Extraction of Palladium from Spent Nuclear Fuel Reprocessing Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. General Remarks
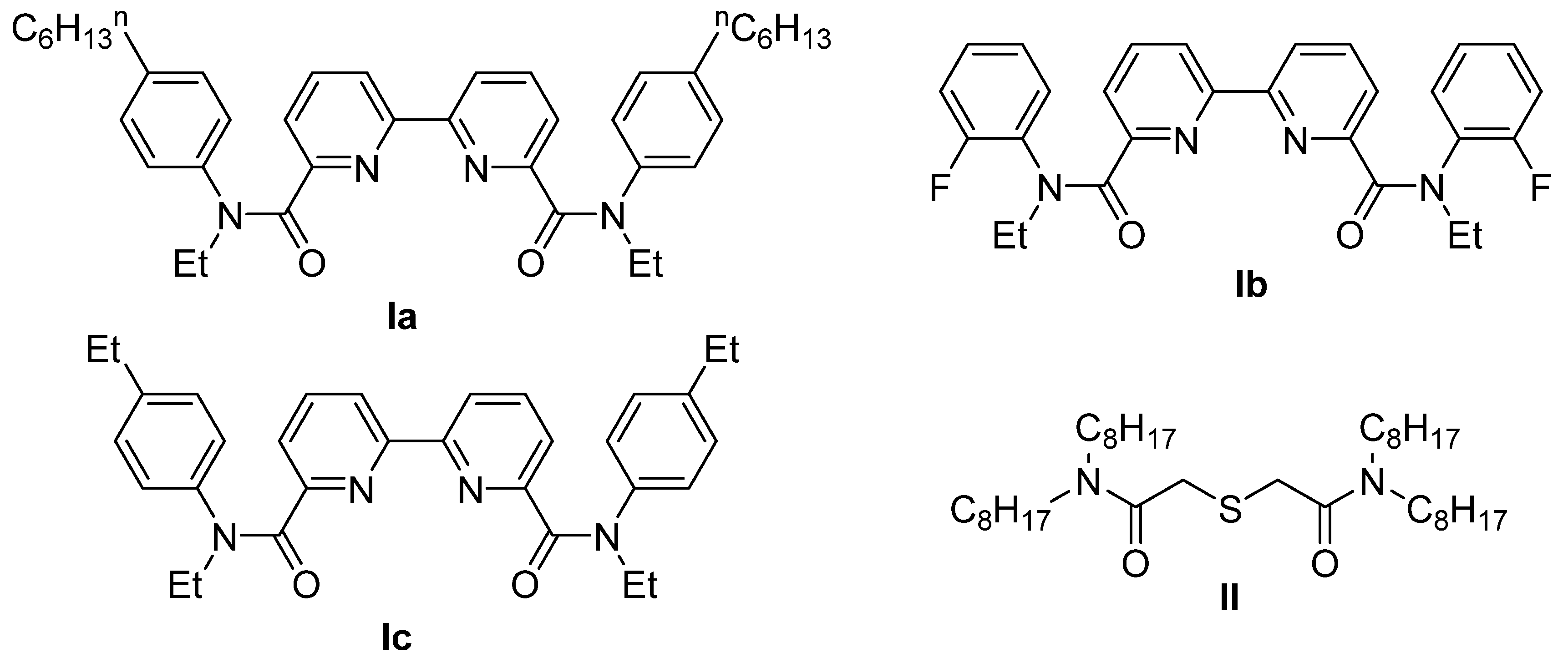
2.2. Synthesis of Amide II
2.2.1. Synthesis of Thiodiglycolic Acid Dichloride
2.2.2. Synthesis of N,N,N′,N′-Tetraoctylthiodiglycolamide
2.3. Synthesis of Silver Complex with Amide Ic
2.4. Measurement of Extraction Coefficients
3. Results and Discussion
3.1. Extraction of Noble Metals from Industrial Waste Solution
3.2. Formation of Silver Complex with 2,2′-bipyridyl-6,6′-dicarboxamide
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Atomic Energy Agency. Feasibility of Separation and Utilization of Ruthenium, Rhodium and Palladium from High Level Wastes; International Atomic Energy Agency: Vienna, Austria, 1989. [Google Scholar]
- Ruhela, R.; Singh, A.K.; Tomar, B.S.; Hubli, R.C. Separation of Palladium from High Level Liquid Waste—A Review. RSC Adv. 2014, 4, 24344–24350. [Google Scholar] [CrossRef]
- Pokhitonov, Y.A.; Tananaev, I.G. Prospects for the Use of Palladium from NPP Spent Nuclear Fuel and Ways to Design the Technology of Its Recovery at a Radiochemical Enterprise. Radiochemistry 2022, 64, 270–280. [Google Scholar] [CrossRef]
- Pokhitonov, Y.A.; Romanovskii, V.N. Palladium in Irradiated Fuel. Are There Any Prospects for Recovery and Application? Radiochemistry 2005, 47, 1–14. [Google Scholar] [CrossRef]
- Gall, N.R.; Gall, L.N.; Berdnikov, A.S.; Semenov, A.A.; Lizunov, A.V.; Safiulina, A.M. Prospects for the Electromagnetic Method of Isotope Separation and Possible Ways of Its Modernization. Issues Nucl. Sci. Technol. 2019, 97, 65–77. [Google Scholar]
- Ruhela, R.; Sharma, J.N.; Tomar, B.S.; Panja, S.; Tripathi, S.C.; Hubli, R.C.; Suri, A.K. N,N,N′,N′-Tetra(2-Ethylhexyl) Thiodiglycolamide T(2EH)TDGA: A Novel Ligand for the Extraction of Palladium from High Level Liquid Waste (HLLW). Radiochim. Acta 2010, 98, 209–214. [Google Scholar] [CrossRef]
- Mezhov, E.A.; Kulikov, I.A.; Teterin, E.G. Study of Extraction of Palladium from Nitric Acid Solutions with Nitrogen-Containing Compounds, as Applied to Recovery of Fission Palladium from Spent Nuclear Fuel of Nuclear Power Plants: 2. Effect of Radioation on Palladium Recovery and Condition of Extraction Systems. Radiochemistry 2002, 44, 141–145. [Google Scholar] [CrossRef]
- Mezhov, E.A.; Druzhenkov, V.V.; Sirotinin, A.N. Study of Extraction of Palladium from Nitric Acid Solutions with Nitrogen-Containing Compounds, as Applied to Recovery of Fission Palladium from Spent Nuclear Fuel of Nuclear Power Plants: 3. Optimization of Extraction Process for Palladium Recovery and Refining. Radiochemistry 2002, 44, 146–150. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, R.; Wei, Y.; Arai, T.; Fujita, T. Electrochemical Recycling of Pd and Ag from Simulated High-Level Liquid Waste. Trans. Nonferrous Met. Soc. China 2022, 32, 1031–1040. [Google Scholar] [CrossRef]
- Dakshinamoorthy, A.; Dhami, P.S.; Naik, P.W.; Dudwadkar, N.L.; Munshi, S.K.; Dey, P.K.; Venugopal, V. Separation of Palladium from High Level Liquid Waste of PUREX Origin by Solvent Extraction and Precipitation Methods Using Oximes. Desalination 2008, 232, 26–36. [Google Scholar] [CrossRef]
- Weng, H.; Wang, Y.; Li, F.; Muroya, Y.; Yamashita, S.; Cheng, S. Recovery of Platinum Group Metal Resources from High-Level Radioactive Liquid Wastes by Non-Contact Photoreduction. J. Hazard. Mater. 2023, 458, 131852. [Google Scholar] [CrossRef]
- Rizvi, G.H.; Mathur, J.N.; Murali, M.S.; Iyer, R.H. Recovery of Fission Product Palladium from Acidic High Level Waste Solutions. Sep. Sci. Technol. 1996, 31, 1805–1816. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, H. Ion Exchange Characteristics of Palladium and Rhodium from a Simulated Radioactive Liquid Waste. J. Nucl. Sci. Technol. 2000, 37, 281–287. [Google Scholar] [CrossRef]
- Parajuli, D.; Hirota, K.; Seko, N. Effective Separation of Palladium from Simulated High Level Radioactive Waste. J. Radioanal. Nucl. Chem. 2011, 288, 53–58. [Google Scholar] [CrossRef]
- Asai, S.; Ohata, M.; Yomogida, T.; Saeki, M.; Ohba, H.; Hanzawa, Y.; Horita, T.; Kitatsuji, Y. Determination of 107 Pd in Pd Purified by Selective Precipitation from Spent Nuclear Fuel by Laser Ablation ICP-MS. Anal. Bioanal. Chem. 2019, 411, 973–983. [Google Scholar] [CrossRef]
- Courson, O.; Lebrun, M.; Malmbeck, R.; Pagliosa, G.; Römer, K.; Sätmark, B.; Glatz, J.P. Partitioning of Minor Actinides from HLLW Using the DIAMEX Process. Part 1—Demonstration of Extraction Performances and Hydraulic Behaviour of the Solvent in a Continuous Process. Radiochim. Acta 2000, 88, 857. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Sasaki, Y.; Suzuki, H.; Suzuki, S.; Kimura, T. Cumulative Study on Solvent Extraction of Elements by N,N,N′, N′-Tetraoctyl-3-Oxapentanediamide (TODGA) from Nitric Acid into n-Dodecane. Anal. Chim. Acta 2004, 527, 163–168. [Google Scholar] [CrossRef]
- Sasaki, Y.; Morita, Y.; Kitatsuji, Y.; Kimura, T. Extraction Behavior of Actinides and Metal Ions by the Promising Extractant, N,N,N′,N′-Tetraoctyl-3,6-Dioxaoctanediamide (DOODA). Solvent Extr. Ion Exch. 2010, 28, 335–349. [Google Scholar] [CrossRef]
- Atanassova, M. Solvent Extraction Chemistry in Ionic Liquids: An Overview of f-Ions. J. Mol. Liq. 2021, 343, 117530. [Google Scholar] [CrossRef]
- Wongsawa, T.; Traiwongsa, N.; Pancharoen, U.; Nootong, K. A Review of the Recovery of Precious Metals Using Ionic Liquid Extractants in Hydrometallurgical Processes. Hydrometallurgy 2020, 198, 105488. [Google Scholar] [CrossRef]
- Ilyas, S.; Kim, H.; Srivastava, R.R. Separation of Platinum Group Metals from Model Chloride Solution Using Phosphonium-Based Ionic Liquid. Sep. Purif. Technol. 2021, 278, 119577. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Yoshida, W.; Hanada, T.; Goto, M. Application of Ionic Liquids in Solvent Extraction of Platinum Group Metals. Solvent Extr. Res. Dev. Japan 2020, 27, 1–24. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Matveev, P.I.; Smirnova, A.A.; Belova, E.V.; Kalmykov, S.N.; Myasoedov, B.F. Screening of the Structure of Americium Extractants Based on a 2,2′-Bipyridyl Scaffold: A Simple Way to a N2,O2-Tetradentate Ligands Library for Rational Design of An/Ln Extractants. ChemistrySelect 2018, 3, 1983–1989. [Google Scholar] [CrossRef]
- Logunov, M.V.; Voroshilov, Y.A.; Babain, V.A.; Skobtsov, A.S. Experience of Mastering, Industrial Exploitation, and Optimization of the Integrated Extraction–Precipitation Technology for Fractionation of Liquid High-Activity Wastes at Mayak Production Association. Radiochemistry 2020, 62, 463. [Google Scholar] [CrossRef]
- Logunov, M.V.; Voroshilov, Y.A.; Babain, V.A. Development of HLW Partitioning Technologies in Extraction Systems Based on Different Radical Phosphine Oxide in Heavy Diluents at “PA Mayak”. Radiochemistry 2022, 64, 581–602. [Google Scholar] [CrossRef]

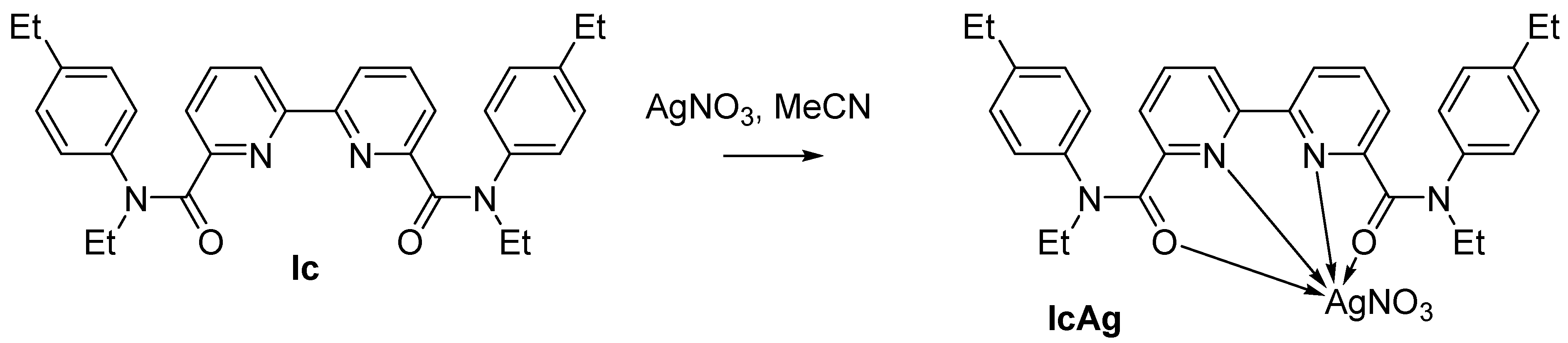
| Metal | Pd a | Ru a | Rh a | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 12.21 | 4.82 | 7.50 | 3.50 | 13.25 | 34.0 | 39.50 | 0.26 | 88.50 | 4.10 | 97.5 |
| Diluents | Degrees of Extraction, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr | |
| PhNO2 | 6.8 | 9.0 | 0 | 8.6 | 3.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| CHCl3 | 2.4 | 2.7 | 0 | 21.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 vol.% TBP in isopar M | 26.6 | 17.3 | 0 | 0 | 7.5 | 8.8 | 1.3 | 7.6 | 12.4 | 7.3 | 30.8 |
| 0.04 M TOMAH in isopar M | 5.2 | 4.8 | 0 | 0 | 7.5 | 0 | 0 | 2.5 | 4 | 0 | 2.1 |
| 0.04 M [BMIM]Tf2N in PhNO2 | 37.2 | 7.5 | 0 | 12.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Solvents | Degrees of Extraction, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr | |
| [BMIM]Tf2N in PhNO2 | 0 | 7.8 | 0 | 0 | 3.8 | 1.5 | 2.5 | 2.9 | 1.1 | 2.4 | 2.6 |
| [BMIM]Tf2N in CHCl3 | 0 | 3.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| [BMIM]Tf2N | 0.04 | 11.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diluents | Degrees of Extraction, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr | |
| PhNO2 | 99.9 | 2.5 | 0 | 99.9 | 7.5 | 4.4 | 0 | 3.6 | 6.8 | 2.4 | 6.2 |
| CHCl3 | 99.9 | 11.2 | 0 | 99.9 | 1.9 | 13.2 | 0 | 1.3 | 5.6 | 0 | 5.1 |
| Diluent | SFPd/M | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pd/Ru | Pd/Rh | Pd/Ag | Pd/Cs | Pd/Ce | Pd/Cr | Pd/Fe | Pd/Mo | Pd/Sr | Pd/Zr | |
| PhNO2 | 4.6 × 104 | - | 17 | 1.4 × 104 | 2.5 × 104 | - | 3.1 × 104 | 1.6 × 104 | 4.7 × 104 | 1.7 × 104 |
| CHCl3 | 7.7 × 103 | - | 14 | 5.1 × 104 | 6.4 × 103 | - | 7.2 × 104 | 1.6 × 104 | - | 1.8 × 104 |
| Pd, g/L | Ru, g/L | Rh, mg/L | Ag, mg/L | Cs, mg/L | Ce, mg/L | Cr, mg/L | Fe, mg/L | Mo, mg/L | Sr, mg/L | Zr, mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| 12.2 | 0.12 | >DL | 3.5 | >DL | 1.5 | >DL | 9.5 | 6.0 | 0.1 | 6.0 |
| The composition of the aqueous solution obtained via back-extraction | ||||||||||
| g/L | mg/L | |||||||||
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr |
| 11.95 | 0.11 | 0 | 2.5 | 0 | 0.25 | 0 | 3.25 | 4.0 | 0.1 | 3.35 |
| Degree of extraction during stripping, % | ||||||||||
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr |
| 97.9 | 93.7 | - | 71.4 | - | 16.7 | - | 34.2 | 66.7 | 100 | 55.8 |
| Solutions | Element Composition of the Solution | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g/L | mg/L | |||||||||||
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr | Total Impurities | |
| Starting | 12.21 | 4.82 | 7.50 | 3.5 | 13.25 | 34 | 39.5 | 262 | 88.5 | 4.1 | 97.5 | 12.86 g/L |
| Stripping | 11.95 | 1.11 | >DL | 2.5 | >DL | 0.25 | >DL | 3.25 | 4.0 | 0.10 | 3.35 | 125 mg/L |
| Diamide | Degrees of Extraction, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pd | Ru | Rh | Ag | Cs | Ce | Cr | Fe | Mo | Sr | Zr | |
| Ia | 99.9 | 42.5 | 0 | 98.5 | 7.5 | 5.6 | 0 | 0 | 0 | 0 | 9.8 |
| Ib | 99.9 | 51.4 | 0 | 98.5 | 7.5 | 0 | 0 | 0 | 0 | 0 | 15.7 |
| II | 99.7 | 21.6 | 0 | 0 | 0 | 0 | 0 | 0 | 88.3 | 0 | 93.9 |
| Diamide | SF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pd/Ru | Pd/Rh | Pd/Ag | Pd/Cs | Pd/Ce | Pd/Cr | Pd/Fe | Pd/Mo | Pd/Sr | Pd/Zr | |
| Ia | 1.8 × 103 | -a | 19 | 1.6 × 104 | 2.2 × 104 | - | - | - | - | 1.2 × 104 |
| Ib | 1.3 × 103 | - | 20 | 1.7 × 104 | - | - | - | - | - | 7.3 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safiulina, A.M.; Borisova, N.E.; Karpyuk, E.A.; Ivanov, A.V.; Lopatin, D.A. Extraction of Palladium from Spent Nuclear Fuel Reprocessing Solutions. Metals 2024, 14, 133. https://doi.org/10.3390/met14020133
Safiulina AM, Borisova NE, Karpyuk EA, Ivanov AV, Lopatin DA. Extraction of Palladium from Spent Nuclear Fuel Reprocessing Solutions. Metals. 2024; 14(2):133. https://doi.org/10.3390/met14020133
Chicago/Turabian StyleSafiulina, Alfiya M., Nataliya E. Borisova, Ekaterina A. Karpyuk, Alexey V. Ivanov, and Dmitry A. Lopatin. 2024. "Extraction of Palladium from Spent Nuclear Fuel Reprocessing Solutions" Metals 14, no. 2: 133. https://doi.org/10.3390/met14020133
APA StyleSafiulina, A. M., Borisova, N. E., Karpyuk, E. A., Ivanov, A. V., & Lopatin, D. A. (2024). Extraction of Palladium from Spent Nuclear Fuel Reprocessing Solutions. Metals, 14(2), 133. https://doi.org/10.3390/met14020133






