Thermo-Physical Properties of Hexavalent Tungsten W6+-Doped Ta-Based Ceramics for Thermal/Environmental Barrier Coating Materials
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Synthesis
2.2. Microstructure Characterization
3. Results and Discussion
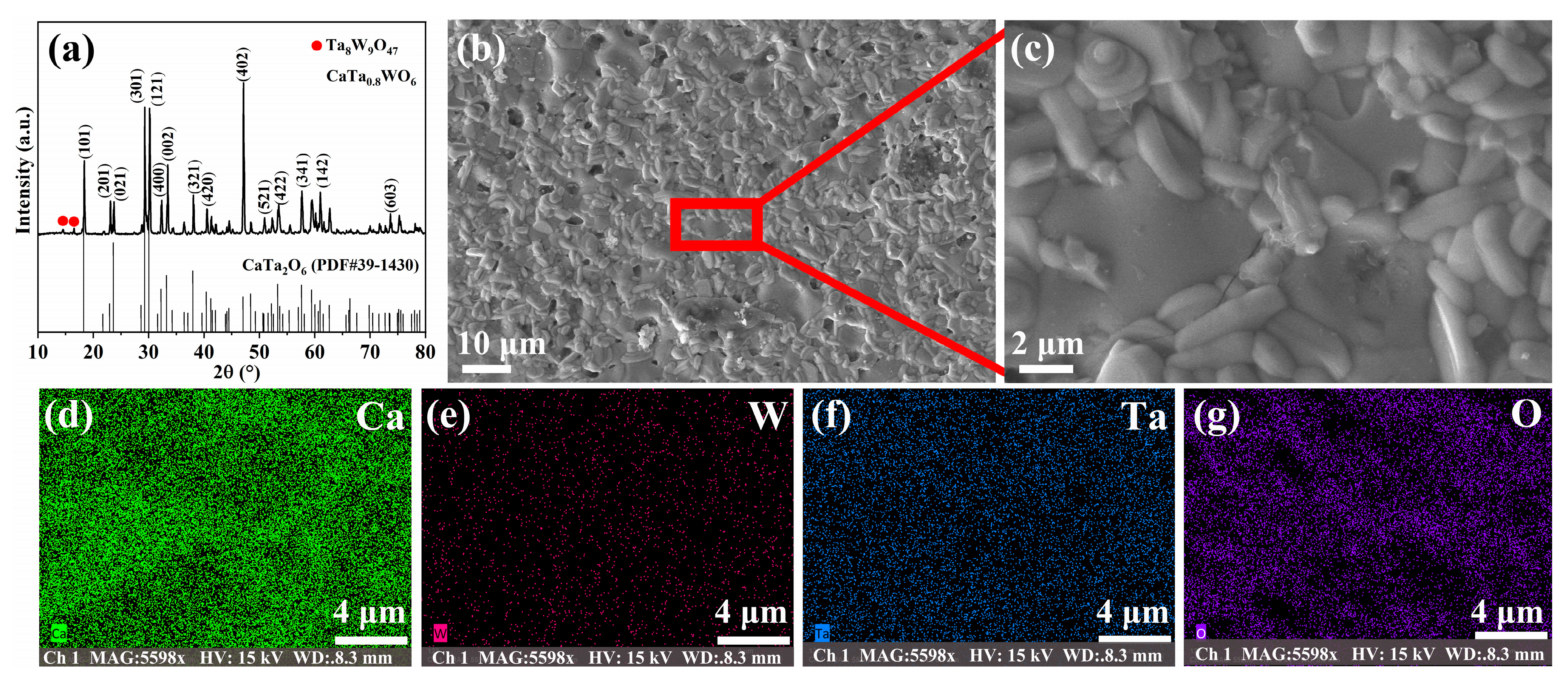
3.1. Microstructure
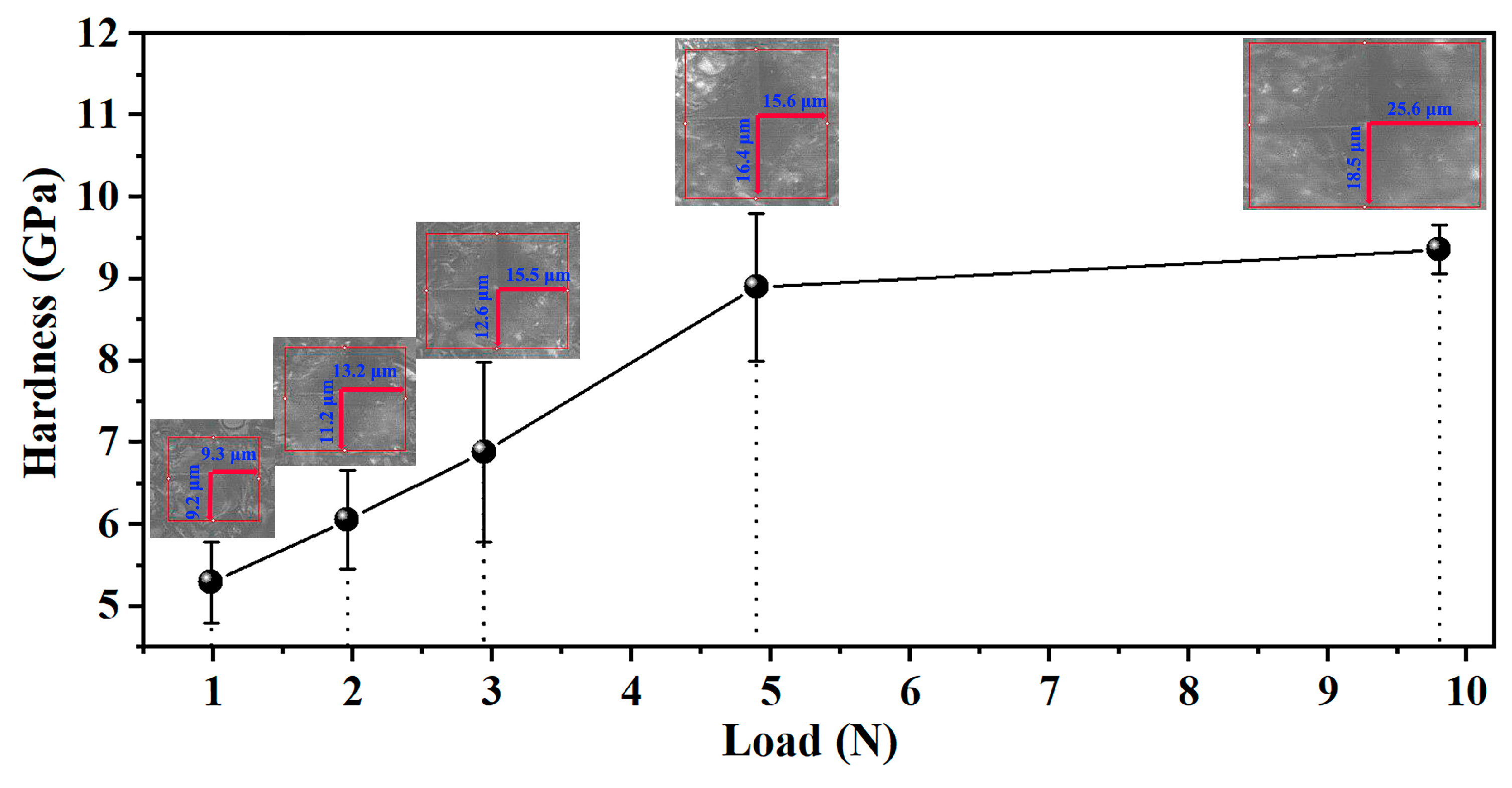
3.2. Mechanical Properties
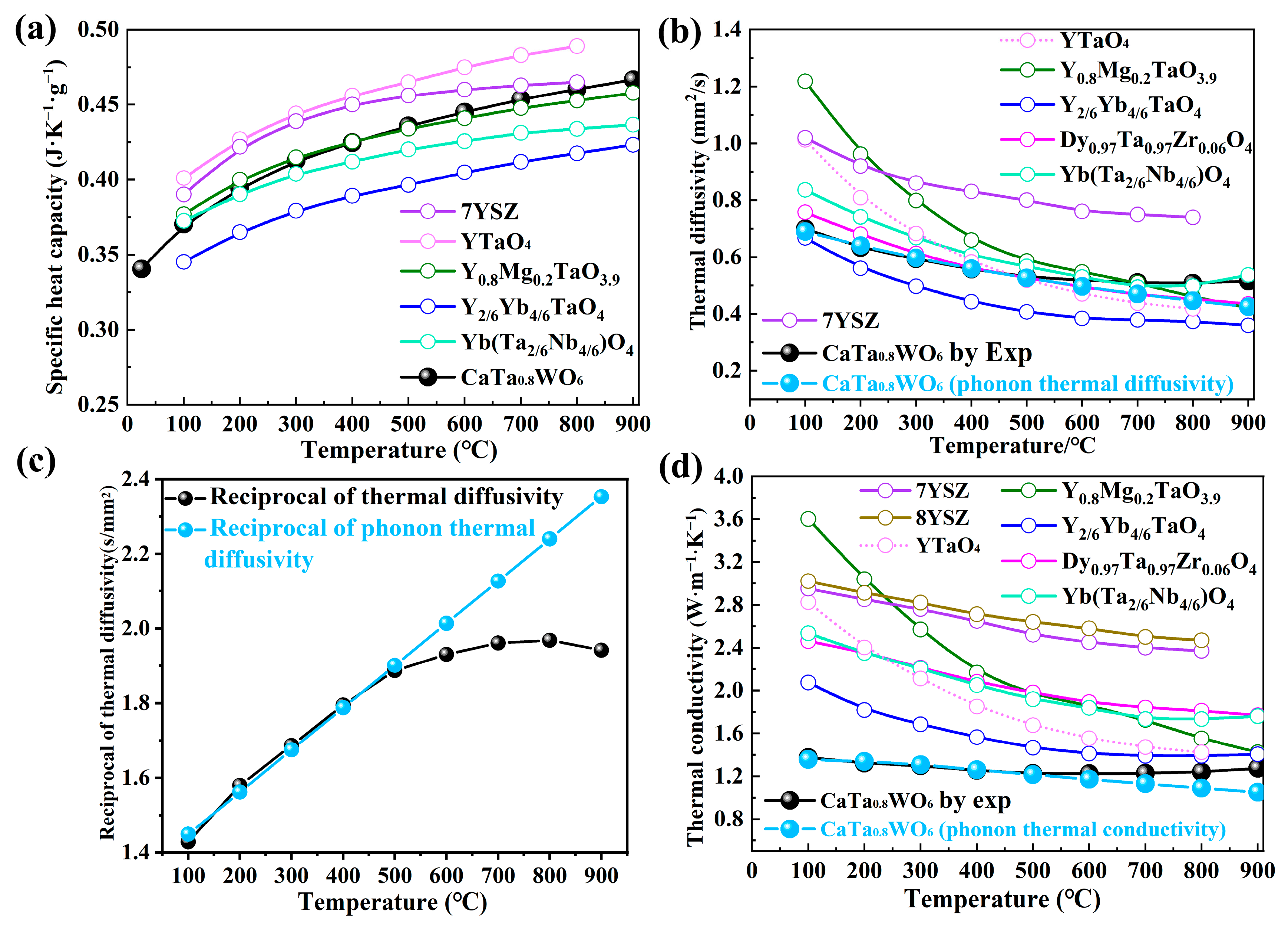
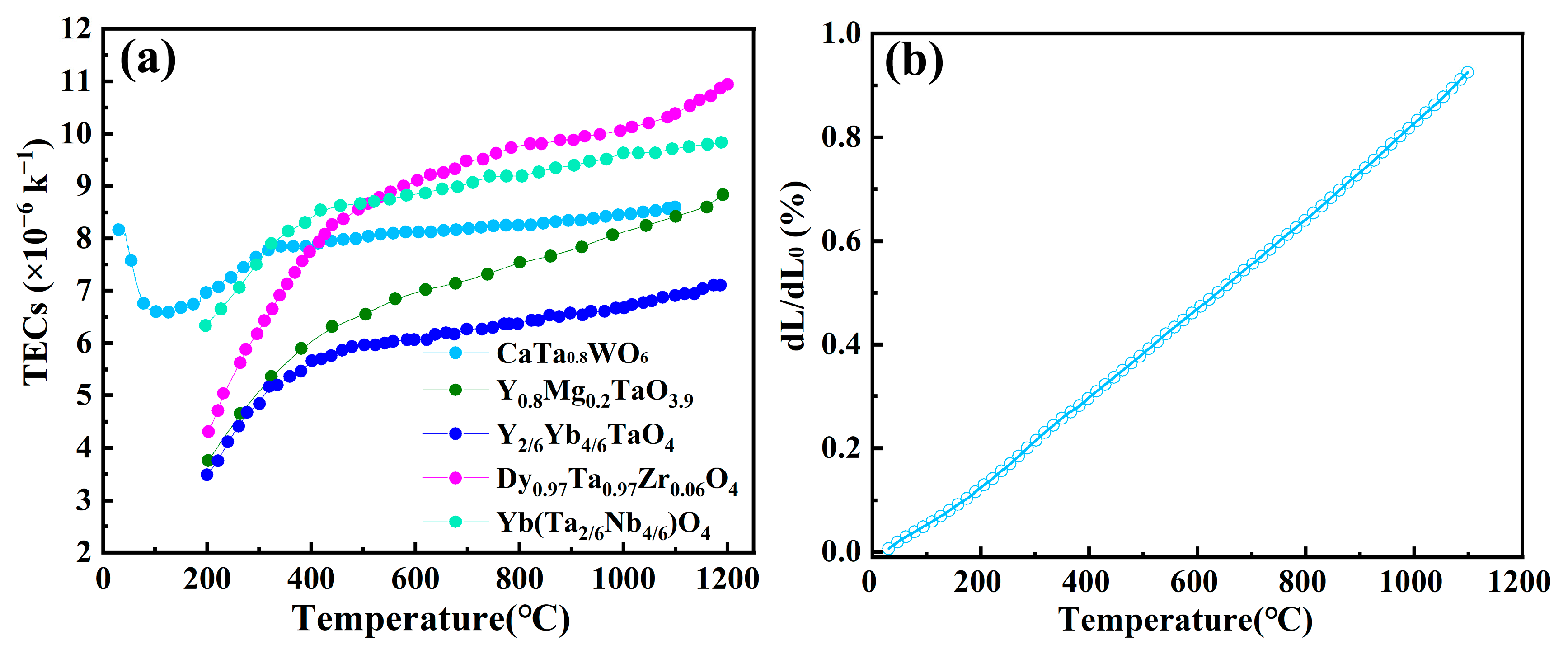
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.-R.; Liu, T.; Luo, X.-T.; Yang, G.-J.; Li, C.-J. Tailoring sintering-resistant thermal barrier coatings by considering critical healing width of two-dimensional interlamellar pores. J. Adv. Ceram. 2023, 12, 1317–1330. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Song, J.; Yang, X.; Ma, X.; Luo, K.; Wu, P.; Chong, X.; Feng, J. Advanced rare earth tantalate RETaO4 (RE = Dy, Gd and Sm) with excellent oxygen/thermal barier performance. J. Rare Earths 2024, 42, 1595–1603. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Wu, L.-H.; Sun, X.-K.; Sun, H.-R.; Ai, B.; Yu-Feng, C. Ultra-low thermal conductivity multilayer composites for insulation in low pressure environments. Adv. Ceram. 2023, 44, 442–450. [Google Scholar]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, J.; Zhang, T.; Ren, X.; Hu, W.; Zheng, L.; Wang, J. Towards thermal barrier coating application for rare earth silicates RE2SiO5 (RE = La, Nd, Sm, Eu, and Gd). J. Eur. Ceram. Soc. 2019, 39, 1463–1476. [Google Scholar] [CrossRef]
- Bogdan, M.; Peter, I. A Comprehensive Understanding of Thermal Barrier Coatings (TBCs): Applications, Materials, Coating Design and Failure Mechanisms. Metals 2024, 14, 575. [Google Scholar] [CrossRef]
- Feng, J.; Ren, X.; Wang, X.; Zhou, R.; Pan, W. Thermal conductivity of ytterbia-stabilized zirconia. Scr. Mater. 2012, 66, 41–44. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, X.; Yang, J.; Pan, W. Thermo-mechanical properties of ThO2-doped Y2O3 stabilized ZrO2 for thermal barrier coatings. Ceram. Int. 2016, 42, 501–508. [Google Scholar] [CrossRef]
- Vassen, R.; Stuke, A.; Stöver, D. Recent developments in the field of thermal barrier coatings. J. Therm. Spray Technol. 2009, 18, 181–186. [Google Scholar] [CrossRef]
- Ghasemi, R.; Vakilifard, H. Plasma-sprayed nanostructured YSZ thermal barrier coatings: Thermal insulation capability and adhesion strength. Ceram. Int. 2017, 43, 8556–8563. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Q.; Song, J.; Zhang, D.; Xu, B.; Ren, Z.; Wang, M.; Yan, S.; Sun, X.; Liu, C.; et al. Revealing the low thermal conductivity of high-entropy rare-earth tantalates via multi-scale defect analysis. J. Adv. Ceram. 2023, 12, 2087–2100. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Q.; Shao, G.; Zhao, X.; Wang, Y. Multicomponent high-entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scr. Mater. 2020, 178, 382–386. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.-X.; Liang, Y.; Zhang, G.-J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef]
- Cao, X.; Vassen, R.; Fischer, W.; Tietz, F.; Jungen, W.; Stöver, D. Lanthanum–cerium oxide as a thermal barrier-coating material for high-temperature applications. Adv. Mater. 2003, 15, 1438–1442. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Zhao, C.; Wang, F.; Sun, Y.J.; Zheng, L.Y.; Wang, X.H. Theoretical Prediction and Experimental Investigation on the Thermal and Mechanical Properties of Bulk β-Yb2Si2O7. J. Am. Ceram. Soc. 2013, 96, 3891–3900. [Google Scholar] [CrossRef]
- Asuvathraman, R.; Gnanasekar, K.; Clinsha, P.; Ravindran, T.; Kutty, K.G. Investigations on the charge compensation on Ca and U substitution in CePO4 by using XPS, XRD and Raman spectroscopy. Ceram. Int. 2015, 41, 3731–3739. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Xu, J.; Zhang, P.; Lou, Z.; Reece, M.J.; Gao, F. Ultra-low thermal conductivity and enhanced mechanical properties of high-entropy rare earth niobates (RE3NbO7, RE = Dy, Y, Ho, Er, Yb). J. Eur. Ceram. Soc. 2021, 41, 1052–1057. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Wang, B.; Sun, L.; Li, F.; Xue, Z.; Zhou, G.; Liu, B.; Nian, H. Theoretical and experimental investigations on high temperature mechanical and thermal properties of BaZrO3. Ceram. Int. 2018, 44, 16475–16482. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Zhang, W.; Zhou, G.; Jiang, D.; Chen, H.; Yang, G.; Nian, H.; Liu, B. High-temperature mechanical and thermal properties of Ca1−xSrxZrO3 solid solutions. J. Am. Ceram. Soc. 2020, 103, 1992–2000. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Meng, G.-H.; Chen, L.; Li, G.-R.; Liu, M.-J.; Zhang, W.-X.; Zhao, L.-N.; Zhang, Q.; Zhang, X.-D.; Wan, C.-L. Progress in ceramic materials and structure design toward advanced thermal barrier coatings. J. Adv. Ceram. 2022, 11, 985–1068. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Chong, X.; Zhou, R.; Feng, J. Microstructure and thermal properties of a promising thermal barrier coating: YTaO4. Ceram. Int. 2016, 42, 13876–13881. [Google Scholar] [CrossRef]
- Wang, J.; Chong, X.; Lv, L.; Wang, Y.; Ji, X.; Yun, H.; Feng, J. High-entropy ferroelastic (10RE0.1)TaO4 ceramics with oxygen vacancies and improved thermophysical properties. J. Mater. Sci. Technol. 2023, 157, 98–106. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Zhang, C.; Xiang, H.; Li, Y.; Fang, C.; Li, M.; Wang, H.; Zhou, Y. Microstructure, elastic/mechanical and thermal properties of CrTaO4: A new thermal barrier material? J. Adv. Ceram. 2024, 13, 373–387. [Google Scholar] [CrossRef]
- Wang, J.; Chong, X.; Zhou, R.; Feng, J. Microstructure and thermal properties of RETaO4 (RE = Nd, Eu, Gd, Dy, Er, Yb, Lu) as promising thermal barrier coating materials. Scr. Mater. 2017, 126, 24–28. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Wang, J.; Li, B.; Feng, J. Dominant mechanisms of thermo-mechanical properties of weberite-type RE3TaO7 (RE = La, Pr, Nd, Eu, Gd, Dy) tantalates toward multifunctional thermal/environmental barrier coating applications. Acta Mater. 2024, 270, 119857. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.; Chong, X.; Feng, J. Synthesis and thermophysical properties of RETa3O9 (RE = Ce, Nd, Sm, Eu, Gd, Dy, Er) as promising thermal barrier coatings. J. Am. Ceram. Soc. 2018, 101, 1266–1278. [Google Scholar] [CrossRef]
- Mahendra, K.; D’Souza, A.; Udayashankar, N. Effect of Zn doping on the structural, optical, photoluminescence and mechanical properties of thiourea barium chloride (TBC) crystal. Mater. Today Commun. 2017, 13, 178–185. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhou, Y.; Wu, P.; Song, P.; Chong, X.-Y.; Feng, J. Thermal properties of Y1−xMgxTaO4−x/2 ceramics via anion sublattice adjustment. Rare Metals 2020, 39, 545–554. [Google Scholar] [CrossRef]
- Wu, P.; Hu, M.; Chen, L.; Chen, W.; Chong, X.; Gu, H.; Feng, J. Investigation on microstructures and thermo-physical properties of ferroelastic (Y1−xDyx)TaO4 ceramics. Materialia 2018, 4, 478–486. [Google Scholar] [CrossRef]
- Wu, P.; Chong, X.; Wu, F.; Hu, M.; Guo, H.; Feng, J. Investigation of the thermophysical properties of (Y1−xYbx)TaO4 ceramics. J. Eur. Ceram. Soc. 2020, 40, 3111–3121. [Google Scholar] [CrossRef]
- Wu, P.; Hu, M.; Chen, L.; Wu, F.; Chong, X.; Feng, J. The effect of ZrO2 alloying on the microstructures and thermal properties of DyTaO4 for high-temperature application. J. Am. Ceram. Soc. 2019, 102, 889–895. [Google Scholar] [CrossRef]
- Yang, K.; Chen, L.; Wu, F.; Zheng, Q.; Li, J.; Song, P.; Wang, Y.; Liu, R.; Feng, J. Thermophysical properties of Yb(TaxNb1−x)O4 ceramics with different crystal structures. Ceram. Int. 2020, 46, 28451–28458. [Google Scholar] [CrossRef]
- Sanditov, D.; Belomestnykh, V. Relation between the parameters of the elasticity theory and averaged bulk modulus of solids. Tech. Phys. 2011, 56, 1619–1623. [Google Scholar] [CrossRef]
- Kurosaki, K.; Kosuga, A.; Muta, H.; Uno, M.; Yamanaka, S. Ag9TiTe5: A high-performance thermoelectric bulk material with extremely low thermal conductivity. Appl. Phys. Lett. 2005, 87, 061919. [Google Scholar] [CrossRef]
- Martin, A.; Thuo, M. Beyond Hume-Rothery Rules. Acc. Mater. Res. 2023, 4, 809–813. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Wu, P.; Feng, J. Thermal expansion performance and intrinsic lattice thermal conductivity of ferroelastic RETaO4 ceramics. J. Am. Ceram. Soc. 2019, 102, 4809–4821. [Google Scholar] [CrossRef]
- He, K.; Chen, J.; Weng, W.; Li, C.; Li, Q. Microstructure and mechanical properties of plasma sprayed Al2O3-YSZ composite coatings. Vacuum 2018, 151, 209–220. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.-Z.; Peng, Z.; Zhou, Y. Preparation and mechanical properties of β-Zr2O(PO4)2: A soft and damage tolerant ceramic with machinability and good thermal shock resistance. J. Eur. Ceram. Soc. 2020, 40, 155–164. [Google Scholar] [CrossRef]
- Petrík, J.; Palfy, P. The Influence of the Load on the Hardness. Metrol. Meas. Syst. 2011, 18, 223–234. [Google Scholar] [CrossRef]
- Clinton, D.; Morrell, R. Hardness testing of ceramic materials. Mater. Chem. Phys. 1987, 17, 461–473. [Google Scholar] [CrossRef]
- Jang, B.-K.; Matsubara, H. Hardness and Young’s modulus of nanoporous EB-PVD YSZ coatings by nanoindentation. J. Alloys Compd. 2005, 402, 237–241. [Google Scholar] [CrossRef]
- Gadag, S.; Subbarayan, G.; Barker, W. Thermo-elastic properties of dense YSZ and porous Ni-ZrO2 monolithic and isotropic materials. J. Mater. Sci. 2006, 41, 1221–1232. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Chen, G.; Zhang, H.; Zou, Y.; Ye, Z.; Ouyang, J.; Jia, D.; Zhou, Y. High-temperature broadband infrared radiation from rare earth monosilicate-based ceramics. J. Eur. Ceram. Soc. 2024, 44, 6510–6517. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Wang, Y.; Chen, G.; Zou, Y.; Wang, M.; Zhao, D.; Jin, R.; Ouyang, J.; Jia, D. Doping engineering for high-temperature broadband high emissivity and low thermal conductivity of ytterbium chromate-based ceramics. Ceram. Int. 2024, 50, 17657–17664. [Google Scholar] [CrossRef]
- Wang, S.; Ye, Z.; Zhang, H.; Wang, Y.; Zhang, T.; Zou, Y.; Ouyang, J.; Jia, D.; Zhou, Y. High-entropy strategy for high-temperature broadband infrared radiation and low thermal conductivity. Ceram. Int. 2024, 50, 18806–18813. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.; Cui, Z.; Wang, L.; Gou, J.; Zhang, Q.; Wang, C. Thermal cycling behavior of nanostructured 8YSZ, SZ/8YSZ and 8CSZ/8YSZ thermal barrier coatings fabricated by atmospheric plasma spraying. Ceram. Int. 2017, 43, 4102–4111. [Google Scholar] [CrossRef]
- Wu, P.; Chong, X.; Feng, J. Effect of Al3+ doping on mechanical and thermal properties of DyTaO4 as promising thermal barrier coating application. J. Am. Ceram. Soc. 2018, 101, 1818–1823. [Google Scholar] [CrossRef]
- Clarke, D.; Levi, C. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]

| Samples | vt (m·s−1) | vl (m·s−1) | vm (m·s−1) | E (GPa) | B (GPa) | G (GPa) | γ | v | θD (K) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| CaTa0.8WO6 | 2618 | 5132 | 2934 | 96.2 | 91.2 | 36.33 | 1.93 | 0.32 | 236 | This work |
| YTaO4 | 2702 | 5286 | 2742 | 138 | 156 | 51 | 2.13 | 0.35 | 354 | [36] |
| Y0.8Mg0.2TaO3.9 | 2369 | 5067 | 2771 | 94.9 | 86.11 | 36.1 | 1.84 | 0.31 | 341 | [28] |
| Y2/6Yb4/6TaO4 | 2249 | 4236 | 2516 | 106 | 78.5 | 41.2 | 1.6 | 0.28 | 325 | [30] |
| Dy0.97Ta0.97Zr0.06O4 | - | - | - | 130 | 98 | 51 | - | 0.28 | - | [31] |
| Yb(Ta2/6Nb4/6)O4 | 2430 | 4943 | 3376 | 120.41 | 126.64 | 44.88 | 2.06 | 0.34 | 438.92 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, G.; Wang, J.; Zhao, Z. Thermo-Physical Properties of Hexavalent Tungsten W6+-Doped Ta-Based Ceramics for Thermal/Environmental Barrier Coating Materials. Metals 2024, 14, 1368. https://doi.org/10.3390/met14121368
Zhang M, Wang G, Wang J, Zhao Z. Thermo-Physical Properties of Hexavalent Tungsten W6+-Doped Ta-Based Ceramics for Thermal/Environmental Barrier Coating Materials. Metals. 2024; 14(12):1368. https://doi.org/10.3390/met14121368
Chicago/Turabian StyleZhang, Manyu, Guangchi Wang, Jun Wang, and Zifan Zhao. 2024. "Thermo-Physical Properties of Hexavalent Tungsten W6+-Doped Ta-Based Ceramics for Thermal/Environmental Barrier Coating Materials" Metals 14, no. 12: 1368. https://doi.org/10.3390/met14121368
APA StyleZhang, M., Wang, G., Wang, J., & Zhao, Z. (2024). Thermo-Physical Properties of Hexavalent Tungsten W6+-Doped Ta-Based Ceramics for Thermal/Environmental Barrier Coating Materials. Metals, 14(12), 1368. https://doi.org/10.3390/met14121368





