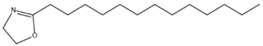
Abstract
The current demand for heat production via geothermal energy is increasingly rising amid concerns surrounding non-renewable forms of energy. The Dogger aquifer in the Paris Basin (DAPB) in France produces saline geothermal waters (GWs), which are as hot as 70–85 °C, anaerobic, slightly acidic (pH 6.1–6.4), and characterized mainly by the presence of Cl−, SO42−, CO2/HCO3−, and H2S/HS−. These GWs are corrosive, and the casings of all geothermal wells are carbon steel. Since 1989, these GWs have been progressively treated using petrosourced organic corrosion inhibitors (PS–OCI) at the bottom of the production wells. Currently, there is a great need to test not only new PS–OCIs but also, and above all, biosourced organic corrosion inhibitors (BS–OCIs) to improve the efficiency and environmental friendliness of this carbon-free geothermal energy source. The main objective of this study is to evaluate the potential performance of biosourced corrosion inhibitor candidates (BS–CICs) in terms of their inhibition efficiency (IE) for carbon steel corrosion. This was achieved using a previously established geochemical and electrochemical method to study the mechanisms and kinetics of the corrosion/scaling of carbon steel and optimize short-term corrosion inhibition in standardized reconstituted geothermal water (SRGW) representative of the DAPB’s waters. Four new molecules from the 2-oxazoline family were evaluated individually and compared based on their behavior and inhibition efficiency. These molecules exhibited a mixed nature (i.e., anodic and cathodic inhibitors), with a slight anodic predominance, and showed a significant IE at a concentration of at 10 mg/L during the first hours of immersion of CS-XC38 in SRGW. The average IEs, obtained via the three electrochemical techniques used for the determination of corrosion current densities, i.e., Jcorr(Rp), Jcorr(Tafel), and Jcorr(Rw), are 51%, 79%, 96%, and 93% for Decenox (C10:1), Decanox (C10:0), Undecanox (C11:0), and Tridecanox (C13:0), respectively.
1. Introduction
Most geothermal installations in France exploit the low-enthalpy geothermal waters (GWs) of the Dogger aquifer in the Paris Basin (DAPB). The aquifer extends to depths of 1600–2200 m below the NGF, the general leveling of France, representing the precise measurement of the altitude of a point relative to mean sea level. The temperatures of anaerobic GWs range from 70 to 85 °C; their pH ranges from 6.1 to 6.4; and their redox potential ranges from −300 to −480 mV versus a standard hydrogen electrode (SHE). The GWs of the DAPB are highly mineralized. The total dissolved solids (TDSs) vary from 6 to 35 g/L (ionic strength, 0.1–0.6 M). Many of these dissolved solids are Na+ (3000–13,000 mg/L), Cl− (5000–20,000 mg/L) [1], or SO42− (300–1200 mg/L) [2]. Dissolved gasses mainly include N2, CO2, H2S, and CH4. The concentrations of CO2/HCO3− range between 250 and 600 mg/L, and the concentrations of H2S/HS− range between 5 and 100 mg/L [2]. Systematic analyses have shown the existence of a sulfide-enriched zone (30–100 mg/L), mapped in 1994 and updated in 2011 [2], which extends from the north to the west of Paris; the rest of the GWs in the DAPB have much lower values (0–30 mg/L). In addition to their specific physical and chemical characteristics, sulfate- and thiosulfate-reducing bacteria are the main bacterial strains present in the GWs of the DAPB [3]. DAPB GWs have been exploited since the 1980s [4], and the vast majority of the 55 installations established in the DAPB since then have used wells made of carbon steel. However, GWs’ geochemical characteristics place them among the foremost corrosive natural waters for carbon steel [5]. The historical problems of corrosion in geothermal installations have been described in several publications.
In order to improve the operating conditions of geothermal exploitation in the Paris region, these geothermal wells have progressively been treated, since 1989, using well-bottom treatment tubes (WBTTs) with various pure or formulated petrosourced organic corrosion inhibitors (PS–OCIs) to prevent corrosion and scaling [6]. By selecting, comparing, and optimizing pure or formulated PS–OCIs using on-site electrochemical techniques, it was found that corrosion inhibition efficiency ranged from 85 to 90% [7]. Basic PS–OCIs include primary, secondary, and ternary amines; ethoxylated fatty amines; benzalkonium chloride; and quaternary ammonium salt-based formulations. Such formulations are still used for the continuous treatment of wells to reduce water–steel interactions and scaling issues. However, although many PS–OCIs are very effective, they are all non-biodegradable. In addition, as they are constantly injected at the bottom of the production wells, they end their journey in the aquifer on the injection side. Although the quantity injected is low—on average, 5 mg of formulated product per liter of water produced (5 mg/L) in the 55 operating wells, with an average operating flow rate of 250 m3/h for almost the entire year—this small quantity represents approximately 15 tons/year per installation, or approximately 600 tons/year of PS–OCI formulations based on ethoxylated fatty amines injected into the DAPB. However, we do not know the fate of these products. Moreover, the use of these products in other applications (very-low-enthalpy geothermal energy via open- or closed-loop heat pumps) is prohibited because of their toxicity and non-biodegradability. Thus, there is a lack of biosourced, biodegradable, eco-designed products that would be economical and effective against corrosion and deposits in all geothermal installations.
However, currently, there are no untreated fluids in the DAPB that can be used for testing the effectiveness of new OCIs, and hence evaluating carbon steel’s corrosion phenomenology and kinetics remains an important challenge. In addition, there is a great need to test new biosourced corrosion inhibitor candidates (BS–CICs) to improve geothermal energy exploitation, particularly since OCIs must be sufficiently effective at economically feasible concentrations; i.e., OCIs must be effective at very low concentrations (2.5 to 10 mg/L) [6] and must be proven to present no risk of promoting localized corrosion. Furthermore, to make these operations more ethical, since they are being introduced into GWs, which are then cycled back into the environment, BS–OCIs should cause no harm to the surrounding ecosystems.
The main objective of this study is to evaluate the potential performance of the proposed BS–CICs in terms of their corrosion inhibition efficiency (IE).
The preselection study begins, above all, with a first phase of selection of potential BS-CICs on the basis of their physicochemical properties, in particular their dipole moment, polarizability, quantum parameters (HOMO and LUMO), hydrophobicity (log P), critical micellar concentration (CMC), interfacial properties, and adsorption capacity (by quartz microbalance) [8,9,10,11]. In a large project (Inhibiosource project, 2018–2022), this preselection was continued by the synthesis of 23 molecules categorized in six series and followed by electrochemical efficiency and full-scale application tests, strictly following the methodology of Betelu et al. [12], in which corrosion experiments were conducted ten times on CS-XC38 for 40 h and in which SRGW in the absence of an inhibitor constitutes the control group. In fact, in a parallel doctoral thesis [13] aimed at the synthesis of biosourced and biodegradable products, the actions of these 23 BS-CICs were compared in terms of the electrochemical behavior of CS-XC38 immersed in standardized reconstituted geothermal water (SRGW) in the absence and presence of BS-CICs. Among the six series (comprising the 23 lead products), one of the series of products to be synthesized was the fatty oxazoline family. Oxazoline is a five-membered heterocyclic (N, O) organic compound with the formula C3H5NO [14,15]. It is the parent of a family of compounds called 2-oxazolines [16]. These are made from non-food vegetable and animal fats and from residues from biodiesel production. They are also degradable and minimally or completely non-toxic, making them compliant with new state standards and guidelines. During the previous far-reaching study [13], strictly following the methodology of Betelu et al. [12], there was only one representative of the oxazoline family: Decenox (C10:1), or decenyl-2-oxazoline. Pre-screening based on the electrochemical behaviors of the different BS-CICs, tested at 160 mg/L, revealed three distinct groups, where Decenox (C10:1) ranked in the best group (presenting an IE greater than 73% at 70 °C). Specifically, in the presence of Decenox (C10:1), the measured corrosion rate was approximately 0.04 mm/year, compared to a rate of approximately 0.57 mm/year in the absence of the inhibitor. In addition, it acts as a mixed anodic and cathodic inhibitor, with an anodic predominance. These results agreed with those of the first phase (based on CMC, surface tension, and adsorption capacity). In a second phase, Decenox (C10:1) and five other compounds were selected for a biodegradability study, where Decenox (C10:1) was found to be biodegradable with a fairly interesting score [13]. The results of Decenox (C10:1) motivated the synthesis of three other BS-CICs derived from the 2-oxazoline family, including Decanox (C10:0) or decanyl, 2-oxazoline and Undecanox (C11:0) or undecanyl, 2-oxazoline and Tridecanox (C13:0) or tridecanyl, 2-oxazoline. Indeed, the presence of nitrogen as a heteroatom (non-bonding doublet) in addition to oxygen, as well as the length of the aliphatic chain and the saturation of this chain on the corrosion inhibition, motivated this investigative work.
2. Materials and Methods
The in-depth investigation and screening of carbon steel corrosion and corrosion inhibition in representative geothermal media on a laboratory scale requires both a systematic and a systemic approach.
2.1. Carbon Steel, Corrosive Medium, and Electrochemical Methodology Used for BS-CIC Evaluation
In a recent study by our team [12], we described how it was possible to simulate the composition of the most representative geothermal waters of the Dogger aquifer of the Paris Basin (DAPB) and, by associating an electrochemical panoply, to carry out valuable laboratory tests using new molecules and formulations. For this purpose, functional carbon steel-based working electrodes were designed, implemented, and adapted to monitor and investigate steel interfaces. Specific materials, as well as analytical equipment and techniques, have been selected and optimized to implement representative experiments, monitor the physical and chemical parameters of the fluid, and investigate corrosion and inhibition. Particular attention was paid to reconstituting a standardized DAPB geothermal water, named SRGW (standardized reconstituted geothermal water), which was a well-balanced water representative of the major elements and dissolved gasses of actual DAPB geothermal waters. The work of Betelu et al. [12] constitutes a reference for investigating corrosion and corrosion inhibition on the laboratory scale, as well as screening and optimizing PS- and BS-OCI formulas for the SRGWs of the DAPB. The methodology in [12] was carefully followed during the present study: the carbon steel electrodes, the methodology for the reconstitution of representative DAPB waters, the experimental setup, and the electrochemical and monitoring apparatus and methodologies were replicated scrupulously.
Corrosion current density, CCD or Jcorr, was determined from the electrochemical measurement of the polarization resistance Rp using linear polarization resistance (LPR), Tafel plots (TPs), and electrochemical impedance spectroscopy (EIS). TPs were used to investigate anodic and cathodic activities, while EIS was used for in-depth investigation of the interactions occurring at the carbon steel–SRGW interface.
Linear polarization resistance (LPR) and Tafel plots (TPs) were measured by linearly polarizing the CS–XC38 electrode at ±20 mV and at ±200 mV, respectively, around OCP at a scan rate of 0.1 mV s−1 and 0.166 mV s−1, respectively. Impedance measurements were performed by electrochemical impedance spectrometry (EIS) at OCP of CS–XC38 over a frequency range of 1 MHz to 1 mHz using perturbation signals with an amplitude of 10 mV.
2.2. Synthesis of Decenox (C10:1)
In a large project (Inhibiosource project, 2018–2022) and doctoral thesis [13] aimed at the synthesis of biosourced and biodegradable products, one of the six series of products to be synthesized was the fatty oxazoline family, with only one representative of this family, Decenox (C10:1), or 2-decenyl-2-oxazoline.
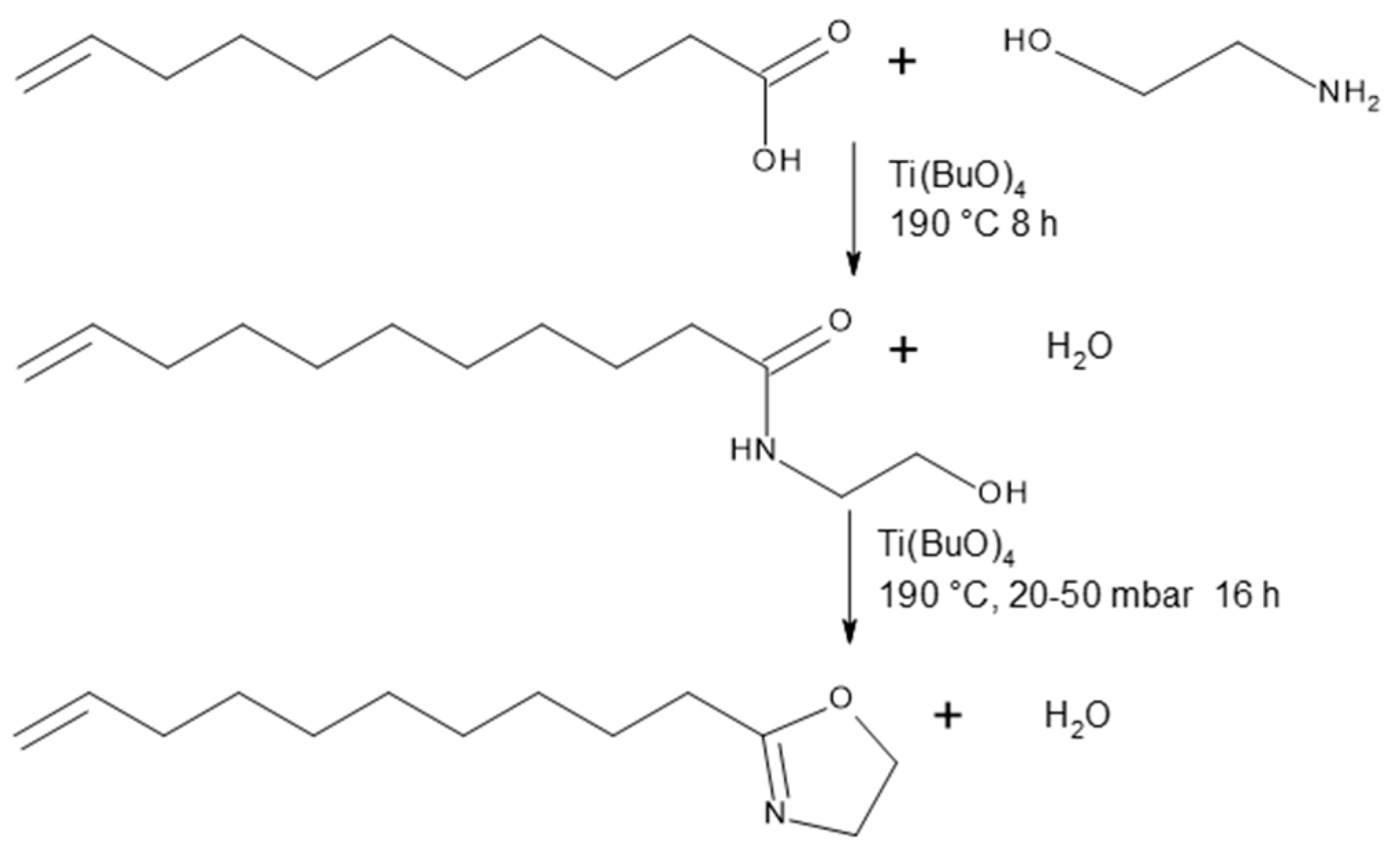
The schematic synthesis of Decenox (C10:1) is illustrated in Figure 1. Decenox (C10:1) is obtained via the amidation of undecylenic acid with ethanolamine in a stirred batch reactor, followed by cyclization. The reactions are catalyzed by titanium tetrabutyl. The product can be distilled in situ during the reaction. The intermediate product, the open amide, can be isolated (hydroxyamide decenoxHAm). Thus, an overall yield of 70% is obtained.
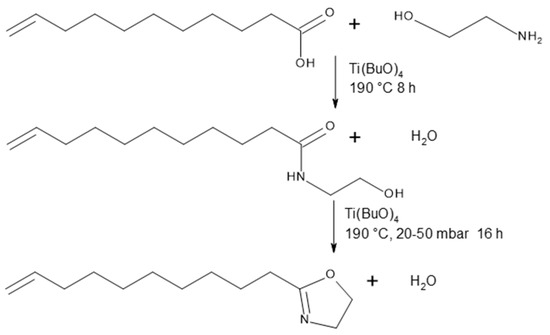
Figure 1.
Synthesis of decenoxHAm and Decenox (C10:1).
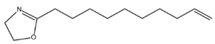
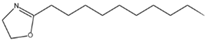
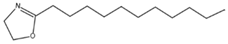
The results of Decenox (C10:1) motivated the synthesis of three other BS-CICs derived from the 2-oxazoline family, including Decanox (C10:0) or 2-decanyl, 2-oxazoline, Undecanox (C11:0) or 2-undecanyl, 2-oxazoline and Tridecanox (C13:0) or 2-tridecanyl, and 2-oxazoline. Thus, in parallel with the selection of Decenox (C10:1) as the main agent, with the aim of improving the effectiveness of oxazolines with subsequent formulations, we undertook modifications aimed at lengthening its aliphatic chain [17], thus strengthening its hydrophobic character. In addition, the removal of the double bond at the end of the aliphatic chain was considered with the three 2-oxazoline derivatives, Decanox, Undecanox, and Tridecanox (Table 1).

Table 1.
The substances biosourced from the 2-oxazoline family and their expanded structures.
The syntheses of the three supplementary 2-oxazoline derivatives will not be given. They may be found in Helali’s thesis manuscript [13]. In fact, under the same reaction conditions of Decenox (C10:1), and after synthesis optimization, the three saturated derivatives were synthesized and purified and are the BS-CICs used in the present study. The effects of the length and saturation (presence of double bond) of the aliphatic chains in these BS-CICs on their IE will be studied by using the methodology of Betelu et al. [12] and surface analyses.
3. Results and Discussion
In this section, we will study, compare, and discuss the electrochemical behavior of the CS-XC38 electrode during its immersion in SRGW in the presence of one of the four compounds—used separately and most often at 10 mg/L—compared to SRGW without an inhibitor. The case of CS-XC38 immersed in SRGW without an inhibitor has been largely investigated and reported in [8]. More precisely, here, in the presence of an inhibitor, we monitored the essential parameters and we determined their mechanism, corrosion current density (CCD or Jcorr), and inhibition effectiveness (IE).
3.1. Temporal Evolution of the OCP, or Ecorr, of the CS-XC38 Electrode
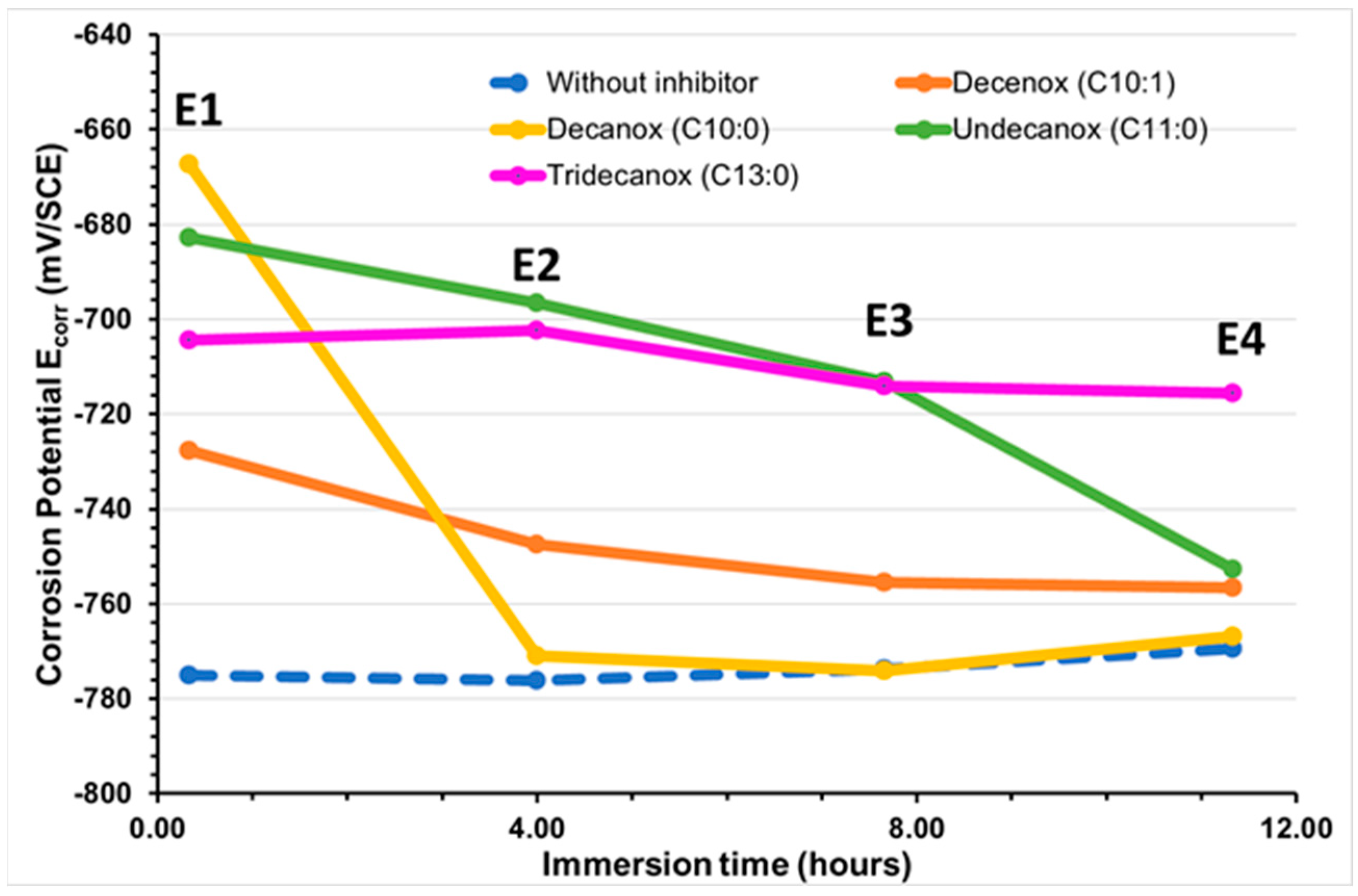
With a concentration of 10 mg/L, the four compounds derived from the oxazoline family induced a similar behavior in the CS-XC38 electrode. During the first hours of immersion, these compounds induce a displacement of Ecorr towards positive values (Table 2 and Figure 2). After 4 h, Ecorr becomes lower than Ecorr(WI), and this tendency is markedly strong for Decanox (C10:0).

Table 2.
Temporal evolution of CS-XC38 potential, Ecorr CS-XC78, during its immersion in SRGW in the presence of each of the four compounds used at 10 mg/L. ΔE1 = Ecorr (4h00) − Ecorr (0.33h); ΔE2 = Ecorr (7h40) − Ecorr (0.33h); ΔE3 = Ecorr (10h59) − Ecorr (0.33h); and ΔE = Ecorr(0.33h) − Ecorr(WI)) (0.33h).
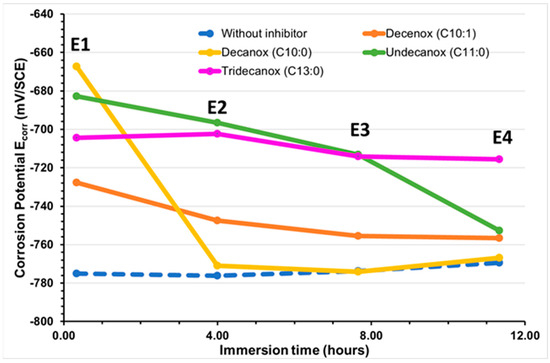
Figure 2.
Temporal evolution of the CS-XC38 electrode’s corrosion potential (Ecorr in mV/SCE) when immersed in SRGW in the presence of each of the four tested oxazolines at 10 mg/L compared to immersion without inhibitor.
Decenox (C10:1), at a concentration of 160 mg/L [13], significantly increased EcorrCSXC38 by approximately ΔE = 121 mV. On the other hand, at a concentration of 10 mg/L, ΔE was weaker (47 mV). This difference can be attributed to the formation of a denser protection film or multi-layer films on the CS-XC38 electrode at concentrations higher than the CMC (75 mg/L) [18,19], which results in the greater effectiveness at these concentrations. This will also be discussed in detail in Section 3.3, where for Decenox (C10:1) at the same immersion time (0 h 57~1 h), the corrosion current density of CS-XC38 decreases and its corrosion potential increases as the Decenox concentration increases (from 5 mg/L to 40 mg/L, at least). In addition, the absence of the double bond in Decanox (C10:0) enables it to move the potential towards greater values. This can lead to a better adsorption under a different orientations on steel, which permits the compound, at an identical concentration, to cover more of the steel’s surface compared to its analog, Decenox (C10:1). In the presence of Tridecanox (C13:0), the potential varies slightly, which suggests that the formed film remains intact and protective. Contrary to the other cases, in the presence of Undecanox (C11:0), the decrease in potential towards negative values indicates the film’s rupture or dissolution [20].
3.2. Temporal Evolution of the Rp of CS-XC38 Electrode
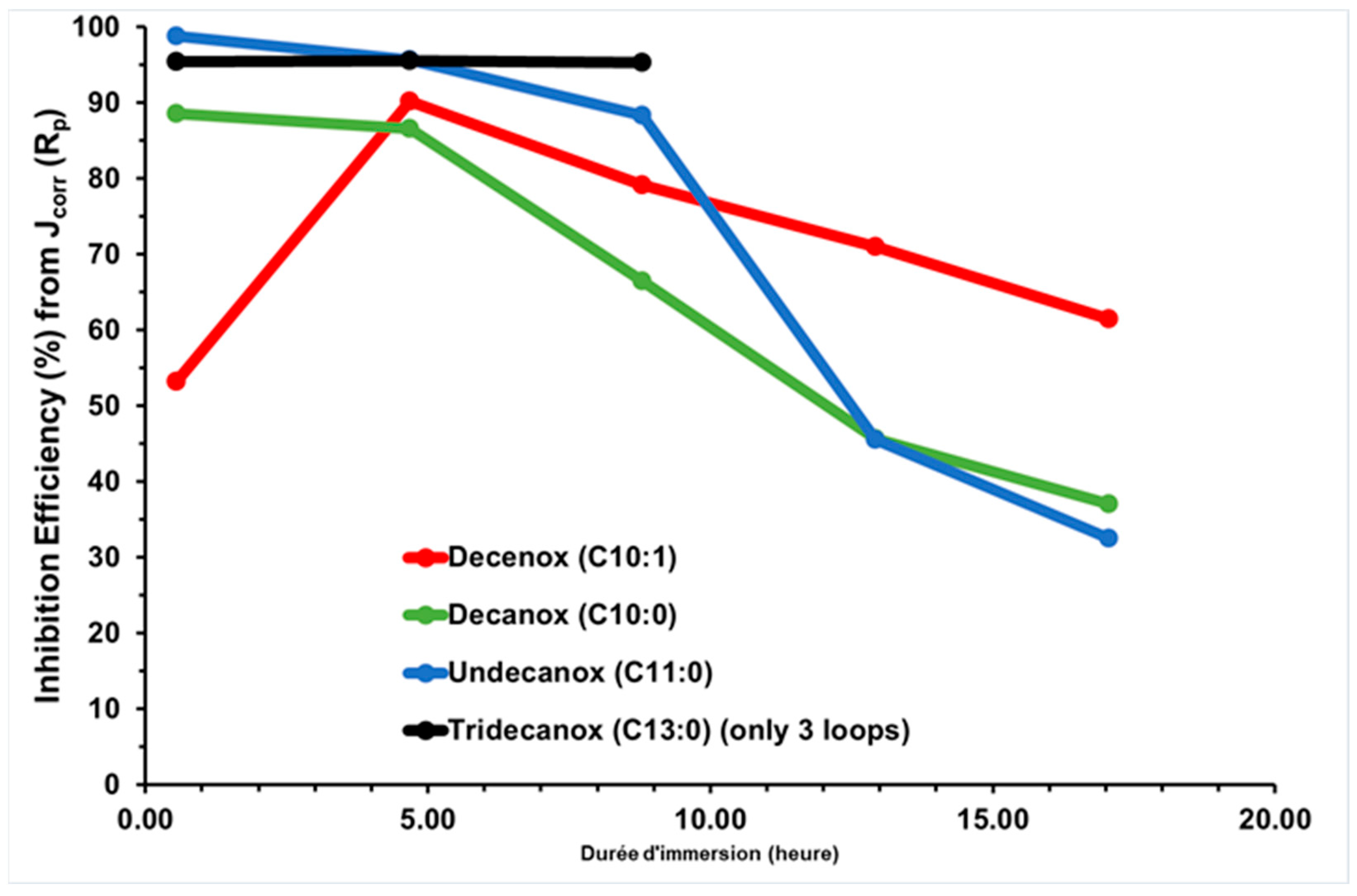
Table 3 presents the Jcorr and Rp values of the CS-X38 electrode in the presence of the four oxazoline derivatives, Decenox (C10:1), Decanox (C10:0), Undecanox (C11:0), and Tridecanox (C13:0), tested at 10 mg/L, and their average IEs (Figure 3). IE is calculated from Jcorr obtained over five measurement loops of approximately 17 h of immersion time per CS-X38 electrode [12], except for Tridecanox (C13:0), for which only three measurement loops of approximately 8 h of immersion time were used. It should be noted that in Table 3, Jcorr represents the CCD calculated from 15 Rp measured over the 17 h period, with three Rp measured per loop.

Table 3.
Summary of IEs obtained via Jcorr (Rp), deduced from Rp on new CS-X38 electrodes immersed in SRGW in the presence of each of the four oxazolines at 10 mg/L and Decenox (C10:0) at 160 mg/L, compared to immersion without inhibitor (WI).
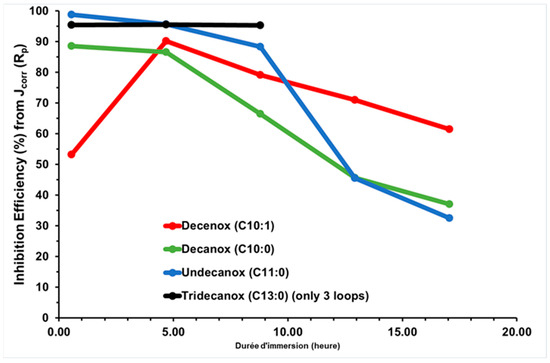
Figure 3.
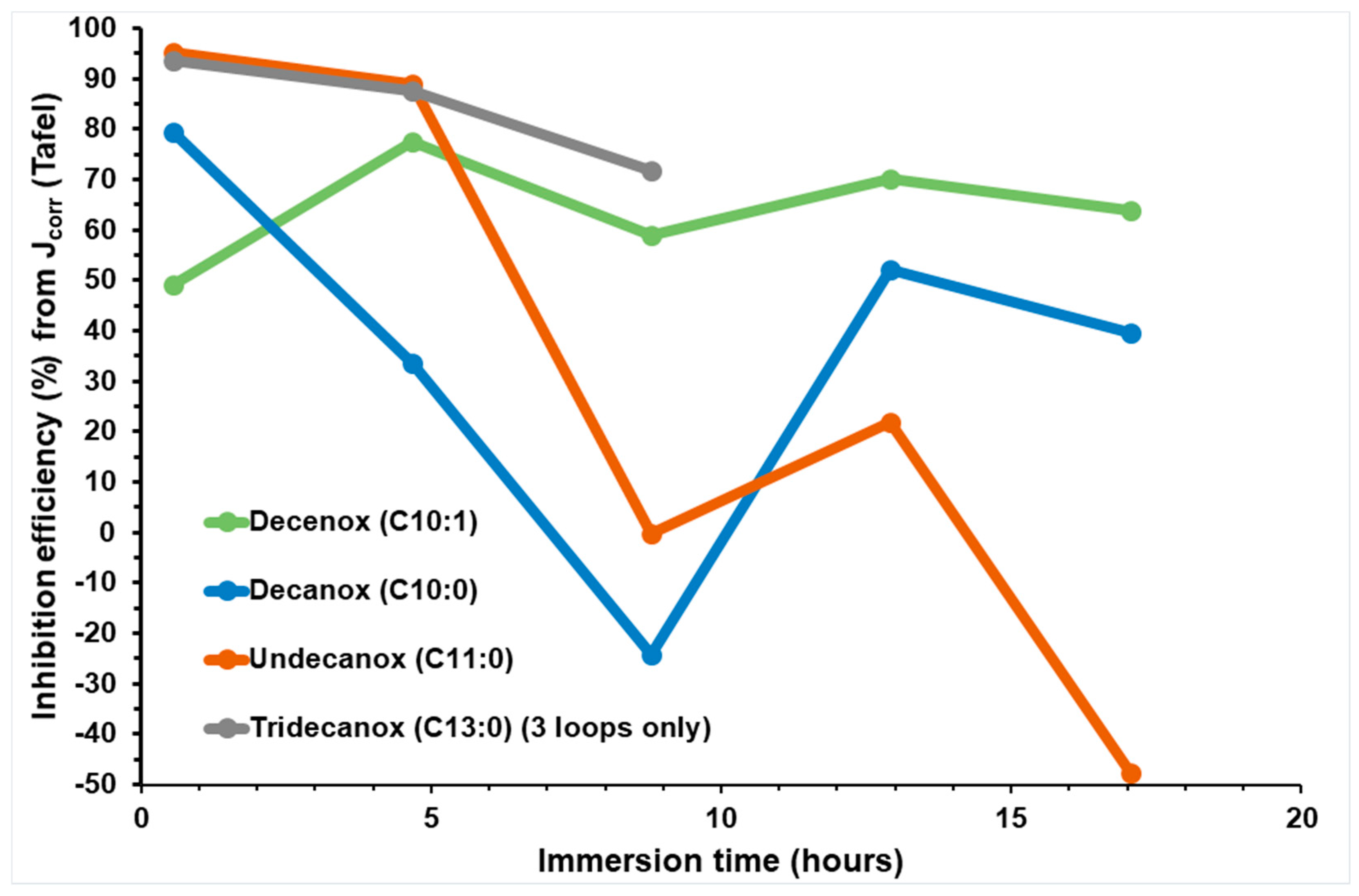
Temporal evolution of IE, obtained via Jcorr (Rp) deduced from Rp on new CS-X38 electrodes immersed in SRGW in the presence of each of the four oxazolines, tested at 10 mg/L.
Undecanox and Tridecanox, at an identical concentration of 10 mg/L, have higher IEs during the first hour of immersion (44 min), and this effectiveness is very similar to that of Decenox at 160 mg/L.
Moreover, these results confirm that lengthening the aliphatic chain via reinforcing its hydrophobic character makes it possible to increase the IE [17], which was attributed to the effect of electron donation by the aliphatic groups [19].
The hydrophobic and hydrophilic characteristics of an organic molecule can be controlled by the addition or elimination of polar and nonpolar substituents. Thus, lengthening the aliphatic chain is the most commonly used method to increase hydrophobicity [21]. This approach produces the same results in acid or alkaline solution [19].
On the other hand, Undecanox presents an effectiveness slightly higher than that of Tridecanox, suggesting a certain limit of improvement. This is attributed to the reduction in the solubility of these compounds due to their very high hydrophobicity [21]. This is why the formulation of the BS-CICs is necessary, although at a temperature of 70 °C, it is possible their solubility is not a limiting factor [18].
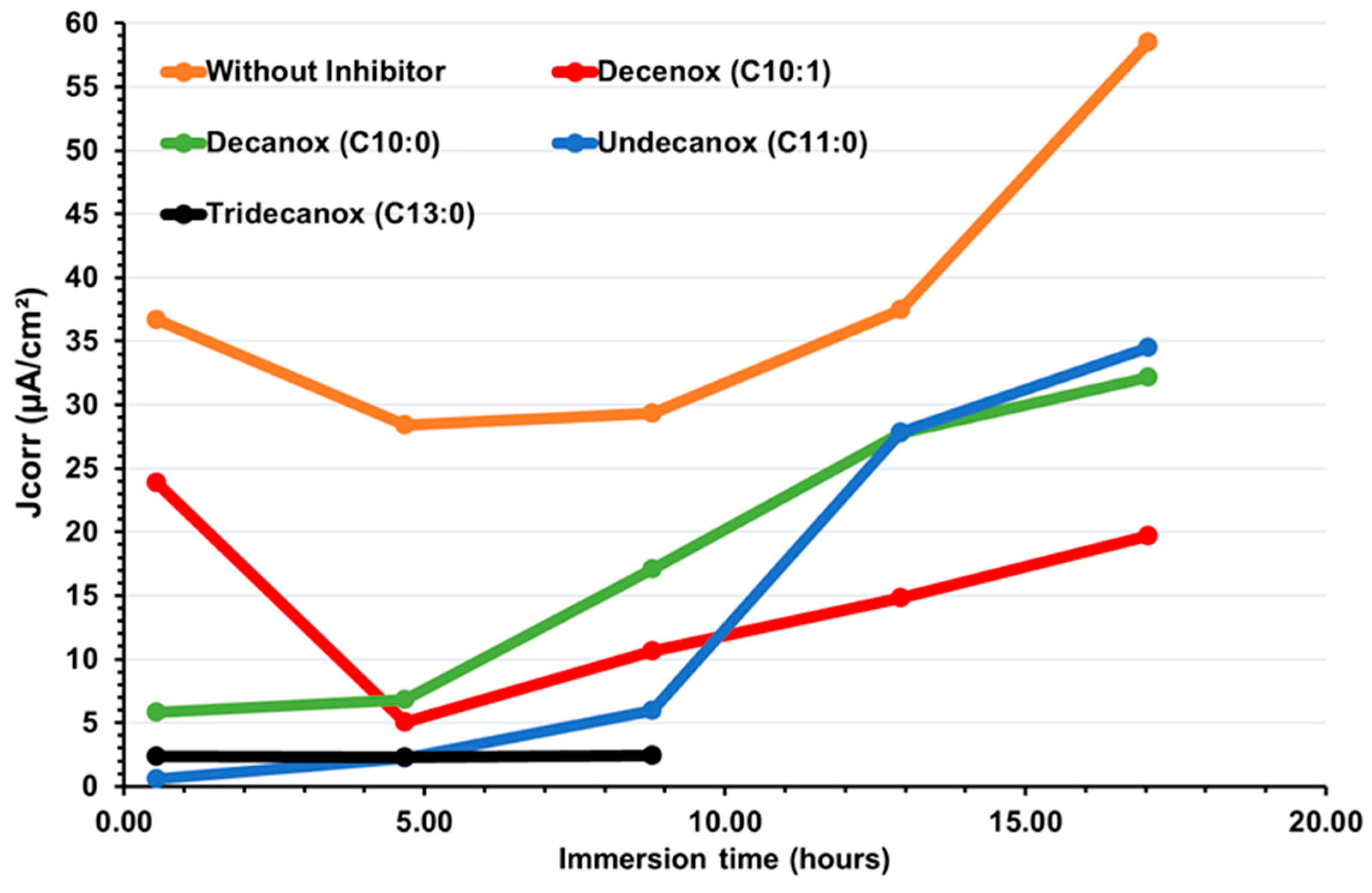
According to Figure 3, over the first hours of immersion, it is clearly noticeable that the absence of unsaturation (of double bond) at the end of the aliphatic chain increases the affinity of the molecule towards the CS-XC38 electrode. This also explains why Decanox (C10:0) has the capacity to move the Ecorr of the CS-XC38 towards higher values (–665 mV/SCE, cf. Figure 2) compared to Decenox (C10:1) (–730 mV/SCE, cf. Figure 2). However, one can note that no improvement in film stability was observed over one 17 h period. This observation applies to all the 2-oxazoline derivatives, except for Tridecanox, which shows a better stability over at least 8 h of immersion. This difference can be related to the presence of a longer aliphatic chain [22]. Stability beyond 8 h could not be confirmed because of lack of data. Despite the instability and the reduction in inhibitory effectiveness, it is important to note that Jcorr remains significantly lower than that measured in the absence of any inhibitor (Figure 4).
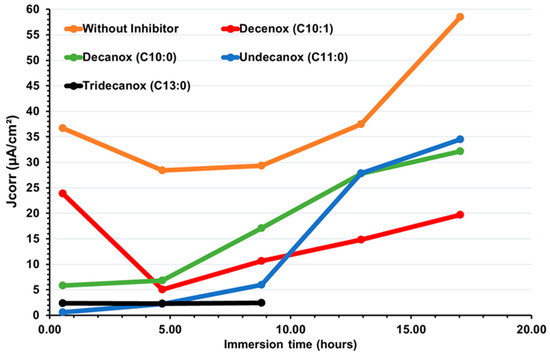
Figure 4.
Temporal evolution of corrosion current density, Jcorr, deduced from Rp on new CS-XC38 electrodes immersed in SRGW in the absence (without an inhibitor) and in the presence of each of the four oxazolines, tested at 10 mg/L.
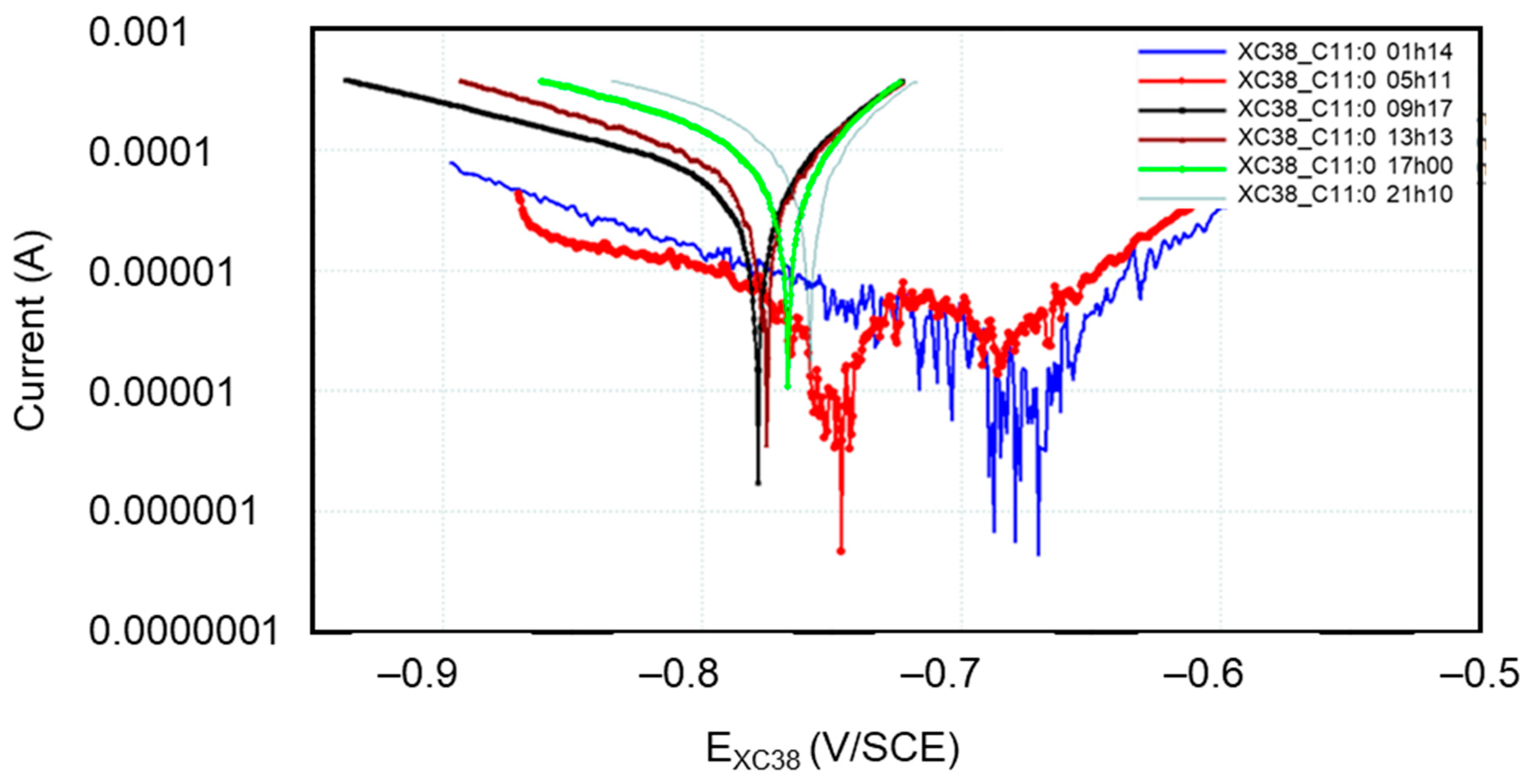
3.3. Temporal Evolution of the Jcorr of the CS-XC38 Electrode, Deduced from Linear Polarization (Tafel Plots)
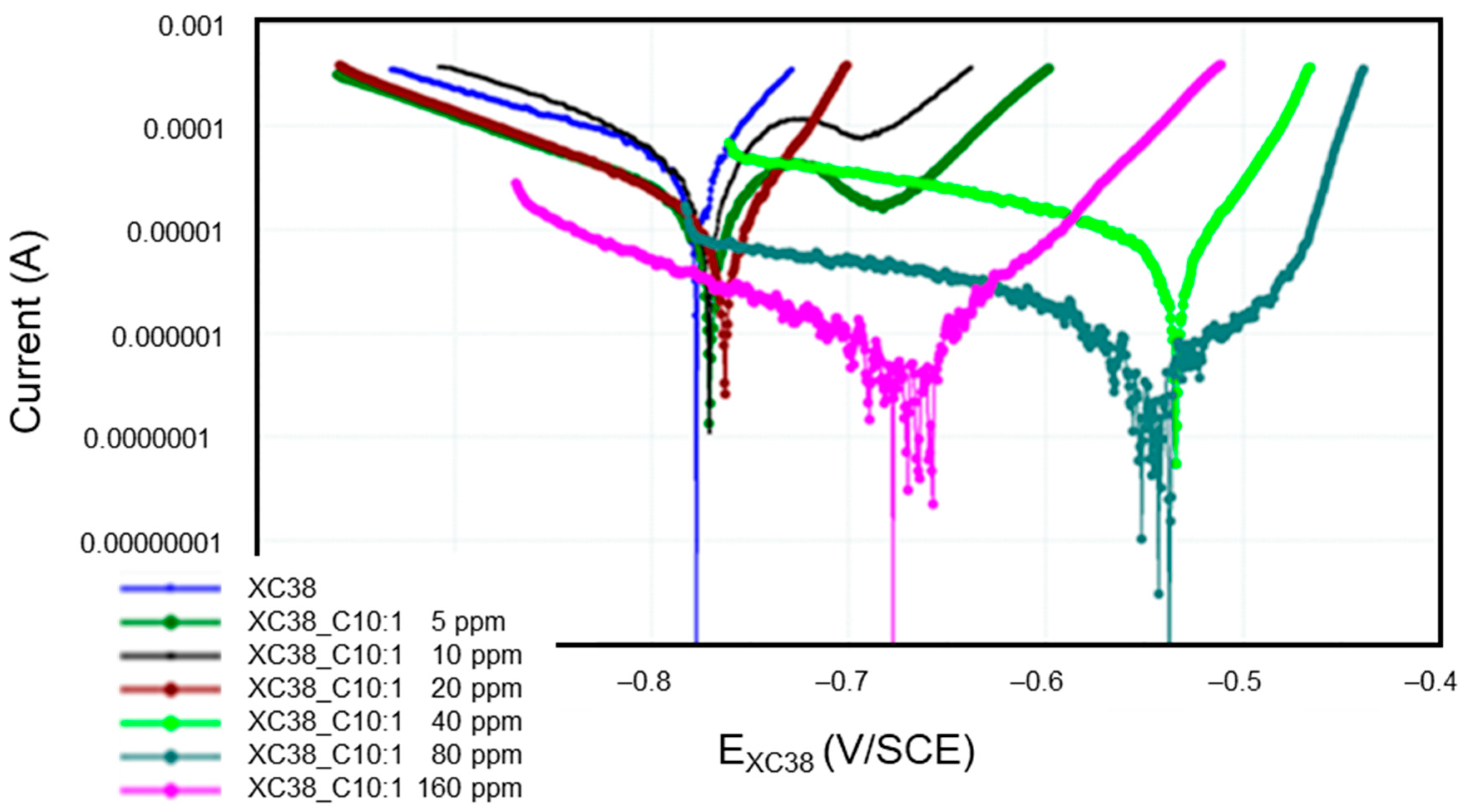
Figure 5 presents the Tafel curves measured on the CS-XC38 electrode when it is immersed in SRGW in the absence of any inhibitor and in the presence of only Decenox (C10:1) at concentrations of 5,10, 20, 40, 80, and 160 mg/L for immersion times between 43 and 57 min (noted as 57 min). It can easily be noted that under Decenox (C10:1), in the same immersion time (0 h 57 min~1 h), the CCD of CS-XC39 decreases and its corrosion potential Ecorr CS-XC38 increases as the Decenox concentration increases (from 5 mg/L to 40 mg/L, at least). What is observed here is that the more concentrated the inhibitor (from 5 to 40 mg/L), the more the steel becomes ennobled (that is to say, its corrosion potential increases).
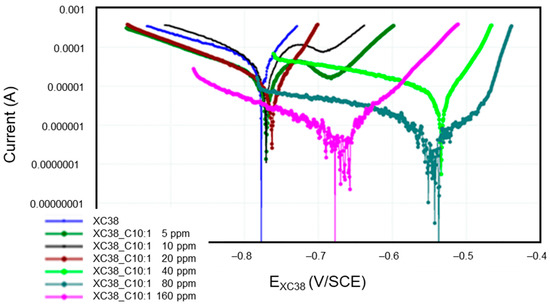
Figure 5.
Evolution of Tafel curves of the CS-XC38 electrode immersed in SRGW in the presence of Decenox (C10:1) at concentrations of 5, 10, 20, 40, 80, and 160 mg/L, compared to immersion without inhibitors at immersion times between 43 and 57 min.
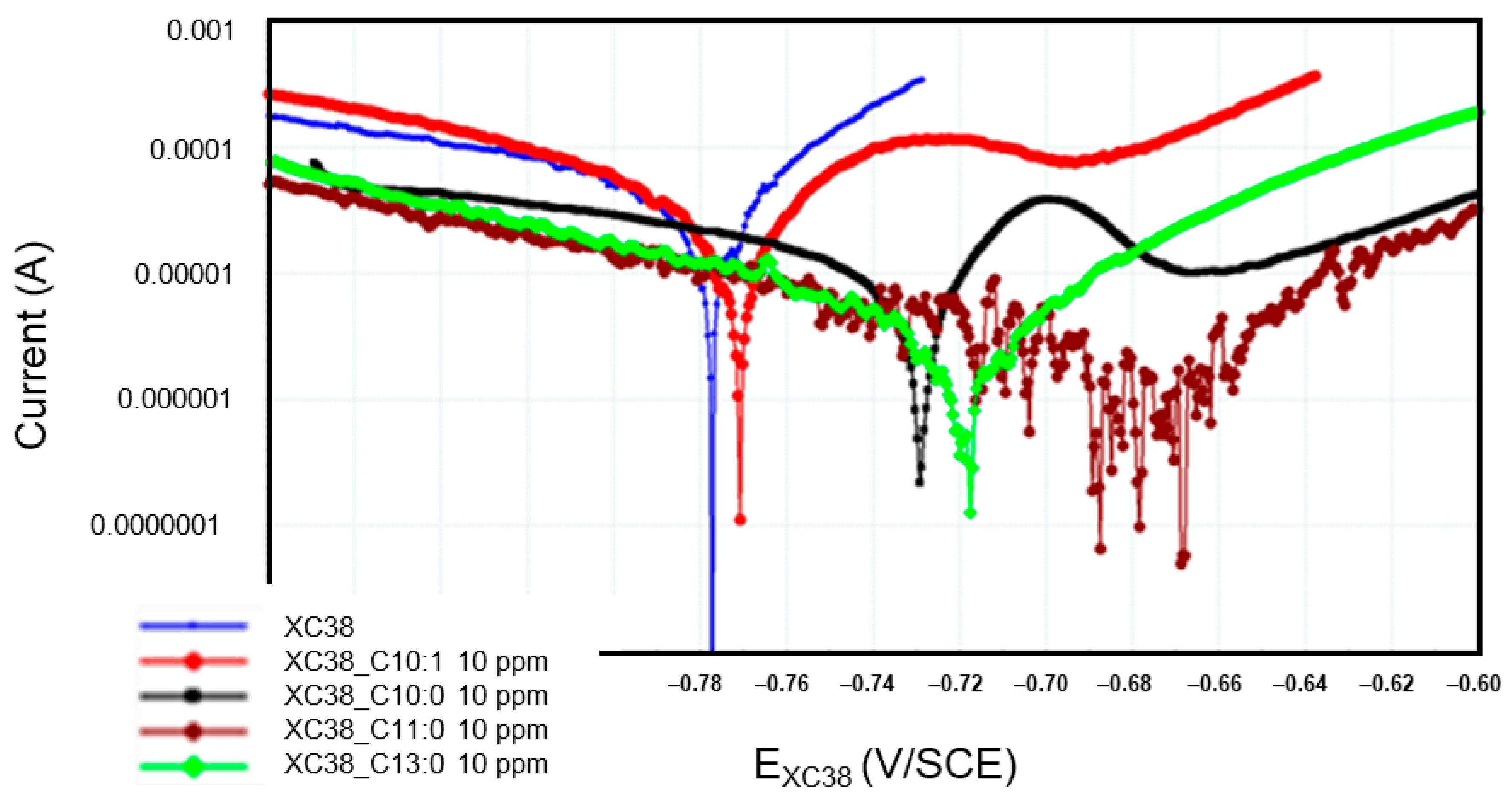
Figure 6 presents the Tafel curves measured on the CS-XC38 electrode when immersed in SRGW in the absence of any inhibitor and in the presence of each of the four oxazolines at 10 mg/L for immersion times between 43 and 57 min (noted as 57 min). To more precisely evaluate the cathodic and anodic behaviors of the CS-XC38 electrode in the presence of the four oxazolines, we investigated the Jcorr measured at the time of the first Tafel curve of the first loop, after 44 min of immersion (Appendix A, Table A1), and the corresponding IE.
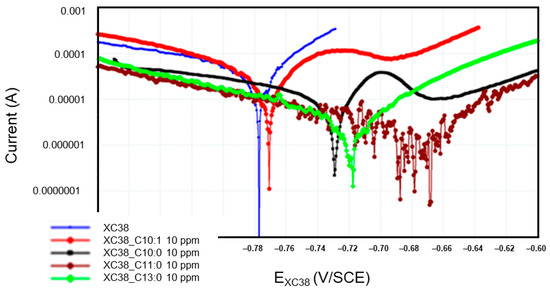
Figure 6.
Evolution of Tafel curves of the CS-XC38 electrode immersed in SRGW in the presence of each of the four oxazolines at 10 mg/L compared to immersion without inhibitors at immersion times between 43 and 57 min.
3.3.1. Cathodic Side Activity
In the presence of Decanox (C10:0), the cathodic activity slightly decreases (see Appendix A, Table A1), reaching an IE of approximately 73% during the first measurement (44 min). On the other hand, in the presence of Decenox (C10:1), the inverse occurs: a light increase in cathodic activity is noted compared to the case without an inhibitor (WI). This observation corresponds to the first measured IE of approximately 34%, although this value remains lower than the IE measured in the presence of Decanox (C10:0). This suggests that the addition of an inhibitor to a corrosive environment does not modify (or modifies them very little) the mechanisms of hydrogen (H2) production and H+ ion reduction on the surface of the metal. The inhibitor adsorbed on the surface of metal forms a film that blocks the reaction sites of the metal. Thus, the surface area available for the corrosive ions such as H+, HS−, and Cl− decreases, while the real mechanism of reaction remains unchanged [23].
3.3.2. Anodic Side Activity
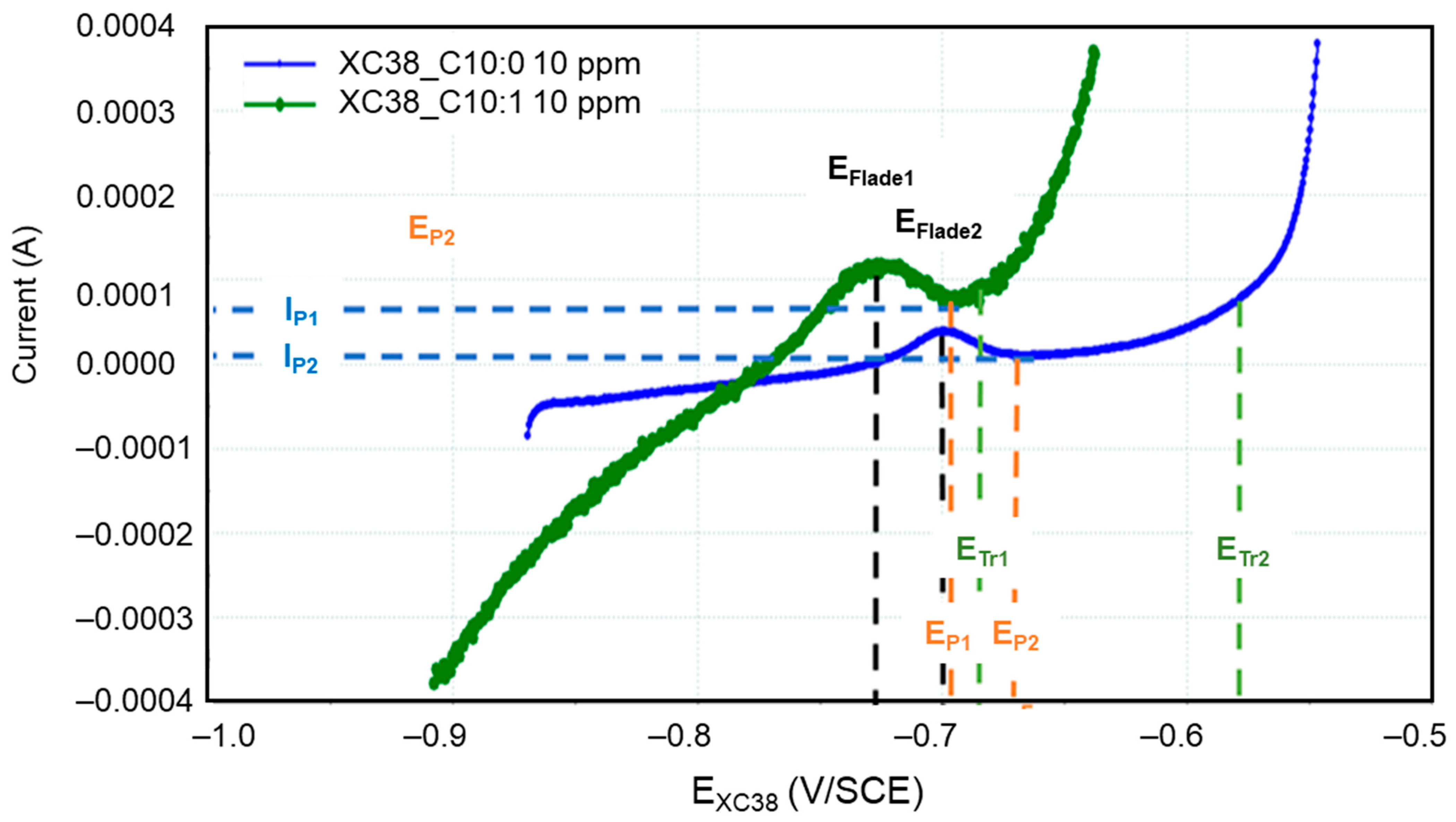
The presence of Decenox (C10:1) and Decanox (C10:0) has an impact on anodic activity, involving a passivation effect in both cases. This passivation is probably due to the formation of a protective coating, which appears more effective in the case of Decanox (C10:0), which results in a broader passivation. This layer causes a fast reduction in the corrosion current (Figure 6). In Figure 7, three zones can be distinguished: a first active zone, located between Ecorr and Ep (passivation potential); a second passive zone, between Ep and ETr (transpassivation potential); and finally, a third zone of transpassivation, beyond ETr. The shape of the anodic branch observed in this case does not seem to be related to a second reaction, such as the oxidation of water or an electroactive reaction implying a species (as described previously in the presence of Decenox (C10:1) at 160 mg/L). This is explained by the fact that the potentials implied in this reaction are definitely more distant and more negative than those of the oxidation of water. Consequently, this phenomenon is ascribable to the rupture of the passivating layer. This rupture is generally due to an attack of chloride ions [19,24]. When the potential E moves towards more anodic values, a peak appears in the active phase, from which we can determine the potential of Flade (EFlade), which represents the potential to which the current reaches its maximum. In the case of Decenox (C10:1), EFlade1 = −725 mV/SCE, while for Decanox (C10:0), EFlade2 = −699 mV/SCE. Beyond this potential, the current decreases, but at Ep, the current falls abruptly, marking the beginning of the passivation phase. However, in this case, it is more about a pseudopassivation [25], with Ep1 = −692 mV/SCE and Ep2 = −659 mV/SCE, respectively. When the potential reaches ETr, which is −680 mV/SCE and −577 mV/SCE for the two cases, a strong corrosion current density is observed. This is related to the pitting potential or transpassivation potential (ETr), beyond which pitting is initiated [26]. Because of this pseudopassivation, for a potential value greater than the passivation potential (Ep), CS-X38 is not completely passive, and dissolution continues, with IP1 = 75 µA and IP2 = 9.98 µA, respectively. The form of passivation can depend on the scanning rate [25]. Thus, in the case of Decenox (C10:1), IP1 > Jcorr, while in the presence of Decanox (C10:0), Ip2 < Jcorr, both of which are considerably smaller than the critical maximum current, IFlade, measured at EFlade [25]. The better-quality passivation film formed by Decenox (C10:1) can probably explain this difference. However, beyond the passivation potential (Ep), the corrosion becomes localized, and the local current density at this site becomes very high [26]. This mechanism is generally observed in the presence of inorganic inhibitors. Inorganic inhibitors have the capacity to be adsorbed onto a metal surface and form a passivation layer, which comprises a mono-oxide or a polyatomic oxide film [27].
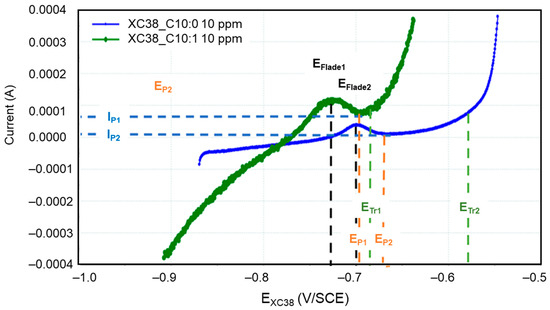
Figure 7.
Polarization curves of CS-XC38 electrode immersed in SRGW in the presence of Decenox (C10:1) (in green) or Decanox (C10:0) (in blue), both at 10 mg/L.
At weak concentrations, the first stage implies the exchange of water molecules adsorbed on the metal surface by the inhibitor. Thereafter, nitrogen atoms and π electrons present in the azole cycle facilitate interactions with the Fe2+ ions generated during iron dissolution. This leads to the formation of Fe(II)–Decenox complexes, which are adsorbed on the metal surface, thus reducing the iron corrosion rate [27]. However, with high positive over-tensions, an increase in the corrosion current is observed, indicating a rupture of the protective coating. This increase is slightly slower in the case of Decanox (C10:0). This passivation phenomenon is not observed in the presence of Decenox (C10:1) at high concentrations (Figure 5), i.e., beyond 10 mg/L. It is possible that at these higher concentrations, the metal surface is covered by a thick layer of Decenox, thus preventing the dissolution of iron. Consequently, the formation of complexes, such as those occurring at weak concentrations, does not occur in this case.
Another possibility is that Decenox is adsorbed onto the metal’s surface by adopting various orientations according to the concentration: plane or horizontal orientations below the optimal concentration (≤10 mg/L), while above the optimal concentration, they are adsorbed by acquiring vertical orientations according to the mode of adsorption proposed by [28]. Moreover, below the optimal concentration, the intermolecular attractive force between the metal surface and the inhibitor molecules prevails, while above the optimal concentration, the intramolecular repulsive force between the inhibitor molecules prevails [18].
A third possible scenario to consider is that the sulfide can enter into a reaction with the azole cycle of Decenox (2-oxazoline), thus causing the opening of the cycle to form a thioamide derivative [15]. In this form, it is probable that this thioamide derivative can induce the passivation of the metal surface. However, the length of the aliphatic chain could stabilize the azole cycle, thus preventing its opening and the passivation, which could occur.
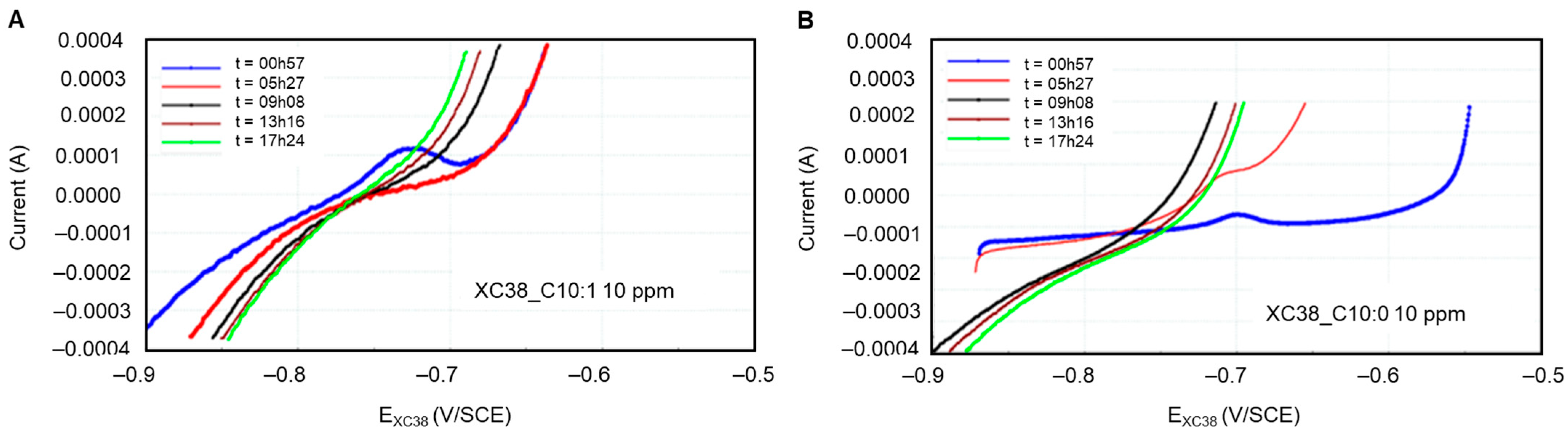
It seems that the passivation observed in the presence of Decanox (C10:0) is present only in the first two loops of measurement over an immersion period of 17 h. However, in the presence of Decenox (C10:1), this characteristic of pseudopassivation appears only in the first measurement (Figure 8). Consequently, in the event of pseudopassivation, the determination of the corrosion current is based on the cathodic curve, for it presents a linear shape, whose extrapolation at the corrosion potential will make it possible to obtain the instantaneous corrosion current [29]. In addition, on the active area, the corrosion current is similar to that given starting from the cathodic curve, even if the anodic polarization curve is more complex because of the non-linearity of the Tafel curve when the potential moves away from approximately 35 mV [26]. Moreover, the values obtained are close to those measured by the LPR technique.
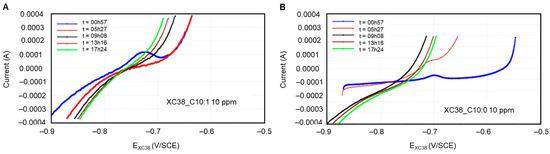
Figure 8.
Polarization curves of CS-XC38 electrode immersed in SRGW in the presence of (A) Decenox (C10:1) on the left and (B) Decanox (C10:0) on the right, both at 10 mg/L, as a function of immersion time.
With regard to Undecanox and Tridecanox, the lengthening of the aliphatic chain is related to the disappearance of passivation. Moreover, just like for the cathodic activity, the anodic activity in the presence of these two molecules remains relatively unchanged. This is probably due to the blocking of the anodic and cathodic sites without major modification to the reaction mechanism because of the covering of the metal’s surface due to their longer aliphatic chains [18,22,30] compared to both compounds carrying a chain of 10 carbon atoms (Figure 6 and Figure 9). In the presence of Tridecanox, the anodic slopes of the Tafel curves present a light deviation from the linearity at all applied potentials, which can be attributed to the adsorption of the inhibitor, the deposit of corrosion products or impurities on the CS-X38 surface [23]. After analysis of the cathodic and anodic behavior of these molecules derived from oxazolines, it was concluded these corrosion inhibitors present a mixed behavior, with a light anodic prevalence, in the presence of Decenox.
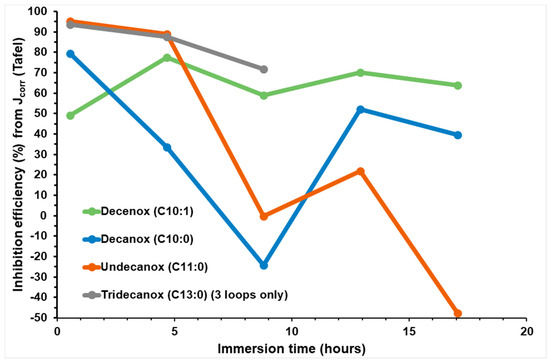
Figure 9.
Evolution of IE (%) determined from Jcorr based on Tafel curves for CS-XC38 electrode immersed in SRGW in the presence of the four oxazolines at 10 mg/L at immersion times from 0 to 17 h.
Figure 9 presents the inhibition efficiency of the four oxazolines obtained from the Tafel plots of Jcorr (TP) as a function of immersion time.
3.4. Temporal Evolution of the Jcorr of CS-XC38 Deduced from Impedance Measurement
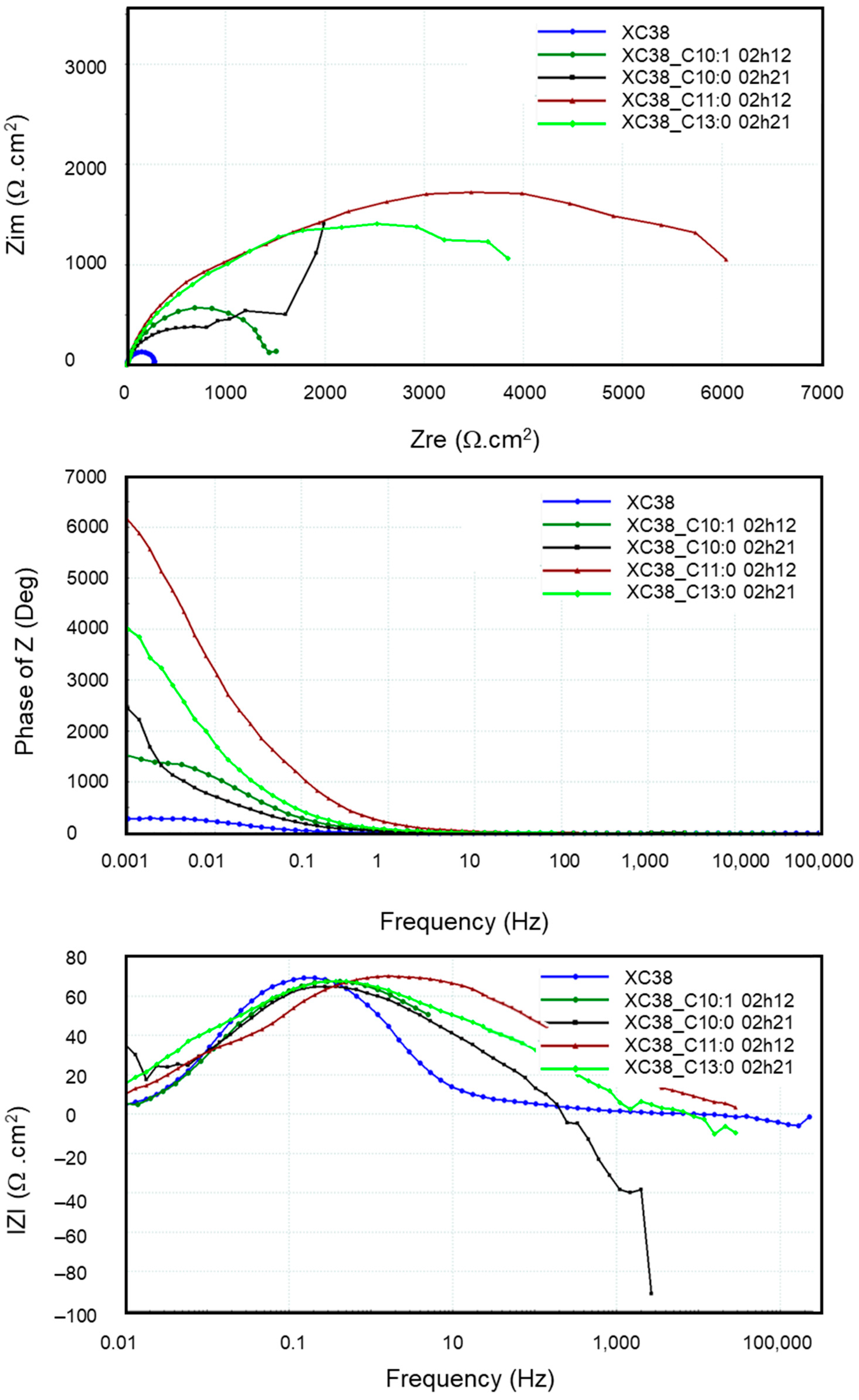
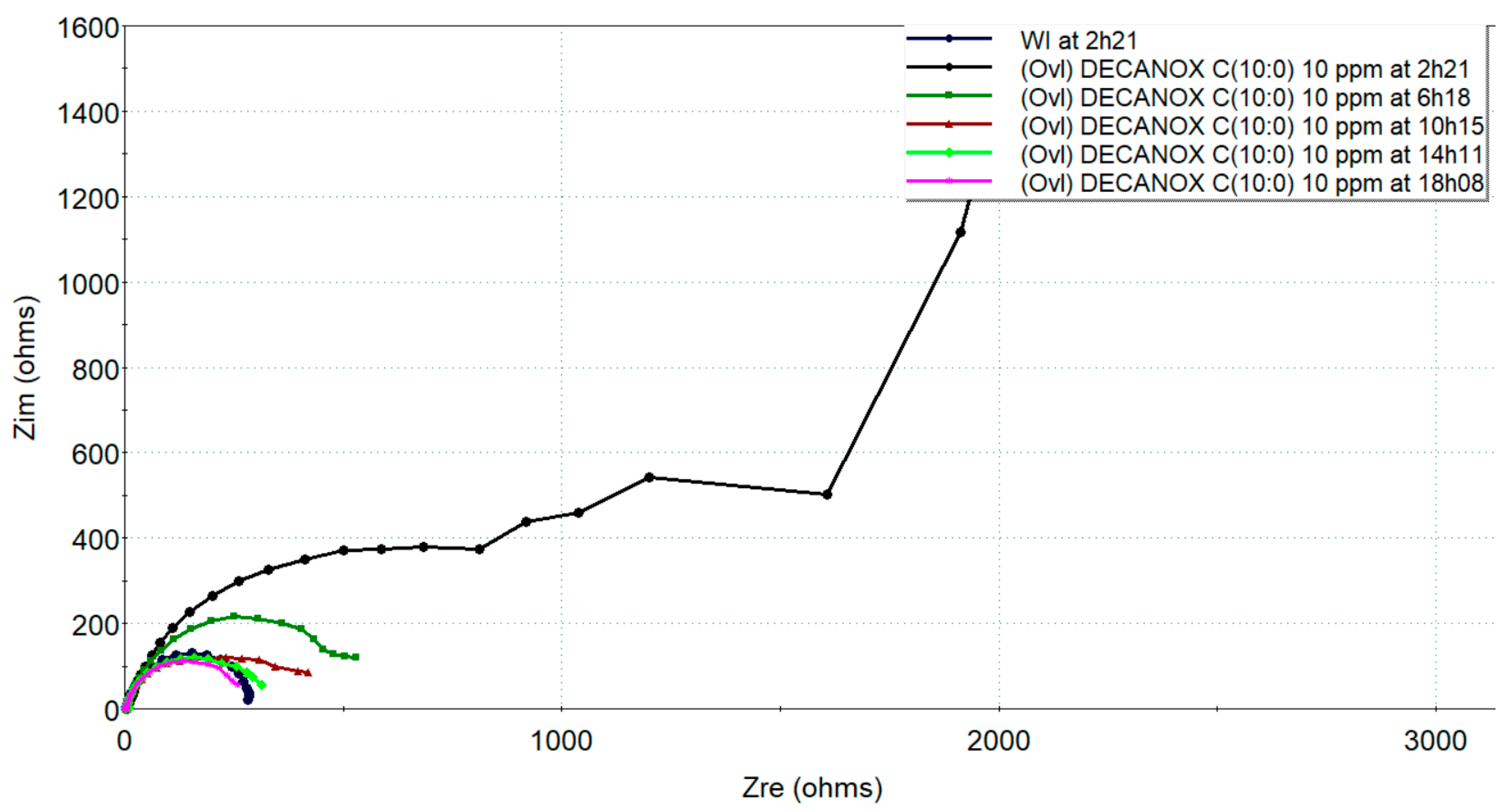
Figure 10 presents the EIS diagrams in Bode and Nyquist mode obtained from the CS-XC38 electrode in the absence and presence of the four oxazolines over one period ranging from 2 to 3 h of immersion. It is observed that the EIS diagrams present only one time-constant in the presence of these inhibiting compounds, except for Decenox (C10:0). The impedance diagram in the presence of Decanox (C11:0) does not systematically form a half-circle (Figure 10), which suggests the possibility of the appearance of a new phenomenon at very low frequencies. However, its exact nature is not clear, even after several attempts at modeling with various equivalent electric circuits (EECs). We should note that the impedance measurement is preceded in the loop by Tafel curve and LRP measurements. This is possibly related to the passivation layer observed at the time of the Tafel plot measurements. Moreover, the other loops obtained at various immersion times do not show notable improvement in IE, which suggests that the phenomenon observed at the very low frequencies (Figure 11) does not correspond to the adsorption of Decanox (C11:0). Consequently, the best adapted EEC model for the comparison of these data seems to be the Re(QRtc) model used previously.
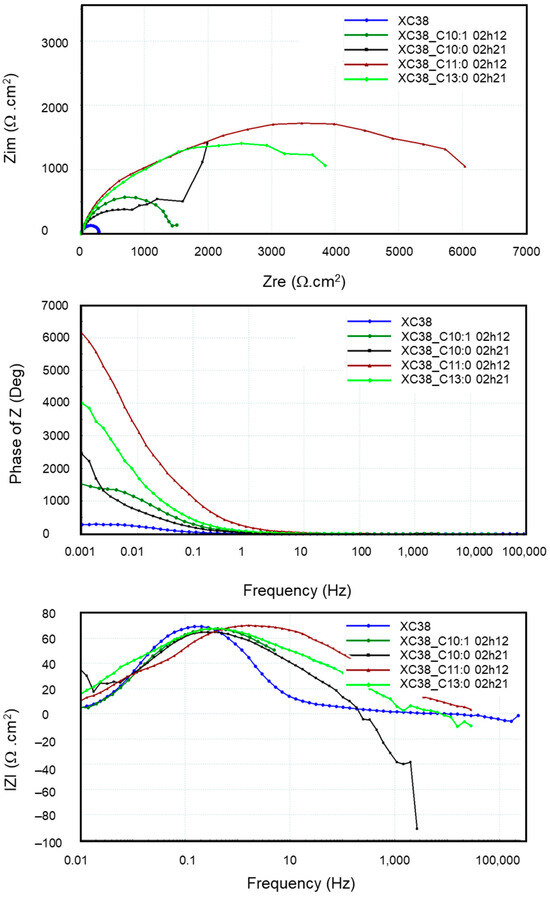
Figure 10.
Evolution of electrochemical impedances, both in the Nyquist and in the Bode planes, measured on new CS-XC38 electrodes in the presence of each of the four oxazolines at 10 mg/L compared to without inhibitors as a function of immersion time in SRGW.
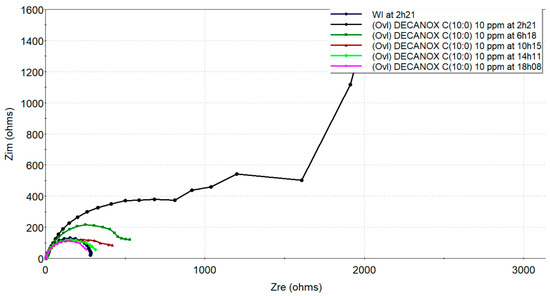
Figure 11.
Evolution of electrochemical impedances, in the Nyquist plane, measured on new CS-XC38 electrodes in the presence of Decanox (C10:0) at 10 mg/L compared to without inhibitors as a function of immersion time in SRGW.
In the presence of Undecanox (C11:0) in Figure 12, it is possible that a new interface begins to form at very low frequencies, different from the interface observed in the presence of Decanox (C10:0). This observation could be due to the long measurement duration at these very low frequencies, where there could be a change in the surface quality of the steel. This evolution was confirmed by the Tafel plot (Figure 13) after approximately 5 h of immersion, where passivation appeared on the anodic branch, which disappears in the rest of Tafel plot. In addition, Rtc decreases to a great extent with time, as in the case of the other molecules, which confirms what was previously discussed about the stability of Undecanox, with both LPR and Tafel plots. It is possible that Undecanox (C11:0) is adsorbed onto the CS-X38 electrode by a physical absorption process, as we know that physical absorption implies forces such as van der Waals interactions and electrostatic interactions [31]. Thus, the high temperatures tend to have an unfavorable effect on this form of adsorption, contrary to the case of chemisorption [32]. Consequently, the desorption of the inhibitor can occur at high temperatures [33,34], and competition with other ions present in the medium can also occur. In addition, inhibitors containing nitrogen atoms such as imidazolines generally show instability at high temperatures [35,36]. However, other factors can take part in this instability, such as the strong disturbance of the sample and the immersion time [34].
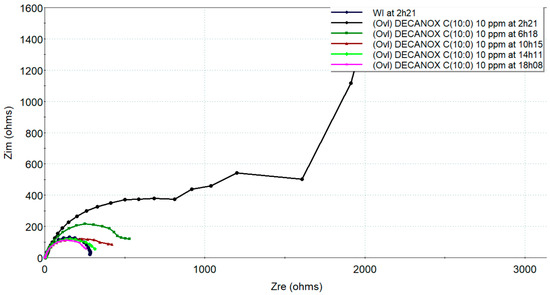
Figure 12.
Evolutions of electrochemical impedance, in the Nyquist plane, measured on new CS-XC38 electrodes in the presence of Undecanox (C11:0) at 10 mg/L compared to without inhibitors as a function of immersion time in SRGW.
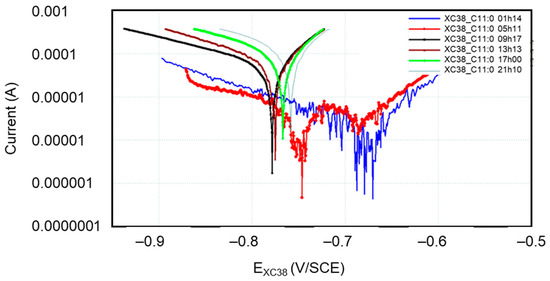
Figure 13.
Evolution of Tafel curves of new CS-XC38 electrodes in the presence of Undecanox (C11:0) at 10 mg/L as a function of immersion time in SRGW.
Some values of IE in % via the corrosion current densities Jcorr Rw deduced from the impedance measurements are presented in Table 4.

Table 4.
Summary of IE in % via Jcorr Rw deduced from the impedance diagrams on new CS-X38 electrodes immersed in SRGW in the presence of each of the four oxazolines, tested at 10 mg/L compared to without inhibitor.
3.5. Study of the Adsorption and Adsorption Isotherms of Decenox (C10:1)
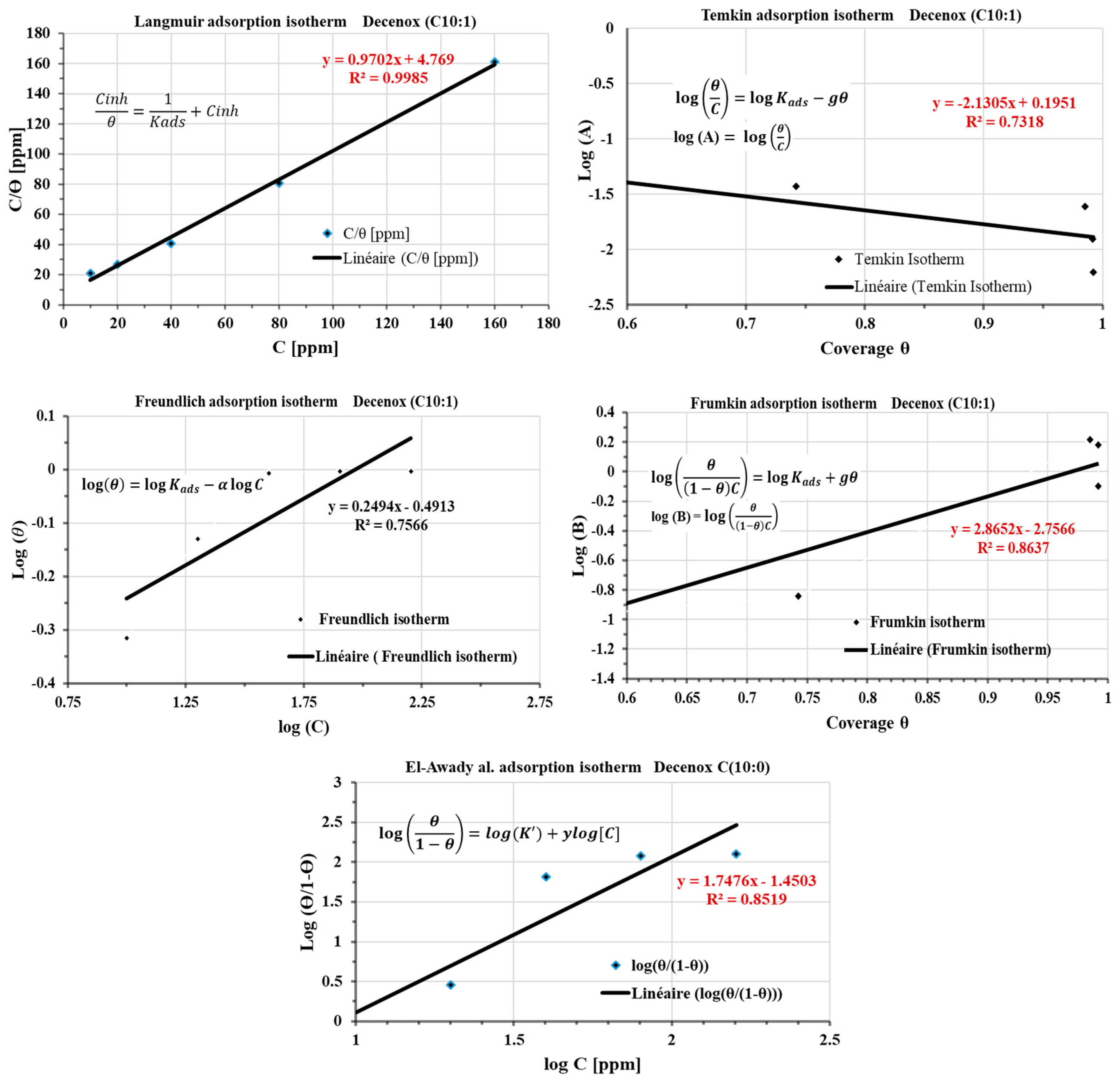
This study aims to deepen the understanding of the mechanisms of adsorption by using isotherms of adsorption of the four oxazolines. However, these isotherms were examined for only one compound, Decenox (C10:1), at various concentrations (10, 20, 40, 80, 160 mg/L) and at a constant temperature of 70 °C. This approach was selected because of the complexity of the medium, i.e., SRGW, characterized by the presence of major anions and cations. It is well known that parameters such as temperature can modify the geochemistry of water (such as the acid–base equilibria and dissolution–precipitation of species), which can, in turn, influence the nature of corrosion, as well as the composition and the morphology of the corrosion products [37,38] (as discussed in the section on the progressive setting up of SRGW [12]), and by extension, the adsorption of the inhibitors. Moreover, the behavior of the inhibitors can vary according to the physicochemical conditions, surface quality, and nature of the formed deposits [39]. The adsorption isotherms make it possible to understand the relationship between the adsorption of Decenox (C10:1) on the CS-XC38’s surface and its concentration [33]. Figure 14 presents the layouts of the various adsorption isotherms in the presence of Decenox (C10:1), in particular the isotherms of Langmuir, Temkin, Freundlich, Frumkin, and El-Awady. The coverage rate, θ, calculated from the value of the first polarization resistance (Rp) measured during the electrochemical tests, allows the homogeneous initial state of the CS-XC38’s surface at the beginning of the immersion to be taken into account. This calculation is carried out using the following formula [27,40]:
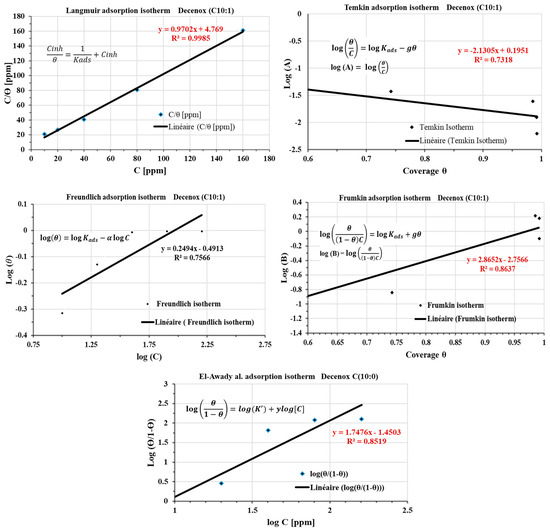
Figure 14.
Langmuir, Temkin, Freundlich, Frumkin, and El-Awady adsorption isotherms plotted from the polarization resistance (1st Rp) of the CS-XC38 electrode in SRGW in the presence of Decenox (C10:1) at 70 °C.
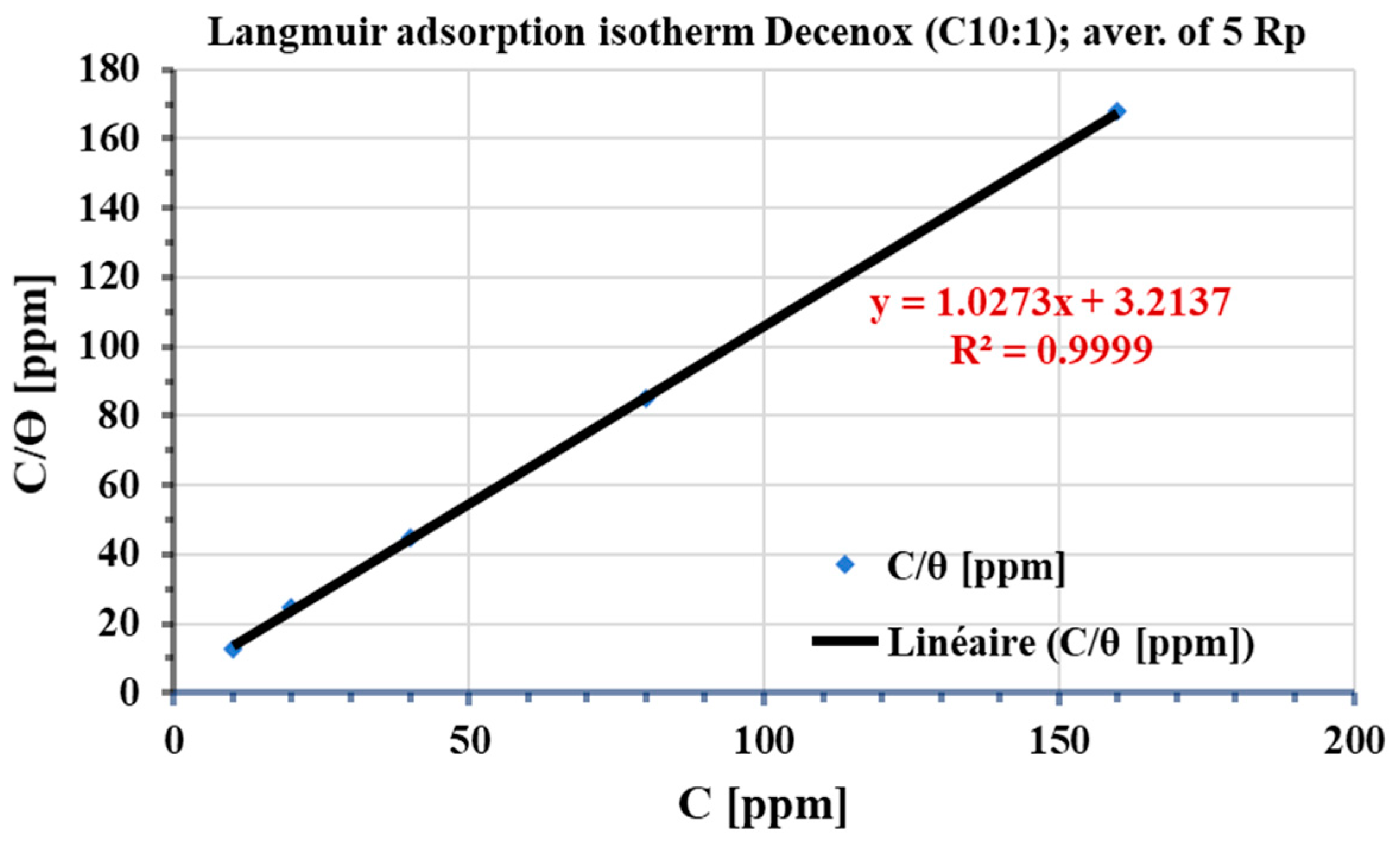
Among the various isotherms evaluated, the Langmuir isotherm showed the best adjustment, with a regression coefficient (R2) close to 1. Generally, the nonionic surfactants follow this isotherm [41]. According to this model, the adsorbed film on the surface of the metal has a thickness roughly equivalent to a monolayer, with a finite number of identical sites [42]. This model, obtained from a linear correlation between θ and the concentration of the inhibitor, assumes that all adsorption sites are uniform in terms of affinity and energy, without there being significant interaction between the adsorbed molecules. Consequently, the process of adsorption occurs in a homogeneous way [42,43]. The potential influence of corrosion products and surface heterogeneity on the adsorption isotherm was investigated. For this, the coverage rate θ was calculated from the average of five electrochemical polarization resistances (average Rp). Figure 15 shows the plot of the Langmuir adsorption isotherm. The variations observed in the thermodynamic parameters are negligible, and the regression coefficient (R2) remains close to unity, thus suggesting the absence of the influence of corrosion products and the heterogeneity of the surface on the adsorption of Decenox (C10:1).
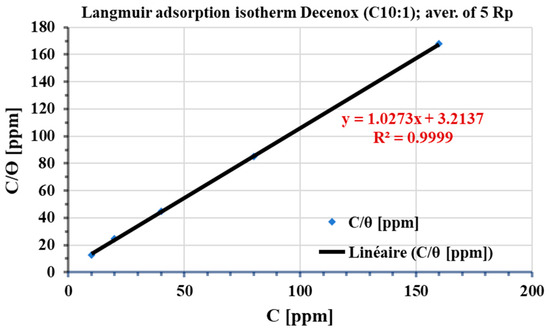
Figure 15.
Langmuir adsorption isotherm, plotted from the average of 5 electrochemical polarization resistances (Rp average) of the CS-XC38 electrode in SRGW in the presence of Decenox (C10:1) at 70 °C.
Kads values were calculated from the intercepts of the graphs. These Kads constants were then used to calculate the values of the standard Gibbs free energy change in adsorption (ΔG°ads) (Table 5). The negativity of ΔG°ads indicates that adsorption occurs faster than desorption, suggesting spontaneous, exothermic adsorption [44]. The absolute value of ΔG°ads is 7 kJ·mol−1, which is well below 41.86 kJ·mol−1, thus indicating physisorption [44]. These observations are consistent with the conclusion drawn from the variation in Eocp as a function of time. Additionally, the Kads value can provide insight into the effect of temperature on the bond strength between the inhibitor and the metal. For temperatures lower than 60 °C (Figure 15), an increase in the Kads value is noted (Kads = 0.77 at 60 °C), indicating that the increase in temperature weakens the bond [45,46]. This effect is generally observed during physisorption [32].

Table 5.
Thermodynamic parameters determined from the Langmuir adsorption isotherm in the presence of Decenox (C10:1) at 70 °C.
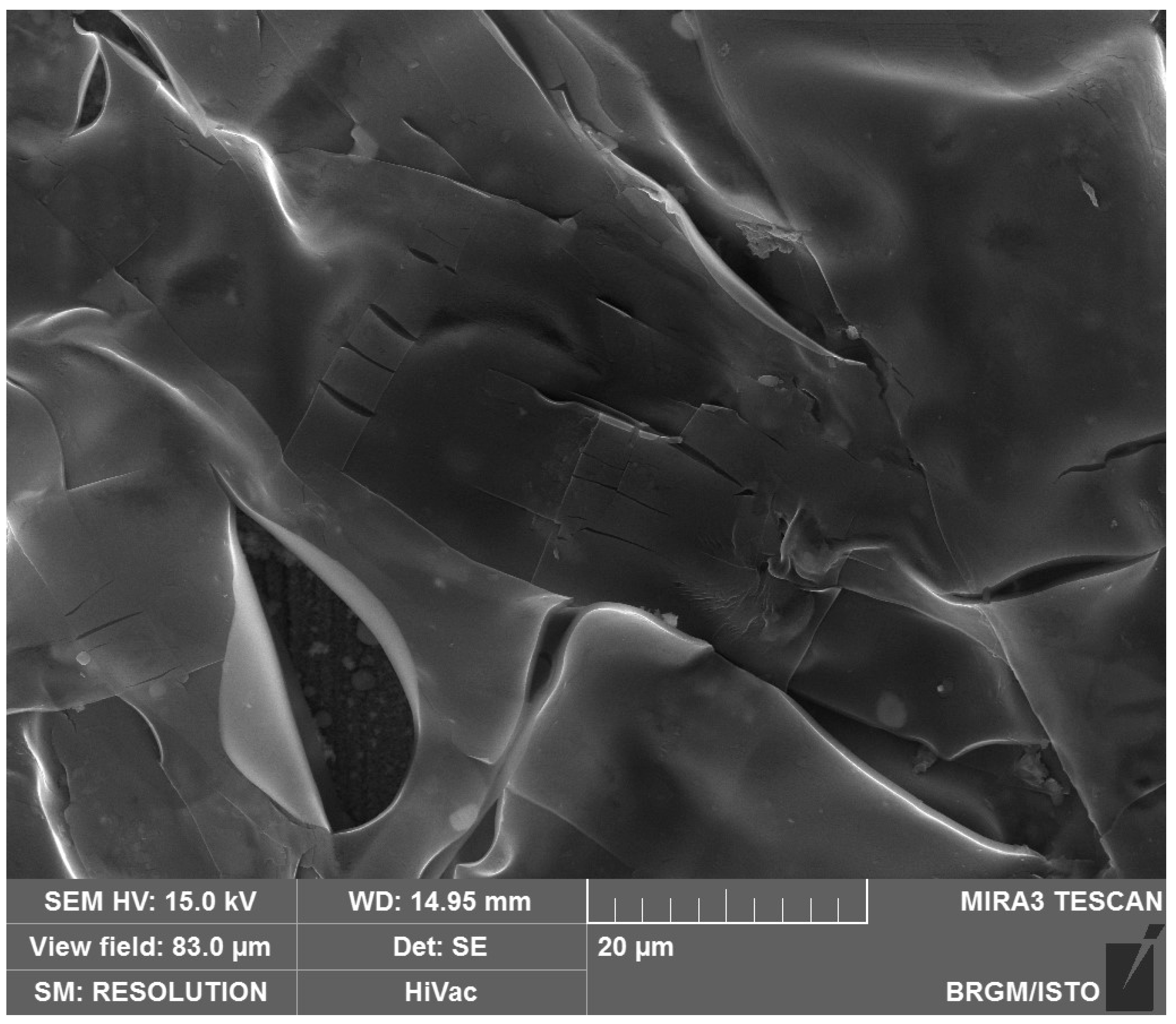
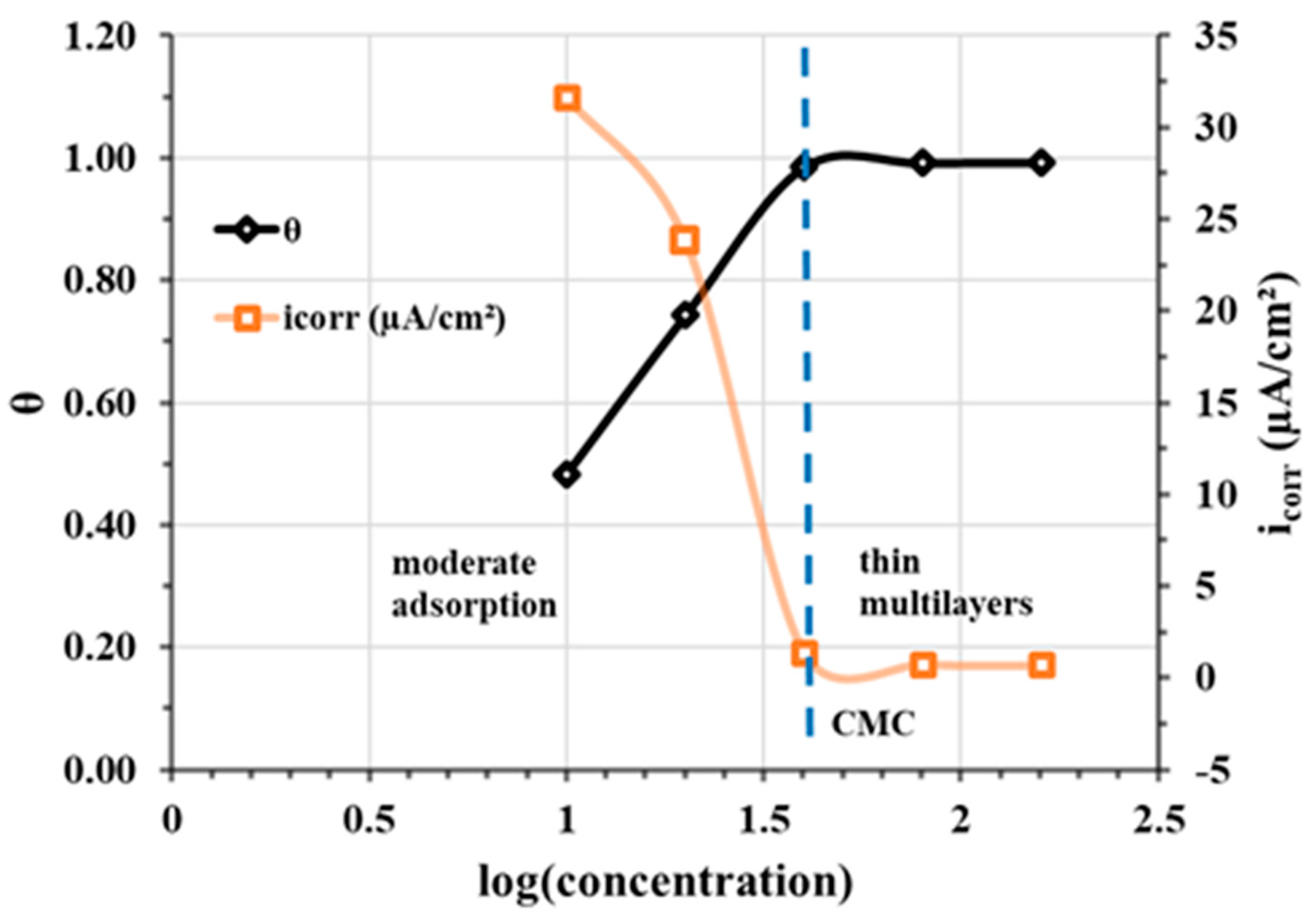
Scanning electron microscope (SEM) analyses (Figure 16) carried out on a CS-XC38 electrode, disturbed by electrochemical studies at 70 °C, reveal the presence of thin multilayers adsorbed on the surface of the CS-XC38 electrode. This is due to the excess of CMC (Figure 17) [33,47,48].
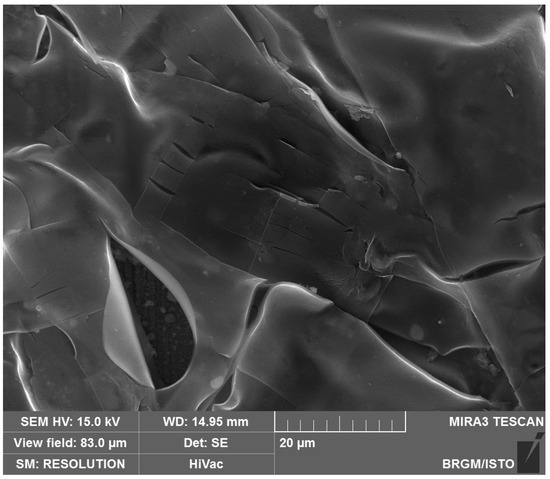
Figure 16.
SEM photograph of the CS-XC38 electrode’s surface in the presence of Decenox (C10:1), showing that the CS-XC38 sample was electrochemically disturbed at 70 °C.
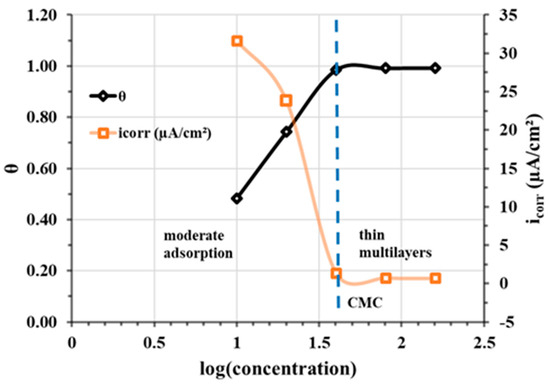
Figure 17.
Variation in coverage rate θ and corrosion current density Jcorr of CS-XC38 electrode in SRGW at 70 °C as a function of log (concentration) of Decenox (C10:1).
3.6. Discussion on the Role of Chain Length and the Presence of Unsaturation on IE
From Table 6, it is obvious that over the first 44 min of immersion, the IE of Decanox (C10:0) (84%) is significantly higher than that of Decenox (C10:1) (35%). This suggests that the presence of unsaturation at the end of the aliphatic chain reduces the IE of the inhibitor and, more precisely, its affinity over the first hours of immersion. The presence of π bonds directly linked to aliphatic chains can influence the wetting properties of a molecule, as well as its adsorption force [18]. It is therefore very likely that Undecanox (C11:0) and Tridecanox (C13:0) will be more effective than their analogs Undecenox (C11:1) and Tridecenox (C13:1), which confirms the correctness of the decision to remove the double bond.

Table 6.
Comparison between BS-CICs of the oxazoline family at 10 mg·L−1.
However, over a period of 17 h of immersion, there was an increase in the average IE value in the presence of Decenox (C10:1) (Figure 3), where the inhibitory effect intensifies after a few hours of immersion.
One hypothesis is that these molecules established on the aliphatic chain have a better adsorption capacity and a more expressed affinity on probably corroded surfaces or in the presence of corrosion products (oxides or hydroxides) on the surface or in the medium, which could indicate a form of synergy by complexation or passivation [27,49]. As was observed in the Tafel plot, passivation was observed on the anodic branch. It is possible that this layer has a more resistant nature over time. Another hypothesis would be that Decenox (C10:1) passes into a protonated form in a manner similar to azole-based inhibitors in acidic solution and would undergo electrostatic attraction with Cl-, HS-, or other halogen ions previously adsorbed on the metal which give the surface a negative charge [27,50]. The mode of action of Decenox (C10:1) may be similar to that of Solamine®129 [51].
The presence of the double bond could also serve as a second adsorption site for the inhibitor [52]. Indeed, when the aliphatic chain is saturated (as in the case of Decanox C10:0), the polar part of the oxazoline is at the metal surface, while the aliphatic chain extends upward into the electrolyte in a more perpendicular manner [21,30,47]. On the other hand, when the aliphatic chain is unsaturated (as in the case of Decenox, C10:1), the π orbital of the double bond can interact with the vacant d orbital of the metal [19], and the surface metal can be covered by flat adsorption of the molecule by both the oxazoline group and the aliphatic chains, thus forming a continuous monolayer that effectively prevents the diffusion of corrosive species. Consequently, corrosion would be strongly inhibited [20,53].
Understanding the physicochemical and electrochemical characteristics of Decenox (C10:1) guided the synthesis of new BS-CICs from the oxazoline family, namely Decanox (C10:0), Undecanox (C11:0), and Tridecanox (C13:0). This family of oxazolines was found to be effective as a corrosion inhibitor during the first hours of immersion of a CS-XC38 electrode. Furthermore, after optimizing the injection concentrations, these BS-CICs showed significant inhibition efficiency (IE) at 10 mg/L (Table 7).

Table 7.
Summary of the IEs (in %) of 2-oxazoline derivatives obtained with the average inhibition rate of three electrochemical techniques (Rp, Tafel, and impedance) at 10 mg/L on new CS-XC38 electrodes immersed in a solution of SRGW.
4. Conclusions
The three oxazolines were found to be effective as corrosion inhibitors during the first hours of the immersion of a CS-XC38 electrode. Furthermore, after optimizing the injection concentrations, they showed significant inhibition efficiency (IE) at 10 mg/L. However, their stability did not reach the level of petroleum-based inhibitors, which requires improvement through different formulations. Among these BS-CICs, Undecanox (C11:0) demonstrated the best IE during the first measurements carried out with the three different electrochemical techniques, with a strong affinity towards the CS-XC38 electrode. However, it was found to be the least stable over the full 17 h of measurements, suggesting the physisorption of this inhibitor to the metal surface. In addition, these BS-CICs have a mixed nature, with a slight anodic predominance in the case of Decenox.
Increasing the hydrophobicity of these oxazolines by lengthening their aliphatic chain has a positive effect on their IE. Additionally, the presence of unsaturation in the aliphatic chain improves stability, while its absence increases affinity. This observation can be attributed to various factors, such as the stability of the cycle, adsorption in the protonated form, or the orientation of the surfactant during adsorption. Furthermore, the extension of the aliphatic chain of oxazolines to Tridecanox (C13:0) improves the stability of the film formed, even in the absence of unsaturation. The mode of action of Decenox (C10:1) varies depending on its concentration. At low concentrations, approximately 10 mg/L, Decenox (C10:1) exhibits a passivating characteristic similar to its analog, Decanox (C10:0). However, at concentrations higher than the critical micellar concentration (CMC), the passivating characteristic disappears, probably giving way to a mode of action based on the formation of a barrier (chemisorption). In addition, the behavior of Decenox (C10:1) follows the Langmuir model, suggesting that the inhibition effect is due to the formation of a monolayer on the carbon steel. This layer results from spontaneous physisorption, thus preventing attacks on the metal by the corrosive agents present in the SRGW. Several hypotheses have been put forward regarding the mechanism of action of these BS-CICs, but it remains difficult to reach a definitive conclusion.
The four molecules have proven effective as corrosion inhibitors at a concentration of 10 mg/L, technically and economically adapted to the concentration ranges of the DAPB geothermal installations (2.5 to 10 mg/L). In addition, the four molecules have a mixed nature, with a slight anodic predominance. At a concentration of 10 mg/L and in the presence of Decenox (C10:1), the corrosion rate is approximately 0.17 mm/year. For Decenox (C10:0), the corrosion rate is approximately 0.27 mm/year; for Undecanox (C11:0), it is approximately 0.26 mm/year; and for Tridecanox (C13:0), it is approximately 0.045 mm/year.
Author Contributions
Conceptualization, S.B., R.V. and I.I.; Methodology, C.H., S.B. and I.I.; Validation, C.H., S.B., R.V., S.T.-R. and I.I.; Formal analysis, C.H., S.B. and I.I.; Investigation, C.H., S.B., R.V. and I.I.; Data curation, C.H., S.B., R.V., S.T.-R. and I.I.; Writing—original draft, C.H., S.B., R.V., S.T.-R. and I.I.; Writing—review and editing, C.H., S.B., R.V., S.T.-R. and I.I.; Visualization, C.H., S.B., R.V., S.T.-R. and I.I.; Supervision, R.V., S.T.-R. and I.I.; Project administration, S.T.-R. and I.I.; Funding acquisition, I.I. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this work was supported by the French National Agency for Environmental and Energy Management (ADEME) as part of the INHIBIOSOURCE project (2017–2022), grant number 1782C0201. This research also was funded by BRGM, Orleans, France (25%), and AQUAPROX Industries SAS, Levallois-Perret, France (75%), under the framework of a three-party scientific convention with the LCA, INRAE of Toulouse INP for the thesis administration (via the doctoral school EDSDM n°482) of Chahinez Helali (2020–2024).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank SCODIP, from Orleans, France (Jean-Yves Leguenic), for fabricating the electrodes.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Summary of the IE (%) deduced from the Tafel plots via the corrosion current JcorrTP for the four oxazolines at 10 mg/L on new CS-XC38 electrodes immersed in SRGW; Jcorr WI applied for the computation of IE (%) is equal to 69.57 µA.
Table A1.
Summary of the IE (%) deduced from the Tafel plots via the corrosion current JcorrTP for the four oxazolines at 10 mg/L on new CS-XC38 electrodes immersed in SRGW; Jcorr WI applied for the computation of IE (%) is equal to 69.57 µA.
| Immersion Time (h) | 1 | 5 | 9 | 13 | 17 | 1 | 5 | 9 | 13 | 17 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor Name | Content (mg/L) | Icorr (Tafel) (µA) After 17 h of Immersion | IE on New Electrode via Jcorr (Rp) (%) | IE (%) Starting from the 1st Icorr Measured via Tafel Versus the 1st Jcorr (WI) over 44 min | Cathodic Constant of Tafel βc (mV) | Cathodic Constant of Tafel βa(mV) | ||||||||
| WI | 0 | 137 | 0.0 | 0.0 | 171 | 210 | 189 | 303 | 424 | 54 | 69 | 70 | 83 | 99 |
| C(10:1) | 10 | 29 | 58 | 34 | 118 | 81 | 859 | 80 | 78 | 73 | 87 | 76 | 69 | 661 |
| C(10:0) | 10 | 52 | 26 | 73 | 234 | 405 | 202 | 145 | 163 | 53 | 84 | 73 | 53 | 59 |
| C(11:0) | 10 | 62 | 10 | 94 | 152 | 122 | 223 | 178 | 240 | 91 | 83 | 70 | 72 | 88 |
| C(13:0) * | 10 | 11 | 84 | 92 | 132 | 144 | 152 | Nd | Nd | 74 | 91 | 80 | Nd | Nd |
WI: Without inhibitor; Nd: Not determined; * Only after 3 loops; Jcorr WI: Jcorr obtained without inhibitor.
References
- Rojas, J.; Giot, D.; Le Nindre, Y.M.; Criaud, A.; Fouillac, C.; Brach, M.; Menjoz; Martin, J.C.; Lambert, M.; Chiles, J.P.; et al. Caractérisation et Modélisation du Réservoir Géothermique du Dogger, Bassin Parisien, France, Rapport Final; BRGM R 30169 IRG SGN 89; BRGM: Orléans, France, 1989; p. 249. [Google Scholar]
- Castillo, C.; Ignatiadis, I. Sulfate-Reduction State of the Geothermal Dogger Aquifer, Paris Basin (France) after 35 Years of Exploitation: Analysis and Consequences of Bacterial Proliferation in Casings and Reservoir. In Proceedings of the 37th Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, CA, USA, 30 January–1 February 2012; Curran Associates, Inc., Ed.; Stanford Geothermal Program, Stanford University: Stanford, CA, USA, 2012. [Google Scholar]
- Fardeau, M.-L.; Goulhen, F.; Bruschi, M.; Khelifi, N.; Cayol, J.-L.; Ignatiadis, I.; Guyot, F.; Ollivier, B. Archaeoglobus fulgidus and Thermotoga elfii, Thermophilic Isolates from Deep Geothermal Water of the Paris Basin. Geomicrobiol. J. 2009, 26, 119–130. [Google Scholar] [CrossRef]
- Lopez, S.; Hamm, V.; Le Brun, M.; Schaper, L.; Boissier, F.; Cotiche, C.; Giuglaris, E. 40 years of Dogger aquifer management in Ile-de-France, Paris Basin, France. Geothermics 2010, 39, 339–356. [Google Scholar] [CrossRef]
- Peter, F.; Ellis, I. A Geothermal Corrosivity Classification System. Trans.-Geotherm. Resour. Counc. 1981, 5, 463–472. [Google Scholar]
- Amalhay, M.; Akar, A.A.; Ignatiadis, I. Study of scale deposition phenomena Geothermal Wells in the Paris Basin. In Proceedings of the International Symposium, Geothermics 94 in Europe, from Research to Development, Orleans, France, 8–9 February 1994; BRGM, Ed.; BRGM: Orléans, France, 1998; pp. 223–232. [Google Scholar]
- Amalhay, M.; Ignatiadis, I. Comparative Study of the Effectiveness of Various Organic Surfactants in Inhibiting Carbon Steel Corrosion in a Natural Geothermal Environment by Using Rapid Electrochemical Tests. Mater. Sci. Forum 1998, 289–292, 169–180. [Google Scholar] [CrossRef]
- Hassan, A.; Numin, M.S.; Jumbri, K.; Kee, K.E.; Borhan, N.; Daud, N.M.R.N.M.; Nor, A.M.; Suhor, M.F.; Wahab, R.A. Density Functional Theory Studies on New Possible Biobased Gemini Corrosion Inhibitors Derived from Fatty Hydrazide Derivatives. ACS Omega 2023, 8, 23945–23952. [Google Scholar] [CrossRef] [PubMed]
- Österberg, M.; Henn, K.A.; Farooq, M.; Valle-Delgado, J.J. Biobased Nanomaterials—The Role of Interfacial Interactions for Advanced Materials. Chem. Rev. 2023, 123, 2200–2241. [Google Scholar] [CrossRef] [PubMed]
- Valentin, R.; Mouloungui, Z. Superhydrophilic surfaces from short and medium chain solvo-surfactants. Oléagineux Corps Gras Lipides 2013, 20, 33–44. [Google Scholar] [CrossRef][Green Version]
- Shenoy, P.; Kedimar, N.; Rao, S.A. A comprehensive review on anticorrosive behaviour of surfactants across diverse metals using multiple techniques: Current insights and future horizons. Chem. Eng. J. Adv. 2024, 20, 100645. [Google Scholar] [CrossRef]
- Betelu, S.; Helali, C.; Ignatiadis, I. Laboratory-Scale Implementation of Standardized Reconstituted Geothermal Water for Electrochemical Investigations of Carbon Steel Corrosion. Metals 2024, 14, 1216. [Google Scholar] [CrossRef]
- Helali, C. Sélection de Tensioactifs Biosourcés Pour L’inhibition de la Corrosion-Dépôt en Géothermie par L’évaluation Comparative de leur Action sur le Comportement Électrochimique de L’acier au Carbone: Du Laboratoire au Démonstrateur In Situ. Ph.D. Thesis, University of Toulouse ENSIACET-INP, Orléans, France, 2024; p. 358. [Google Scholar]
- Wiley, R.H.; Bennett, L.L. The Chemistry of the Oxazolines. Chem. Rev. 1949, 44, 447–476. [Google Scholar] [CrossRef]
- Frump, J.A. Oxazolines. Their preparation, reactions and applications. Chem. Rev. 1949, 44, 483–505. [Google Scholar] [CrossRef]
- Gant, T.G.; Meyers, A.I. The chemistry of 2-oxazolines (1985–present). Tetrahedron 1994, 50, 2297–2360. [Google Scholar] [CrossRef]
- Vračar, L.M.; Dražić, D.M. Adsorption and corrosion inhibitive properties of some organic molecules on iron electrode in sulfuric acid. Corros. Sci. 2002, 44, 1669–1680. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Verma, C.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Experimental and computational insights. J. Mol. Struct. 2021, 1227, 129374. [Google Scholar] [CrossRef]
- Lin, B.; Zuo, Y. Corrosion inhibition of carboxylate inhibitors with different alkylene chain lengths on carbon steel in an alkaline solution. RSC Adv. 2019, 9, 7065–7077. [Google Scholar] [CrossRef] [PubMed]
- Porcayo-Calderon, J.; Regla, I.; Vazquez-Velez, E.; de la Escalera, L.M.M.; Canto, J.; Casales-Diaz, M. Effect of the Unsaturation of the Hydrocarbon Chain of Fatty-Amides on the CO2 Corrosion of Carbon Steel Using EIS and Real-Time Corrosion Measurement. J. Spectrosc. 2015, 2015, 184140. [Google Scholar] [CrossRef]
- Alrefaee, S.H. Effect of alkyl chain length and halide ions on the corrosion inhibition potential of imidazolium and pyridinium based ionic liquids: Computational studies. J. Mol. Liq. 2021, 344, 117848. [Google Scholar] [CrossRef]
- Frignani, A.; Zucchi, F.; Trabanelli, G.; Grassi, V. Protective action towards aluminium corrosion by silanes with a long aliphatic chain. Corros. Sci. 2006, 48, 2258–2273. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Desai, P.D.; Pawar, C.B.; Avhad, M.S.; More, A.P. Corrosion inhibitors for carbon steel: A review. Vietnam J. Chem. 2023, 61, 15–42. [Google Scholar] [CrossRef]
- Viramontes-Gamboa, G.; Rivera-Vasquez, B.F.; Dixon, D.G. The Active-Passive Behavior of Chalcopyrite: Comparative Study Between Electrochemical and Leaching Responses. J. Electrochem. Soc. 2007, 154, C299. [Google Scholar] [CrossRef]
- Gabrielli, C.; Takenouti, H. Méthodes électrochimiques appliquées à la corrosion–Techniques dynamiques. Tech. L’ingénieur 2010, 810, 1–16. [Google Scholar] [CrossRef]
- Caldona, E.B.; Zhang, M.; Liang, G.; Hollis, T.K.; Webster, C.E.; Smith, D.W.; Wipf, D.O. Corrosion inhibition of mild steel in acidic medium by simple azole-based aromatic compounds. J. Electrochem. Soc. 2021, 880, 114858. [Google Scholar] [CrossRef]
- Srisuwan, N. Propriétés Inhibitrices D’un Mélange D’amines Grasses et de Sébaçate de Sodium Vis-à-Vis de La Corrosion D’un Acier Au Carbone; Institut National Polytechnique: Toulouse, France, 2008; p. 274. [Google Scholar]
- Reboul, M. Corrosion des alliages d’aluminium. Tech. L’ingénieur 2005, 325, 1–19. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Rhee, K.Y. Hydrophilicity and hydrophobicity consideration of organic surfactant compounds: Effect of alkyl chain length on corrosion protection. Adv. Colloid Interface Sci. 2022, 306, 102723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Popova, A.; Sokolova, E.; Raicheva, S.; Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 2003, 45, 33–58. [Google Scholar] [CrossRef]
- de Damborenea, J.; Bastidas, J.M.; Vázquez, A.J. Adsorption and inhibitive properties of four primary aliphatic amines on mild steel in 2 M hydrochloric acid. Electrochim. Acta 1997, 42, 455–459. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Deng, S.; Du, G. Two phenylpyrimidine derivatives as new corrosion inhibitors for cold rolled steel in hydrochloric acid solution. Corros. Sci. 2014, 87, 27–39. [Google Scholar] [CrossRef]
- Hong, T.; Jepson, W.P. Corrosion inhibitor studies in large flow loop at high temperature and high pressure. Corros. Sci. 2001, 43, 1839–1849. [Google Scholar] [CrossRef]
- Saji, V.S. A review on recent patents in corrosion inhibitors. Recent Pat. Corros. Sci. 2010, 2, 6–12. [Google Scholar] [CrossRef]
- Buyuksagis, A.; Dilek, M.; Kargioglu, M. Corrosion inhibition of st37 steel in geothermal fluid by Quercus robur and pomegranate peels extracts. Prot. Met. Phys. Chem. Surf. 2015, 51, 861–872. [Google Scholar] [CrossRef]
- Aristia, G.; Hoa, L.Q.; Bäßler, R. Corrosion of Carbon Steel in Artificial Geothermal Brine: Influence of Carbon Dioxide at 70 °C and 150 °C. Materials 2019, 12, 3801. [Google Scholar] [CrossRef] [PubMed]
- El Brahim, I.; Lei, G. Azole-Based Compounds as Corrosion Inhibitors for Metallic Materials. In Azoles; Aleksey, K., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 5. [Google Scholar]
- Gutiérrez, E.; Rodríguez, J.A.; Cruz-Borbolla, J.; Alvarado-Rodríguez, J.G.; Thangarasu, P. Development of a predictive model for corrosion inhibition of carbon steel by imidazole and benzimidazole derivatives. Corros. Sci. 2016, 108, 23–35. [Google Scholar] [CrossRef]
- Gutig, C.; Grady, B.P.; Striolo, A. Experimental Studies on the Adsorption of Two Surfactants on Solid−Aqueous Interfaces: Adsorption Isotherms and Kinetics. Langmuir 2008, 24, 4806–4816. [Google Scholar] [CrossRef] [PubMed]
- Resende, G.O.; Teixeira, S.F.; Figueiredo, I.F.; Godoy, A.A.; Lougon, D.J.F.; Cotrim, B.A.; Souza, F.C. Synthesis of 1,2,3-Triazole Derivatives and Its Evaluation as Corrosion Inhibitors for Carbon Steel. Int. J. Electrochem. 2019, 2019, 6759478. [Google Scholar] [CrossRef]
- Srivastava, V.; Salman, M.; Chauhan, D.S.; Abdel-Azeim, S.; Quraishi, M.A. (E)-2-styryl-1H-benzo[d]imidazole as novel green corrosion inhibitor for carbon steel: Experimental and computational approach. J. Mol. Liq. 2021, 324, 115010. [Google Scholar] [CrossRef]
- Martinez, S.; Stern, I. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl. Surf. Sci. 2002, 199, 83–89. [Google Scholar] [CrossRef]
- Sliem, M.H.; Afifi, M.; Radwan, A.B.; Fayyad, E.M.; Shibl, M.F.; Heakal, F.E.-T.; Abdullah, A.M. AEO7 Surfactant as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl solution. Sci. Rep. 2019, 9, 2319. [Google Scholar] [CrossRef] [PubMed]
- Fragoza-Mar, L.; Olivares-Xometl, O.; Domínguez-Aguilar, M.A.; Flores, E.A.; Arellanes-Lozada, P.; Jiménez-Cruz, F. Corrosion inhibitor activity of 1,3-diketone malonates for mild steel in aqueous hydrochloric acid solution. Corros. Sci. 2012, 61, 171–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Yi, G. The effects of surfactant concentration, adsorption, aggregation, and solution conditions on steel corrosion inhibition and associated modeling in aqueous media. Corros. Sci. 2016, 102, 233–250. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashim, M.A.; Nabi, F.; Al-Thabaiti, S.A.; Khan, Z. Anti-corrosion Ability of Surfactants: A Review. Int. J. Electrochem. Sci. 2011, 6, 1927–1948. [Google Scholar] [CrossRef]
- Kern, P.; Landolt, D. Adsorption of organic corrosion inhibitors on iron in the active and passive state. A replacement reaction between inhibitor and water studied with the rotating quartz crystal microbalance. Electrochim. Acta 2001, 47, 589–598. [Google Scholar] [CrossRef]
- Öztürk, S.; Gerengi, H.; Solomon, M.M.; Gece, G.; Yıldırım, A.; Yıldız, M. A newly synthesized ionic liquid as an effective corrosion inhibitor for carbon steel in HCl medium: A combined experimental and computational studies. Mater. Today Commun. 2021, 29, 102905. [Google Scholar] [CrossRef]
- Ignatiadis, I.; Garnier, F.; Betelu, S. Étude Comparative de l’action de Différents Inhibiteurs de Corrosion-Dépôts Sur Le Comportement Électrochimique d’un Acier Au Carbone Dans Un Fluide Géothermal Reconstitué; Représentatif Du Dogger Du Bassin Parisien; Final BRGM/RP-64364-FR; BRGM: Orléans, France, 2015; p. 146. [Google Scholar]
- Hackerman, N.; Makrides, A.C. Action of Polar Organic Inhibitors in Acid Dissolution of Metals. Ind. Eng. Chem. 1954, 46, 523–527. [Google Scholar] [CrossRef]
- Yoon, S.K.; Choban, E.; Kane, C.; Tzedakis, T.; Kenis, P. Laminar flow based electrochemical microreactor for efficient regeneration of Nicotinamide cofactors for biocatalysis. J. Am. Chem. Soc. 2005, 127, 10466–10467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).