Innovative Solid-State Recycling of Aluminum Alloy AA6063 Chips Through Direct Hot Rolling Process
Abstract
1. Introduction
2. Materials and Methods
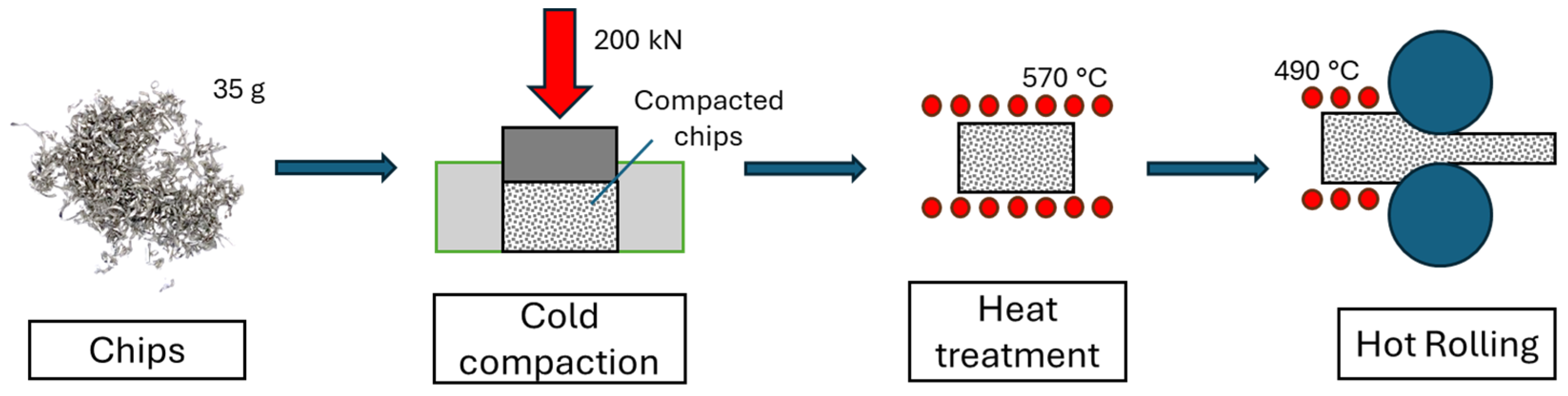
2.1. Chip Production and Cold Compaction
2.2. Heat Treatment and Direct Hot Rolling Process
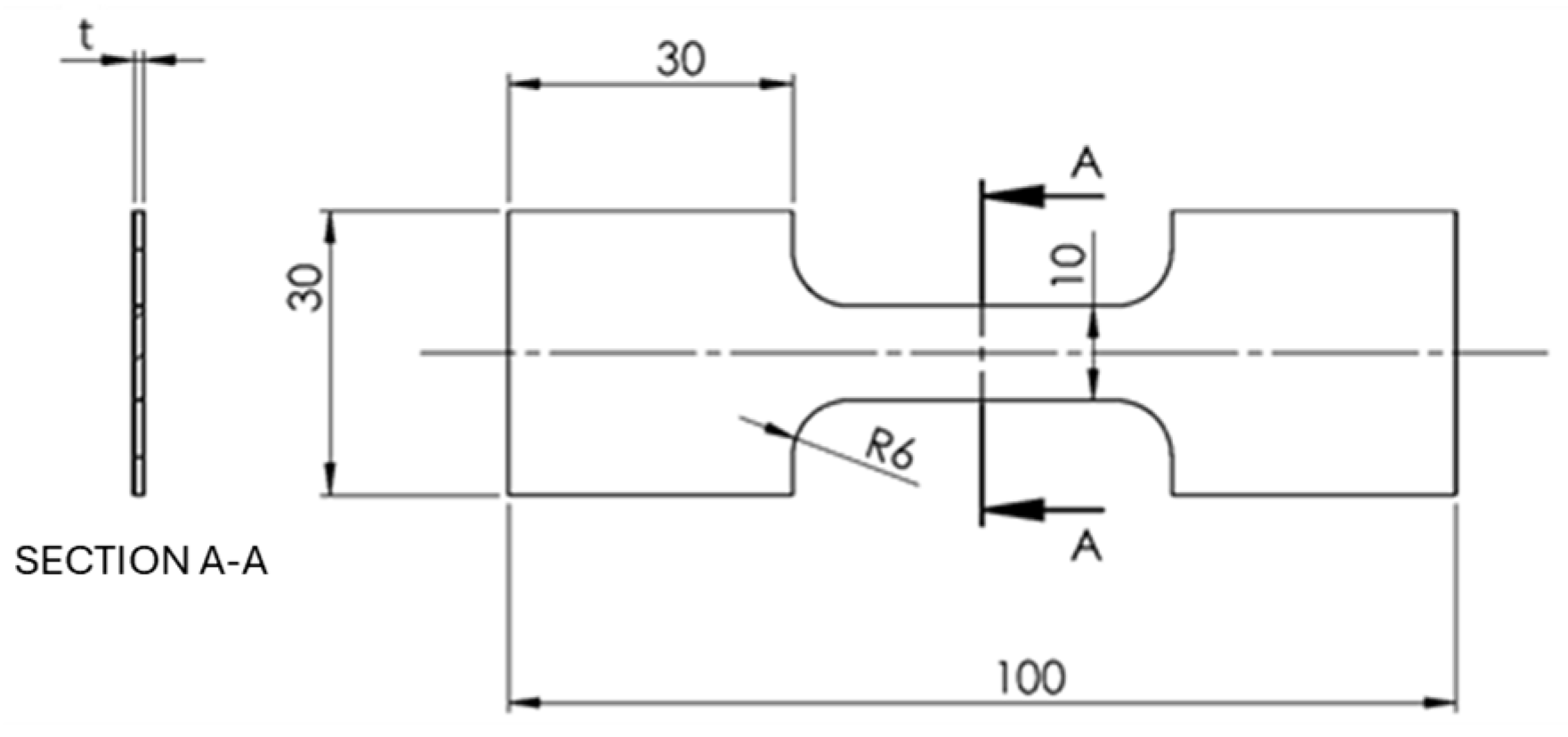
2.3. Mechanical Properties
2.4. Microstructure Analysis
3. Results and Discussions
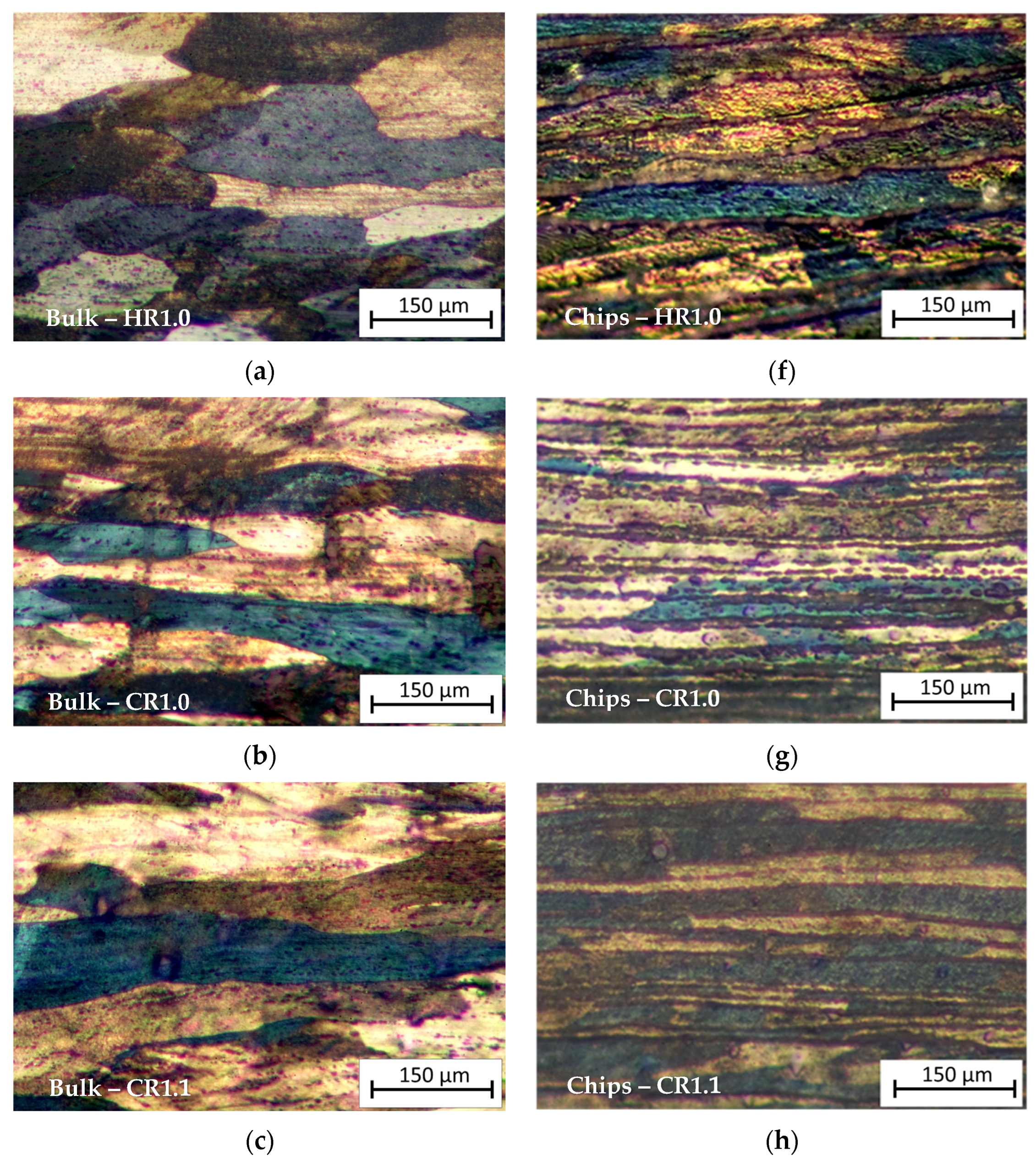
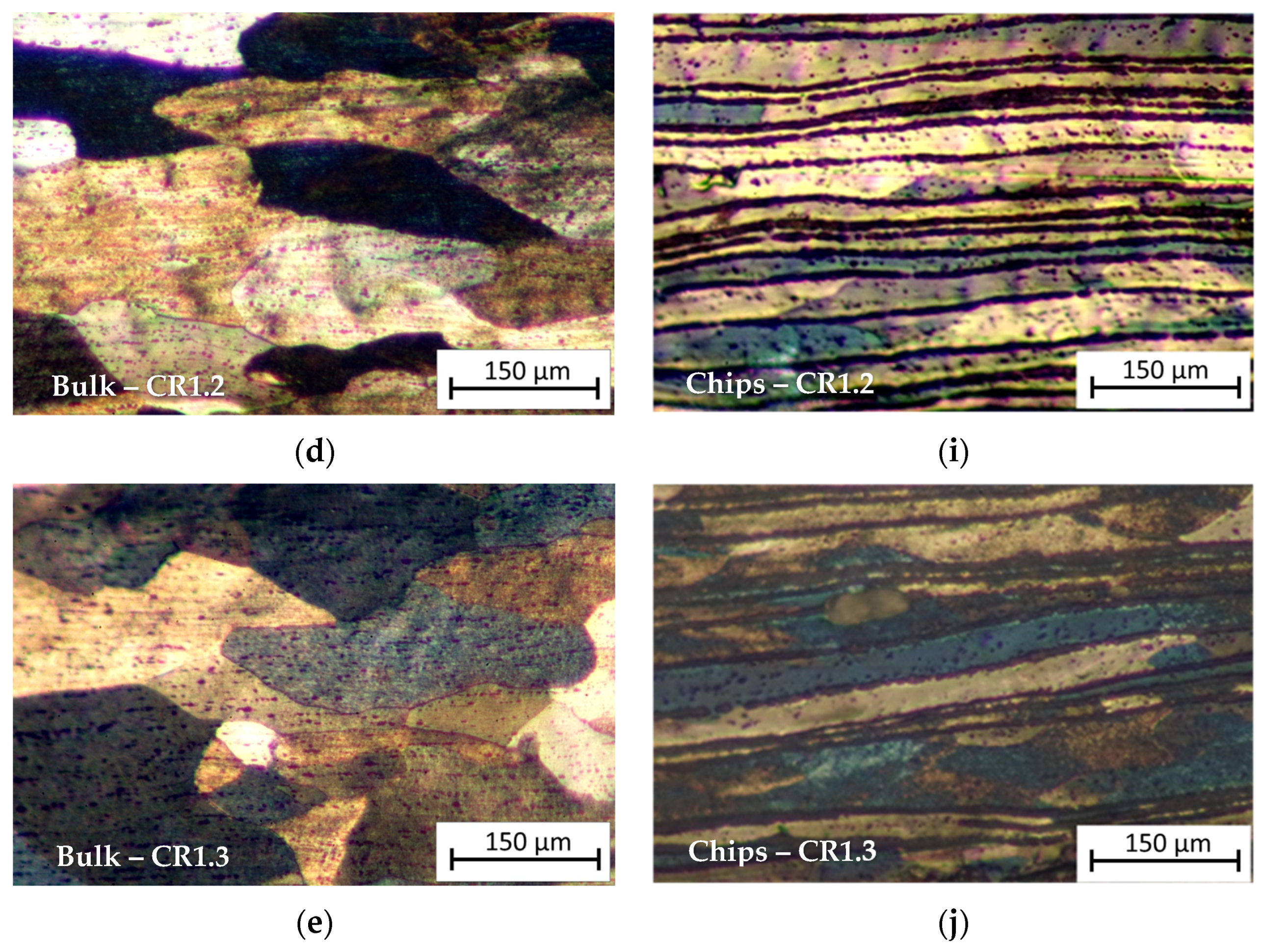
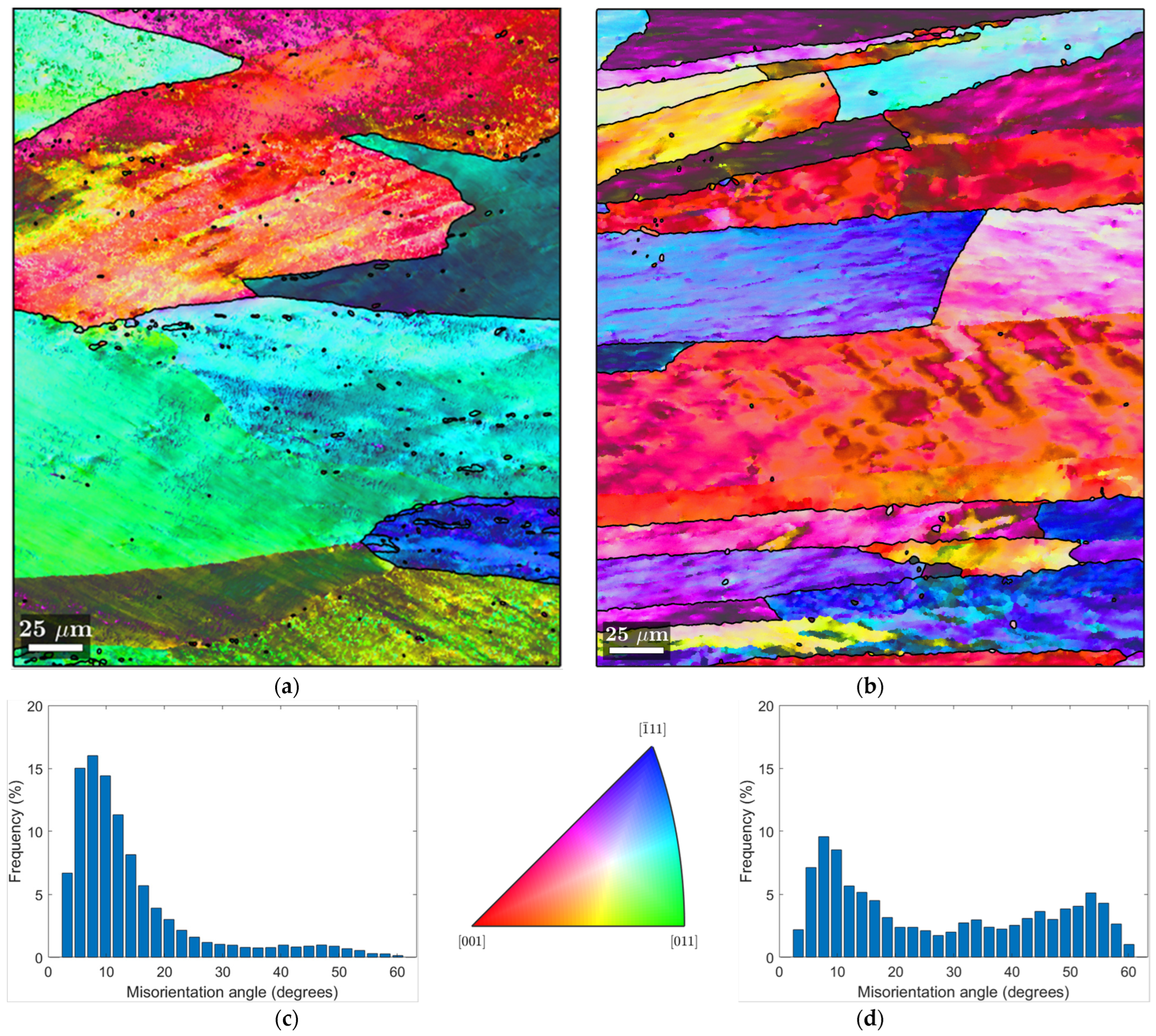
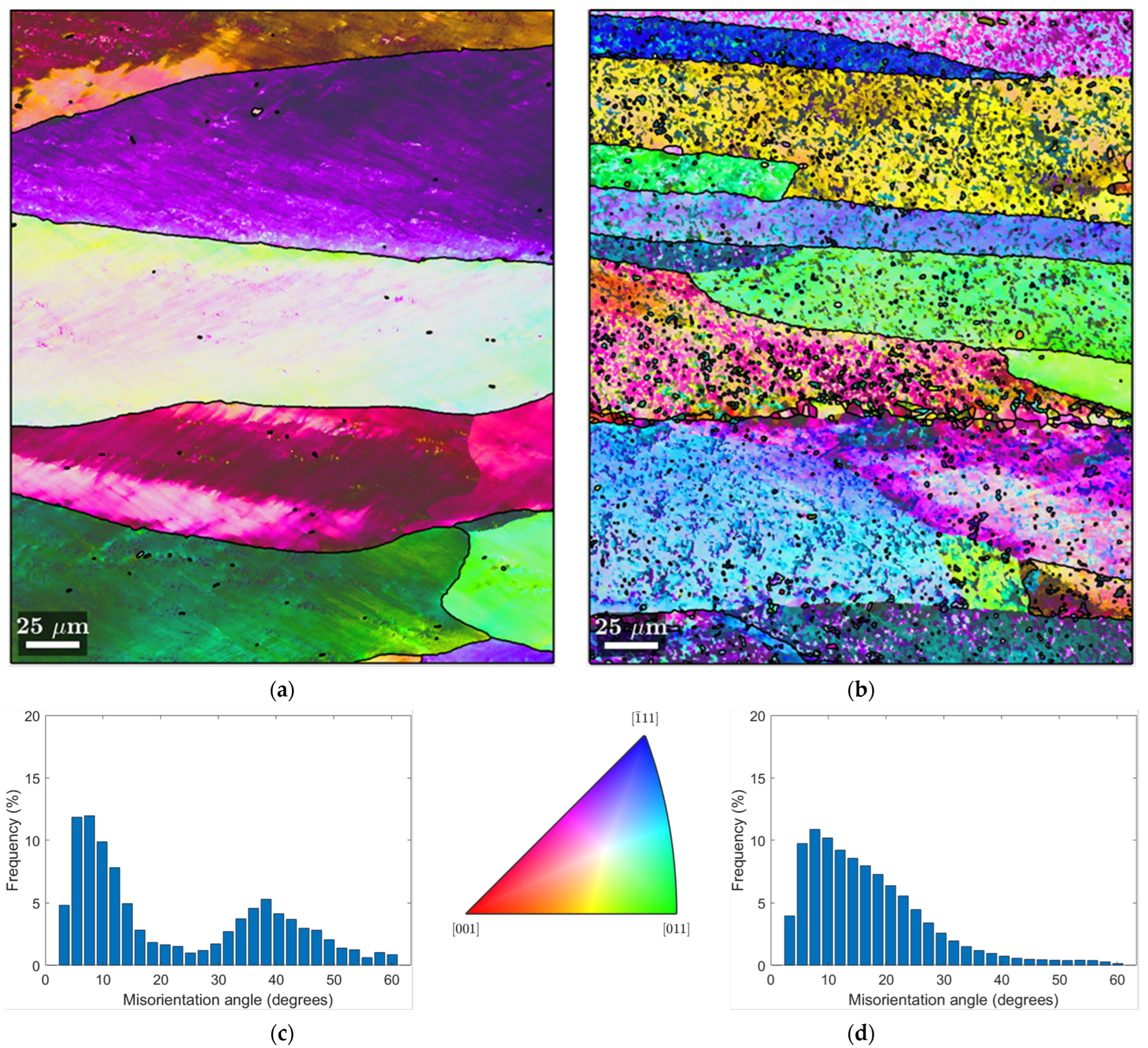
3.1. Metallographic Analysis
3.2. EBSD-SEM Analysis
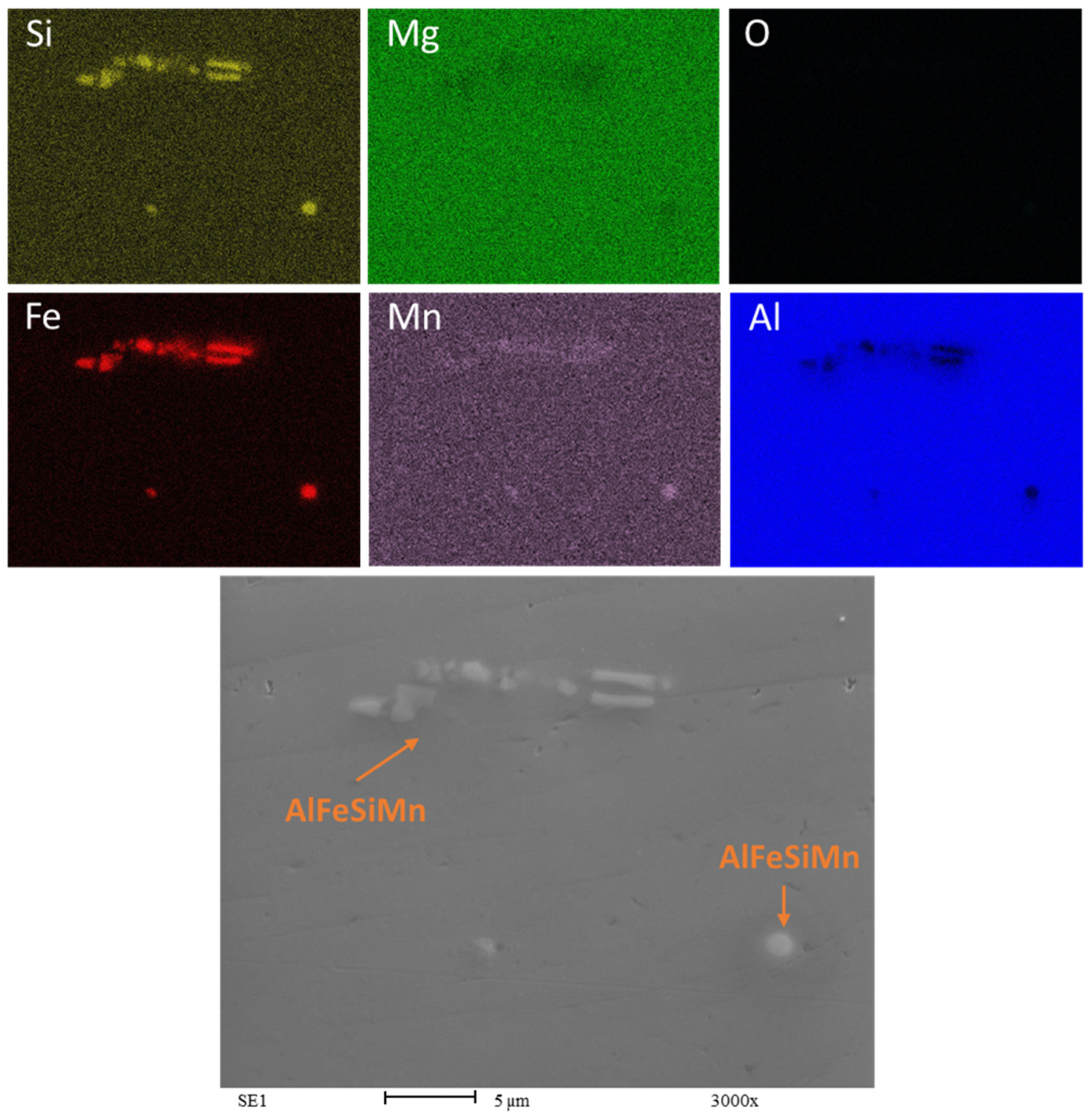
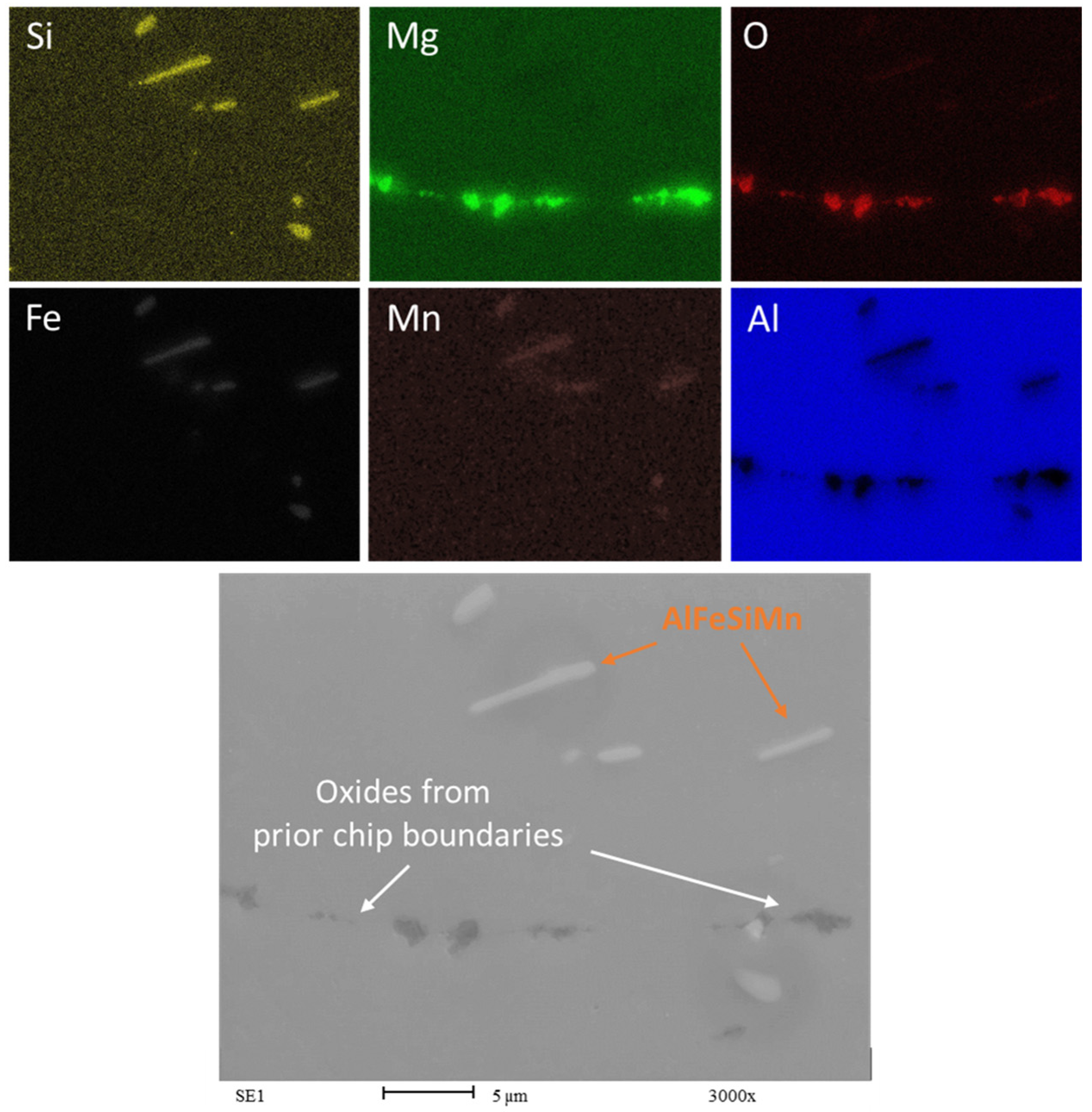
3.3. SEM and EDS Analysis
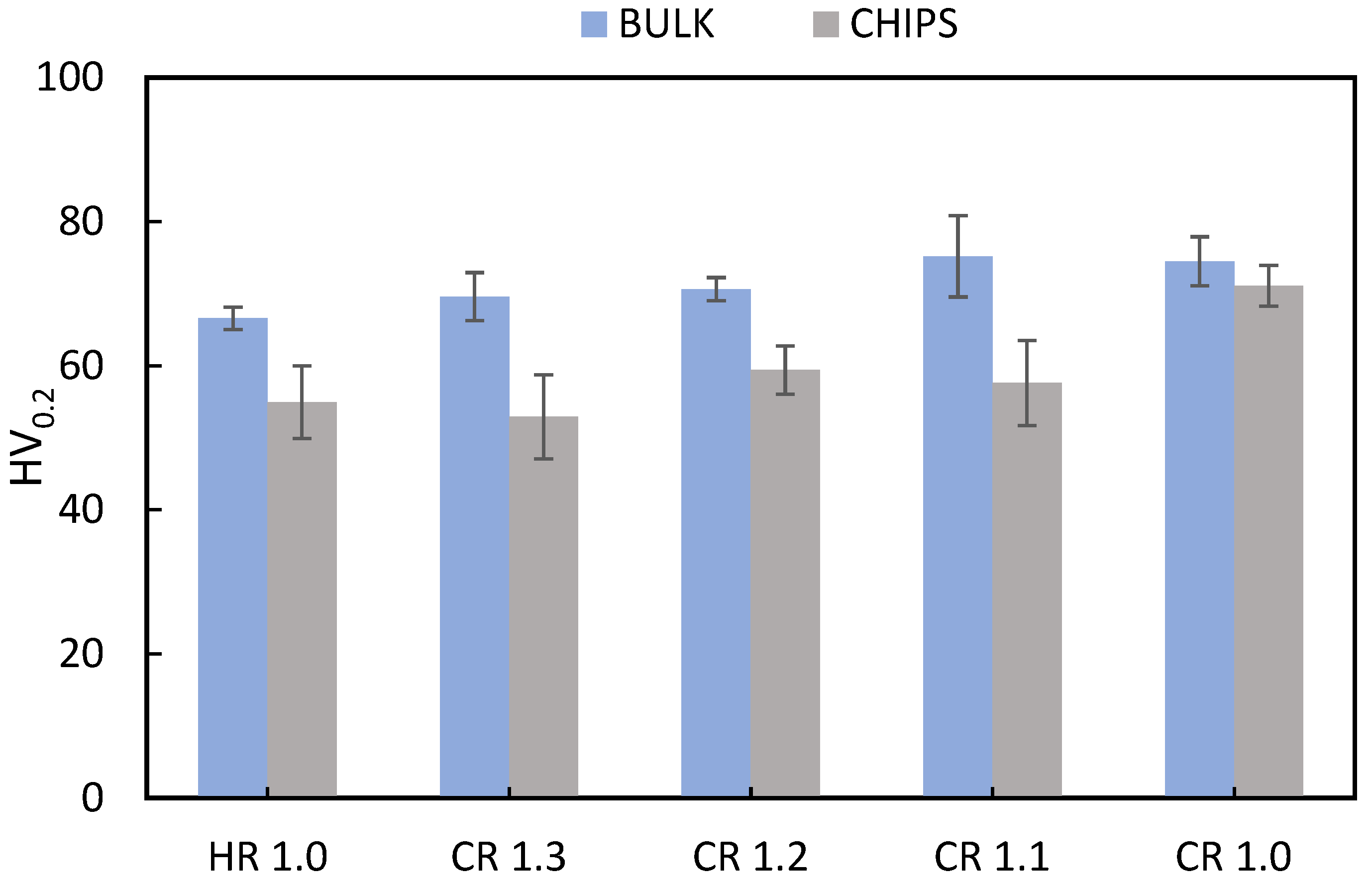
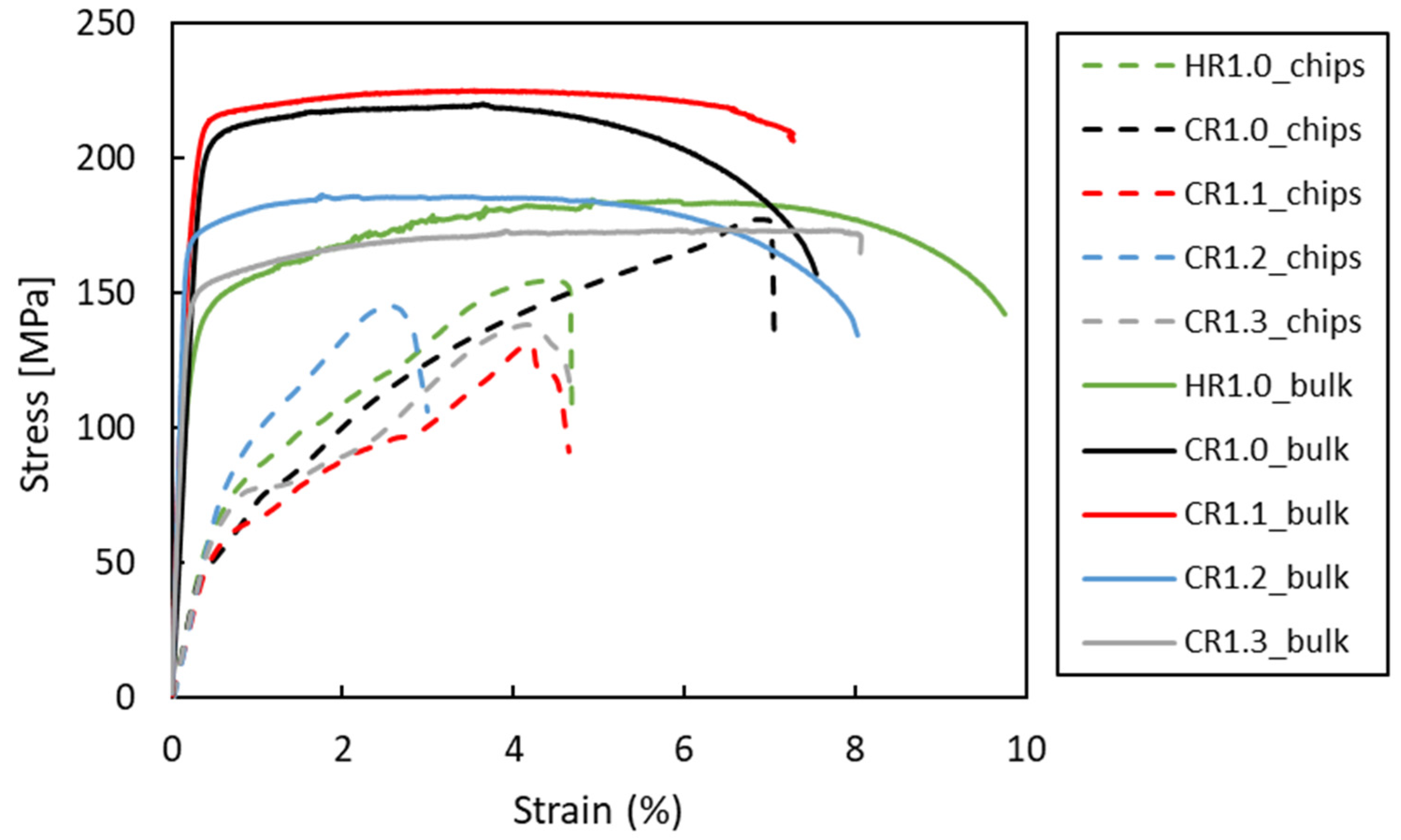
3.4. Mechanical Properties
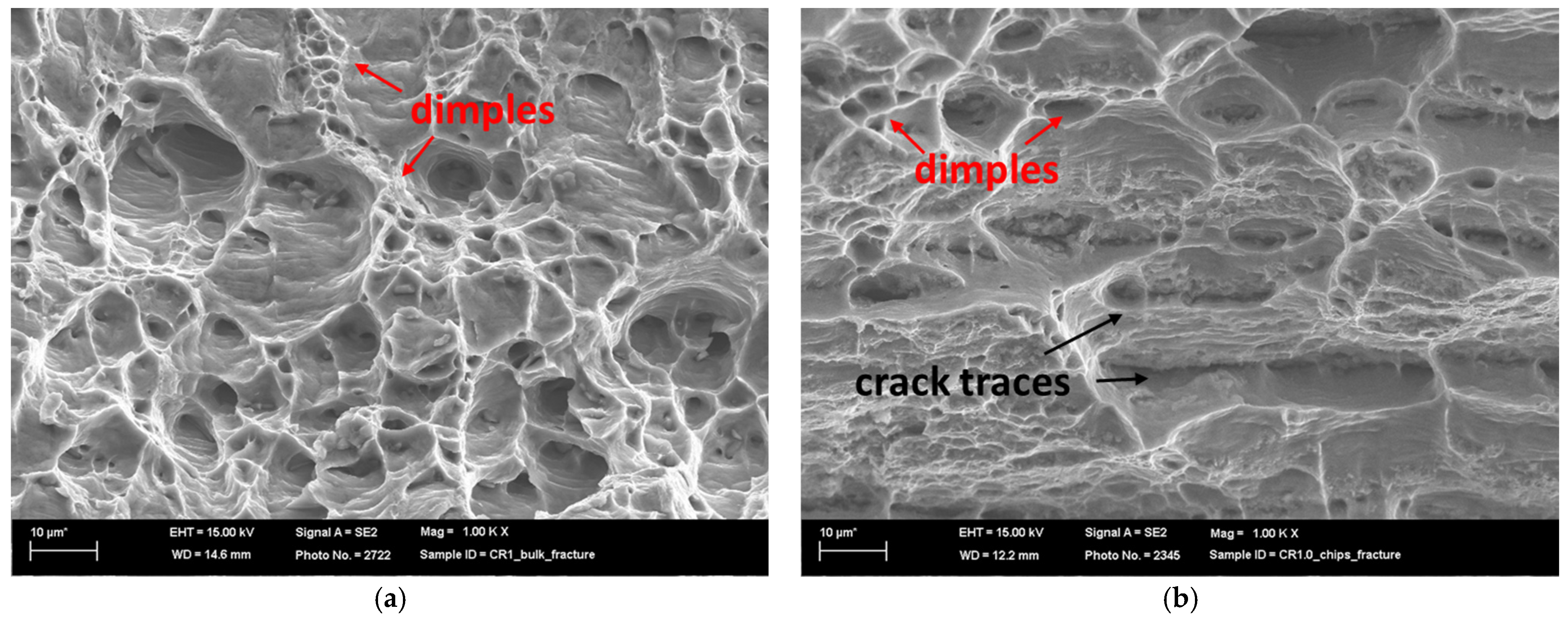
3.5. Fracture Surface
4. Conclusions
- The recycled chip samples achieved good mechanical properties, particular in the CR1.0 condition, where the bulk exhibited a UTS of 218 MPa, and the recycled chips reached 177 MPa with similar elongation.
- Microstructural analysis revealed a layered structure in all recycled chips. This is due to the inherent nature of the base material. SEM and EDS analysis showed broken oxide layers on the recycled material, facilitating aluminum matrix continuity and aligning with the rolling direction. The presence of Mg in the alloy leads to the formation of a mixed oxide layer (aluminum and magnesium oxides) on the chips.
- By varying rolling schedules, in the recycled samples, it was found that cold rolling in the final pass enhanced the mechanical properties to a UTS of 177 MPa (CR1.0 condition) compared with just hot rolling (155 MPa in the HR1.0 condition).
- SEM analysis revealed distinct fractures in the bulk and chips. The bulk material fractured in a ductile manner, while the chips showed a mixed mode due to consolidation challenges.
- Given the oxidation of magnesium, recycling aluminum–silicon–magnesium alloys from chips requires the addition of magnesium to ensure the effectiveness of the aging treatment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Das, S.K.; Yin, W. The Worldwide Aluminum Economy: The Current State of the Industry. JOM 2007, 59, 57–63. [Google Scholar] [CrossRef]
- Brough, D.; Jouhara, H. The Aluminium Industry: A Review on State-of-the-Art Technologies, Environmental Impacts and Possibilities for Waste Heat Recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Wagiman, A.; Mustapa, M.S.; Asmawi, R.; Shamsudin, S.; Lajis, M.A.; Mutoh, Y. A Review on Direct Hot Extrusion Technique in Recycling of Aluminium Chips. Int. J. Adv. Manuf. Technol. 2020, 106, 641–653. [Google Scholar] [CrossRef]
- Balomenos, E.; Panias, D.; Paspaliaris, I. Energy and Exergy Analysis of the Primary Aluminum Production Processes: A Review on Current and Future Sustainability. Miner. Process. Extr. Metall. Rev. 2011, 32, 69–89. [Google Scholar] [CrossRef]
- Tan, R.B.H.; Khoo, H.H. An LCA Study of a Primary Aluminum Supply Chain. J. Clean. Prod. 2005, 13, 607–618. [Google Scholar] [CrossRef]
- Güley, V.; Ben Khalifa, N.; Tekkaya, A.E. Direct Recycling of 1050 Aluminum Alloy Scrap Material Mixed with 6060 Aluminum Alloy Chips by Hot Extrusion. Int. J. Mater. Form. 2010, 3, 853–856. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Paraskevas, D.; Vanmeensel, K.; Vleugels, J.; Dewulf, W.; Duflou, J.R. Solid State Recycling of Aluminium Sheet Scrap by Means of Spark Plasma Sintering. Key Eng. Mater. 2015, 639, 493–498. [Google Scholar] [CrossRef]
- Gronostajski, J.; Marciniak, H.; Matuszak, A. New Methods of Aluminium and Aluminium-Alloy Chips Recycling. J. Mater. Process. Technol. 2000, 106, 34–39. [Google Scholar] [CrossRef]
- Shamsudin, S.; Lajis, M.; Zhong, Z. Solid-State Recycling of Light Metals: A Review. Adv. Mech. Eng. 2016, 8, 168781401666192. [Google Scholar] [CrossRef]
- Pantke, K.; Güley, V.; Khalifa, N.B.; Heilmann, M.; Biermann, D.; Tekkaya, A.E. Aluminum Scrap Recycling by Hot Extrusion without Melting Process. In Proceedings of the 12th International Conference on Aluminium Alloys, Yokohama, Japan, 5–9 September 2010; p. 6. [Google Scholar]
- Tekkaya, A.E.; Güley, V.; Haase, M.; Jäger, A. Hot Extrusion of Aluminum Chips. In ICAA13 Pittsburgh: Proceedings of the 13th International Conference on Aluminum Alloys; Springer International Publishing: Cham, Switzerland, 2016; pp. 1559–1573. [Google Scholar]
- Mehtedi, M.E.; Forcellese, A.; Mancia, T.; Simoncini, M.; Spigarelli, S. A New Sustainable Direct Solid State Recycling of AA1090 Aluminum Alloy Chips by Means of Friction Stir Back Extrusion Process. Procedia CIRP 2019, 79, 638–643. [Google Scholar] [CrossRef]
- Carta, M.; Buonadonna, P.; El Mehtedi, M. Numerical Analysis of Process Parameters and Tool Geometry in Friction Stir Back Extrusion of Pure Aluminum. Procedia CIRP 2024, 126, 710–714. [Google Scholar] [CrossRef]
- Tahmasbi, K.; Mahmoodi, M.; Tavakoli, H. Corrosion Resistance of Aluminum Alloy AA7022 Wire Fabricated by Friction Stir Extrusion. Trans. Nonferrous Met. Soc. China 2019, 29, 1601–1609. [Google Scholar] [CrossRef]
- Li, X.; Baffari, D.; Reynolds, A.P. Friction Stir Consolidation of Aluminum Machining Chips. Int. J. Adv. Manuf. Technol. 2018, 94, 2031–2042. [Google Scholar] [CrossRef]
- Wahed, S.N.A.; Hussein, S.K.; Al-Saadi, M.H. Disc Forming by Friction Stir Consolidation of AA2024 Chips. J. Technol. 2022, 4, 1–8. [Google Scholar] [CrossRef]
- Al-Alimi, S.; Lajis, M.A.; Shamsudin, S. Solid-State Recycling of Light Metal Reinforced Inclusions by Equal Channel Angular Pressing: A Review. MATEC Web Conf. 2017, 135, 00013. [Google Scholar] [CrossRef]
- Krolo, J.; Lela, B.; Dumanić, I.; Kozina, F. Statistical Analysis of the Combined ECAP and Heat Treatment for Recycling Aluminum Chips Without Remelting. Metals 2019, 9, 660. [Google Scholar] [CrossRef]
- Paraskevas, D.; Dadbakhsh, S.; Vleugels, J.; Vanmeensel, K.; Dewulf, W.; Duflou, J.R. Solid State Recycling of Pure Mg and AZ31 Mg Machining Chips via Spark Plasma Sintering. Mater. Des. 2016, 109, 520–529. [Google Scholar] [CrossRef]
- Paraskevas, D.; Vanmeensel, K.; Vleugels, J.; Dewulf, W.; Deng, Y.; Duflou, J. Spark Plasma Sintering as a Solid-State Recycling Technique: The Case of Aluminum Alloy Scrap Consolidation. Materials 2014, 7, 5664–5687. [Google Scholar] [CrossRef]
- Altharan, Y.M.; Shamsudin, S.; Al-Alimi, S.; Saif, Y.; Zhou, W. A Review on Solid-State Recycling of Aluminum Machining Chips and Their Morphology Effect on Recycled Part Quality. Heliyon 2024, 10, e34433. [Google Scholar] [CrossRef]
- Paraskevas, D.; Kellens, K.; Deng, Y.; Dewulf, W.; Kampen, C.; Duflou, J.R. Solid State Recycling of Aluminium Alloys via a Porthole Die Hot Extrusion Process: Scaling up to Production. AIP Conf. Proc. 2017, 1896, 140008. [Google Scholar] [CrossRef]
- Bochniak, W.; Ostachowski, P.; Korbel, A.; Łagoda, M. Potential of the KOBO Extrusion Process for Nonferrous Metals in the Form of Solids and Chips. Int. J. Adv. Manuf. Technol. 2023, 127, 733–750. [Google Scholar] [CrossRef]
- Suzuki, K.; Huang, X.S.; Watazu, A.; Shigematsu, I.; Saito, N. Recycling of 6061 Aluminum Alloy Cutting Chips Using Hot Extrusion and Hot Rolling. Mater. Sci. Forum 2007, 544–545, 443–446. [Google Scholar] [CrossRef]
- Chiba, R.; Nakamura, T.; Kuroda, M. Solid-State Recycling of Aluminium Alloy Swarf through Cold Profile Extrusion and Cold Rolling. J. Mater. Process. Technol. 2011, 211, 1878–1887. [Google Scholar] [CrossRef]
- Allwood, J.M.; Huang, Y.; Barlow, C.Y. Recycling Scrap Aluminium by Cold-Bonding. In Proceedings of the 8th International Conference on Technology Plasticity, Verona, Italy, 9–13 October 2005. [Google Scholar]
- Kore, A.S.; Nayak, K.C.; Date, P.P. Formability of Aluminium Sheets Manufactured by Solid State Recycling. J. Phys. Conf. Ser. 2017, 896, 012007. [Google Scholar] [CrossRef]
- El Mehtedi, M.; Buonadonna, P.; Carta, M.; El Mohtadi, R.; Mele, A.; Morea, D. Sustainability Study of a New Solid-State Aluminum Chips Recycling Process: A Life Cycle Assessment Approach. Sustainability 2023, 15, 11434. [Google Scholar] [CrossRef]
- Carta, M.; Ben Khalifa, N.; Buonadonna, P.; Mele, A.; El Mehtedi, M. Life Cycle Assessment (LCA) of a Novel Solid-State Recycling Process for Aluminum Alloy AA6063 Chips via Direct Hot Rolling. In Proceedings of the Material Forming—ESAFORM 2024, Toulouse, France, 25 May 2024; pp. 2881–2890. [Google Scholar]
- El Mehtedi, M.; Buonadonna, P.; El Mohtadi, R.; Loi, G.; Aymerich, F.; Ben Khalifa, N.; Carta, M. Feasibility Study of Solid-State Recycling through Direct Hot Rolling of AA5754 Aluminum Chips for Automotive Applications. Mater. Sci. Forum 2024, 1130, 3–12. [Google Scholar] [CrossRef]
- De Caro, D.; Tedesco, M.M.; Pujante, J.; Bongiovanni, A.; Sbrega, G.; Baricco, M.; Rizzi, P. Effect of Recycling on the Mechanical Properties of 6000 Series Aluminum-Alloy Sheet. Materials 2023, 16, 6778. [Google Scholar] [CrossRef]
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2024.
- BS EN 895:1997; British Standards Institution: London, UK, 1997.
- Hielscher, R.; Schaeben, H.; Siemes, H. Orientation Distribution Within a Single Hematite Crystal. Math. Geosci. 2010, 42, 359–375. [Google Scholar] [CrossRef]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX—Free and Open Source Software Toolbox. Solid State Phenom. 2010, 160, 63–68. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, J.; Xia, T.; Xie, Y.; Chan, S.L.I.; Wang, J.; Zhang, D. Effects of Oxide Fragments on Microstructure and Mechanical Properties of AA6061 Aluminum Alloy Tube Fabricated by Thermomechanical Consolidation of Machining Chips. Materials 2023, 16, 1384. [Google Scholar] [CrossRef]
- Kuijpers, N.C.W.; Vermolen, F.J.; Vuik, C.; Koenis, P.T.G.; Nilsen, K.E.; Zwaag, S.V.D. The Dependence of the β-AlFeSi to α-Al(FeMn)Si Transformation Kinetics in Al–Mg–Si Alloys on the Alloying Elements. Mater. Sci. Eng. A 2005, 394, 9–19. [Google Scholar] [CrossRef]
- Que, Z.; Mendis, C.L. Heterogeneous Nucleation and Phase Transformation of Fe-Rich Intermetallic Compounds in Al–Mg–Si Alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]
- Alvarez-Antolin, F.; Asensio-Lozano, J.; Cofiño-Villar, A.; Gonzalez-Pociño, A. Analysis of Different Solution Treatments in the Transformation of β-AlFeSi Particles into α-(FeMn)Si and Their Influence on Different Ageing Treatments in Al–Mg–Si Alloys. Metals 2020, 10, 620. [Google Scholar] [CrossRef]
- Laurent-Brocq, M.; Lilensten, L.; Pinot, C.; Schulze, A.; Duchaussoy, A.; Bourgon, J.; Leroy, E.; Tekkaya, A.E. Solid State Recycling of Aluminium Chips: Multi-Technique Characterization and Analysis of Oxidation. Materialia 2023, 31, 101864. [Google Scholar] [CrossRef]






| Element | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.585 | 0.231 | 0.027 | 0.030 | 0.489 | 0.007 | 0.007 | 0.033 | 0.018 | Bal. |
| Number of Passes | h0 | h1 | h2 | h3 | h4 | h5 | h6 | h7 | h8 |
|---|---|---|---|---|---|---|---|---|---|
| Chip billet [mm] | 10.5 | 9 | 6 | 4.5 | 3.3 | 2.5 | 2 | 1.5 | 1.3/1.2/1.1/1.0 |
| Bulk billet [mm] | 10.5 | 9 | 6 | 4.5 | 3.3 | 2.5 | 2 | 1.5 | 1.3/1.2/1.1/1.0 |
| Material | Tensile Properties | HR 1.0 | CR 1.3 | CR 1.2 | CR 1.1 | CR 1.0 | As Received (O) |
|---|---|---|---|---|---|---|---|
| Bulk | UTS [MPa] | 182.3 ± 3.7 | 175.0 ± 1.2 | 185.4 ± 1.6 | 221.1 ± 6.0 | 219.1 ± 1.7 | 186 |
| A% | 8.7 ± 1.6 | 8.1 ± 2.0 | 8.0 ± 1.5 | 7.5 ± 1.8 | 7.5 ± 2.0 | 22 | |
| Chips | UTS [MPa] | 154.9 ± 14.2 | 138.4 ± 26.0 | 145.5 ± 8.2 | 131.3 ± 13.4 | 177.4 ± 7.1 | - |
| A% | 4.7 ± 3.3 | 4.7 ± 3.2 | 3.0 ± 2.7 | 4.7 ± 2.7 | 7.0 ± 3.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carta, M.; Ben Khalifa, N.; Buonadonna, P.; El Mohtadi, R.; Bertolino, F.; El Mehtedi, M. Innovative Solid-State Recycling of Aluminum Alloy AA6063 Chips Through Direct Hot Rolling Process. Metals 2024, 14, 1442. https://doi.org/10.3390/met14121442
Carta M, Ben Khalifa N, Buonadonna P, El Mohtadi R, Bertolino F, El Mehtedi M. Innovative Solid-State Recycling of Aluminum Alloy AA6063 Chips Through Direct Hot Rolling Process. Metals. 2024; 14(12):1442. https://doi.org/10.3390/met14121442
Chicago/Turabian StyleCarta, Mauro, Noomane Ben Khalifa, Pasquale Buonadonna, Rayane El Mohtadi, Filippo Bertolino, and Mohamad El Mehtedi. 2024. "Innovative Solid-State Recycling of Aluminum Alloy AA6063 Chips Through Direct Hot Rolling Process" Metals 14, no. 12: 1442. https://doi.org/10.3390/met14121442
APA StyleCarta, M., Ben Khalifa, N., Buonadonna, P., El Mohtadi, R., Bertolino, F., & El Mehtedi, M. (2024). Innovative Solid-State Recycling of Aluminum Alloy AA6063 Chips Through Direct Hot Rolling Process. Metals, 14(12), 1442. https://doi.org/10.3390/met14121442







