kearns, J.J. Terminal solubility and partitioning of hydrogen in the alpha phase of zirconium, Zircaloy-2 and Zircaloy-4. J. Nucl. Mater. 1967, 22, 292–303. [Google Scholar] [CrossRef]
- Simpson, C.J.; Ells, C.E. Delayed hydrogen embrittlement in Zr-2.5 wt % Nb. J. Nucl. Mater. 1974, 52, 289–295. [Google Scholar] [CrossRef]
- Chu, H.C.; Wu, S.K.; Kuo, R.C. Hydride reorientation in Zircaloy-4 cladding. J. Nucl. Mater. 2008, 373, 319–327. [Google Scholar] [CrossRef]
- Simon, P.A.; Frank, C.; Chen, L.; Tonks, M.R.; Motta, A.T. Quantifying the effect of hydride microstructure on zirconium alloys embrittlement using image analysis. J. Nucl. Mater. 2021, 547, 152817. [Google Scholar] [CrossRef]
- Puls, M.P.; Shi, S.; Rabier, J. Experimental studies of mechanical properties of solid zirconium hydrides. J. Nucl. Mater. 2005, 336, 73–80. [Google Scholar] [CrossRef]
- Gopalan, A.; Bind, A.K.; Sunil, S.; Murty, T.N.; Sharma, R.K.; Samanta, A.; Soren, A.; Singh, R.N. Effect of radial hydride on room temperature fracture toughness of Zr-2.5Nb pressure tube material. J. Nucl. Mater. 2021, 544, 152681. [Google Scholar] [CrossRef]
- Carpenter, G.J.C. The precipitation of γ-zirconium hydride in zirconium. Acta Metall. 1978, 26, 1225–1235. [Google Scholar] [CrossRef]
- Carpenter, G.J.C. The dilatational misfit of zirconium hydrides precipitated in zirconium. J. Nucl. Mater. 1973, 48, 264–266. [Google Scholar] [CrossRef]
- Jia, Y.-J.; Han, W.-Z. Effect of external stress on hydride reorientation in zirconium. Acta Mater. 2022, 235, 118100. [Google Scholar] [CrossRef]
- Simon, P.A.; Aagesen, L.K.; Jokisaari, A.M.; Chen, L.; Daymond, M.R.; Motta, A.T.; Tonks, M.R. Investigation of δ zirconium hydride morphology in a single crystal using quantitative phase field simulations supported by experiments. J. Nucl. Mater. 2021, 557, 153303. [Google Scholar] [CrossRef]
- Lin, X.-H.; Beyerlein, I.J.; Han, W.-Z. Annealing cracking in Zr and a Zr-alloy with low hydrogen concentration. J. Mater. Sci. Technol. 2024, 182, 165–175. [Google Scholar] [CrossRef]
- Long, F.; Kerr, D.; Domizzi, G.; Wang, Q.; Daymond, M.R. Microstructure characterization of a hydride blister in Zircaloy-4 by EBSD and TEM. Acta Mater. 2017, 129, 450–461. [Google Scholar] [CrossRef]
- Liu, S.-M.; Li, S.-H.; Han, W.-Z. Effect of ordered helium bubbles on deformation and fracture behavior of α-Zr. J. Mater. Sci. Technol. 2019, 35, 1466–1472. [Google Scholar] [CrossRef]
- Hong, X.Y.; Ma, F.Q.; Zhang, J.Y.; Du, D.H.; Tian, H.; Xu, Q.; Zhou, J.; Gong, W.J. Effect of hydride orientation on tensile properties and crack formation in zirconium alloy cladding tubes. J. Nucl. Mater. 2024, 596, 155120. [Google Scholar] [CrossRef]
- Auzoux, Q.; Bouffioux, P.; Machiels, A.; Yagnik, S.; Bourdiliau, B.; Mallet, C.; Mozzani, N.; Colas, K. Hydride reorientation and its impact on mechanical properties of high burn-up and unirradiated cold-worked stress-relieved Zircaloy-4 and ZirloTM fuel cladding. J. Nucl. Mater. 2022, 568, 153893. [Google Scholar] [CrossRef]
- Plyasov, A.A.; Novikov, V.V.; Devyatko, Y.N. A Review of Hydride Reorientation in Zirconium Alloys for Water-Cooled Reactors. Phys. At. Nucl. 2020, 83, 1407–1424. [Google Scholar] [CrossRef]
- Woo, D.; Lee, Y. Understanding the mechanical integrity of Zircaloy cladding with various radial and circumferential hydride morphologies via image analysis. J. Nucl. Mater. 2023, 584, 154560. [Google Scholar] [CrossRef]
- Bang, S.; Kim, H.; Noh, J.; Keum, K.; Lee, Y. Temperature-dependent axial mechanical properties of Zircaloy-4 with various hydrogen amounts and hydride orientations. Nucl. Eng. Technol. 2022, 54, 1579–1587. [Google Scholar] [CrossRef]
- Kim, D.; Woo, D.; Lee, Y. Radial hydride fraction with various rod internal pressures and hydrogen contents for Zr-Nb alloy cladding tube. J. Nucl. Mater. 2022, 572, 154036. [Google Scholar] [CrossRef]
- Kearns, J.J.; Woods, C.R. Effect of texture, grain size, and cold work on the precipitation of oriented hydrides in Zircaloy tubing and plate. J. Nucl. Mater. 1966, 20, 241–261. [Google Scholar] [CrossRef]
- Alam, A.; Hellwig, C. Cladding Tube Deformation Test for Stress Reorientation of Hydrides. In Zirconium in the Nuclear Industry: 15th International Symposium; ASTM International: West Conshohocken, PA, USA, 2009; 136p. [Google Scholar]
- Kim, J.-S.; Kim, Y.-J.; Kook, D.-H.; Kim, Y.-S. A study on hydride reorientation of Zircaloy-4 cladding tube under stress. J. Nucl. Mater. 2015, 456, 246–252. [Google Scholar] [CrossRef]
- Chu, L.H.; Yuan, G.H.; Yao, M.Y.; Gao, B.; Xv, S.T.; Zhou, B.X. Effect of Impurity Elements (C, Si) on Microstructure and Corrosion Resistance of Zircaloy-4 Sheet. Rare Met. Mater. Eng. 2020, 49, 3338–3346. [Google Scholar]
- Hui, B.N.; Liang, W.; Zhou, J.; Liu, H.M.; Wen, H.M.; Li, F.; Shang, X.D.; Wei, Q.; Liu, J.; Yang, Y.H. The Invention Relates to a High Pressure Gas Permeating Device and Method for Nuclear Zirconium Alloy Material. CN202210587863.8, 26 May 2022. [Google Scholar]
- ASTM B811-13(2022)e1; B10 Committee, Wrought Zirconium Alloy Seamless Tubes for Nuclear Reactor Fuel Cladding. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Bradbrook, J.S.; Lorimer, G.W.; Ridley, N. The precipitation of zirconium hydride in zirconium and zircaloy-2. J. Nucl. Mater. 1972, 42, 142–160. [Google Scholar] [CrossRef]
- Maric, M.; Thomas, R.; Davis, A.; Lunt, D.; Donoghue, J.; Gholinia, A.; De Graef, M.; Ungar, T.; Barberis, P.; Bourlier, F.; et al. Identification, classification and characterisation of hydrides in Zr alloys. Scr. Mater. 2024, 238, 115768. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Wu, H.; Chen, G. Microstructural and crystallographic analysis of hydride reorientation in a zirconium alloy cladding tube. J. Nucl. Mater. 2020, 537, 152232. [Google Scholar] [CrossRef]
- Qin, W.; Kumar, N.A.P.K.; Szpunar, J.A.; Kozinski, J. Intergranular δ-hydride nucleation and orientation in zirconium alloys. Acta Mater. 2011, 59, 7010–7021. [Google Scholar] [CrossRef]
- UNE, K.; Ishimoto, S. Terminal Solid Solubility of Hydrogen in Unalloyed Zirconium by Differential Scanning Calorimetry. J. Nucl. Sci. Technol. 2004, 41, 949–952. [Google Scholar] [CrossRef]
- Ells, C.E.; Simpson, C.J. Hydrogen in Metals; ASM: Metals Park, OH, USA, 1974. [Google Scholar]
- Plus, M.P. The Effect of Hydrogen and Hydrides on the Integrity of Zirconium Alloy Components: Delayed Hydride Cracking; Springer: London, UK, 2012. [Google Scholar]
- Nantes, K.R.B.; Jin, M.; Motta, A.T. Modeling hydrogen localization in Zircaloy cladding subjected to temperature gradients. J. Nucl. Mater. 2024, 589, 154853. [Google Scholar] [CrossRef]
- Motta, A.T.; Capolungo, L.; Chen, L.-Q.; Cinbiz, M.N.; Daymond, M.R.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simon, P.-C.A.; Tonks, M.R.; et al. Hydrogen in zirconium alloys: A review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef]





| Zr | Sn | Nb | Fe | O |
|---|---|---|---|---|
| Matrix | 1.0% | 1.0% | 0.3% | 0.11% |
| Sample | Heating Rate (°C/min) | Holding Temperature (°C) | Hold Time (h) | Cooling Rate (°C/min) |
|---|---|---|---|---|
| No. 1 | 5 | 400 | 2 | 2 |
| No. 2 | 5 | 500 | 2 | 2 |
| No. 3 | 5 | 600 | 2 | 2 |
| No. 4 | 10 | 400 | 2 | 2 |
| No. 5 | 15 | 400 | 2 | 2 |
| No. 6 | 20 | 400 | 2 | 2 |
| No. 7 | 5 | 400 | 2 | 5 |
| No. 8 | 5 | 400 | 2 | 10 |
| No. 9 | 5 | 400 | 2 | 20 |
| Sample | Heating Rate (°C/min) | Cooling Rate (°C/min) | WH (%) | |
|---|---|---|---|---|
| No. 1 | 5 | 2 | 0.071 ± 0.0010 | 0.0190 ± 0.0006 |
| No. 4 | 10 | 2 | 0.122 ± 0.0036 | 0.0451 ± 0.0010 |
| No. 5 | 15 | 2 | 0.461 ± 0.0032 | 0.0460 ± 0.0008 |
| No. 6 | 20 | 2 | 0.482 ± 0.0010 | 0.0464 ± 0.0010 |
| No. 7 | 5 | 5 | 0.070 ± 0.0012 | 0.0190 ± 0.0006 |
| No. 8 | 5 | 10 | 0.112 ± 0.0006 | 0.0170 ± 0.0007 |
| No. 9 | 5 | 20 | 0.159 ± 0.0036 | 0.0149 ± 0.0007 |
| Sample | Intra-Granular Proportion | Inter-Granular Proportion |
|---|---|---|
| No. 1 | 67.19% | 32.80% |
| No. 6-Inside | 47.33% | 52.67% |
| Sample Region | No. 1 | No. 4 | No. 6 |
|---|---|---|---|
| Outside | 9.38% | 14.05% | 23.04% |
| Inside | 10.58% | 12.25% | 10.77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, B.; Chen, M.; Li, X.; Chen, B.; Li, Y.; Zhou, J.; Tang, R.; Li, J. Effect of Heating Rate on Hydride Reorientation Behavior of Zirconium Alloy Tubes under Non-Stress Loading. Metals 2024, 14, 1126. https://doi.org/10.3390/met14101126
Hui B, Chen M, Li X, Chen B, Li Y, Zhou J, Tang R, Li J. Effect of Heating Rate on Hydride Reorientation Behavior of Zirconium Alloy Tubes under Non-Stress Loading. Metals. 2024; 14(10):1126. https://doi.org/10.3390/met14101126
Chicago/Turabian StyleHui, Boning, Mingju Chen, Xinyi Li, Biao Chen, Yuli Li, Jun Zhou, Rongtao Tang, and Jinshan Li. 2024. "Effect of Heating Rate on Hydride Reorientation Behavior of Zirconium Alloy Tubes under Non-Stress Loading" Metals 14, no. 10: 1126. https://doi.org/10.3390/met14101126








