Direct In Situ Fabrication of Strong Bonding ZIF-8 Film on Zinc Substrate and Its Formation Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
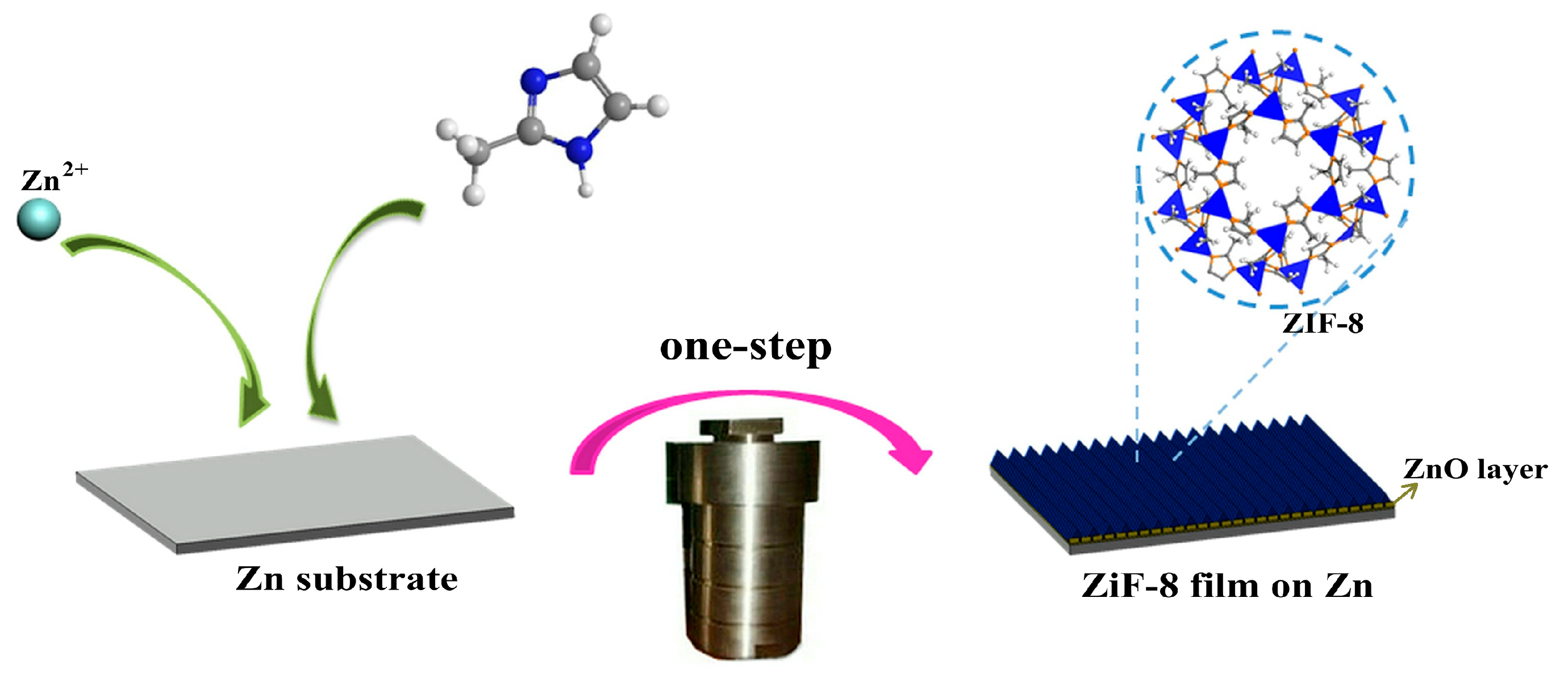
2.2. Preparation of the ZIF-8 Film
2.3. Characterization
3. Results and Discussion
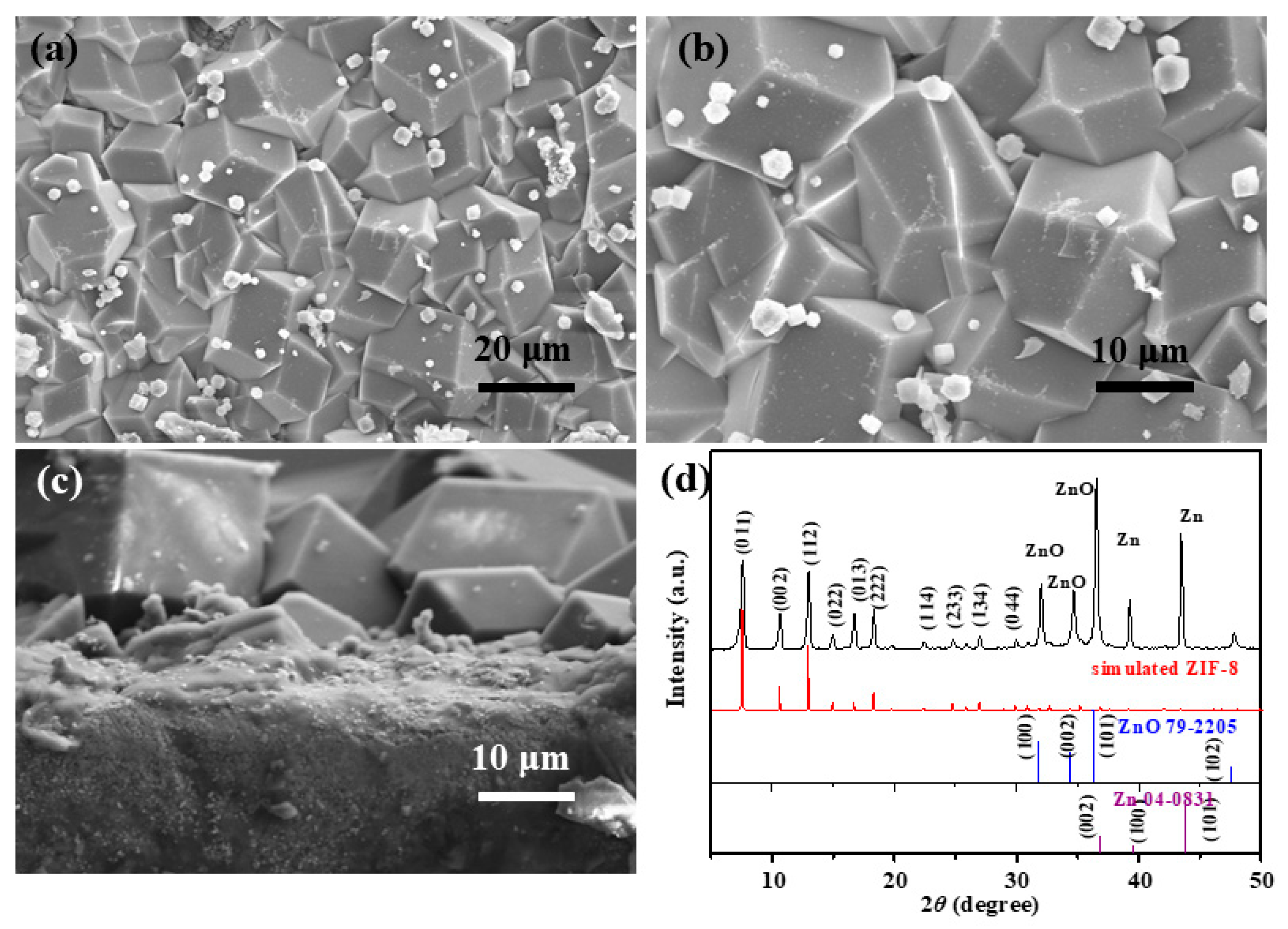
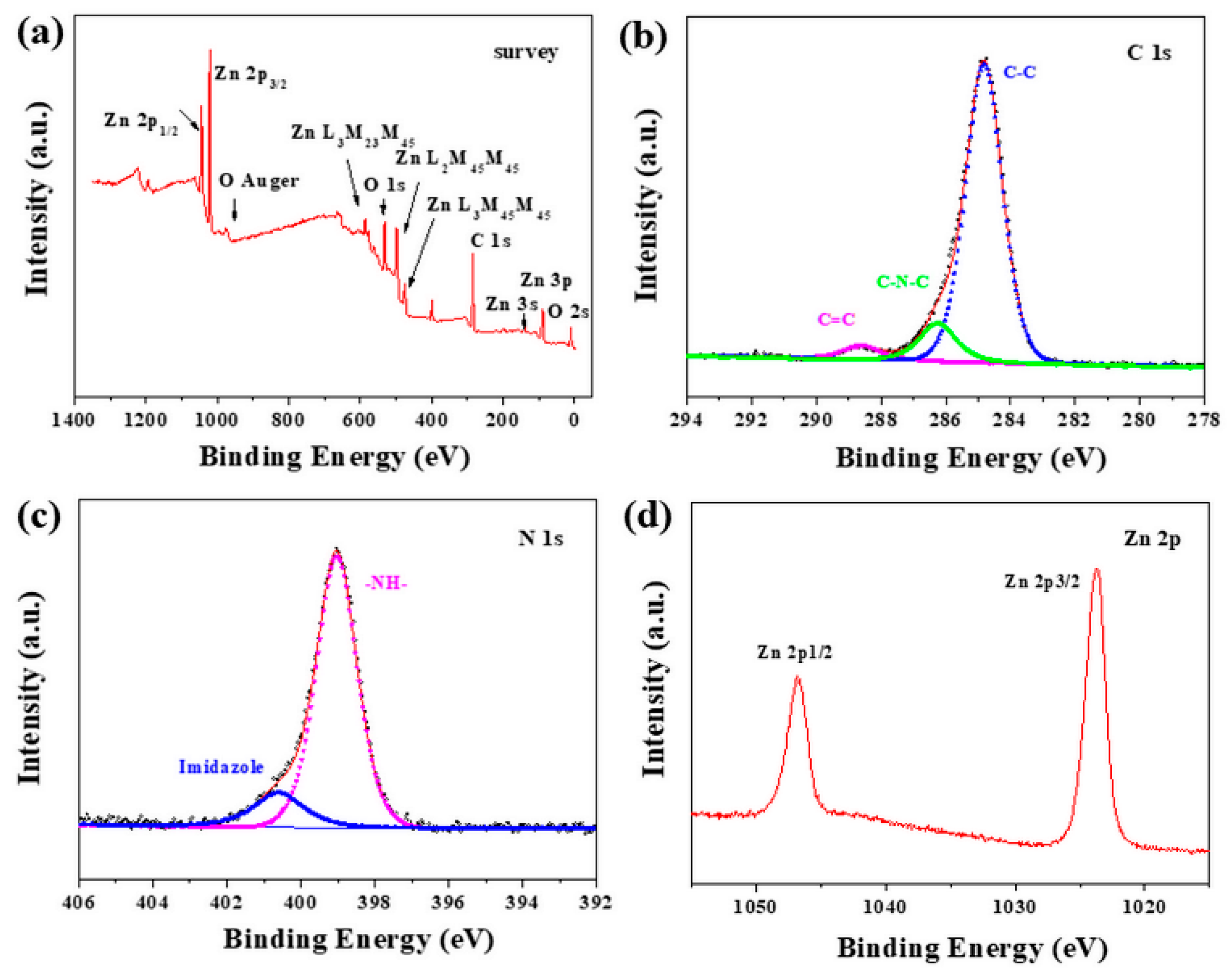
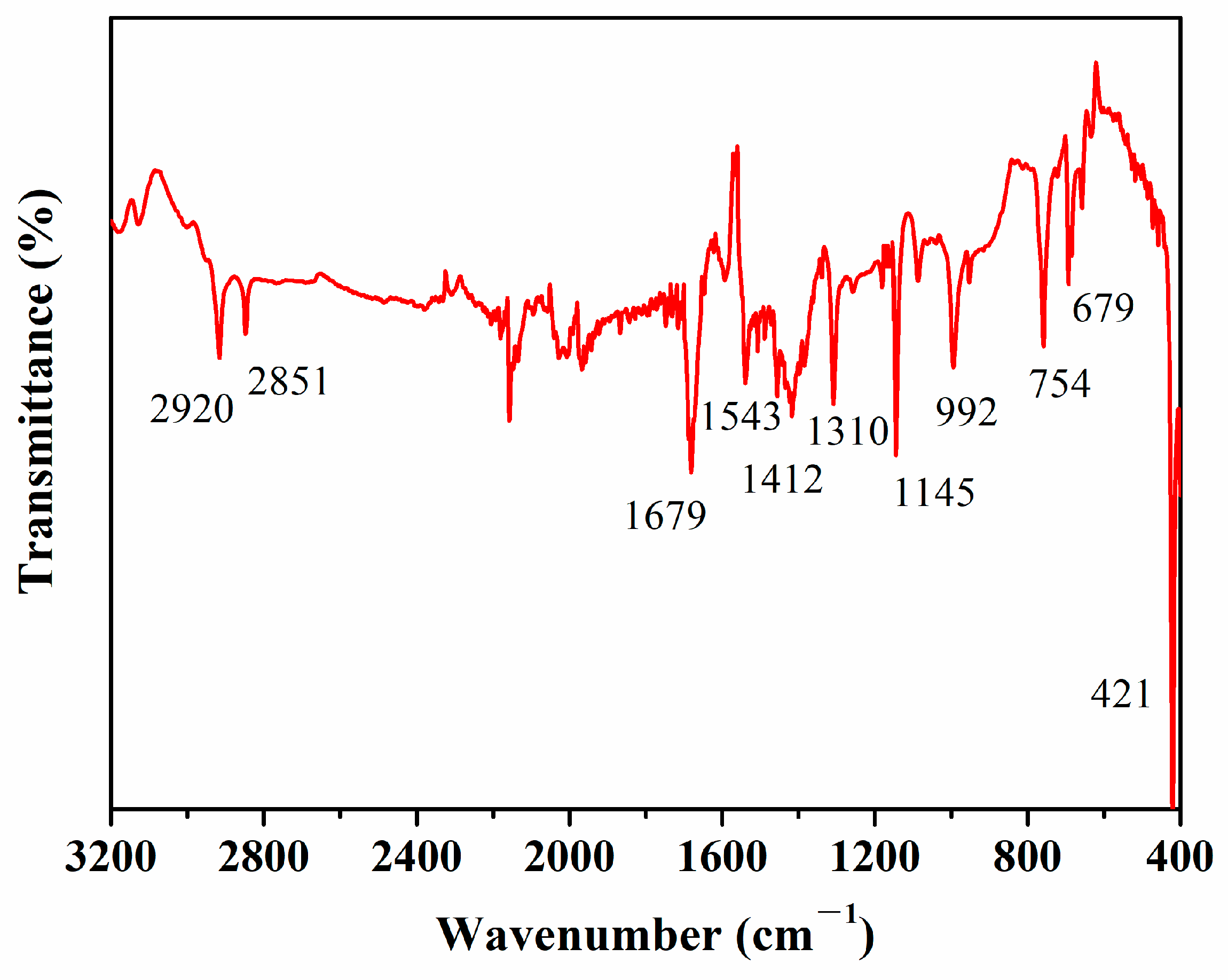
3.1. Synthesis and Characterization of ZIF-8 Film
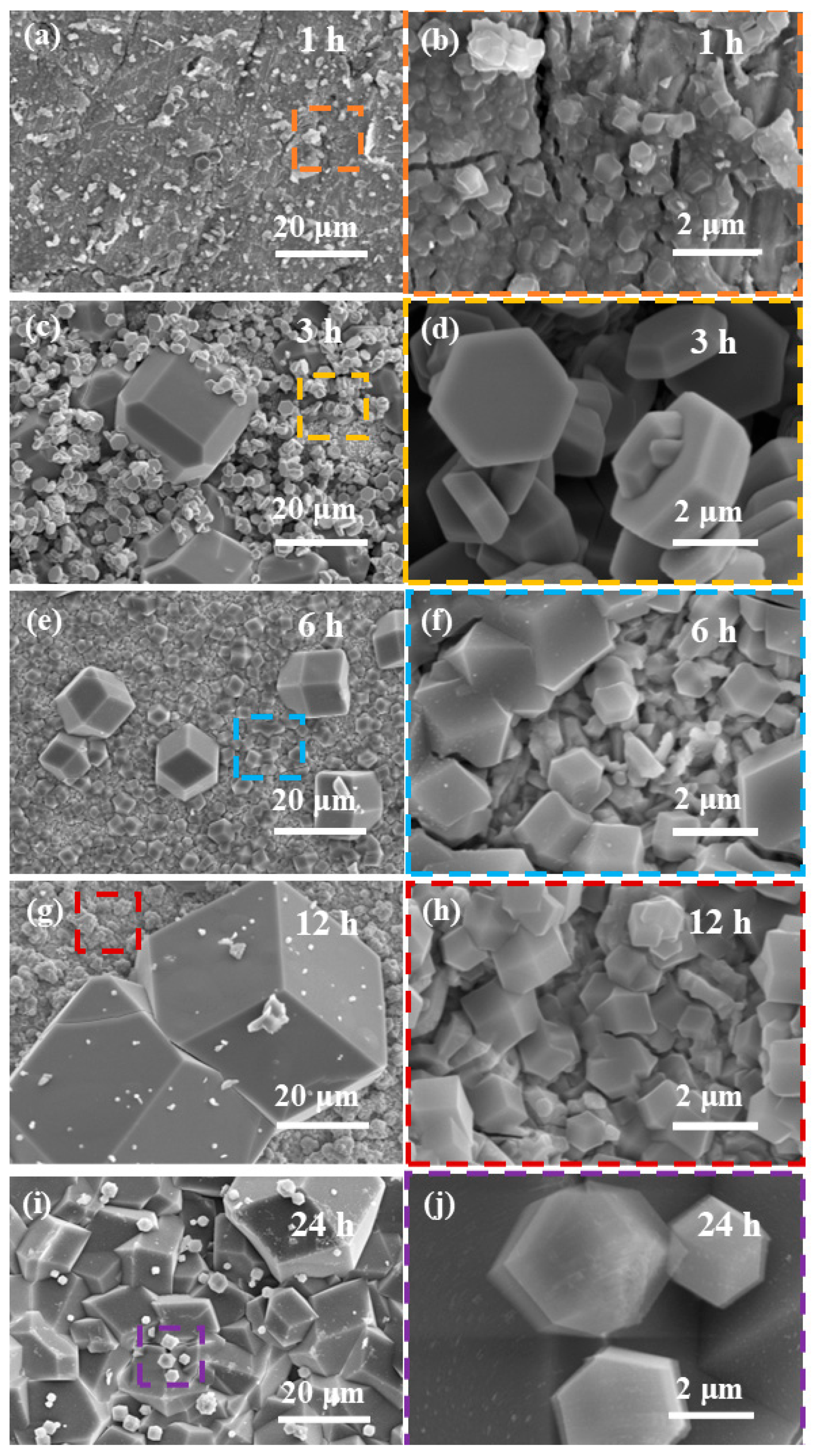
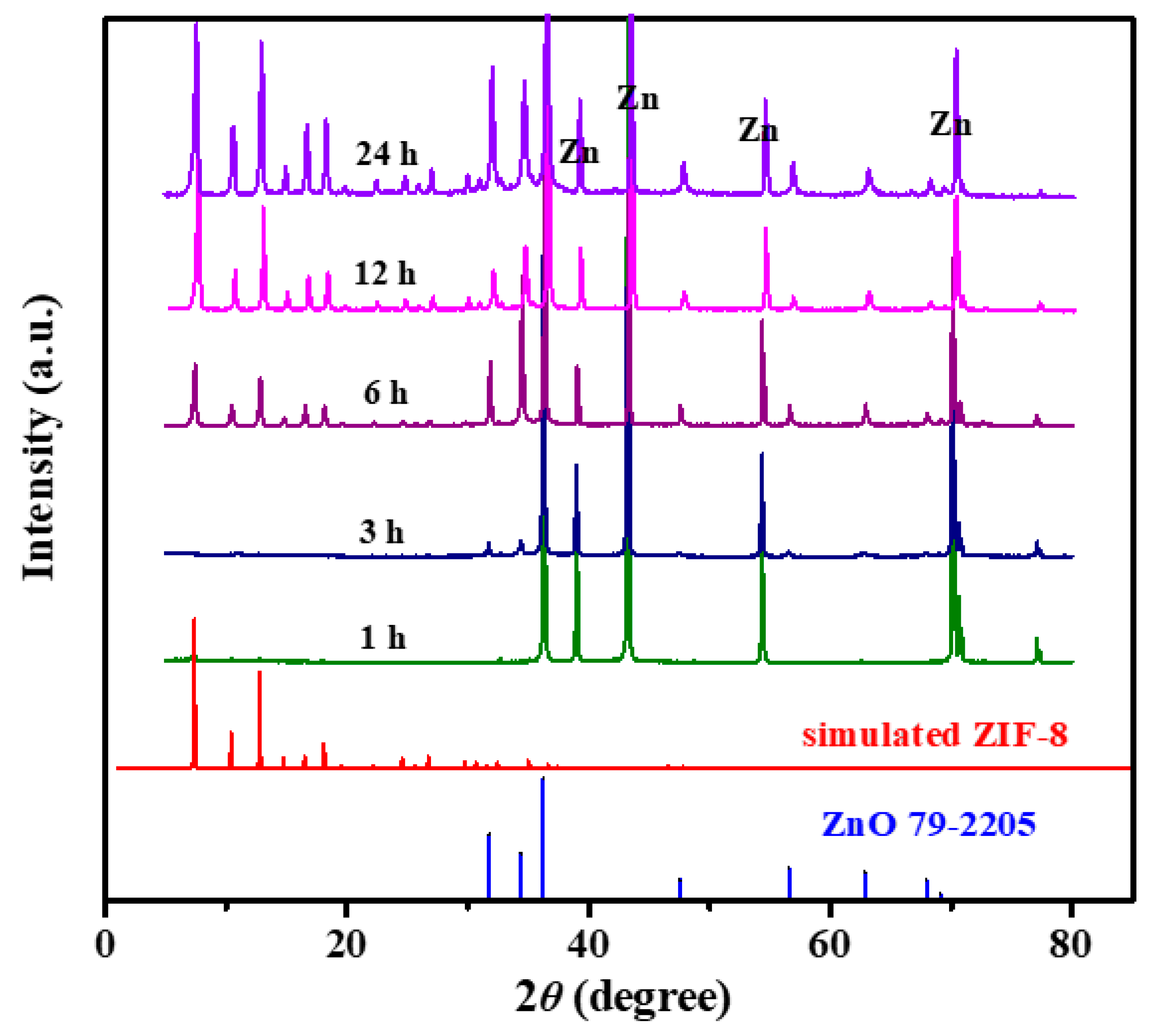
3.2. Evolution of the ZIF-8 Film Formation
3.3. Effect of Solvent and Reactives on ZnO Formation
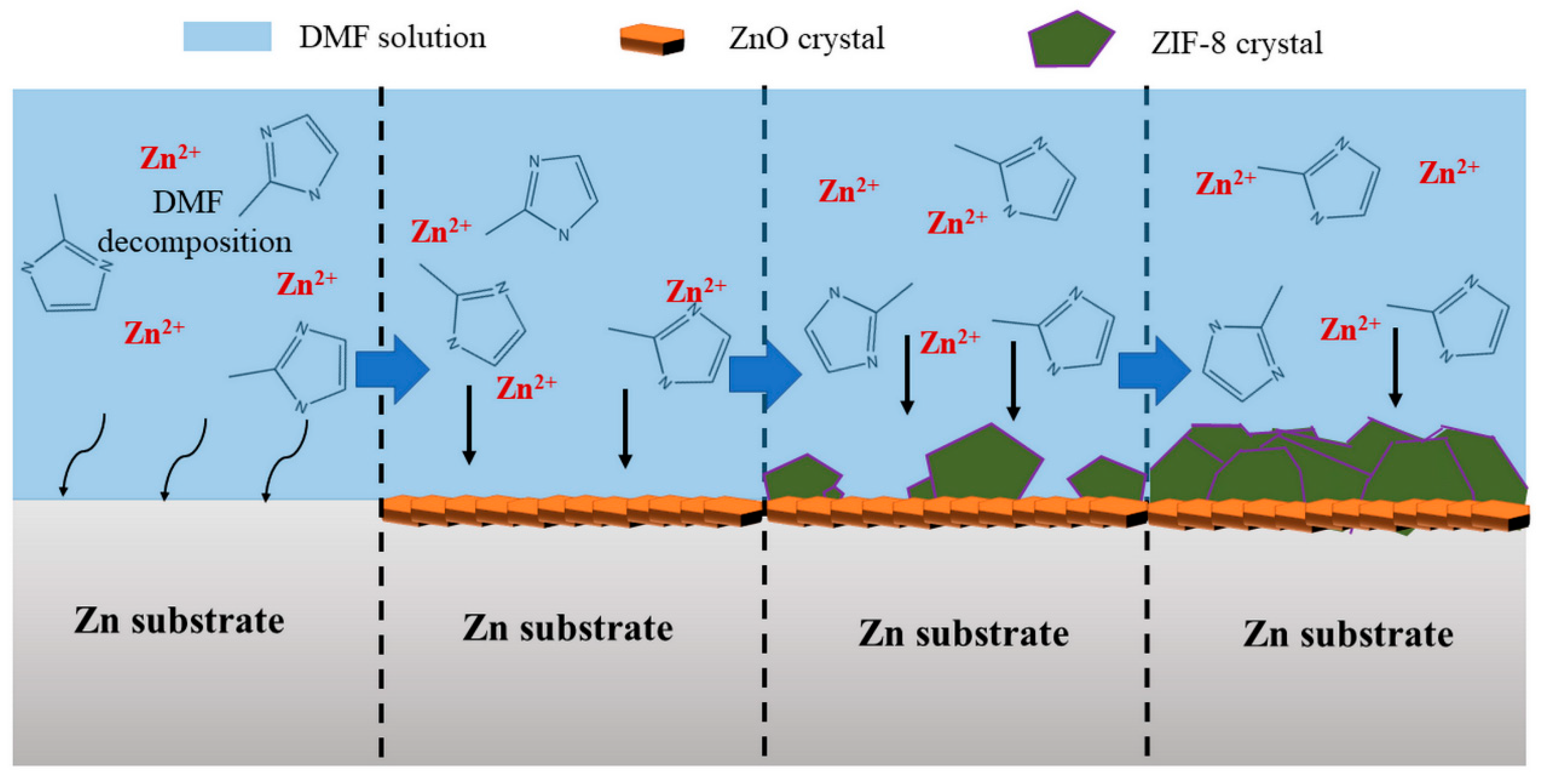
3.4. Formation Mechanisms of ZIF-8 Film
3.5. Adhesion Test of the ZIF-8 Film on Metal Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammadkhani, R.; Sharifi, K.; Fedel, M.; Ramezanzadeh, B. Fabricating epoxy composite coating having self-healing/barrier anti-corrosion functions utilizing ion-exchange/pH-sensitive phosphate-doped ZIF8 MOF decorated Zn-Al-LDH nano-layers. Surf. Coat. Technol. 2024, 477, 130284. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, D.; Luo, J.; Xiang, Y. First-principles study of ZIF-8 as anode for Na and K ion batteries. Colloids Surf. A 2023, 659, 130802. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Li, X.; Wang, R.; Zhang, Y.; Wang, G.; Shi, G. ZIF-8/ZIF-67 solid electrolyte ozone sensor at room temperature. Sens. Actuators A Phys. 2023, 354, 114281. [Google Scholar] [CrossRef]
- Lai, N.; Chang, G.; Yang, Y.; He, M.; Tang, W.; Huang, Q.; Liao, J.; Yang, Y.; Wang, C.; Wang, R. CsPbX3 quantum dots@ZIF-8 composites with enhanced luminescence emission and stability. J. Luminesc. 2024, 266, 120280. [Google Scholar] [CrossRef]
- Liu, L.; Ji, T.; Hu, W.; Sun, Y.; He, Y.; Yan, J.; He, G.; Liu, Y. Epitaxial supercritical fluid processing of ZIF-8 membranes towards efficient C3H6/C3H8 separation. J. Membr. Sci. 2023, 669, 121300. [Google Scholar] [CrossRef]
- Fatima, H.; Azharb, M.R.; Zhong, Y.; Arafat, Y.; Khiadani, M.; Shao, Z. Rational design of ZnO-zeolite imidazole hybrid nanoparticles with reduced charge recombination for enhanced photocatalysis. J. Colloid Interface Sci. 2022, 614, 538–546. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Wang, J.; Xiang, L. Conductometric methane gas sensors based on ZnO/Pd@ZIF-8: Effect of dual filtering of ZIF-8 to increase the selectivity. Sens. Actuators B Chem. 2023, 383, 133600. [Google Scholar] [CrossRef]
- Huang, W.; Li, Y.; Chang, N.; Hong, W.; Wang, C.; Huang, C. Highly stable and selective H2 gas sensors based on light-activated a-IGZO thin films with ZIF-8 selective membranes. Sens. Actuators B Chem. 2024, 417, 136175. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, J.; Zhang, X.; Ren, B.; Fu, S.; Sun, Y.; Luo, Z. Accordion-like ZIF-8/MoO3 composite gas sensor for highly selective and sensitive H2S detection. Ceram. Int. 2024, 50, 38253–38262. [Google Scholar] [CrossRef]
- Peng, C.; Wang, C.; Li, Z.; Wang, Z. A novel approach to detecting doping agents in food using electrochemical sensor based on zinc oxide/graphene oxide nanocomposites. J. Food Meas. Charact. 2024, 18, 6770–6781. [Google Scholar] [CrossRef]
- Nguyen, T.; Chen, J.; Pham, M.; Bui, H.; Hu, C.; You, S.; Wang, Y. A high-performance ZIF-8 membrane for gas separation applications: Synthesis and characterization. Environ. Technol. Innov. 2023, 31, 103169. [Google Scholar] [CrossRef]
- Shan, Y.; He, M.; Zhang, F.; Wang, Y.; Liu, Y.; Yang, Y.; Wang, Z.; Chen, X. Plasma-assisted synthesis of ZIF-8 membrane for hydrogen separation. Sep. Purif. Technol. 2023, 317, 123871. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, L.; Zhang, H.; Liu, C.; Wang, Z. ZIF-8 membranes on ZIF-8-PVDF/PVDF dual-layer polymeric hollow fiber supports for gas separation. Sep. Purif. Technol. 2024, 335, 126209. [Google Scholar] [CrossRef]
- Liu, Y.; Matsuda, R.; Kusaka, S.; Hori, A.; Ma, Y.; Kitagawa, S. Insights into inorganic buffer layer-assisted in situ fabrication of MOF films with controlled microstructures. CrystEngComm 2018, 20, 6995–7000. [Google Scholar] [CrossRef]
- Shi, P.; Wang, C.; Wang, H.; Lei, X.; Wang, B.; Liu, X.; You, J.; Guo, R. Characteristics of zeolitic imidazolate framework-L and application of its derivatives in oxygen evolution reaction: Recent trends. J. Alloys Compd. 2024, 1006, 176293. [Google Scholar] [CrossRef]
- Sadughi, M.M.; Mazani, A.; Varnaseri, M. Synthesis of magnetic nanocomposites based on imidazole zeolite-8 framework doped with silver nanoparticles for effective removal of norfloxacin from effluents. J Clust. Sci. 2024, 35, 2991–3009. [Google Scholar] [CrossRef]
- Su, Z.; Shaw, W.L.; Miao, Y.R.; You, S.; Dlott, D.D.; Suslick, K.S. Shock wave chemistry in a metal–organic framework. J. Am. Chem. Soc. 2017, 139, 4619. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sutrisna, P.D.; Zhang, Y.; Chen, V. Formation of ultrathin, continuous metal-organic framework membranes on flexible polymer substrates. Angew. Chem. Int. Ed. 2016, 55, 3947. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Fang, X.; Liao, Z.; Wang, D.; Sun, X.; Shen, J.; Han, W.; Wang, L. Interfacial growth of metal–organic framework membranes on porous polymers via phase transformation. Chem. Commun. 2018, 54, 3590–3593. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Park, S.; Kim, J.S.; Lee, Y.M.; Jeong, H.-K. In situ formation of zeolitic-imidazolate framework thin films and composites using modified polymer substrates. J. Mater. Chem. A 2019, 7, 9680. [Google Scholar] [CrossRef]
- Li, B.; Xu, C.; Yu, D.; Qi, Z.; Wang, Y.; Peng, Y. Enhanced phosphate remediation of contaminated natural water by magnetic zeolitic imidazolate framework-8@engineering nanomaterials (ZIF8@ENMs). J. Colloid Interface Sci. 2022, 613, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Papporello, R.L.; Miro, E.E.; Zamaro, J.M. Secondary growth of ZIF-8 films onto copper-based foils. Insight into surface interactions. Microporous Mesoporous Mater. 2015, 211, 64–72. [Google Scholar] [CrossRef]
- Bradshaw, D.; Garai, A.; Huo, J. Metal–organic framework growth at functional interfaces: Thin films and composites for diverse applications. Chem. Soc. Rev. 2012, 41, 2344–2381. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Bux, H.; Feldhoff, A.; Yang, W.; Caro, J. Molecular sieve membrane: Supported metal–organic framework with high hydrogen selectivity. Angew. Chem. Int. Ed. 2010, 49, 548–551. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, F.; Wei, Q.; Wang, N.; Qin, X.; Zhang, S.; Wang, B.; Nie, Z.; Ji, S.; Yan, H.; et al. Oriented nano-microstructure-assisted controllable fabrication of metal-organic framework membranes on nickel foam. Adv. Mater. 2016, 28, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ricco, R.; Tokudome, Y.; Styles, M.J.; Hill, A.J.; Takahashi, M.; Falcaro, P. Copper conversion into Cu(OH)2 nanotubes for positioning Cu3(BTC)2 MOF crystals: Controlling the growth on flat plates, 3D architectures, and as patterns. Adv. Funct. Mater. 2014, 24, 1969–1977. [Google Scholar] [CrossRef]
- Abuzalat, O.; Wong, D.; Elsayed, M.; Park, S.; Kim, S. Sonochemical fabrication of Cu(II) and Zn(II) metal-organic framework films on metal substrates. Ultrason. Sonochem. 2018, 45, 180–188. [Google Scholar] [CrossRef]
- Zhu, C.; Hou, J.; Wang, X.; Wang, S.; Xu, H.; Hu, J.; Jing, L.; Wang, S. Optimizing ligand-to-metal charge transfer in metal–organic frameworks to enhance photocatalytic performance. Chem. Eng. J. 2024, 499, 156527. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, X.; Li, J.; Wang, N. Bifunctional polyimide/ZIF8 composite nanofibrous membranes with controllable bilayer structure for bioprotective application and high-efficiency oil/water separation. J. Environ. Chem. Eng. 2023, 11, 110913. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, S.; Kong, L.; Liu, H.; Li, Y.; Han, W.; Yeung, K.L.; Zhu, W.; Yang, W.; et al. New membrane architecture with high performance: ZIF-8 membrane supported on vertically aligned ZnO nanorods for gas permeation and separation. Chem. Mater. 2014, 26, 1975–1981. [Google Scholar] [CrossRef]
- Tian, L.; Sun, Y.; Huang, H.; Guo, X.; Qiao, Z.; Meng, J.; Zhong, C. Porous ZIF-8 thin layer coating on ZnO hollow nanofibers for enhanced acetone sensing. Chemistryselect. 2020, 5, 2401–2407. [Google Scholar] [CrossRef]
- Dong, X.L.; Lin, Y.S. Synthesis of an organophilic ZIF-71 membrane for pervaporationsolvent separation. Chem. Commun. 2013, 49, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of zeolitic imidazolate framework-78 molecular-sieve membrane: Defect formation and elimination. J. Mater. Chem. 2012, 22, 19222–19227. [Google Scholar] [CrossRef]
- Kasik, A.; James, J.; Lin, Y.S. Synthesis of ZIF-68 membrane on a ZnO modified α-Alumina support by a modified reactive seeding method. Ind. Eng. Chem. Res. 2016, 55, 2831–2839. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Meng, B.; Tan, X.; Liu, J.; Wang, S.; Liu, S. Formation of continuous and highly permeable ZIF-8 membranes on porous alumina and zinc oxide hollow fibers. Chem. Commun. 2016, 52, 13448–13451. [Google Scholar] [CrossRef]
- Meckler, S.M.; Li, C.; Queen, W.L.; Williams, T.E.; Long, J.R.; Buonsanti, R.; Milliron, D.J.; Helms, B.A. Sub-micron polymer–zeolitic imidazolate framework layered hybrids via controlled chemical transformation of naked ZnO nanocrystal films. Chem. Mater. 2015, 27, 7673–7679. [Google Scholar] [CrossRef]
- Wang, R.; Chang, Y.; Li, J.; Yang, S.; Zhu, T.; Bi, Y.; Cui, J. Carbonic anhydrase-embedded ZIF-8 membrane reactor with improved the recycling and stability for efficient CO2 capture. Int. J. Biol. Macromol. 2024, 280, 136083. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Liu, H.; Qiu, J. Synthesis of a highly stable ZIF-8 membrane on a macroporous ceramic tube by manual-rubbing ZnO deposition as a multifunctional layer. J. Membr. Sci. 2015, 490, 354–363. [Google Scholar] [CrossRef]
- Tian, F.; Cerro, A.M.; Mosier, A.M.; Wayment-Steele, H.K.; Shine, R.S.; Park, A.; Webster, E.R.; Johnson, L.E.; Johal, M.S.; Benz, L. Surface and stability characterization of a nanoporous ZIF-8 thin Film. J. Phys. Chem. C 2014, 118, 14449–14456. [Google Scholar] [CrossRef]
- Ai, S.; Guo, X.; Zhao, L.; Yang, D.; Ding, H. Zeolitic imidazolate framework-supported Prussian blue analogues as an efficient Fenton-like catalyst for activation of peroxymonosulfate. Colloids Surf. A 2019, 581, 123796. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances. J. Am. Chem. Soc. 2015, 137, 12304–12311. [Google Scholar] [CrossRef]
- Hillman, F.; Brito, J.; Jeong, H.-K. Rapid one-pot microwave synthesis of mixed-linker hybrid zeolitic-imidazolate framework membranes for tunable gas separations. ACS Appl. Mater. Interfaces 2018, 10, 5586–5593. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Mei, Y.; Xiao, Z.; Kang, Z.; Wang, R.; Sun, D. Monitoring thermally induced structural deformation and framework decomposition of ZIF-8 through in situ temperature dependent measurements. Phys. Chem. Chem. Phys. 2017, 19, 27178–27183. [Google Scholar] [CrossRef] [PubMed]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. CrystEngComm 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Cravillon, J.; Schröder, C.A.; Bux, H.; Rothkirch, A.; Caro, J.; Wiebcke, M. Formate modulated solvothermal synthesis of ZIF-8 investigated using time-resolved in situ X-ray diffraction and scanning electron microscopy. CrystEngComm 2012, 14, 492–498. [Google Scholar] [CrossRef]
- Hillman, F.; Zimmerman, J.M.; Paek, S.-M.; Hamid, M.R.A.; Lim, W.T.; Jeong, H.-K. Rapid microwave-assisted synthesis of hybrid zeolitic–imidazolate frameworks with mixed metals and mixed linkers. J. Mater. Chem. A 2017, 5, 6090–6099. [Google Scholar] [CrossRef]
- Lee, M.J.; Hamid, M.R.A.; Lee, J.; Kim, J.S.; Lee, Y.M.; Jeong, H.-K. Ultrathin zeolitic-imidazolate framework ZIF-8 membranes on polymeric hollow fibers for propylene/propane separation. J. Membr. Sci. 2018, 559, 28–34. [Google Scholar] [CrossRef]
- Enomoto, T.; Ueno, S.; Hosono, E.; Hagiwara, M.; Fujihara, S. Size-controlled synthesis of ZIF-8 particles and their pyrolytic conversion into ZnO aggregates as photoanode materials of dye-sensitized solar cells. CrystEngComm 2017, 19, 2844–2851. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on metal–organic framework engenders high sensitivity for enzymatic electrochemical detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef]
- Muzart, J. N,N-Dimethylformamide: Much more than a solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar] [CrossRef]
- Zhu, M.; Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Room-temperature synthesis of ZIF-8: The coexistence of ZnO nanoneedles. Chem. Mater. 2011, 23, 3590–3592. [Google Scholar] [CrossRef]
- Nian, P.; Liu, H.; Zhang, X. Bottom-up synthesis of 2D Co-based metal–organic framework nanosheets by an ammonia-assisted strategy for tuning the crystal morphology. CrystEngComm 2019, 21, 3199–3208. [Google Scholar] [CrossRef]
- Khan, M.A.; Magnone, E.; Kang, Y.M. Elucidation of optoelectronic properties of the sol-gel-grown Al-doped ZnO nanostructures. J. Sol-Gel Sci. Technol. 2015, 77, 642–649. [Google Scholar] [CrossRef]
- Gobo, F.; Goto, T.; Long, T.; Yin, S.; Sato, T. Mild solution synthesis of plate-like and rod-like ZnO crystals. Res. Chem. Intermed. 2011, 37, 211–217. [Google Scholar] [CrossRef]
- Xu, F.; Lu, Y.; Xie, Y.; Liu, Y. Controllable morphology evolution of electrodeposited ZnO nano/micro-scale structures in aqueous solution. Mater. Des. 2009, 30, 1704–1711. [Google Scholar] [CrossRef]
- Ji, H.; Hwang, S.; Kim, K.; Kim, C.; Jeong, N.C. Direct in situ conversion of metals into metal–organic frameworks: A strategy for the rapid growth of MOF films on metal substrates. ACS Appl. Mater. Interfaces 2016, 8, 32414–32420. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Yang, J.; Yang, R. Investigation on hydrogenation of metal–organic frameworks HKUST-1, MIL-53, and ZIF-8 by hydrogen spillover. J. Phys. Chem. C 2013, 117, 7565–7576. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling zeolitic imidazolate framework nano-and microcrystal formation: Insight into crystal growth by time-sesolved in situ static light scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, X.; Liu, W.; Hou, Q.; Wang, Y. Advances in bioinspired and multifunctional biomaterials made from chiral cellulose nanocrystals. Chem. Eng. J. 2023, 474, 145980. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, J.; Liu, B.; Zhang, Z.; Ren, X.; Wang, X.; Wu, P.; Zhang, Y. Direct In Situ Fabrication of Strong Bonding ZIF-8 Film on Zinc Substrate and Its Formation Mechanism. Metals 2024, 14, 1403. https://doi.org/10.3390/met14121403
Wang H, Liu J, Liu B, Zhang Z, Ren X, Wang X, Wu P, Zhang Y. Direct In Situ Fabrication of Strong Bonding ZIF-8 Film on Zinc Substrate and Its Formation Mechanism. Metals. 2024; 14(12):1403. https://doi.org/10.3390/met14121403
Chicago/Turabian StyleWang, Haidong, Jie Liu, Baosheng Liu, Zhechao Zhang, Xiaoxia Ren, Xitao Wang, Pengpeng Wu, and Yuezhong Zhang. 2024. "Direct In Situ Fabrication of Strong Bonding ZIF-8 Film on Zinc Substrate and Its Formation Mechanism" Metals 14, no. 12: 1403. https://doi.org/10.3390/met14121403
APA StyleWang, H., Liu, J., Liu, B., Zhang, Z., Ren, X., Wang, X., Wu, P., & Zhang, Y. (2024). Direct In Situ Fabrication of Strong Bonding ZIF-8 Film on Zinc Substrate and Its Formation Mechanism. Metals, 14(12), 1403. https://doi.org/10.3390/met14121403





