Deformation Behaviors and Microstructure Evolution of Mg-Zn-Y-Zr Alloys During Hot Compression Process
Abstract
1. Introduction
2. Materials and Methods
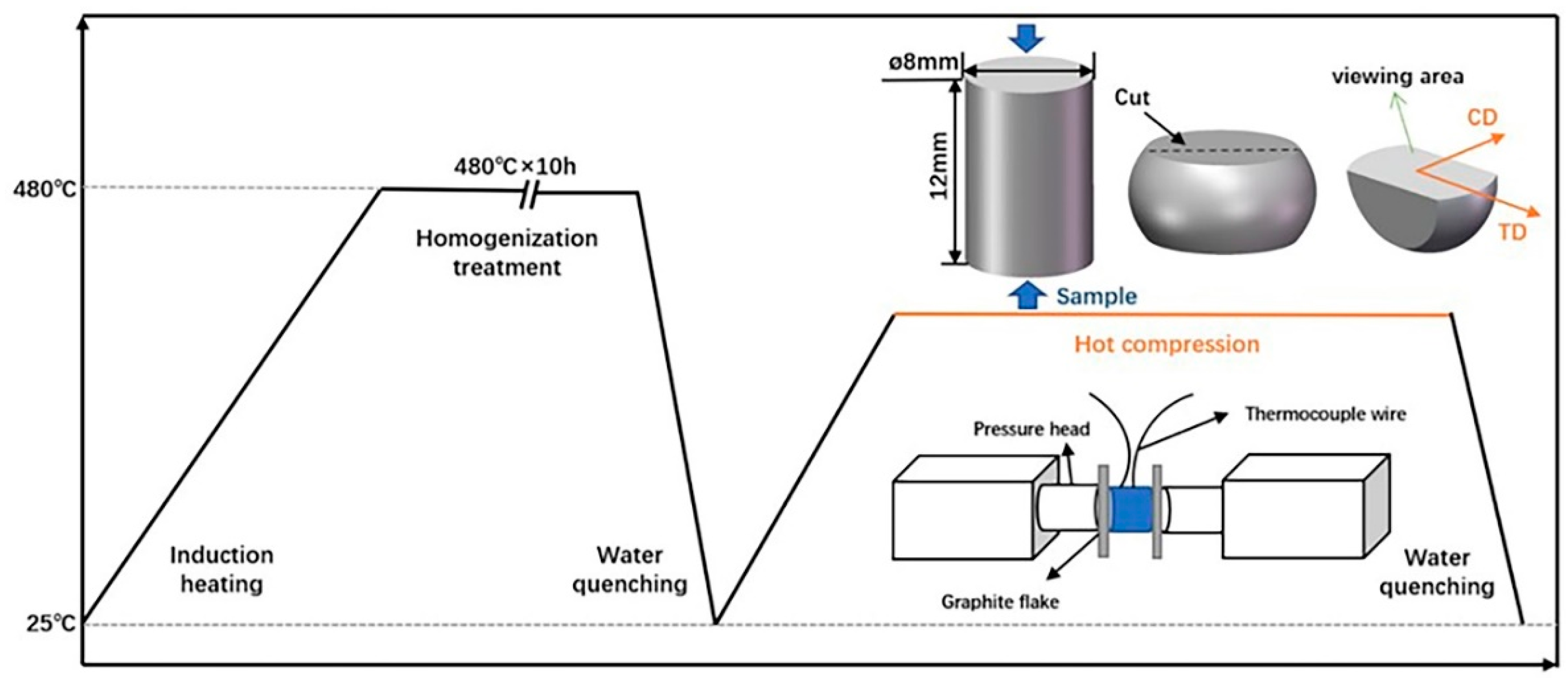
2.1. Materials Preparation
2.2. Thermal Compression Test
2.3. Characterization
3. Results and Discussion
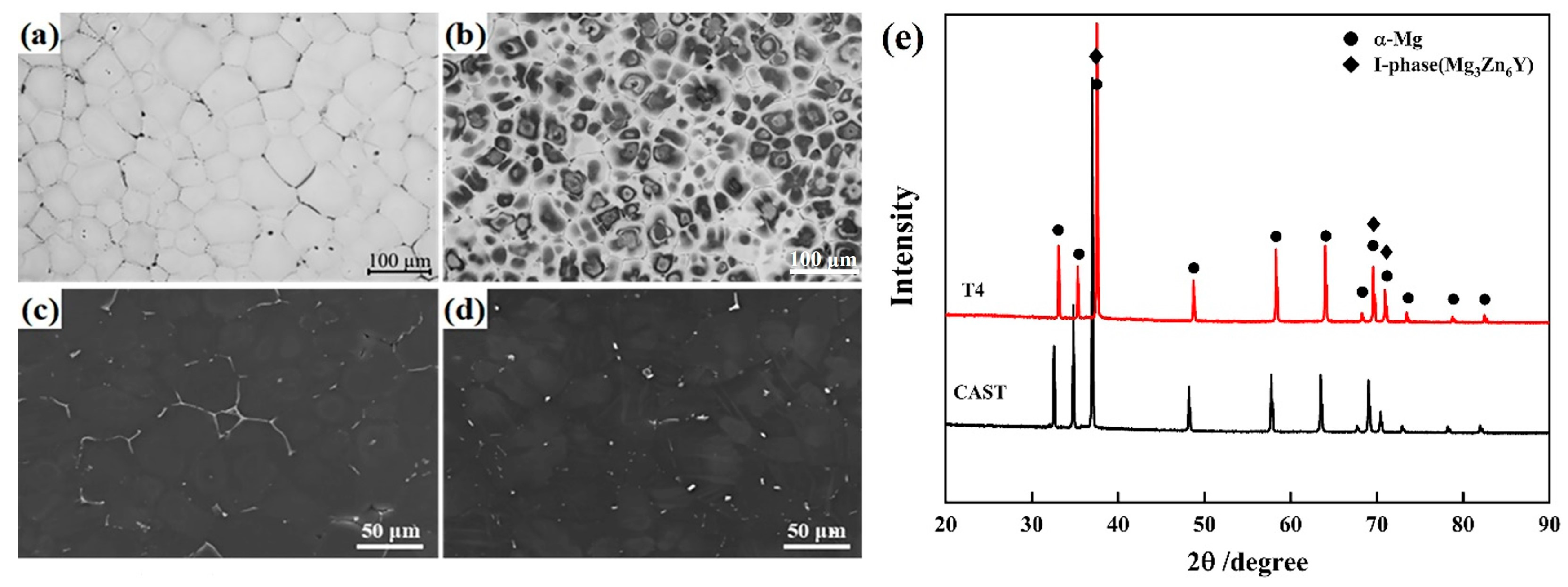
3.1. Microstructure of Initial State
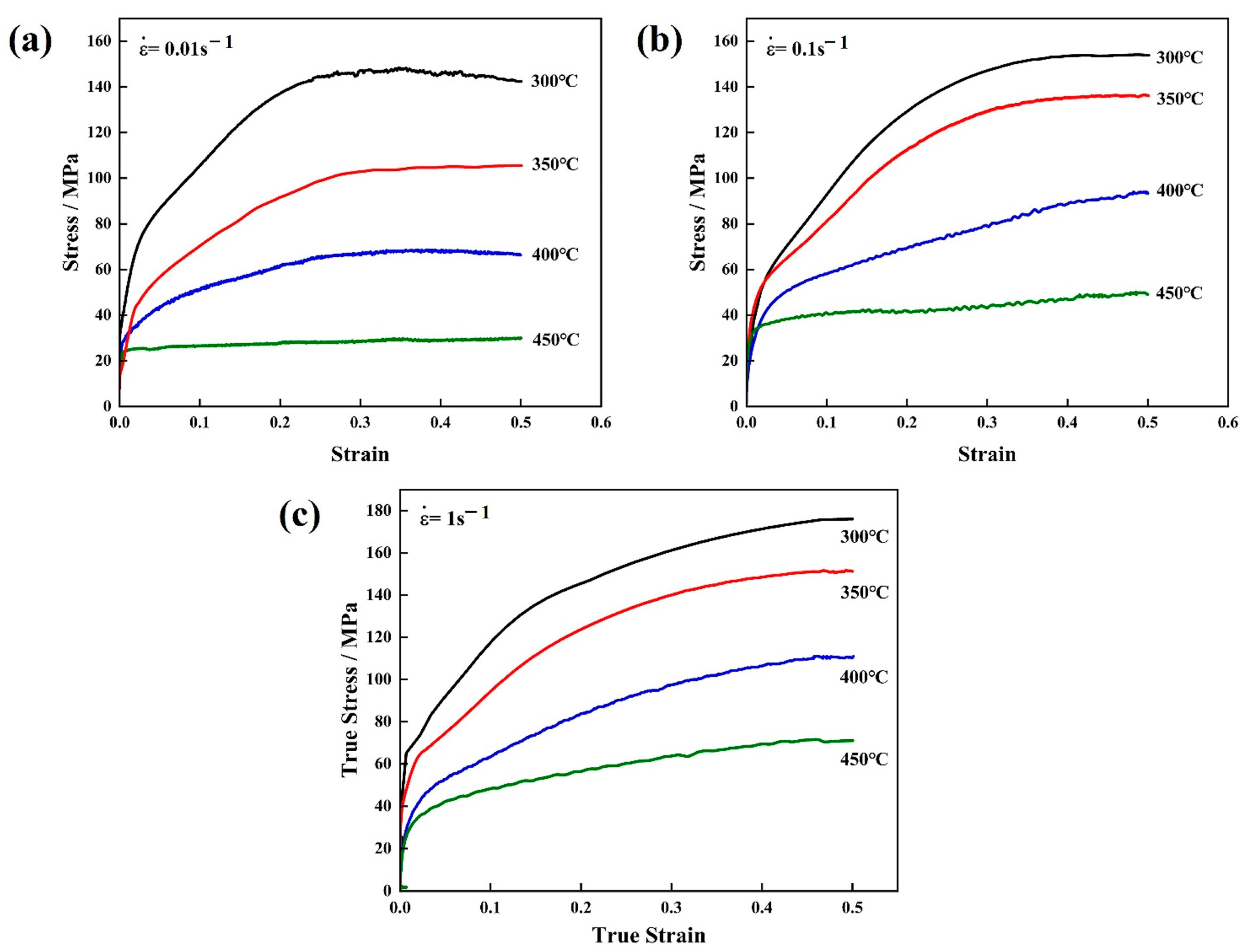
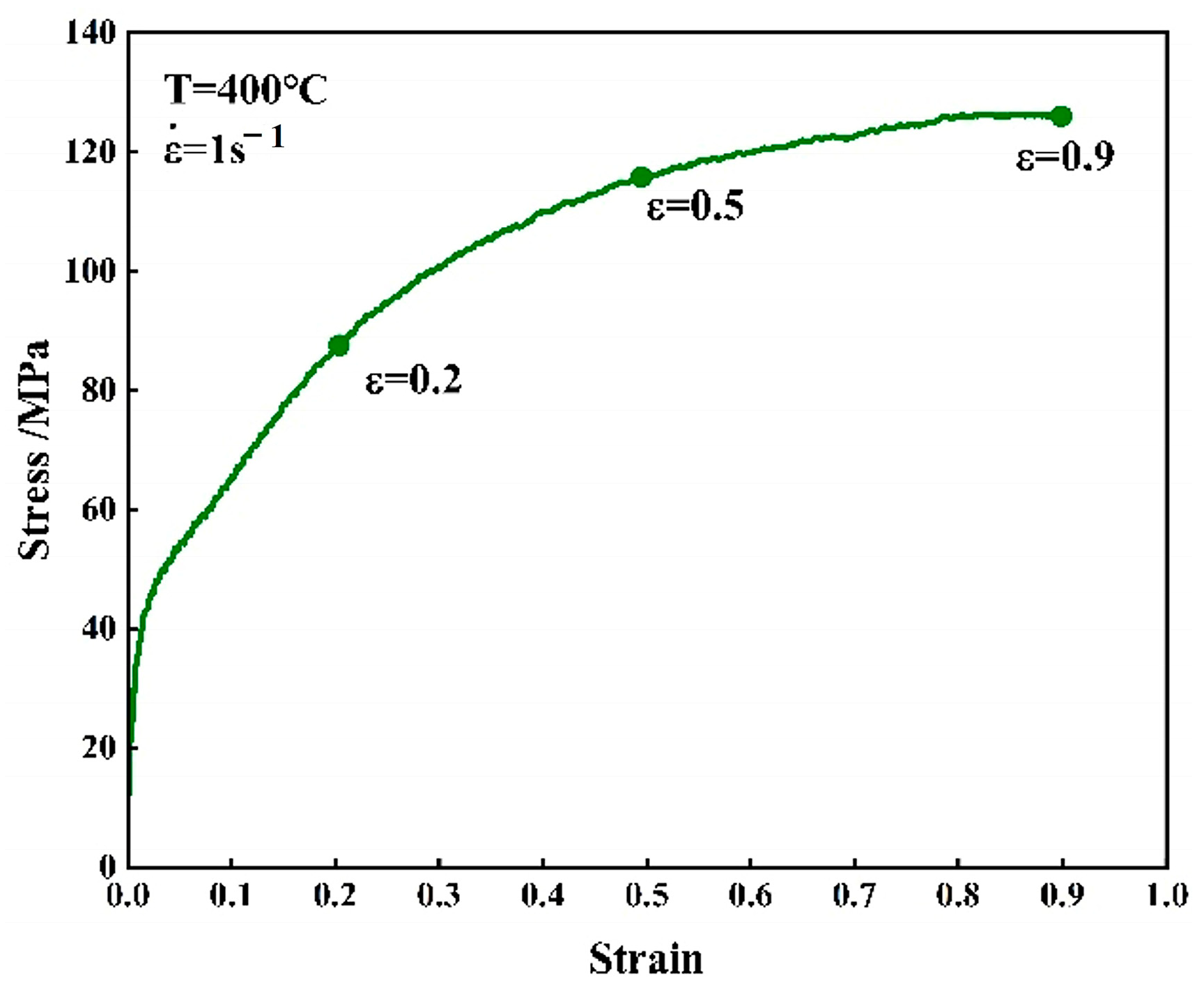
3.2. True Stress–Strain Curves
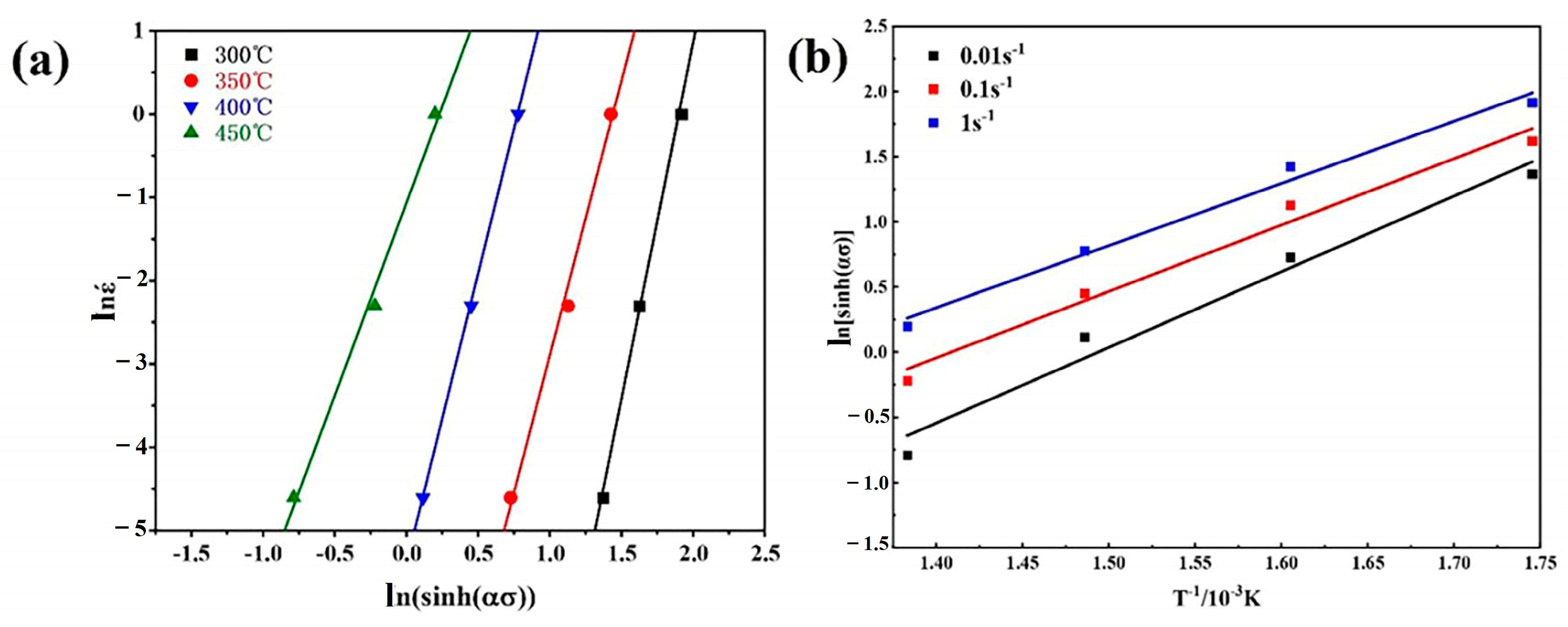
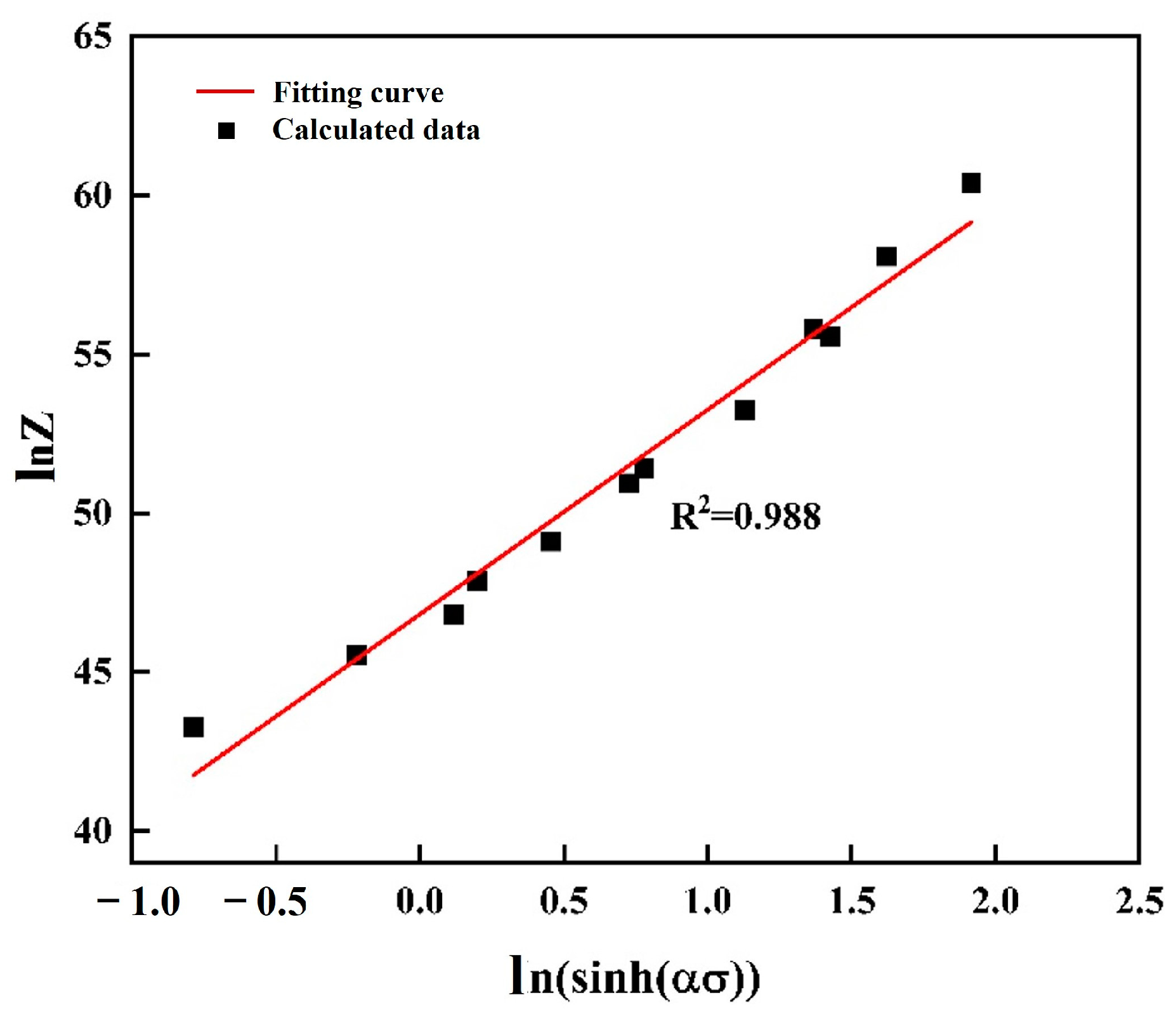
3.3. Constitutive Strain-Dependent Equation
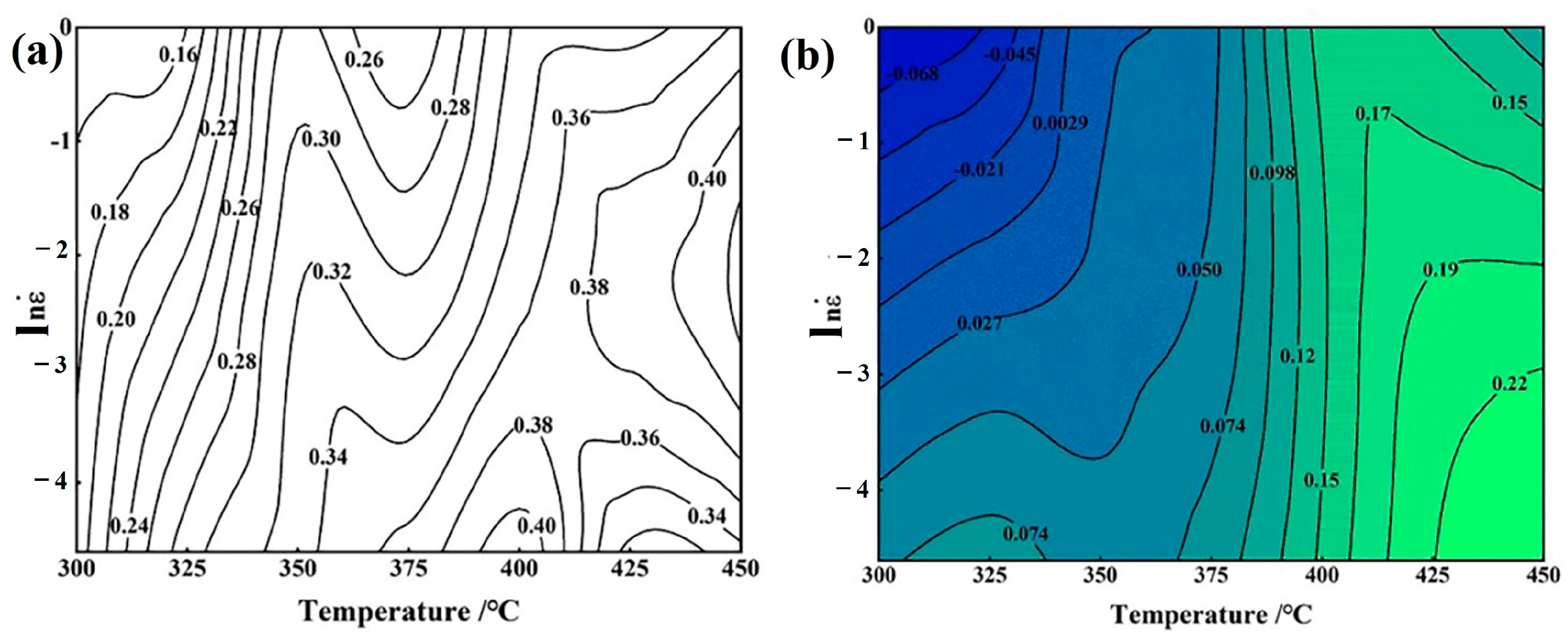
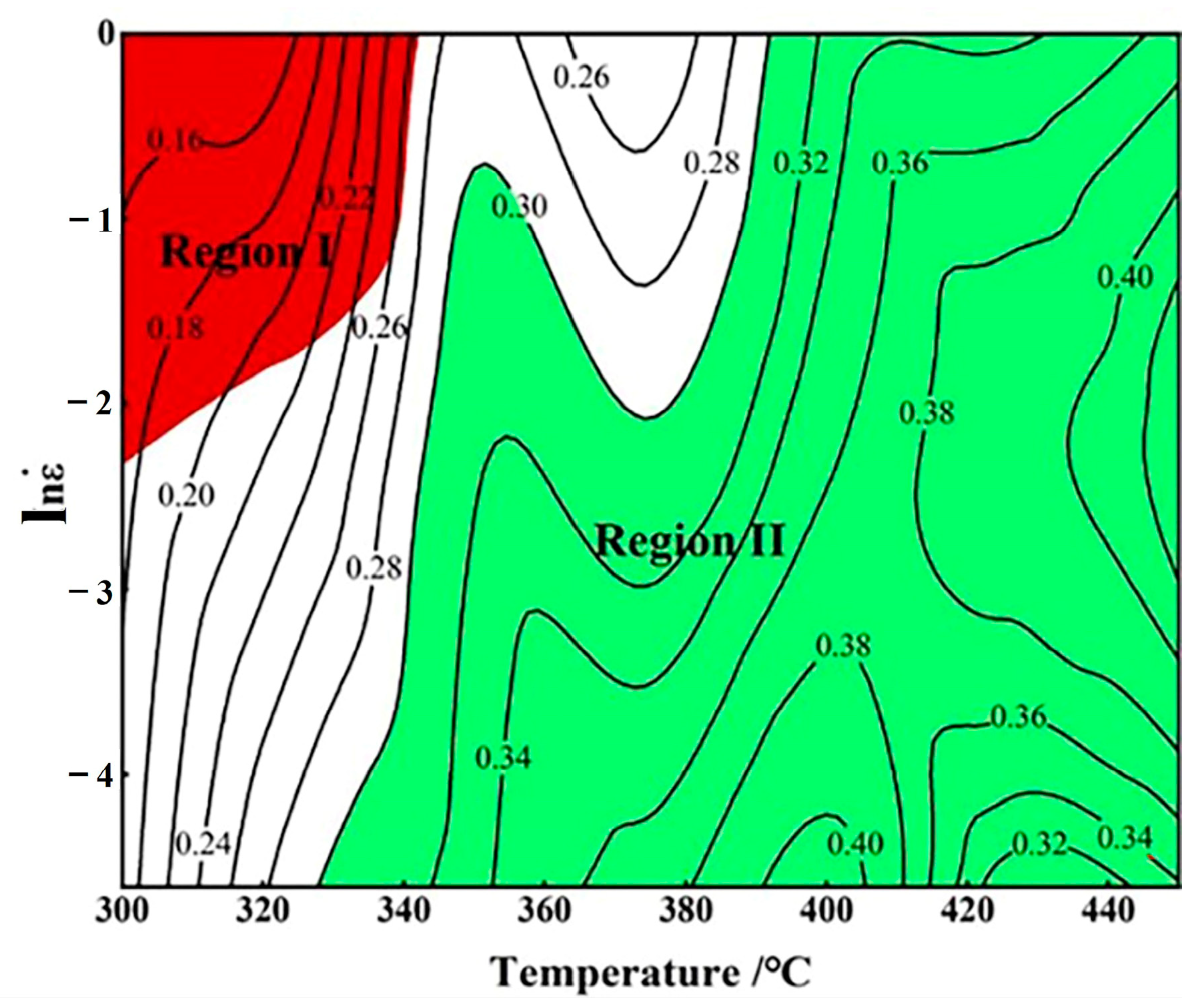
3.4. Thermal Processing Maps
3.5. Microstructure Evolution
3.5.1. Degree of Deformation
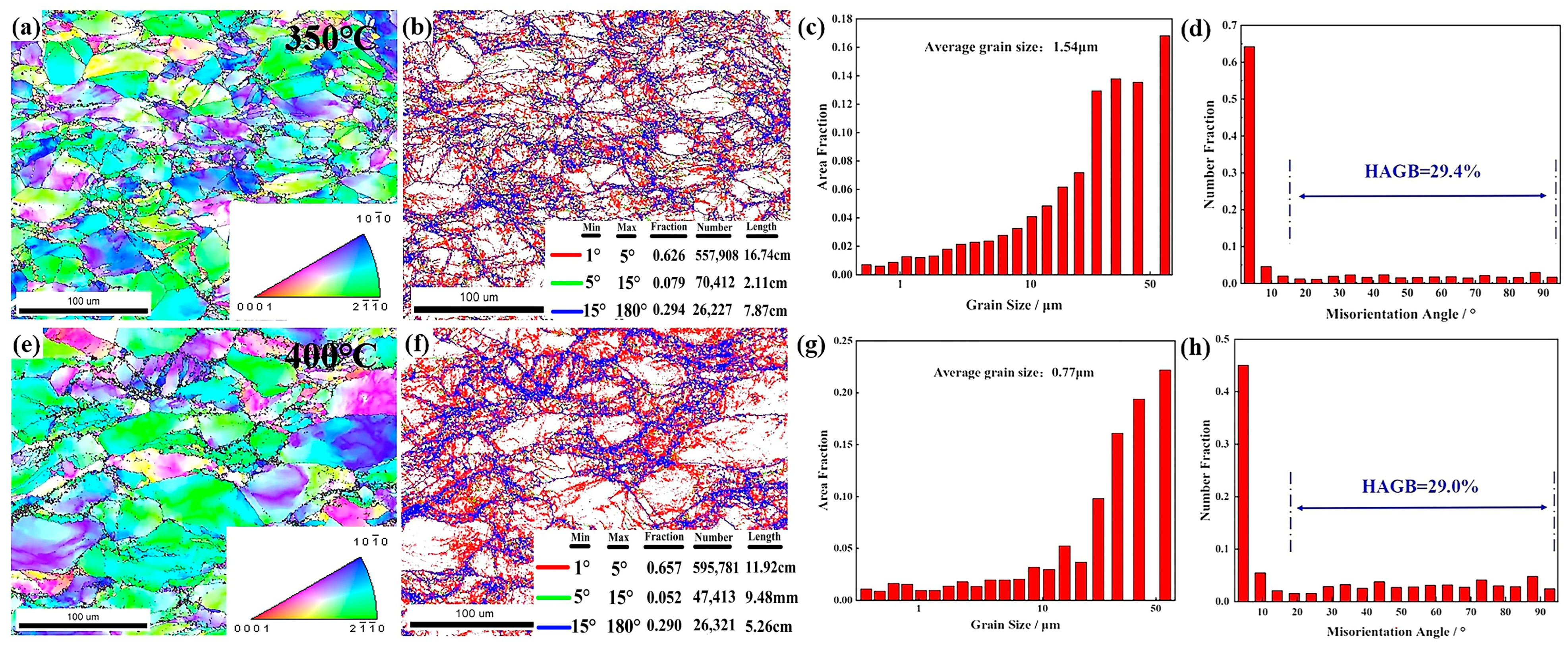
3.5.2. Deformation Temperature
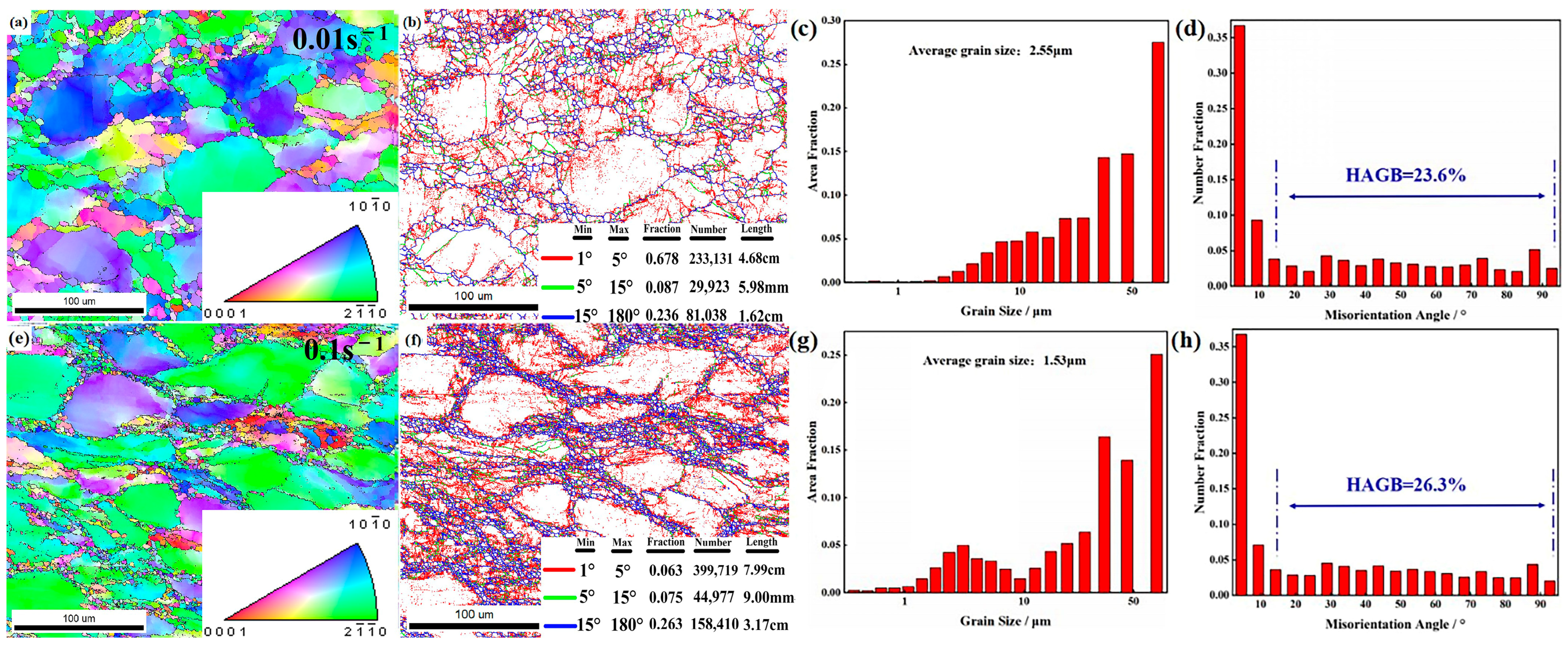
3.5.3. Strain Rate
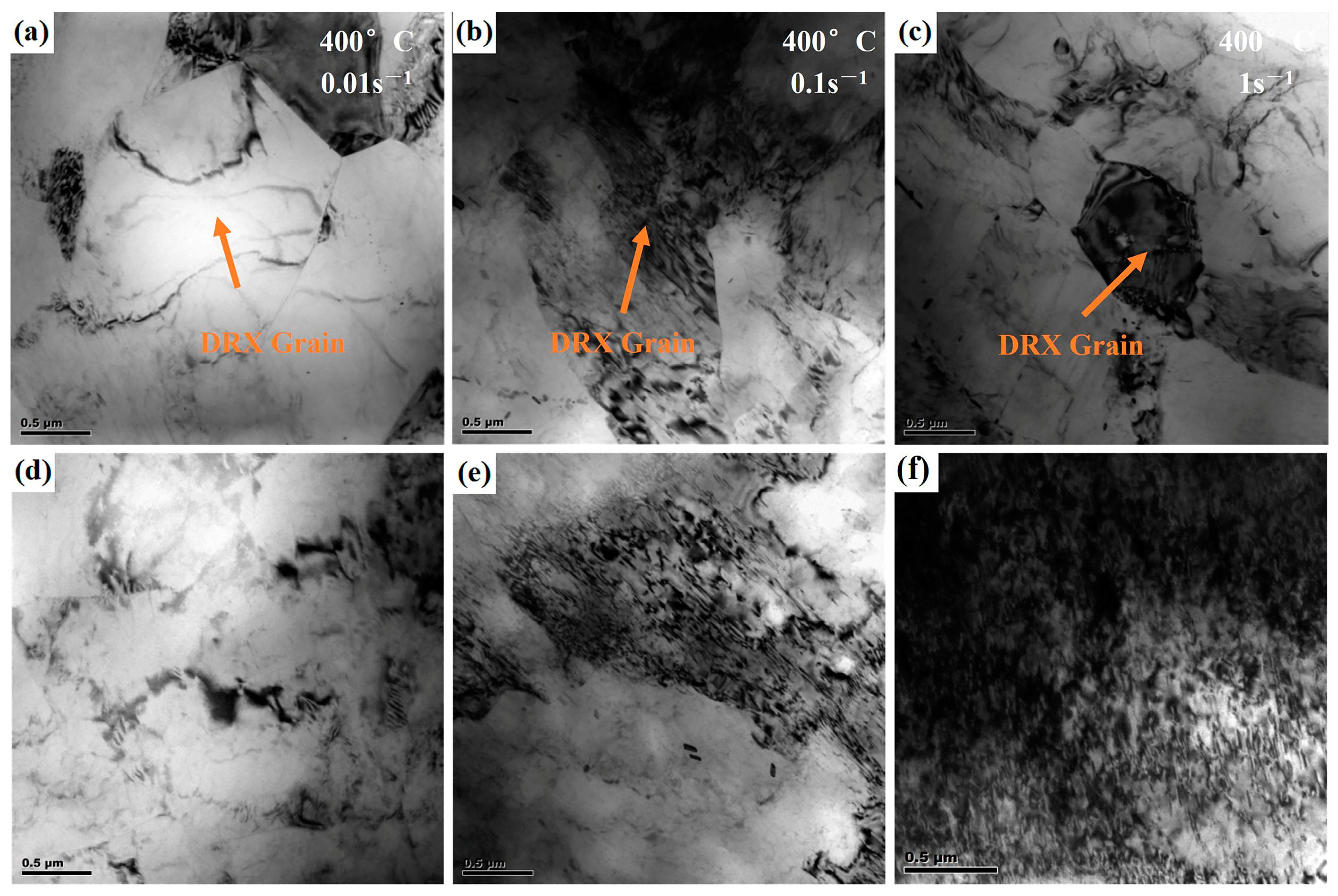
3.6. Dynamic Recrystallization Mechanism Analysis
4. Conclusions
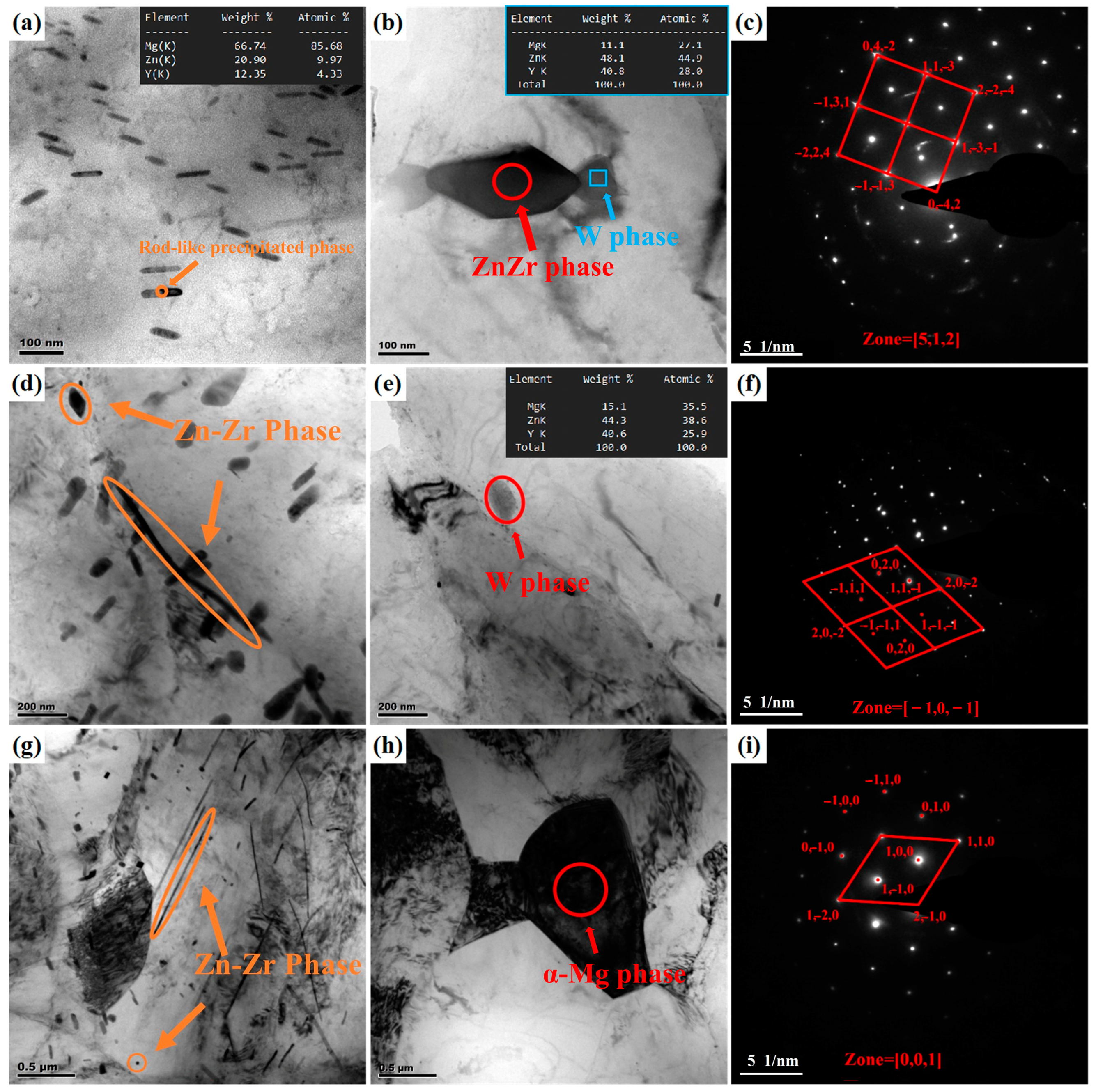
- As-cast ZW305K alloy is mainly composed of α-Mg matrix and I-phase and Zn-Zr phase distributed at the grain boundaries. After solution treatment, most of the I-phase on the grain boundary is dissolved into the matrix, and the Zn-Zr phase exists in α-Mg matrix in the form of a precipitated phase. After hot compression deformation, a large number of dispersed second phases appear, which hinders dislocation movement and strengthens the alloy. As the strain rate decreases, the I-phase in the microstructure gradually transforms into the W-phase, and its inhibitory effect on the growth of DRX grains gradually weakens.
- The activation energy for deformation of ZW305K was determined to be Q = 287 kJ/mol. The constitutive equation obtained is as follows:
- Based on the hot processing map, material instability primarily occurs at low temperatures and high strain rates. An increase in temperature or a decrease in strain rate is conducive to the occurrence of dynamic recrystallization, leading to improved processability of the material. The optimal processing range is 400 °C at 0.01 s−1 and 450 °C at 0.1 s−1.
- The higher the deformation temperature, the lower the strain rate, and the greater the amount of deformation, the higher the degree of recrystallization. At 350 °C and 1 s−1, TDRX nucleation occurs in the microstructure. As the temperature increases and the strain rate decreases, CDRX and DDRX, primarily nucleating at grain boundaries, become the dominant phenomena in the microstructure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, J.; Lin, T.; Shao, H.; Wang, H.; Wang, X.; Song, K.; Li, Q. Advances in degradation behavior of biomedical magnesium alloys: A review. J. Alloys Compd. 2022, 908, 164600. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Wang, Z.; Hu, C.; Yu, H.; Chen, C. Research progress of biodegradable magnesium-based biomedical materials: A review. J. Alloys Compd. 2022, 923, 166377. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Song, J.; Pan, F.; Willumeit-Römer, R.; Kainer, K.U.; Hort, N. Development and prospects of degradable magnesium alloys for structural and functional applications in the fields of environment and energy. J. Magnes. Alloys 2023, 11, 3926–3947. [Google Scholar] [CrossRef]
- Bai, H.; He, X.; Ding, P.; Liu, D.; Chen, M. Fabrication, microstructure, and properties of a biodegradable Mg-Zn-Ca clip. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Ikeo, N.; Nakamura, R.; Naka, K.; Hashimoto, T.; Yoshida, T.; Urade, T.; Fukushima, K.; Yabuuchi, H.; Fukumoto, T.; Ku, Y.; et al. Fabrication of a magnesium alloy with excellent ductility for biodegradable clips. Acta Biomater. 2016, 29, 468–476. [Google Scholar] [CrossRef]
- Savaedi, Z.; Motallebi, R.; Mirzadeh, H.; Mehdinavaz Aghdam, R.; Mahmudi, R. Superplasticity of fine-grained magnesium alloys for biomedical applications: A comprehensive review. Curr. Opin. Solid State Mater. Sci. 2023, 27, 101058. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Wu, R.; Hou, L.; Zhang, M. Recent developments in high-strength Mg-RE-based alloys: Focusing on Mg-Gd and Mg-Y systems. J. Magnes. Alloys 2018, 6, 277–291. [Google Scholar] [CrossRef]
- Li, H.-z.; Liu, H.-t.; Li, F.-b.; Wang, H.-j.; Liang, X.-p.; Liu, C.-m. Microstructures and Properties of Extruded Mg-Gd-Y-Zr Alloys Containing Zn. J. Mater. Eng. Perform. 2011, 21, 1056–1060. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wang, Z.; Le, Q.; Hu, W.; Bao, L.; Cui, J. Effects of phase composition and content on the microstructures and mechanical properties of high strength Mg–Y–Zn–Zr alloys. Mater. Des. 2015, 88, 915–923. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Koltygin, A.V.; Sung, M.C.; Park, S.H.; Tselovalnik, Y.V.; Stepashkin, A.A.; Rizhsky, A.A.; Belov, M.V.; Belov, V.D.; Malyutin, K.V. Development of Mg–Zn–Y–Zr casting magnesium alloy with high thermal conductivity. J. Magnes. Alloys 2021, 9, 1567–1577. [Google Scholar] [CrossRef]
- Li, P.; Sun, Y.; Zhu, S.; Wang, L.; Guan, S.; Wang, J. Microstructure, corrosion and mechanical properties of as-cast Mg-Zn-Y with different Zn-to-Y ratios. Mater. Today Commun. 2023, 37, 107562. [Google Scholar] [CrossRef]
- Singh, A.; Watanabe, M.; Kato, A.; Tsai, A.P. Strengthening in magnesium alloys by icosahedral phase. Sci. Technol. Adv. Mater. 2016, 6, 895–901. [Google Scholar] [CrossRef][Green Version]
- Huang, Z.H.; Liang, S.M.; Chen, R.S.; Han, E.H. Solidification pathways and constituent phases of Mg–Zn–Y–Zr alloys. J. Alloys Compd. 2009, 468, 170–178. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hashimoto, K.; Hagihara, K.; Kawamura, Y. Effect of multimodal microstructure evolution on mechanical properties of Mg–Zn–Y extruded alloy. Acta Mater. 2011, 59, 3646–3658. [Google Scholar] [CrossRef]
- Huang, H.; Miao, H.; Yuan, G.; Wang, Z.; Ding, W. Fabrication of ultra-high strength magnesium alloys over 540 MPa with low alloying concentration by double continuously extrusion. J. Magnes. Alloys 2018, 6, 107–113. [Google Scholar] [CrossRef]
- Pan, H.; Qin, G.; Huang, Y.; Ren, Y.; Sha, X.; Han, X.; Liu, Z.-Q.; Li, C.; Wu, X.; Chen, H.; et al. Development of low-alloyed and rare-earth-free magnesium alloys having ultra-high strength. Acta Mater. 2018, 149, 350–363. [Google Scholar] [CrossRef]
- Hu, K.; Li, C.; Xu, G.; Guo, R.; Le, Q.; Liao, Q. Effect of extrusion temperature on the microstructure and mechanical properties of low Zn containing wrought Mg alloy micro-alloying with Mn and La-rich misch metal. Mater. Sci. Eng. A 2019, 742, 692–703. [Google Scholar] [CrossRef]
- Nakata, T.; Mezaki, T.; Xu, C.; Oh-ishi, K.; Shimizu, K.; Hanaki, S.; Kamado, S. Improving tensile properties of dilute Mg-0.27Al-0.13Ca-0.21Mn (at.%) alloy by low temperature high speed extrusion. J. Alloys Compd. 2015, 648, 428–437. [Google Scholar] [CrossRef]
- Stanford, N.; Callaghan, M.D.; de Jong, B. The effect of rare earth elements on the behaviour of magnesium-based alloys: Part 1—Hot deformation behaviour. Mater. Sci. Eng. A 2013, 565, 459–468. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, K.; Li, X.; Ma, M.; Li, Y. Microstructure and texture of coarse-grained Mg–Gd–Y–Nd–Zr alloy after hot compression. Mater. Des. 2013, 44, 521–527. [Google Scholar] [CrossRef]
- Jonas, J.J.; Aranas, C.; Fall, A.; Jahazi, M. Transformation softening in three titanium alloys. Mater. Des. 2017, 113, 305–310. [Google Scholar] [CrossRef]
- Ning, Y.Q.; Luo, X.; Liang, H.Q.; Guo, H.Z.; Zhang, J.L.; Tan, K. Competition between dynamic recovery and recrystallization during hot deformation for TC18 titanium alloy. Mater. Sci. Eng. A 2015, 635, 77–85. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Huang, Y.; Zhou, H.; Qin, S.; Liu, J. Hot deformation behavior and processing map of vanadium particles reinforced AZ31 composite. J. Alloys Compd. 2024, 999, 175047. [Google Scholar] [CrossRef]
- Yin, L.; Wu, Y. Comparison of Constitutive Models and Microstructure Evolution of GW103K Magnesium Alloy during Hot Deformation. Materials 2022, 15, 4116. [Google Scholar] [CrossRef]
- Xia, X.; Chen, Q.; Huang, S.; Lin, J.; Hu, C.; Zhao, Z. Hot deformation behavior of extruded Mg–Zn–Y–Zr alloy. J. Alloys Compd. 2015, 644, 308–316. [Google Scholar] [CrossRef]
- Singh, A.; Somekawa, H.; Mukai, T. High temperature processing of Mg–Zn–Y alloys containing quasicrystal phase for high strength. Mater. Sci. Eng. A 2011, 528, 6647–6651. [Google Scholar] [CrossRef]
- Singh, A.; Watanabe, M.; Kato, A.; Tsai, A.P. Formation of icosahedral–hexagonal H phase nano-composites in Mg–Zn–Y alloys. Scr. Mater. 2004, 51, 955–960. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, Y.; Li, H.; Zhang, D. Creep behavior and dynamic precipitation of highly heat-resistant Mg alloy with low rare-earth content. J. Alloys Compd. 2024, 997, 174960. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, R.; Yan, C.; Xin, Y.; Feng, B.; Xu, J.; Huang, G.; Zhao, L. Mechanical properties and corrosion behavior of a ZK60 magnesium alloy containing profuse twins and precipitates. J. Mater. Res. Technol. 2024, 29, 1767–1778. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Qi, M.; Liao, L.; Gao, Z. Enhanced mechanical properties of Mg-Zn-Y-Zr alloy by low-speed indirect extrusion. J. Mater. Res. Technol. 2020, 9, 9856–9867. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, M.; Li, Q.; Chen, X.; Zheng, Z. Effect of long-period stacking ordered phase on dynamic recrystallization in Mg-Y-Zn-Zr alloy processed by backward extrusion. J. Mater. Res. Technol. 2023, 26, 2384–2393. [Google Scholar] [CrossRef]
- Sun, W.; Deng, Y.; Zhan, H.; Zhou, X.; Yao, Y.; Liu, W.; Zeng, G. Interaction of dynamic precipitation and dynamic recrystallization of a Mg-4Sn-3Al-1Zn alloy during hot compression. J. Alloys Compd. 2024, 970, 172434. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, S.; Wang, J.; Chen, L.; Jin, P. Hot deformation behavior, processing map, microstructure evolution and dynamic recrystallization mechanism of Mg-5Al-0.6Sc alloy. J. Alloys Compd. 2022, 922, 166244. [Google Scholar] [CrossRef]
- Li, H.Z.; Wang, H.J.; Li, Z.; Liu, C.M.; Liu, H.T. Flow behavior and processing map of as-cast Mg–10Gd–4.8Y–2Zn–0.6Zr alloy. Mater. Sci. Eng. A 2010, 528, 154–160. [Google Scholar] [CrossRef]
- Ebrahimpourghandi, B.; Mahmudi, R. Hot deformation constitutive analysis and processing maps of the as-cast and wrought Mg–2.5Gd–0.5Zr alloy. J. Alloys Compd. 2023, 942, 169132. [Google Scholar] [CrossRef]
- Savaedi, Z.; Motallebi, R.; Mirzadeh, H. A review of hot deformation behavior and constitutive models to predict flow stress of high-entropy alloys. J. Alloys Compd. 2022, 903, 163964. [Google Scholar] [CrossRef]
- Tahreen, N.; Zhang, D.F.; Pan, F.S.; Jiang, X.Q.; Li, D.Y.; Chen, D.L. Texture evolution and deformation activity of an extruded magnesium alloy: Effect of yttrium and deformation temperature. J. Alloys Compd. 2016, 688, 270–284. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Q.; Chen, R.; Wang, X.; Chen, D.; Fu, H. Hot deformation and constitutive equation for ultrasonic treated Nb-Si-Ti-Gd refractory alloy. Int. J. Refract. Met. Hard Mater. 2023, 112, 106129. [Google Scholar] [CrossRef]
- Zhou, H.T.; Li, Q.B.; Zhao, Z.K.; Liu, Z.C.; Wen, S.F.; Wang, Q.D. Hot workability characteristics of magnesium alloy AZ80—A study using processing map. Mater. Sci. Eng. A 2010, 527, 2022–2026. [Google Scholar] [CrossRef]
- Prasad, Y.V.R.K.; Gegel, H.L.; Doraivelu, S.M.; Malas, J.C.; Morgan, J.T.; Lark, K.A.; Barker, D.R. Modeling of dynamic material behavior in hot deformation: Forging of Ti-6242. Metall. Trans. A 1984, 15, 1883–1892. [Google Scholar] [CrossRef]
- Li, C.; Huang, L.; Zhao, M.; Guo, S.; Su, Y.; Li, J. Characterization of hot workability of Ti-6Cr-5Mo-5V-4Al alloy based on hot processing map and microstructure evolution. J. Alloys Compd. 2022, 905, 164161. [Google Scholar] [CrossRef]
- Long, J.; Deng, L.; Jin, J.; Zhang, M.; Tang, X.; Gong, P.; Wang, X.; Xiao, G.; Xia, Q. Enhancing constitutive description and workability characterization of Mg alloy during hot deformation using machine learning-based Arrhenius-type model. J. Magnes. Alloys 2024, 12, 3003–3023. [Google Scholar] [CrossRef]
- Ding, N.; Du, W.; Li, S.; Liu, K.; Du, X.; Yu, Z. Strain rate dependence of dynamic recrystallization and texture evolution in hot compressed Mg-Gd-Er-Zr alloy. J. Magnes. Alloys 2023. [Google Scholar] [CrossRef]
- Li, Z.; Wu, G.; Yu, J.; Wang, J.; Han, J.; Cui, H.; Zhang, Z. Influence of strain rate on grain refinement and texture evolution under complex shear stress conditions. J. Magnes. Alloys 2022, 11, 2558–2584. [Google Scholar] [CrossRef]
- Liu, J.F.; Yang, Z.Q.; Ye, H.Q. In situ transmission electron microscopy investigation of quasicrystal-crystal transformations in Mg–Zn–Y alloys. J. Alloys Compd. 2015, 621, 179–188. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Meng, X.; Wan, L.; Feng, J. Microstructural evolution and mechanical properties of Mg Zn Y Zr alloy during friction stir processing. J. Alloys Compd. 2017, 696, 875–883. [Google Scholar] [CrossRef]
- Liao, Q.; Hu, W.; Chen, R.; Le, Q. Effect of Zn/Y atomic ratio on precipitation behavior and dynamic recrystallization behavior of Mg–Zn–Y alloy under different extrusion temperature. J. Mater. Res. Technol. 2023, 27, 48–62. [Google Scholar] [CrossRef]
- Liu, Q.; Roy, A.; Silberschmidt, V.V. Temperature-dependent crystal-plasticity model for magnesium: A bottom-up approach. Mech. Mater. 2017, 113, 44–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, Y.; He, Q.; Zhang, J.; Luo, R.; Di, H.; Huang, G.; Liu, Q. Three-dimensional hot processing map of a nickel-based superalloy (Alloy 925) established by modified artificial neural network model. Intermetallics 2022, 141, 107433. [Google Scholar] [CrossRef]
- Qi, H.N.; Zhang, Z.M.; Yu, J.M.; Yin, X.Y.; Du, Z.Y. Dynamic Recrystallization of Mg-8Gd-3Y-1Nd-0.5Zr Alloy during Hot Deformation. Mater. Sci. Forum 2017, 898, 311–322. [Google Scholar] [CrossRef]
- del Valle, J.A.; Carreño, F.; Ruano, O.A. Influence of texture and grain size on work hardening and ductility in magnesium-based alloys processed by ECAP and rolling. Acta Mater. 2006, 54, 4247–4259. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, P.; Wang, H.; Ji, J.; Wang, T.; Wang, Y.; Wang, X. Thermal Deformation Characteristics and Dynamic Recrystallization Mechanism of Incoloy 800H Alloy under Different Deformations. Adv. Eng. Mater. 2023, 25. [Google Scholar] [CrossRef]
- Beer, A.G.; Barnett, M.R. Microstructural Development during Hot Working of Mg-3Al-1Zn. Metall. Mater. Trans. A 2007, 38, 1856–1867. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, F.; Shuang, Y.; Ma, L.; Chu, Z.; Zhao, C. Characterization of hot deformation behavior of as-extruded AZ31 alloy through kinetic analysis and processing maps. J. Mater. Process. Technol. 2020, 276, 116325. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhang, H.; Wang, H.; Shin, K.S. The dynamic recrystallization and mechanical property responses during hot screw rolling on pre-aged ZM61 magnesium alloys. Mater. Sci. Eng. A 2020, 798, 140126. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, K.; Wang, L.; Ma, K.; Zhang, D.; Li, Y.; Zhang, Z.; Zhao, Z.; Bai, P. Hot deformation behavior and microstructural evolutions of a Mg-Gd-Y-Zn-Zr alloy prepared by hot extrusion. J. Alloys Compd. 2024, 1009, 176916. [Google Scholar] [CrossRef]



| Element | Mg | Zn | Y | Zr | Fe |
|---|---|---|---|---|---|
| Content | Bal | 2.80 | 0.32 | 0.65 | 0.0081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Yang, B.; Wu, Y.; Peng, B.; He, M. Deformation Behaviors and Microstructure Evolution of Mg-Zn-Y-Zr Alloys During Hot Compression Process. Metals 2024, 14, 1332. https://doi.org/10.3390/met14121332
Jiang H, Yang B, Wu Y, Peng B, He M. Deformation Behaviors and Microstructure Evolution of Mg-Zn-Y-Zr Alloys During Hot Compression Process. Metals. 2024; 14(12):1332. https://doi.org/10.3390/met14121332
Chicago/Turabian StyleJiang, Hong, Bin Yang, Yujuan Wu, Biyou Peng, and Meifeng He. 2024. "Deformation Behaviors and Microstructure Evolution of Mg-Zn-Y-Zr Alloys During Hot Compression Process" Metals 14, no. 12: 1332. https://doi.org/10.3390/met14121332
APA StyleJiang, H., Yang, B., Wu, Y., Peng, B., & He, M. (2024). Deformation Behaviors and Microstructure Evolution of Mg-Zn-Y-Zr Alloys During Hot Compression Process. Metals, 14(12), 1332. https://doi.org/10.3390/met14121332





